Abstract
Background
Little is known about the prevalence of undiagnosed cognitive impairment and its impact on instrumental activities of daily living (IADL) among people with HIV (PWH) in primary care.
Methods
PWH were recruited from an integrated health care setting in the United States. PWH were eligible for recruitment if they were ≥50 years old, taking antiretroviral therapy (ie, ≥1 antiretroviral therapy [ART] prescription fill in the past year), and had no clinical diagnosis of dementia. Participants completed a cognitive screen (St. Louis University Mental Status exam) and a questionnaire on IADL (modified Lawton-Brody).
Results
Study participants (n = 47) were mostly male (85.1%), 51.1% White, 25.5% Black, 17.0% Hispanic, and the average age (SD) was 59.7 (7.0) years. Overall, 27 (57.5%) participants were categorized as cognitively normal, 17 (36.2%) as having mild cognitive impairment, and 3 (6.4%) as having possible dementia. Of the 20 participants with mild cognitive impairment or possible dementia, 85.0% were men, the average age (SD) was 60.4 (7.1) years; 45.0% were White, 40.0% were Black, 10.0% were Hispanic, and 30.0% reported difficulty with at least 1 IADL. Most (66.7%) attributed difficulty with IADL primarily (33.3%) or in part (33.3%) to cognitive problems.
Conclusions
Undiagnosed cognitive impairment is frequent among ART-treated PWH, with possible elevated risk among Black PWH, and may be accompanied by difficulty with IADL. Efforts are needed to optimize identification of factors contributing to cognitive and IADL difficulties among ART-treated PWH in primary care.
Keywords: activities of daily living, aging, cognitive impairment, dementia, everyday functioning, function
Cognitive impairment is common in people with HIV infection (PWH) and may adversely affect everyday activities [1]. Data from observational research cohorts estimate that 30%–50% of PWH have some degree of cognitive impairment, but little is known about the prevalence of cognitive and functional impairments among PWH engaged in primary care and taking antiretroviral therapy (ART) [2–8]. Recent electronic health record (EHR)–based studies in primary care settings report elevated risk of diagnosed dementia among PWH [9–11]. However, EHR-based analyses typically underestimate the prevalence of cognitive impairment and dementia as providers do not routinely assess cognition, and patients may not recognize or report cognitive problems until they substantially impact everyday life [12, 13]. Among PWH, cognitive issues may not be prioritized given other important clinical goals such as achieving HIV suppression and limited time to address multiple concerns during clinic encounters. To better understand cognitive impairment among ART-treated PWH in primary care and complement recent retrospective EHR-based analyses of diagnosed dementia [9, 10], we conducted a cross-sectional study to estimate the prevalence of undiagnosed cognitive impairment and associated difficulties with instrumental activities of daily living (IADL) in a diverse sample of PWH.
METHODS
We recruited a sample of PWH at Kaiser Permanente Northern California (KPNC), an integrated health care system in the United States; participants were recruited between March 2020 and June 2022 (with periodic suspension due to the coronavirus disease 2019 [COVID-19] pandemic). Inclusion criteria mirrored those from prior EHR-based studies in the same setting [9, 10]. PWH were eligible for recruitment if they were ≥50 years old, taking ART (defined as ≥1 ART prescription fill in the past year), and had no prior clinical diagnosis of dementia recorded in the EHR. PWH recruited for this study were simultaneously being recruited for another study that aimed to evaluate substance use and mental health (PIs: Silverberg/Satre, U01AA026230), but substance use and mental health status were not inclusion criteria for either study. Target enrollment was 50 participants. Study participation consisted of a brief cognitive screen, a questionnaire about IADL, and a questionnaire about depressive symptoms. The study protocol was approved by the KPNC Institutional Review Board. Study participants were provided with a study information form before agreeing to participate, and a waiver of documented informed consent was received as no identifying information was collected with study questionnaires.
Cognition
Cognitive status was assessed using the St. Louis University Mental Status (SLUMS) exam, which was administered in-person by trained staff [14]. The SLUMS exam was chosen for this study given its ease of administration and scoring based on level of education [15, 16]. Cognitive status was scored numerically from 1 to 30 and categorized as normal, mild cognitive impairment, or possible dementia, with thresholds differing by high school completion status (yes/no) [14]. Prior clinical diagnosis of mild cognitive impairment was ascertained from the EHR.
IADL
Difficulty with IADL was assessed via a self-administered questionnaire. IADL included housekeeping, managing finances, buying groceries, cooking, transportation, using the telephone, doing laundry, managing medications, and maintaining performance at work. The questionnaire was based on the Lawton-Brody IADL Scale [17]. As we expected study participants to be relatively young and not yet retired (although all at least 50 years old), the questionnaire was modified to include a question about ability to maintain attention and finish tasks at work, as done in other HIV cohorts [18]. Participants were also asked whether difficulty with IADL was primarily due to cognitive problems, physical problems, or both.
Depressive Symptoms
Depressive symptoms were assessed via a self-administered Patient Health Questionnaire (PHQ-2) given its accuracy, efficiency, and utility in screening for depression in HIV clinics [20, 21]. The PHQ-2 consists of the first 2 items of the PHQ-9: (1) feeling down, depressed, or hopeless and (2) little interest or pleasure in doing things [19]. Depression was defined as a score of 3 or higher, consistent with prior studies [20–22].
Analyses
Descriptive analyses were conducted examining cognition, IADL (presence and attribution), and depressive symptoms in the overall sample and among participants with any cognitive impairment (ie, mild cognitive impairment or possible dementia according to the SLUMS exam). The likelihood of cognitive and IADL impairments was compared within sex and race/ethnicity categories using χ2 tests. Fisher exact tests were used when cell sizes were <10. Analyses were conducted in Stata 18 (StataCorp, College Station, TX, USA).
RESULTS
In total, 59 eligible individuals were approached for participation in the study, and 47 agreed to participate. Participants were mostly male (85.1%), the average age (SD) was 59.7 (7.0) years, 97.9% reported completing high school, and 78.7% were not yet retired. Participants were 51.1% White, 25.5% Black, 17.0% Hispanic, 4.3% Asian or other race, and 2.1% unknown race. Individuals who declined to participate were slightly older (63.5 years) but comparable in terms of sex (83.3% male), education level (91.7% completed high school), and race/ethnicity (58.3% White, 25% Black, and 16.7% Hispanic). Overall, 27 (57.5%) participants were categorized as cognitively normal, 17 (36.2%) as having mild cognitive impairment, and 3 (6.4%) as having possible dementia. None of these participants had a prior diagnosis of mild cognitive impairment in the EHR.
Of the 20 participants with any cognitive impairment (ie, mild cognitive impairment or possible dementia), 85.0% were men; the mean age (SD) was 60.4 (7.1) years; 45.0% were White, 40.0% were Black, and 10.0% were Hispanic; 80.0% were not yet retired; and 30.0% reported difficulty with at least 1 IADL. Though a large proportion of participants with cognitive impairment were men (reflecting the overrepresentation of men in the sample), the likelihood of cognitive impairment among men was not different than among women (relative risk [RR], 1.00 and 1.01, respectively; P = .99). The likelihood of having cognitive impairment was elevated among Black participants (with vs without cognitive impairment; RR, 2.70; P = .09), but not among White participants (RR, 0.81; P = .56), Hispanic participants (RR, 0.45; P = .44), or participants of Asian or other race (RR, 0.68; P = 1.0).
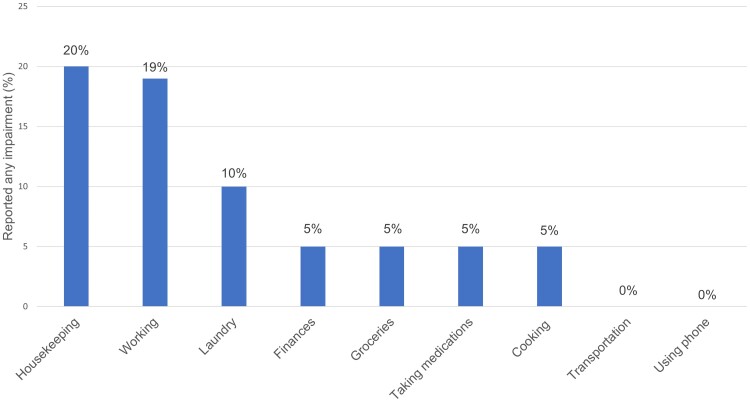
Among participants with any cognitive impairment (n = 20), difficulty with IADL was reported for housekeeping (20.0%), working (18.8%), doing laundry (10.0%), managing finances (5%), doing groceries (5%), managing medications (5%), and cooking (5%) (Figure 1). None of these participants reported difficulty with transportation or using their phone. Most (66.7%) reported that difficulty with IADL was due primarily (33.3%) or in part (33.3%) to cognitive problems. All 3 of the women with cognitive impairment reported difficulty with IADL, compared with 17.6% (3 out of 17) of the men with cognitive impairment. Black participants with cognitive impairment were more likely to report difficulty with IADL (62.5%) than those who were White (11.1%). None of the participants who were Hispanic, Asian, or other race/ethnicity and who had cognitive impairment reported difficulty with IADL.
Figure 1.
Self-reported difficulty with IADL among PWH with any cognitive impairment (n = 20). Calculation of difficulty at work excluded 4 participants who were retired. Abbreviations: IADL, instrumental activities of daily living; PWH, people with HIV.
Difficulty with IADL was also reported by 9 PWH who were categorized as having normal cognition. Like participants with cognitive impairment, most (66.7%) attributed difficulty with IADL primarily (55.6%) or in part (11.1%) to cognitive problems. Possible depression was identified in 3 (15.8%) participants with any cognitive impairment, in 3 (20.0%) participants who reported any difficulty with IADL, and in 2 participants (33.3%) with both cognitive impairment and difficulty with IADL.
DISCUSSION
In a sample of ART-treated PWH who had no prior clinical diagnosis of mild cognitive impairment or dementia, cognitive impairment was common (42.6%). Undiagnosed cognitive impairment was more than twice as likely among Black PWH and was frequently accompanied by self-reported difficulty with IADL (62.5%). Our data also suggest that women with cognitive impairment may be more likely to have difficulty with IADL than men with cognitive impairment, a finding that should be explored in larger studies. Overall, most participants (66.7%) who had difficulty with IADL reported that the difficulties were attributable at least in part to cognitive problems. PWH with cognitive impairment were on average 60 years old. These findings add to recent EHR-based analyses from this same setting that found that PWH were diagnosed with age-associated dementia on average 10 years earlier than their HIV-uninfected peers and remained at 58% elevated risk of dementia after adjustment for confounding sociodemographic and clinical factors [9, 10].
This study contributes knowledge on undiagnosed cognitive impairment and its potential impact on everyday functioning among a diverse sample of ART-treated PWH in a US health care system. It extends prior studies reporting high frequency of cognitive impairment among PWH conducted in observational research cohorts or among PWH with advanced HIV disease, immunodeficiency, or taking older ART regimens with possible suboptimal HIV suppression [4, 23–28]. It also adds to the literature on cognitive impairment among PWH taking modern ART regimens, including a study of cognitive impairment in 290 PWH age ≥50 years (80% of whom were on ART) participating in a clinical trial of HIV and aging, which also reported high prevalence of cognitive impairment (34.5% using the global deficit score and 30.0% using Frascati criteria) but only included Black and White participants in the United Kingdom and Ireland [29].
The high prevalence of cognitive impairment in our study population (42.6%) is consistent with prior literature reporting that many PWH experience cognitive issues, occurring as early as middle age and despite well-controlled HIV infection [2–7]. Although our study population was restricted to PWH with evidence of ART dispensation in the EHR, we were not able to measure HIV RNA or CD4+ T-cell levels on the day of cognitive and IADL screening. Therefore, participants who had cognitive impairment may have been those who were nonadherent to ART at the time of assessment. Regarding comparability of our study to others, we note several important considerations. First, cognitive assessment was done using a brief cognitive screen used in clinical practice to identify individuals who may require further evaluation. Cognitive status assigned therefore does not correspond to the subtypes of HIV-associated neurocognitive disorders (HANDs) diagnosed in research settings following full neuropsychological and functional assessment [30]. Second, comparability of cognitive impairment prevalence across studies is generally limited as there is no widely used and validated cognitive screening tool for ART-treated PWH, though this is an active area of research [31–34]. Previously validated brief cognitive tests for PWH were designed for detection of more severe cognitive deficits and are no longer suitable for use in ART-treated PWH [34, 35]. The SLUMS exam was normed in a general geriatric population and has not been validated in PWH, so it may be less sensitive to cognitive impairments among our relatively younger and relatively highly educated (ie, 97.9% high school education or greater) HIV-infected study population, resulting in underestimation of cognitive impairment. However, prior studies indicate that the SLUMS exam is superior in detecting dementia in its early stages compared with other brief cognitive assessments such as the Mini-Mental State Examination (MMSE) [15, 16]. Lastly, comparability to other studies may be limited since we excluded PWH previously diagnosed with dementia and/or not taking ART.
Most PWH attributed difficulty with IADL to cognitive problems, consistent with other studies reporting that severity of cognitive impairment positively correlates with objective measures of functional impairment [23, 24, 36, 37]. Some people with normal cognition reported difficulty with IADL, and most of them attributed it to cognitive problems. This may be because people who were less cognitively impaired were more aware of having difficulty with everyday activities. Another explanation is that the SLUMS exam was insensitive to cognitive deficits among PWH, thus misclassifying some PWH as cognitively normal [29]. Use of a subjective IADL instrument based on self-report instead of an objective assessment may also have contributed to potential misclassification of functional status. Few PWH screened positive on the PHQ-2. Therefore, comorbid depression at the time of cognitive and IADL assessment was unlikely to have substantially affected participants’ responses.
As PWH are living to older ages on ART, cognitive impairment and age-associated dementia are important emerging health concerns [38–41]. Cognitive impairment has significant clinical implications because it is associated with poor retention in HIV care, reduced ART adherence, lower quality of life, and increased all-cause mortality, all of which threaten the significant advances in HIV health and survival made in recent decades [42, 43]. Treatment failure among cognitively impaired PWH could also jeopardize progress in the “Ending the HIV Epidemic” Initiative, an effort led by the US Department of Health and Human Services to end the persistent national HIV epidemic by 2030 [44]. By identifying IADL difficulty among older PWH with undiagnosed cognitive impairment and potential disparities by race/ethnicity and sex, this study contributes to ongoing discussions regarding the value and utility of clinical cognitive screening in this population [27, 34, 45]. Brief cognitive and IADL screenings could potentially be used to proactively identify PWH requiring more focused care including intervention on contributing causes (eg, polypharmacy, substance use), more attention to ART adherence, assistance with managing comorbidities, and referral to support services. Limitations of this study include (1) the small sample size (n = 47), (2) primarily male (85%) and employed (79% not yet retired) study sample, which could limit generalizability, (3) self-report of IADL and depressive symptoms, (4) lack of longitudinal measurement, and (5) lack of data on behavioral and clinical risk factors for cognitive impairment, such as treatable psychiatric conditions or other causes of cognitive impairment including traumatic brain injury or past prolonged loss of consciousness. Due to COVID-19 pandemic–related suspensions in recruitment, the enrollment target of 50 participants was not reached. Strengths of the study include assessment of both cognitive and functional status, capture of cognitive impairment related to work, assessment of depression, and restriction of participation to PWH on ART.
CONCLUSIONS
Undiagnosed cognitive impairment is frequent among ART-treated PWH engaged in primary care, with possible higher risk among Black PWH, and may be accompanied by difficulty with IADL. Cognitive impairment among younger PWH of working age could adversely impact employment, independence, and quality of life. Efforts are needed to optimize identification of factors contributing to cognitive and IADL difficulties among ART-treated PWH and investigate potential racial, ethnic, and/or sex disparities.
Acknowledgments
We thank the study participants. We also thank Dana Morales, Julien Van Den Berg, Tory Levine, and Nicole Hood for assistance with data collection.
Financial support. This work was supported by the Kaiser Permanente Northern California Community Health Program (J.O.L.; no grant number), the National Institute of Allergy and Infectious Diseases (grant number K01AI157849 to J.O.L.), and the National Institute on Alcohol Abuse and Alcoholism (grant number U01AA026230 to M.J.S. and D.D.S.).
Author contributions. J.O.L. conceptualized the study, contributed to study design and data collection, conducted the data analysis, drafted the manuscript, and provided funding support. C.E.H. contributed to study design and data collection and provided critical input on the manuscript. P.G. and C.L. provided critical input on the manuscript. A.N.L. provided administrative support, contributed to data collection, and provided critical input on the manuscript. D.D.S. provided funding support and critical input on the manuscript. M.J.S. contributed to study design and provided funding support and critical input on the manuscript.
Patient consent. This study was approved by the Kaiser Permanente Northern California Institutional Review Board. A waiver of documented informed consent was received for all patients who agreed to participate.
Contributor Information
Jennifer O Lam, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Craig E Hou, South San Francisco Medical Center, Kaiser Permanente Northern California, South San Francisco, California, USA.
Paola Gilsanz, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Catherine Lee, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Alexandra N Lea, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Derek D Satre, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA; Department of Psychiatry and Behavioral Sciences, Weill Institute for Neurosciences, University of California, San Francisco, California, USA.
Michael J Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
References
- 1. Alford K, Vera JH. Cognitive impairment in people living with HIV in the ART era: a review. Br Med Bull 2018; 127:55–68. [DOI] [PubMed] [Google Scholar]
- 2. Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–21. [DOI] [PubMed] [Google Scholar]
- 3. Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cysique LA, Heaton RK, Kamminga J, et al. HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. J Neurovirol 2014; 20:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sacktor N, Skolasky RL, Seaberg E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makinson A, Dubois J, Eymard-Duvernay S, et al. Increased prevalence of neurocognitive impairment in aging people living with human immunodeficiency virus: the ANRS EP58 HAND 55–70 study. Clin Infect Dis 2020; 70:2641–8. [DOI] [PubMed] [Google Scholar]
- 8. Eggers C, Arendt G, Hahn K, et al. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 2017; 264:1715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lam JO, Hou CE, Hojilla JC, et al. Comparison of dementia risk after age 50 between individuals with and without HIV infection. AIDS 2021; 35:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam JO, Lee C, Gilsanz P, et al. Comparison of dementia incidence and prevalence between individuals with and without HIV infection in primary care from 2000 to 2016. AIDS 2022; 36:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bobrow K, Xia F, Hoang T, Valcour V, Yaffe K. HIV and risk of dementia in older veterans. AIDS 2020; 34:1673–9. [DOI] [PubMed] [Google Scholar]
- 12. Alzheimer's Association. Alzheimer's Disease Facts and Figures. Vol. 18. Alzheimer’s Association; 2022. [DOI] [PubMed] [Google Scholar]
- 13. Liss JL, Seleri Assunção S, Cummings J, et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer's disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med 2021; 290:310–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. St. Louis University School of Medicine . St. Louis University Mental Status exam. Available at: https://www.slu.edu/medicine/internal-medicine/geriatric-medicine/aging-successfully/assessment-tools/mental-status-exam.php. Accessed May 3, 2019.
- 15. Szcześniak D, Rymaszewska J. The usefulness of the SLUMS test for diagnosis of mild cognitive impairment and dementia. Psychiatr Pol 2016; 50:457–72. [DOI] [PubMed] [Google Scholar]
- 16. Tariq SH, Tumosa N, Chibnall JT, Perry MH III, Morley JE. Comparison of the Saint Louis University Mental Status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–10. [DOI] [PubMed] [Google Scholar]
- 17. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–86. [PubMed] [Google Scholar]
- 18. D'Souza G, Bhondoekhan F, et al. Characteristics of the MACS/WIHS combined cohort study: opportunities for research on aging with HIV in the longest US observational study of HIV. Am J Epidemiol 2021; 190:1457–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–92. [DOI] [PubMed] [Google Scholar]
- 20. Choi SK, Boyle E, Burchell AN, et al. Validation of six short and ultra-short screening instruments for depression for people living with HIV in Ontario: results from the Ontario HIV Treatment Network Cohort Study. PLoS One 2015; 10:e0142706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staples LG, Dear BF, Gandy M, et al. Psychometric properties and clinical utility of brief measures of depression, anxiety, and general distress: the PHQ-2, GAD-2, and K-6. Gen Hosp Psychiatry 2019; 56:13–8. [DOI] [PubMed] [Google Scholar]
- 22. Parry S, Zetler S, Kentridge A, Petrak J, Barber T. Simple screening for neurocognitive impairment in routine HIV outpatient care: is it deliverable? AIDS Care 2017; 29:1275–9. [DOI] [PubMed] [Google Scholar]
- 23. Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004; 10:317–31. [DOI] [PubMed] [Google Scholar]
- 24. Gandhi NS, Skolasky RL, Peters KB, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol 2011; 17:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heaton RK, Franklin DR Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015; 60:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Justice AC, McGinnis KA, Atkinson JH, et al. Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS 2004; 18(Suppl 1):S49–59. [PubMed] [Google Scholar]
- 27. Ripamonti E, Clerici M. Living with chronic HIV disease in the antiretroviral era: the impact of neurocognitive impairment on everyday life functions. Top Antivir Med 2021; 29:386–96. [PMC free article] [PubMed] [Google Scholar]
- 28. Delle Donne V, Ciccarelli N, Massaroni V, et al. The University of California San Diego performance-based skills assessment: a useful tool to detect mild everyday functioning difficulties in HIV-infected patients with very good immunological condition. J Neurovirol 2020; 26:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Underwood J, De Francesco D, Post FA, et al. Associations between cognitive impairment and patient-reported measures of physical/mental functioning in older people living with HIV. HIV Med 2017; 18:363–9. [DOI] [PubMed] [Google Scholar]
- 30. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis 2011; 53:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Underwood J, De Francesco D, Cole JH, et al. Validation of a novel multivariate method of defining HIV-associated cognitive impairment. Open Forum Infect Dis 2019; 6:ofz198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zipursky AR, Gogolishvili D, Rueda S, et al. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS 2013; 27:2385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan P, Valcour V. Neurocognition and the aging brain in people with HIV: implications for screening. Top Antivir Med 2022; 29:423–9. [PMC free article] [PubMed] [Google Scholar]
- 35. Underwood J, Winston A. Guidelines for evaluation and management of cognitive disorders in HIV-positive individuals. Curr HIV/AIDS Rep 2016; 13:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woods SP, Iudicello JE, Morgan EE, et al. Health-related everyday functioning in the internet age: HIV-associated neurocognitive disorders disrupt online pharmacy and health chart navigation skills. Arch Clin Neuropsychol 2016; 31:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woods SP, Iudicello JE, Morgan EE, Verduzco M, Smith TV, Cushman C. Household everyday functioning in the internet age: online shopping and banking skills are affected in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc 2017; 23:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis 2013; 13:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valcour VG. HIV, aging, and cognition: emerging issues. Top Antivir Med 2013; 21:119–23. [PMC free article] [PubMed] [Google Scholar]
- 40. Wing EJ. HIV and aging. Int J Infect Dis 2016; 53:61–8. [DOI] [PubMed] [Google Scholar]
- 41. Gutierrez J. The persistent disparity in brain health among aging people with HIV. AIDS 2022; 36:475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nweke M, Mshunqane N, Govender N, Akinpelu AO, Ukwuoma M. Impact of HIV-associated cognitive impairment on functional independence, frailty and quality of life in the modern era: a meta-analysis. Sci Rep 2022; 12:6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence 2019; 13:475–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 45. Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013; 159:601–12. [DOI] [PubMed] [Google Scholar]