Abstract
Objective
Candida species are responsible for fungal diseases and the development of nosocomial bloodstream infections. Treatment is resource-intensive and economically challenging for healthcare systems. Cost analyses of drugs against candidiasis, such as rezafungin, are thus of great interest to healthcare payers.
Methods
We conducted a cost-of-illness study of patients with Candida infections based on real-word data of the Department I of Internal Medicine, University Hospital Cologne (Germany) between 2016 and 2021. Health-economic parameters were analysed to describe the economic impact of Candida infections. Potential cost savings due to the administration of rezafungin were modelled for patients with invasive candidiasis or candidaemia based on a 5 day reduction of ICU length of stay (LOS) shown by the STRIVE study.
Results
We found 724 cases (652 patients) with Candida infections, of which 61% received ICU treatment (n = 442) and 29% were mechanically ventilated (n = 207). Twenty-six percent died during hospitalization (n = 185). Median LOS was 25 and 15 days, on normal wards and ICU, respectively. Median total treatment costs per case accounted for €22 820. Based on the ICU LOS reduction, the retrospective model showed a median cost-saving potential of €7175 per hospital case with invasive candidiasis or candidaemia. Accumulated cost savings for 37 patients of €283 335 were found.
Conclusions
Treatment of candidiasis is cost intensive due to increased hospital LOS. The ICU LOS reduction rezafungin showed in STRIVE would lead to sustainable cost savings.
Introduction
Candida spp. are the leading fungal pathogens.1Candida spp. are further the fourth most common cause of nosocomial bloodstream infections, among which Candida albicans is most frequently identified.2,3 Nosocomial infections worsen patients’ morbidity, prolong length of hospital stay (LOS) as ICU treatment is often required, and thus increase treatment costs.4 Immunocompromised patients with preceding cancer treatment or stem cell transplantation are particularly prone to develop candidaemia.2 Further risk factors for the development of candidaemia are, for instance, long-term use of broad-spectrum antibiotics, indwelling central venous catheters (CVCs) or haemodialysis.5,6
To date, antifungals of four different classes are approved for the treatment of superficial and systemic Candida infections, including polyenes, azoles, echinocandins and nucleoside analogues. The localization and severity of infection, and patient’s morbidity guide the therapeutic decision.7 According to the ESCMID guideline, however, echinocandins are recommended as first-line therapy for Candida infections.8 Rezafungin is a novel, once-weekly echinocandin, which was designated as an orphan drug for the treatment of invasive candidiasis by the European Commission in the beginning of 2021.9 The FDA approved rezafungin as a Qualified Infectious Disease Product (QIDP) and orphan drug, designated for the treatment of invasive candidiasis including candidaemia.10 Granting was based on the Phase 2 STRIVE study, which showed comparable safety and efficacy profiles for rezafungin and caspofungin in the treatment of invasive candidiasis and candidaemia.11 It showed further that rezafungin was associated with a reduced ICU LOS compared with caspofungin.12 The administration of rezafungin may thus be potentially accompanied by savings in treatment costs. This is especially relevant as hospital treatment costs for invasive candidiasis and candidaemia impose a significant economic burden on healthcare systems, whereby LOS is considered an important cost driver.13,14 Therefore, the aim of this study was 2-fold: first, to analyse the cost-of-illness of Candida infections in general; second, to model the cost-saving potential for the treatment of invasive candidiasis and candidaemia under the use of rezafungin.
Patients and methods
Ethics
This study was based on anonymized medical chart data. According to the Health Data Protection Act of North-Rhine Westphalia (GDSG NW), no patient informed consent was needed.15
Statistical analyses
Parameters of descriptive statistics, such as arithmetic mean, 95% CI, median and value ranges, were included in both parts of the study. To make treatment costs of the 5 year period comparable with regard to inflation and to determine the cost value of the year 2022, a yearly discount rate of 3% was applied.16
Analytical approach
Reimbursement data from the data warehouse and hospital information system of the Department I of Internal Medicine, University Hospital Cologne (Germany), served as the basis for the current analysis. From a healthcare payer perspective, flat rates of German diagnosis-related groups (G-DRGs) were assumed as costs incurred by the hospital treatment. Data bases were evaluated for a 5 year period from June 2016 to June 2021. To conduct a cost-of-illness study on patients with Candida infections, databases were searched for the International Statistical Classification of Diseases (ICD-10) basis code B37, including different subforms and localization specifications (ICD-10 codes B37.0 to B37.9).17 Patient characteristics and health-economic parameters were described to demonstrate the economic burden of Candida infections using the example of the University Hospital Cologne, a comprehensive cancer centre. Health-economic parameters involved total LOS and ICU LOS, treatment costs (including G-DRG flat rates, potential extra-budgetary fees, and outlier surcharges) and case-mix. The latter reflects case severity and its resource consumption. The cost-of-illness study of Candida infections was evaluated based on hospital cases, as multiple hospitalizations per patient were possible during the study period.
The second part of the study aimed at modelling potential cost savings through the ICU LOS reduction by 5 days shown by the STRIVE study.12
Based on the population of the cost-of-illness study, patients with invasive candidiasis or candidaemia (IC/C) were identified as they were eligible for rezafungin treatment.9,10 Rezafungin eligibility was defined by the ICD-10 code B37.7 (candidaemia). ICD-10 codes B37.5 (Candida meningitis), B37.88 (candidiasis in other locations) and B37.9 (candidiasis with unspecified location) were used to reflect invasive candidiasis through the coding system.17 Further inclusion criteria were oncological indication as main or side diagnosis (ICD-10 codes C00 to C97, including malignant neoplasms), IV treatment with an antifungal agent identified through operation and procedure (OPS) codes, and ICU treatment with an LOS of at least 19 days based on the caspofungin cohort of the STRIVE trial.12 Based on the German guideline for the treatment of Candida infections, the following antifungal agents and OPS codes were considered: itraconazole (6-002.c), caspofungin (6-002.p), liposomal amphotericin B (6-002.q), voriconazole (6-002.r), anidulafungin (6-003.k), micafungin (6-004.5), posaconazole (6-007.k) and isavuconazole (6-008.g).7,18 The use of fluconazole could not be evaluated as it is not associated with a specific OPS code in the current catalogue. Medical chart data of eligible cases were checked regarding risk factors for candidaemia, including antibiotic therapy, haemodialysis, indwelling CVC, immunosuppression and mechanical ventilation.5,6 Included cases were further checked for eligibility by the treating physician based on case-by-case review in the hospital information system, which included further clinical information, such as diagnostic results. Potential cost savings per case were modelled assuming a linear resource consumption during the entire hospital stay. This approach thus followed the rationale of DRG flat rates. Rezafungin was not yet launched in the German market by the time of conducting the study. Its acquisition costs were thus indeterminate.
To verify the robustness of the cost-saving approach, a subgroup analysis with specific focus on candidaemia cases was conducted (ICD-10 code B37.7). This further detected potential differences in cost savings for ICD-10 codes with regard to ICD-10 codes B37.5, B37.88 and B37.9.
Results
Cost-of-illness study of candidiasis
Searching the databases regarding patients with Candida infections in the 5 year period, 801 individual hospital cases were found. Seventy-seven cases were excluded as their datasets were incomplete (Table 1). Therefore, 724 cases were included in the cost-of-illness study of Candida infections, reflecting a total of 652 patients. The study population had a median age of 58 years (range: 13–89 years), was mostly male (n = 413; 57.0%) and affiliated to statutory health insurances (n = 625; 86.3%). Two-thirds of the cases were treated on the ICU (n = 442; 61.1%), while approximately half of them were mechanically ventilated (n = 207; 28.6%). One-quarter of the population died during their hospitalization (n = 185; 25.6%).
Table 1.
Baseline case characteristics
| Characteristic | |
| Cases (patients), n | 724 (652) |
| ICU treatment, n (%) | 442 (61.05) |
| Invasive ventilation, n (%) | 207 (28.59) |
| Age, years | |
| Median (range) | 58 (13–89) |
| Mean (95% CI) | 55.7 (54.6–56.8) |
| Gender | |
| Female, n (%) | 331 (42.96) |
| Male, n (%) | 413 (57.04) |
| Health insurance, n (%) | |
| Statutory | 625 (86.33) |
| Private | 67 (9.25) |
| Self-payer | 23 (3.18) |
| Others | 9 (1.24) |
| Death in hospital, n (%) | 185 (25.55) |
Health-economic and treatment-cost parameters were evaluated to fully assess the cost of illness with Candida infections (Table 2). Total LOS of eligible cases was a median of 25 days with a median ICU LOS of 15 days when treated in the ICU. Both LOS values ranged widely (1–252 days and 1–203 days, respectively). Among affected cases, the median duration of ventilation was 227 h (range: 1–1412 h), reflecting approximately 9.5 days. Median total treatment costs per case including G-DRG tariffs, and extra-budgetary payments for cost-intensive procedures and drugs, accounted for €22 820. Large outliers, both below (€527) and above (€514 297) median costs, were identified. Due to its mutual dependence, the same applied for the case-mix (median: 5.225 points; range: 0.192–123.654 points). Considering the distribution of underlying Candida indications, Candida stomatitis with the ICD-10 code B37.0 was most frequently found (n = 256; 35.4%). Candidiasis of other sites (B37.88) and Candida oesophagitis (B37.81) were the second and third most frequent indications (n = 124; 17.1% and n = 108; 14.9%, respectively). Candidaemia was identified in 45 cases (6.2%).
Table 2.
Health-economic and treatment cost parameters
| Parameter | |
| Total LOS, days | |
| Median (range) | 25 (1–252) |
| Mean (95% CI) | 34.06 (31.74–36.39) |
| ICU LOS, days | |
| Median (range) | 15 (1–203) |
| Mean (95% CI) | 22.33 (20.07–24.58) |
| Mechanical ventilation when affected, h | |
| Median (range) | 227 (1–1412) |
| Mean (95% CI) | 303.84 (265.34–342.33) |
| Total treatment costs, EURa | |
| Median (range) | 22 819.66 (527.12–514 297.45) |
| Mean (95% CI) | 39 427.16 (35 440.74–43 413.59) |
| Sensitivity analysis of total treatment costs with different discount rates, EUR | |
| 0%, median (range) | 25 330.91 (629.41–561 986.71) |
| 5%, median (range) | 21 071.91 (496.68–485 465.25) |
| 10%, median (range) | 17 906.25 (355.29–422 228.93) |
| Case-mix index, points | |
| Median (range) | 5.225 (0.192–123.654) |
| Mean (95% CI) | 8.853 (8.012–9.693) |
| Candidiasis indication with ICD-10 code, n (%) | |
| B37.0 (Candidal stomatitis) | 256 (35.36) |
| B37.1 (Pulmonary candidiasis) | 76 (10.50) |
| B37.2 (Candidiasis of skin and nail) | 26 (3.59) |
| B37.3 (Candidiasis of vulva and vagina) | 23 (3.18) |
| B37.4 (Candidiasis of other urogenital sites) | 59 (8.15) |
| B37.5 (Candidal meningitis) | 3 (0.41) |
| B37.6 (Candidal endocarditis) | 0 (0.00) |
| B37.7 (Candidal sepsis/candidaemia) | 45 (6.22) |
| B37.81 (Candidal oesophagitis) | 108 (14.92) |
| B37.88 (Candidiasis of other sites) | 124 (17.13) |
| B37.9 (Candidiasis, unspecified) | 4 (0.55) |
Adjusted to inflation to the year 2022 by 3% discount rate as recommended by the German Institute for Quality and Cost Effectiveness in the Health Care Sector (IQWiG).
Modelling of potential cost savings due to rezafungin
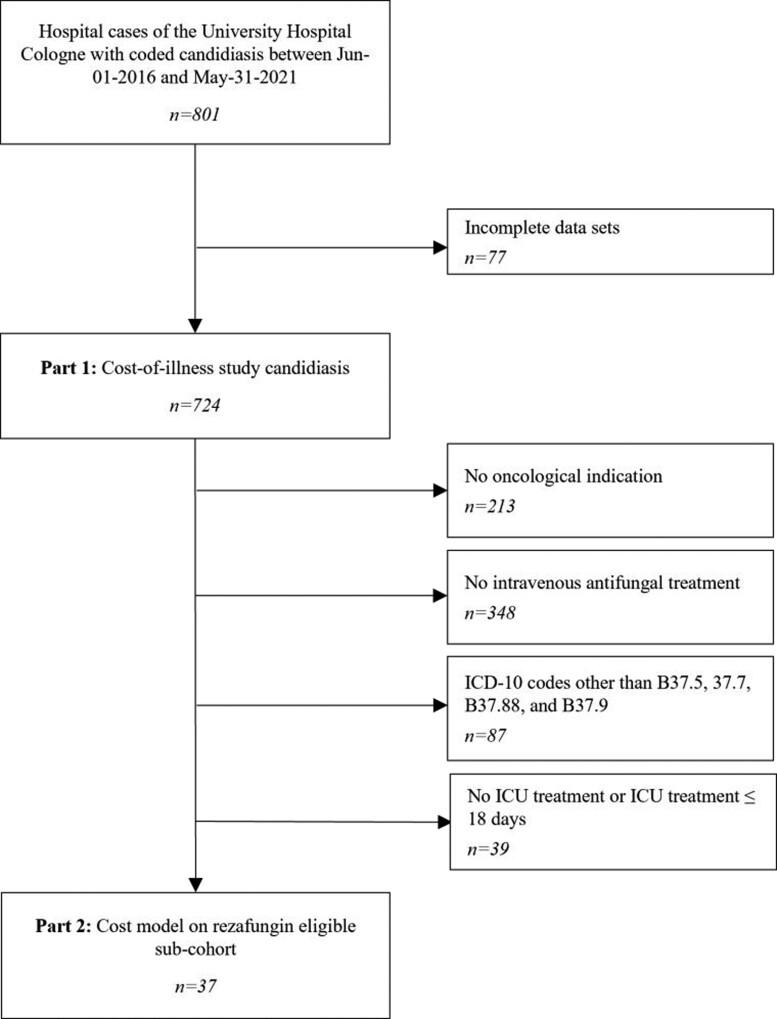
The population of the cost-of-illness study was adjusted by excluding hospital cases without oncological indication (n = 213), without IV antifungal treatment (n = 348), with ICD-10 codes other than B37.5, B37.7, B37.88 and B37.9 (n = 87), and with ICU treatment between 0 and 18 days (n = 39). After applying the predefined exclusion criteria, no candidal meningitis case (B37.5) was identified as eligible. Cases with codes B37.88 (n = 24) and B37.9 (n = 2) were considered eligible after case-by-case review as all of them presented invasive Candida infections.
Thirty-seven individual patients were identified as being theoretically eligible for treatment with rezafungin and were thus included in the retrospective cost model (Figure 1).
Figure 1.
Flow chart summarizing eligibility hospital cases for both part 1 and part 2 of the study. n, number of hospital cases.
Eligible patients were evaluated regarding risk factors for IC/C. Most of them received antibiotic treatment (n = 35; 94.6%) and had an indwelling CVC (n = 32; 89.2%). Haemodialysis was performed in 12 patients (32.4%) and 15 patients were immunocompromised (40.5%). Most patients were mechanically ventilated (n = 26; 70.3%). In total, eligible patients were exposed to a median of 3 out of 5 investigated risk factors, ranging from 1 to 5 occurrences.
The retrospective economic model suggested that the use of rezafungin and its incorporated ICU LOS reduction of 5 days may lead to a median cost-saving potential of €7175 per hospital case with IC/C (Table 3). Assuming linear resource consumption, accumulated cost savings of 37 included patients accounted for €283 335.
Table 3.
Retrospective cost-saving model
| Cases, n | Basic G-DRG code | Case mix, points, median (range) | Candidiasis indication (n) | Total LOS, days, median (range) | Total G-DRG treatment costs, EUR, median (range) | Daily G-DRG treatment costs, EUR, median (range) | Cost savings due to reduced (ICU) LOS by 5 days, median (range) |
|---|---|---|---|---|---|---|---|
| 3 | A07 | 51.543 (35.563–123.654) | B37.88 (5) | 121 (51–252) | 181 380.01 (142 191.64–429 602.39) | 1704.77 (1499.01–2788.07 | 8523.86 (7495.04–13 940.36) |
| 5 | A09 | 22.364 (14.854–33.711) | B37.88 (5) | 57 (36–101) | 78 362.17 (69 519.06–187 364.40) | 1855.09 (1362.20–2062.16) | 9275.47 (6811.00–10 310.81) |
| 4 | A11 | 27.503 (14.077–47.713) | B37.7 (1), B37.88 (3) | 67 (19–110) | 101 617.64 (46 183.30–514 297.45) | 2125.41 (1230.84–4675.43) | 10 627.04 (6154.18–23 377.16) |
| 6 | A13 | 16.035 (11.294–33.555) | B37.7 (3), B37.88 (3) | 33 (31–92) | 63 184.20 (52 983.52–132 673.97) | 1944.00 (1151.82–2085.77) | 9719.98 (5759.08–10 428.87) |
| 2 | A15 | 6.609 (5.257–7.961) | B37.88 (1), B37.9 (1) | 26.5 (19–34) | 25 083.67 (15 357.44–34 809.89) | 916.06 (808.29–1023.82) | 4580.27 (4041.43–5119.10) |
| 1 | A18 | 62.725 | B37.88 | 84 | 216 948.56 | 2582.72 | 12 913.60 |
| 3 | A36 | 17.665 (16.624–30.968) | B37.7 (1), B37.88 (2) | 75 (69–88) | 122 878.99 (82 507.28–126 288.67) | 1435.10 (1195.76–1638.39) | 7175.49 (5978.79–8191.93) |
| 1 | B61 | 56.144 | B37.88 (1) | 232 | 156 309.90 | 673.75 | 3.368.75 |
| 1 | E36 | 14.486 | B37.88 (1) | 57 | 45 542.65 | 798.99 | 3994.97 |
| 3 | G36 | 14.419 (9.954–5.335) | B37.7 (1), B37.88 (2) | 47 (45–60) | 57 165.72 (39 371.13–62 352.33) | 1039.21 (874.91–1216.29) | 5196.03 (4374.57–6081.46) |
| 1 | I08 | 9.788 | B37.9 (1) | 119 | 36 147.36 | 303.76 | 1518.80 |
| 1 | I26 | 29.504 | B37.7 (1) | 114 | 86 620.40 | 759.83 | 3799.14 |
| 3 | R36 | 32.122 (12.571–37.715) | B37.7 (2), B37.88 (1) | 109 (21–127) | 121 160.74 (47 851.87–139 857.19) | 1111.57 (1101.24–2278.66) | 5557.83 (5506.19–11 393.30) |
| 1 | R60 | 4.912 | B37.88 (1) | 50 | 45 547.34 | 910.95 | 4554.73 |
| 2 | R61 | 5.389 (4.198–6.579) | B37.7 (2) | 43.5 (37–50) | 26 021.62 (18 901.34–33 141.89) | 586.85 (510.85–662.84) | 2934.22 (2554.24–3314.19) |
| Total (median) | / | 949.987 (16.624) | / | 2682 (57) | 3 967 894.16 (77 645.35) | 56 666.94 (1435.10) | 283 334.72 (7175.49) |
Subgroup analysis specifically focusing on candidaemia cases (ICD-10 code B37.7) resulted in median cost savings of €7211 and thus differed €36 from the basic model including the combination of ICD-10 codes B37.7, B37.88 and B37.9 (Table S1, available as Supplementary data at JAC-AMR Online).
Discussion
We analysed the cost of illness of patients with Candida infections based on real-life data of the University Hospital Cologne. In the second part, the dataset was further used to theoretically model the cost-saving potential for the treatment of IC/C when administering rezafungin. In total, 724 hospital cases with Candida infections were identified; median treatment costs accounted for approximately €23 000 per case. A recent review reported mean total costs per patient with IC/C (included costs, i.e. for hospital stay, antifungal therapy and adverse drug reactions) in Western countries to range between $48 487 and $157 574, with mean cost per hospitalization of $10 216 to $37 715.19 As we found a great number of hospital cases with non-invasive infections and with lower median treatment costs, the large economic burden of treatment of IC/C is highlighted.
The retrospective model was based on findings of the STRIVE study12 and included a total of 37 hospital cases with IC/C. Compared with other antifungal agents, we found a median cost-saving potential of €7175 in cases who received ICU treatment for at least 19 days. To our knowledge, this is the first retrospective model to quantify potential cost savings due to rezafungin. Recent decision analysis models analysed the cost-effectiveness of different antifungals for patients with IC/C in various countries from a hospital perspective.20–23 For instance, a Spanish study identified anidulafungin to be more cost-effective compared with fluconazole.20 Although higher antifungal acquisition costs were quantified for anidulafungin, the overall treatment costs were lower (€40 047 versus €41 350) due to the reduction in other costs, i.e. in ICU costs (€18 985 versus €20 916).20 Similarly, an Australian study reported anidulafungin as a cost-effective option compared with fluconazole.21
Other studies examined the cost-effectiveness of different antifungal agents in the UK.22 Thereby, it was shown that the cost-effectiveness ratio of anidulafungin dominated both caspofungin and micafungin due to lower treatment costs (£28 216 for anidulafungin versus £28 905 for caspofungin and £28 721 for micafungin), especially triggered by lower ICU and drug acquisition costs, and better effectiveness parameters (life-years gained were 7.23 for anidulafungin versus 6.03 for caspofungin and 5.55 for micafungin).22 Another UK study found similar results for the cost-effectiveness of caspofungin and micafungin.23
Economic evaluations such as cost-effectiveness analyses can support decision-makers and healthcare payers in balancing quality and value of care.19 To date, cost-effectiveness analyses including rezafungin are lacking.
This study has limitations. The cost-of-illness analysis included all Candida infections treated in the University Hospital Cologne in the given time period. It therefore did not differentiate between Candida infections coded as main diagnosis or as side diagnosis. Furthermore, a distinction between invasive and superficial Candida infections cannot be derived by the medical coding system. Thus, investigated Candida infections may not necessarily be the reason for hospital admission but may only be co-treated during hospitalization. Analysing data from a sole medical controlling approach, it cannot be ruled out that coded Candida infections in the cost-of-illness analysis may be restricted to Candida colonizations. Additionally, uniform criteria for pulmonary candidiasis diagnosis and coding, for instance, remain to be defined.24 Thus, findings of the cost-of-illness analysis may be overestimated.
Further, the modelled cost-saving potential may be underestimated as only hospital cases with an ICU LOS more than 18 days were included. Patients with a shorter ICU LOS may also benefit from the administration of rezafungin. Further, ICU treatment demands more resources than treatment on a normal ward. However, reimbursement data only allowed us to assume linear resource consumption during the hospital stay. Thus, cost-saving potential may be higher than identified in this model. As ICD-10 codes other than B37.5, B37.7, B37.88 and B37.9 have not been considered for the cost-saving model, the number of cases being eligible for rezafungin treatment may be underestimated. Moreover, total G-DRG treatment costs included acquisition costs of administered echinocandins or antifungals. An exclusion of acquisition costs was not feasible as the reimbursement for most of the drugs was negotiated by each hospital individually. As acquisition costs of rezafungin were not considered, costs for both administered drugs and rezafungin were assumed to be equal.
Conclusions
The resource intensity of the treatment of Candida infections is of health-economic relevance. The results of our cost-of-illness study confirm the high treatment costs of Candida infections stated by recent studies.19–23 Yet, future research that merges clinical information (e.g. the classification according to EORTC/MSGERC definitions) with medical coding is needed for further validation. As shown by the retrospective model, reduced ICU LOS due to the novel echinocandin rezafungin may lead to sustainable cost savings in the hospital treatment of patients with IC/C. The administration of rezafungin may thus contribute to counteracting resource scarcity in healthcare resources. To gain further health-economic information, we encourage future research to analyse direct costs associated with the resource saving due to rezafungin and to investigate potential cost savings on normal wards. Cost-effectiveness analyses including rezafungin and other antifungals besides caspofungin are needed to set the findings into context.
Supplementary Material
Contributor Information
Julia Jeck, Department I of Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 62, 50937 Cologne, Germany; VITIS GmbH, Am Morsdorfer Hof 12, 50933 Cologne, Germany.
Florian Jakobs, Department of Haematology and Stem Cell Transplantation, Faculty of Medicine and Essen University Hospital, University of Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany.
Melina S Kurte, VITIS GmbH, Am Morsdorfer Hof 12, 50933 Cologne, Germany.
Oliver A Cornely, Department I of Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 62, 50937 Cologne, Germany; Center for Integrated Oncology Cologne (CIO ABCD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 28 62, 50937 Cologne, Germany; Clinical Trials Centre Cologne (ZKS Köln), Faculty of Medicine and University Hospital Cologne, University of Cologne, Gleueler Straße 269, 50935 Cologne, Germany; CECAD Cologne, Translational Research, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Joseph-Stelzmann-Straße 26, 50931 Cologne, Germany; Excellence Centre for Medical Mycology (ECMM), Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 62, 50937 Cologne, Germany.
Florian Kron, Department I of Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 62, 50937 Cologne, Germany; VITIS GmbH, Am Morsdorfer Hof 12, 50933 Cologne, Germany; Center for Integrated Oncology Cologne (CIO ABCD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 28 62, 50937 Cologne, Germany; FOM University of Applied Sciences, Leimkugelstraße 6, 45141 Essen, Germany.
Funding
This work was supported by an unrestricted grant from Mundipharma Research Limited.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Pfaller MA. Epidemiology of candidiasis. J Hosp Infect 1995; 30Suppl: 329–38. 10.1016/0195-6701(95)90036-5 [DOI] [PubMed] [Google Scholar]
- 2. Ruhnke M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr Drug Targets 2006; 7: 495–504. 10.2174/138945006776359421 [DOI] [PubMed] [Google Scholar]
- 3. Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 1995; 20: 1526–30. 10.1093/clinids/20.6.1526 [DOI] [PubMed] [Google Scholar]
- 4. Pappas PG. Invasive candidiasis. Infect Dis Clin North Am 2006; 20: 485–506. 10.1016/j.idc.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 5. Wey SB, Mori M, Pfaller MAet al. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch Intern Med 1989; 149: 2349–53. 10.1001/archinte.1989.00390100145030 [DOI] [PubMed] [Google Scholar]
- 6. Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 2014; 10: 95–105. 10.2147/TCRM.S40160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Groll AH, Buchheidt D, Heinz Wet al. S1 Leitlinie Diagnose und Therapie von Candida Infektionen: Gemeinsame Empfehlungen der Deutschsprachigen Mykologischen Gesellschaft (DMykG) und der Paul-Ehrlich-Gesellschaft für Chemotherapie (PEG) ICD 10: B37. https://www.awmf.org/uploads/tx_szleitlinien/082-005l_S1_Diagnose-Therapie-Candida-Infektionen_2020-09.pdf.
- 8. Cornely OA, Bassetti M, Calandra Tet al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18Suppl 7: 19–37. 10.1111/1469-0691.12039 [DOI] [PubMed] [Google Scholar]
- 9. EMA . EU/3/20/2385: Orphan Designation for the Treatment of Invasive Candidiasis. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3202385.
- 10. US FDA . Search Orphan Drug Designations and Approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=507215.
- 11. Thompson GR, Soriano A, Skoutelis Aet al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis 2021; 73: e3647–e55. 10.1093/cid/ciaa1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soriano A, Dickerson S, Das AFet al. Early outcomes and intensive care unit length of stay with rezafungin once-weekly echinocandin in invasive Candida disease. 40th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 2021. Abstract P038. [Google Scholar]
- 13. Wilke M. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin and its impact on use and costs: review of the literature. Eur J Med Res 2011; 16: 180–6. 10.1186/2047-783X-16-4-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neoh CF, Slavin M, Chen SCAet al. Echinocandins in the treatment of candidaemia and invasive candidiasis: clinical and economic perspectives. Int J Antimicrob Agents 2014; 43: 207–14. 10.1016/j.ijantimicag.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 15. State law of North Rhine-Westphalia . §6 Gesetz zum Schutz personenbezogener Daten im Gesundheitswesen—Datenverarbeitung für wissenschaftliche Zwecke. In: Gesundheitsdatenschutzgesetz (GSDG NW). http://www.lexsoft.de/cgi-bin/lexsoft/justizportal_nrw.cgi?t=168606749760700054&xid=146653,7.
- 16. German Institute for Quality and Efficiency in Health Care . General Methods Version—6.0 of 05.11.2020. https://www.iqwig.de/methoden/general-methods_version-6-0.pdf. [PubMed]
- 17. Bundesinstitut für Arzneimittel und Medizinprodukte . Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme, 10. Revision, German Modification, Version 2022. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2022/.
- 18. Bundesinstitut für Arzneimittel und Medizinprodukte . OPS Version 2022: Operationen- und Prozedurenschlüssel, Version 2022. https://www.dimdi.de/static/de/klassifikationen/ops/kode-suche/opshtml2022/.
- 19. Ismail WNAW, Jasmi N, Khan TMet al. The economic burden of candidemia and invasive candidiasis: a systematic review. Value Health Reg Issues 2020; 21: 53–8. 10.1016/j.vhri.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 20. Grau S, Salavert M, Carlos Pozo Laderas Jet al. Cost-effectiveness of anidulafungin in confirmed candidaemia and other invasive Candida infections in Spain. J Mycol Med 2013; 23: 155–63. 10.1016/j.mycmed.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 21. Neoh CF, Liew D, Slavin Met al. Cost-effectiveness analysis of anidulafungin versus fluconazole for the treatment of invasive candidiasis. J Antimicrob Chemother 2011; 66: 1906–15. 10.1093/jac/dkr186 [DOI] [PubMed] [Google Scholar]
- 22. Auzinger G, Playford EG, Graham CNet al. Cost-effectiveness analysis of anidulafungin for the treatment of candidaemia and other forms of invasive candidiasis. BMC Infect Dis 2015; 15: 463. 10.1186/s12879-015-1143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sidhu MK, van Engen AK, Kleintjens Jet al. Cost-effectiveness analysis of micafungin versus caspofungin for treatment of systemic Candida infections in the UK. Curr Med Res Opin 2009; 25: 2049–59. 10.1185/03007990903072565 [DOI] [PubMed] [Google Scholar]
- 24. El-Ebiary M, Torres A, Fàbregas Net al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med 1997; 156: 583–90. 10.1164/ajrccm.156.2.9612023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.