Abstract
Background
Apple watch-derived electrocardiogram (awECG) may help identify prolongation of corrected QT (QTc) interval. This study aimed to determine its usefulness for assessment of prolongation of QTc interval in children and adolescents with long QT syndrome (LQTS).
Methods
Children and adolescents with and without LQTS were recruited for measurement of QTc intervals based on standard 12-lead (sECG) and awECG lead I, II and V5 tracings. Bland-Altman analysis of reproducibility, concordance assessment of T wave morphologies, and receiver operating characteristic (ROC) analysis of sensitivity and specificity of awECG-derived QTc interval for detecting QTc prolongation were performed.
Results
Forty-nine patients, 19 with and 30 without LQTS, aged 3–22 years were studied. The intraclass correlation coefficient was 1.00 for both intra- and inter-observer variability in the measurement of QTc interval. The awECG- and sECG-derived QTc intervals correlated strongly in all three leads (r = 0.90–0.93, all p < 0.001). Concordance between awECG and sECG in assessing T wave morphologies was 84% (16/19). For detection of QTc prolongation, awECG lead V5 had the best specificity (94.4% and 87.5%, respectively) and positive predictive value (87.5% and 80.0%, respectively), and for identification of patients with LQTS, awECG leads II and V5 had the greatest specificity (92.3%–94.1%) and positive predictive value (85.7% to 91.7%) in both males and females.
Conclusions
Apple Watch leads II and V5 tracings can be used for reproducible and accurate measurement of QTc interval, ascertainment of abnormal T wave morphologies, and detection of prolonged QTc interval in children and adolescents with LQTS.
Keywords: Long QT syndrome, QTc interval, Apple watch electrocardiogram
1. Introduction
Congenital long QT syndrome (LQTS) is characterized by prolongation of the QT interval on electrocardiogram (ECG), associated with life-threatening arrhythmias and sudden death [1]. The prevalence of congenital LQTS has been reported to be at least 1 in 2534 [2]. There has been concern of underdiagnosis of this condition in asymptomatic individuals and under-recognition of symptomatic LQTS patients presenting with recurrent seizure and unexplained syncope [3], [4]. While electrocardiographic screening for congenital LQTS has been proposed [2], [5], population-wide screening is difficult to implement as ECG is not usually performed in the paediatric population beyond the hospital setting.
Smartwatches have been popularized in recent years as mobile tools for health surveillance. The commercially available Apple Watch ECG (awECG) has been approved by the US Food and Drug Administration for detecting atrial fibrillation in users aged 22 years or above [6]. Recent studies in the adult population have provided evidence that awECG may be useful for assessment of QT interval [7], [8]. Furthermore, studies in adults have demonstrated feasibility of using the Apple Watch to record multiple ECG leads including praecordial leads with good signal qualities [9], [10], [11]. This is of particular relevance in the context of LQTS as measurement of QT interval in leads II and V5 is preferred given the repeatability and correlation with the genetic variants in LQTS [12], [13].
Data supporting the use of awECG in the paediatric population are, however, scarce and limited to the evaluation of lead I [14], [15]. No studies have to date evaluated the use of smartwatch for the detection of prolongation of heart rate-corrected QT interval (QTc) in children and adolescents with congenital LQTS. This study tested the hypothesis that awECG enables the detection of prolongation of QTc interval in paediatric patients with congenital LQTS. The aims of the present study are 1) to validate the reproducibility and accuracy of using awECG compared with standard ECG (sECG) for the measurement of QTc interval, 2) to ascertain the use of awECG to assess abnormal T wave morphologies, and 3) to assess the use of awECG to detect prolongation of QTc interval in children and adolescents with congenital LQTS.
2. Methods
2.1. Subjects
This prospective study enrolled children and adolescents followed up at the paediatric cardiac arrhythmia clinic at the Hong Kong Children’s Hospital between April and October 2022 for congenital LQTS and other types of cardiac arrhythmias. Congenital LQTS in our patients was diagnosed based on documented pathogenic mutation, signification prolongation of QTc interval, or the Schwartz score [16]. Exclusion criteria included i) the presence of severe dysmelia or amelia of the upper extremities, ii) the absence of sinus or atrial rhythm at the time of study, iii) the presence of ventricular bigeminy, and iv) the presence of widened QRS complexes due to preexcitation or bundle branch block. The following data were collected: demographic data, primary cardiac diagnosis, type of cardiac arrhythmia, genotype, and current medications. The body weight and height of all patients were measured. The study was approved by Institutional Review Board of the Hong Kong Children’s Hospital, Hong Kong, China. All parents of minors and patients aged 18 or above gave written informed consent and patients aged less than 18 years provided additional assent to the study.
2.2. Acquisition of ECG
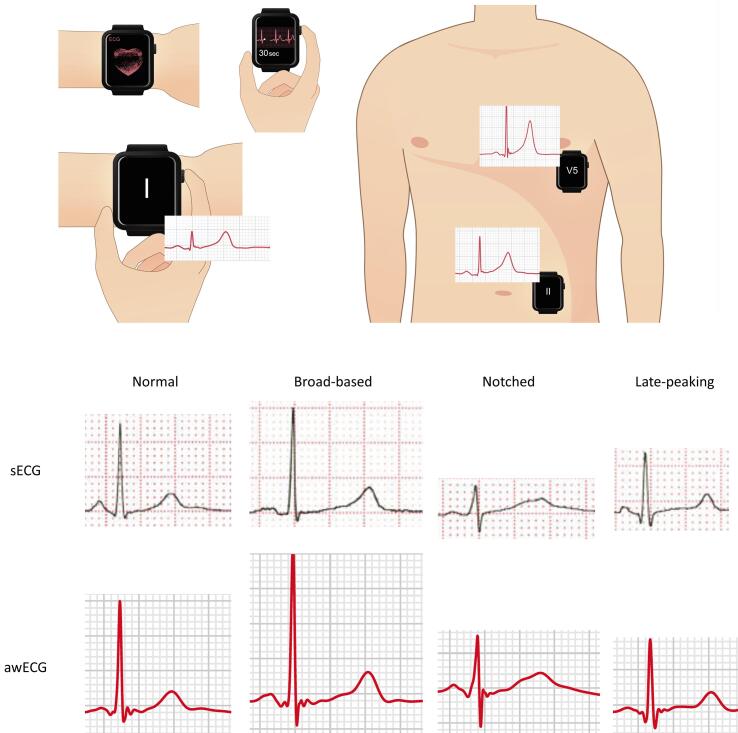
All ECGs were acquired from patients after a resting period of at least five minutes. The procedure of ECG acquisition was explained to all patients and parents of minors. Standard 12-lead ECG was performed first using the ECG machine with a paper speed of 25 mm/s. Ten-second tracings of leads I, II, and V5 were obtained and scanned for measurement of QTc interval. This was followed immediately by awECG recordings using the Apple Watch Series 7 (Apple Inc, Cupertino, CA, USA) to obtain 30-second tracings of lead I (watch on left wrist), II (watch on left lower abdomen), and V5 (watch over the fifth intercostal space at the anterior axillary line) (Fig. 1 upper panel). The recorded awECG tracings were stored digitally in anonymized portable document format using the health application on an iPhone 13 and sent via email to the desktop computer for measurement of the QTc interval. For sECG or awECG tracings with suboptimal qualities, the recordings were repeated and the ones with the least interference were used for analysis.
Fig. 1.
Upper panel: Illustrations to show the recording of 30-second electrocardiographic tracings using Apple Watch. The watch was placed on the left wrist with the right index finger on the crown for recording lead I tracing, over the left lower abdomen for recording lead II tracing, and over the fifth intercostal space at the anterior axillary line for recording lead V5 tracing. Lower panel: Assessment of different T wave morphologies using Apple Watch electrocardiogram (awECG) and standard electrocardiogram (sECG).
2.3. Determination of QTc interval
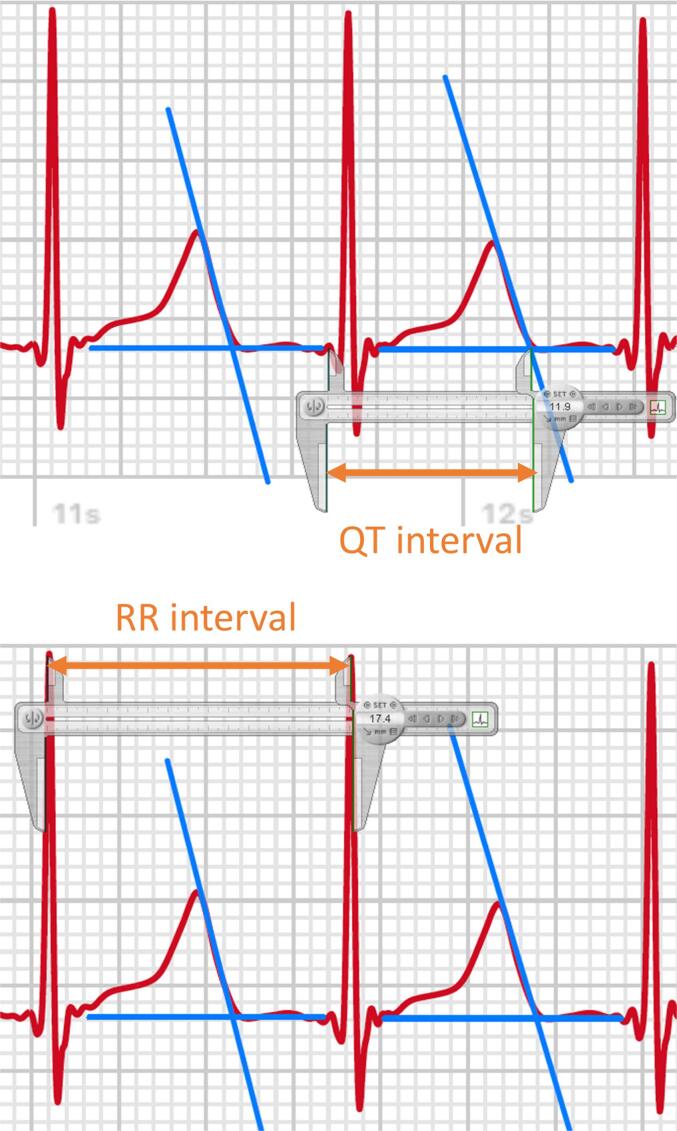
The QTc intervals of awECG and sECG tracings were analyzed independently by two investigators (JL and SYK) blinded to the underlying diagnosis. A commercially available software Cardio Calipers (Iconico Inc, NYC, USA) was used for measurement of the QT and RR intervals (supplementary Fig. 1). The QT interval was measured from the beginning of the QRS complex to the end of the T wave. The end of the T wave was ascertained using the tangent technique [12]. The second peak of a bifid T wave or a large U wave was included when it was at least half the amplitude of the first T wave.
Three consecutive QT intervals and the corresponding preceding RR intervals were measured in leads I, II, and V5. In the presence of sinus arrhythmia, three consecutive QRS complexes that included the shortest RR interval were selected. The QTc interval was calculated using the Bazett formula (QT interval/square root of the preceding RR interval). For each of the leads, the average of three individually calculated QTc intervals was used for statistical analysis.
2.4. Assessment of T wave morphology
T wave morphologies on the sECG and awECG tracings (Fig. 1 lower panel) in patients with congenital LQTS were evaluated and compared by a single assessor (ST) blinded to the subtypes of LQTS. The awECG tracings were further assessed by two assessors (SYK and ST), blinded to the LQTS subtypes, for determination of agreement in identification of abnormal T wave morphologies.
2.5. Statistical analysis
Data are presented as mean ± standard deviation where appropriate. Intraclass correlation coefficient and Bland-Altman analysis, for determination of bias and 95% limits of agreement (LoA), were used to assess intra- and inter-observer variability in the measurement of awECG-QTc and sECG-QTc obtained from lead II in 20 subjects (10 with and 10 without congenital LQTS). Agreement between the measurement of QTc interval by awECG and sECG was assessed using Pearson correlation coefficient and Bland-Altman analysis. Receiver operating characteristic (ROC) analysis was performed to determine the usefulness of awECG tracings in detecting prolongation of QTc interval (based on sECG-derived QTc ≥ 450 ms for male and ≥460 ms for female [17]) and identifying patients with LQTS, and to determine the optimal cutoff values with respective sensitivity, specificity, and positive and negative predictive values in males and females. The Cohen’s kappa coefficient was calculated for assessment of interobserver agreement in the identification of abnormal T wave morphologies based on awECG tracings in patients with congenital LQTS. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics version 26 (SPSS, Inc., Chicago, Illinois).
3. Results
3.1. Subjects
A total of 49 patients, 19 (3–22 years) with and 30 (6–18 years) without congenital LQTS, were recruited (Table 1). There were no significant differences in age, sex distribution, weight, and height between the two groups. Of the 19 patients with congenital LQTS, 14 (74%) were symptomatic, 4 (21%) were diagnosed during family screening, and 1 (5%) was detected incidentally during follow-up for another medical problem (supplementary Table 1). All of the patients had QTc prolongation at diagnosis and 14 patients (74%) had identifiable pathogenic genetic variants. All LQTS patients were on beta-blocker therapy, while two had pacemakers and two had implantable cardioverter defibrillators. Of the 30 patients without congenital LQTS, 18 (60%) were followed up for history of supraventricular tachycardia and the others were followed for history of other types of arrhythmias including ventricular tachycardia, premature ventricular and atrial contraction, and first- and second-degree heart block (Table 1). All patients were in sinus or atrial rhythm at the time of study.
Table 1.
Demographic and clinical parameters.
| Congenital LQTS (n = 19) | Non-congenital LQTS (n = 30) | p | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 13.5 ± 5.1 | 12.7 ± 3.3 | 0.52 |
| Sex (M:F) | 8:11 | 17:13 | 0.39 |
| Body weight (kg) | 48.8 ± 21.5 | 46.0 ± 15.5 | 0.60 |
| Body height (cm) | 153.8 ± 23.2 | 154.1 ± 16.8 | 0.95 |
| Body mass index (kg/m2) | 19.5 ± 4.8 | 18.8 ± 3.3 | 0.57 |
| Body surface area (m2) | 1.4 ± 0.4 | 1.4 ± 0.3 | 0.78 |
| Type of arrhythmia | |||
| LQTS | 19 | ||
| SVT | 18 | ||
| VT | 2 | ||
| PVC | 2 | ||
| PAC | 1 | ||
| First-degree heart block | 1 | ||
| Second-degree heart block | 3 | ||
| No arrhythmias | 3 | ||
| Genetic variants | |||
| KCNQ1 (LQT1) | 5 | ||
| KCNH2 (LQT2) | 6 | ||
| SCN5A (LQT3) | 2 | ||
| CACNA1C (LQT8) | 1 | ||
| VUS | 3 | ||
| Negative | 2 | ||
| Medical therapy | |||
| Nadolol | 10 | ||
| Atenolol | 5 | 2 | |
| Propranolol | 2 | 1 | |
| Metoprolol | 2 | ||
| Sotalol | 1 | ||
| Mexiletine | 3 | ||
| Amiodarone | 1 | ||
| Flecainide | 2 | ||
| Propafenone | 1 | ||
| None | 23 | ||
| Cardiac implantable device | |||
| ICD | 2 | ||
| Pacemaker | 2 | ||
| None | 15 | 30 |
|
Abbreviations: ICD, implantable cardioverter defibrillator, LQTS, long QT syndrome, PAC, premature atrial contraction, PVC, premature ventricular contraction, SVT, supraventricular tachycardia, VT, ventricular tachycardia, VUS, variant of uncertain significance.
3.2. Usability of awECG and sECG tracings for QTc measurement
All patients had at least one of the three leads from awECG and sECG feasible for measurement of QTc interval. For awECG, suboptimal tracings unsuitable for analysis were noted in lead I in 2/49 (4.1%), lead II in 2/49 (4.1%), and lead V5 in 1/49 (2.0%). For sECG, suboptimal tracings unsuitable for analysis were noted in lead I in 1/49 (2.0%), lead II in 1/49 (2.0%), and lead V5 in 0/49 (0%) (p = 0.98). The reasons included low T wave amplitude (n = 4 in awECG and n = 2 in sECG) and poor tracing quality (n = 1 in awECG).
3.3. Reproducibility of QTc measurements
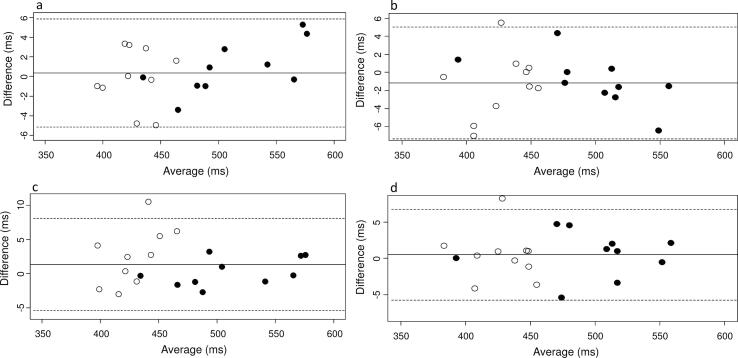
For intraobserver variability, the intraclass correlation coefficient was 1.00 for the measurement of both awECG-QTc and sECG-QTc intervals in lead II. The Bland-Altman plots are shown in supplementary Fig. 2a and Fig. 2b. There was minimal difference between readings of 0.23 ms (95% LoA −5.45 to 5.91) for awECG-QTc interval and −1.17 ms (95% LoA −7.37 to 5.03) for sECG-QTc interval. There was no trend to suggest that the difference increased with QTc interval in subjects with congenital LQTS.
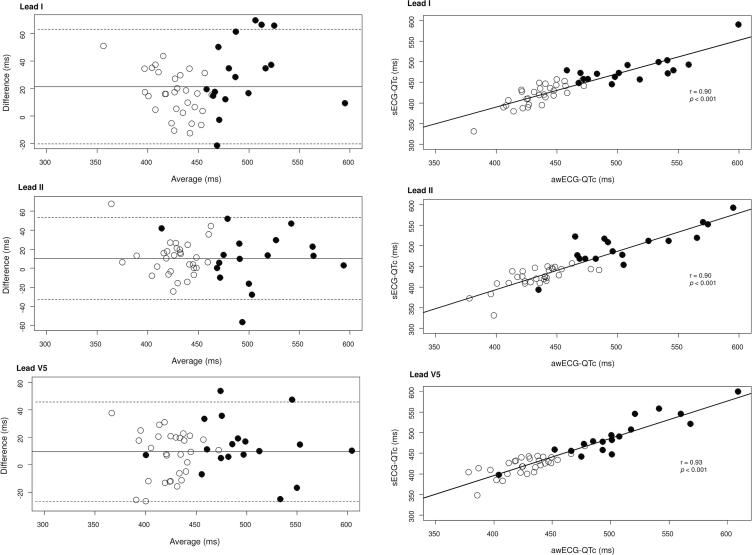
Fig. 2.
Left panel: Scatter plots showing QTc intervals as measured by Apple Watch ECG (awECG-QTc) and standard ECG (sECG-QTc) in leads I, II and V5 (solid circles, subjects with congenital long QT syndrome, open circles, subjects without congenital long QT syndrome). Right panel: Bland-Altman plots showing reproducibility of measuring QTc interval by Apple Watch ECG and standard ECG in leads I, II and V5. The x-axis is the average of the awECG-QTc and sECG-QTc intervals, while the y-axis is the difference between awECG-QTc and sECG-QTc intervals. Solid circles are subjects with congenital long QT syndrome, and open circles are subjects without congenital long QT syndrome.
For interobserver variability, the intraclass correlation coefficient was 1.00 for the measurement of both awECG-QTc and sECG-QTc intervals in lead II. The Bland-Altman plots are shown in supplementary Fig. 2c and Fig. 2d. There was only a small difference between readings of 1.35 ms (95% LoA −5.41 to 8.11) for awECG-QTc interval and 0.51 ms (95% LoA −5.73 to 6.76) for sECG-QTc interval. There was similarly no trend to suggest that the difference increased with QTc interval in subjects with congenital LQTS.
3.4. Agreement between awECG-QTc and sECG-QTc
Fig. 2 (right panel) demonstrates strong associations between awECG-QTc and sECG-QTc for leads I (r = 0.90, p < 0.001), II (r = 0.90, p < 0.001), and V5 (r = 0.93, p < 0.001). One patient with congenital LQTS, who was taking nadolol, had normal QTc intervals in leads II and V5 based on both awECG and sECG measurements.
Bland-Altman analysis found that awECG overestimated the QTc by 21.47 ms (95% LoA −20.23 to 63.16) for lead I, 10.31 ms (95% LoA –32.82 to 53.44) for lead II, and 9.37 ms (95% LoA −26.70 to 45.44) for lead V5 (Fig. 2, left panel). While there was no obvious trend to suggest that the difference increased with QTc interval in subjects with congenital LQTS, the 95% LoA of difference between awECG-QTc and sECG-QTc appeared to be wider, although not statistically significant, in these patients. For subjects with LQTS, the 95% LoA of difference between awECG-QTc and sECG-QTc was −20.78 to 81.20 ms for lead I, −44.17 to 63.68 ms for lead II, and −25.69 to 52.44 ms for lead V5. On the other hand, for subjects without LQTS, the 95% LoA of difference between awECG-QTc and sECG-QTc was −15.95 to 48.97 ms for lead I, −26.06 to 47.31 ms for lead II, and −26.99 to 40.93 ms for lead V5.
3.5. T Wave morphologies in awECG and sECG
The T wave morphologies in the sECG and awECG tracings are shown in supplementary Table 2. The concordance in findings in terms of the absence or presence of abnormal T wave morphologies between the two tracings was 95% (18/19), and in terms of the exact T wave morphologies was 84% (16/19).
Table 2.
Cutoff QTc interval, sensitivity, specificity, and positive and negative predictive values of using awECG for the diagnosis of prolongation of QTc interval and identification of patients with congenital LQTS.
| Cutoff (ms) | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Detection of prolongation of QTc | ||||||
| Male | ||||||
| I | 454 | 0.98 | 100 | 88.2 | 80.0 | 100 |
| II | 467 | 0.99 | 100 | 94.4 | 85.7 | 100 |
| V5 | 451 | 0.99 | 100 | 94.4 | 87.5 | 100 |
| Female | ||||||
| I | 478 | 0.97 | 100 | 88.3 | 75.0 | 100 |
| II | 464 | 0.92 | 100 | 80.0 | 75.0 | 100 |
| V5 | 476 | 0.95 | 100 | 87.5 | 80.0 | 100 |
| Identification of patients with congenital LQTS | ||||||
| Male | ||||||
| I | 454 | 0.98 | 100 | 88.2 | 80.0 | 100 |
| II | 467 | 0.89 | 87.5 | 94.1 | 87.5 | 94.1 |
| V5 | 451 | 0.87 | 85.7 | 94.1 | 85.7 | 94.1 |
| Female | ||||||
| I | 463 | 0.99 | 100 | 92.3 | 91.7 | 100 |
| II | 464 | 0.97 | 100 | 92.3 | 91.7 | 100 |
| V5 | 460 | 0.98 | 100 | 92.3 | 91.7 | 100 |
Abbreviations: AUC, area under receiver operating characteristic curve, awECG, Apple Watch electrocardiogram, LQTS, long QT syndrome, NPV, negative predictive value, PPV, positive predictive value.
There was substantial interobserver agreement on the interpretation of T wave morphologies in the awECG tracings with a Cohen’s kappa coefficient of 0.68. The concordance in findings in terms of the absence or presence of abnormal T wave morphologies between the two observers was 89% (17/19), and in terms of the exact T wave morphologies was 68% (13/19).
3.6. Detection of QTc prolongation and identification of patients with LQTS based on awECGs
Receiver operating characteristic analysis revealed that the three leads of awECG tracings had excellent abilities with areas under the ROC curve ranging from 0.87 to 0.99 (Table 2) to discriminate between patients with and without prolongation of QTc interval, and those with and without congenital LQTS.
Table 2 shows the sensitivity, specificity, and positive and negative predictive values of the optimal awECG-derived QTc interval cutoff values, which were higher in females than males. For detection of prolongation of QTc interval, in both males and females, all three leads had a sensitivity and negative predictive value of 100%. Among the three leads, the awECG lead V5 had the best specificity (94.4% and 87.5%, respectively) and positive predictive value (87.5% and 80.0%, respectively) in both males and females. For identification of patients with congenital LQTS, awECG lead I had a sensitivity and negative predictive value of 100% in both males and females. On the other hand, the awECG lead II and V5 had the greatest specificity (92.3% to 94.1%) and positive predictive value (85.7% to 91.7%) in both males and females.
4. Discussion
The present study demonstrates that awECG tracings can be used 1) for the measurement of QTc interval in a reproducible and accurate fashion in children and adolescents, 2) to ascertain abnormal T wave morphologies in congenital LQTS with substantial agreement between observers, and 3) to detect prolongation of QTc interval and identification of patients with congenital LQTS with high sensitivity and specificity (both >85%) based on awECG lead V5 in both males and females. To the best of our knowledge, this is the first study to evaluate the usefulness of Apple Watch to detect prolongation of QTc interval in paediatric patients with congenital LQTS.
Data on the potential usefulness of Apple Watch in adults for the measurement of QTc interval are accumulating. Strik et al. measured QTc intervals using Apple Watch in adult patients from the outpatient or emergency department and reported high interobserver agreement, with an intraclass correlation coefficient of 0.92, and a measurement bias of ≤ 11 ms (with 95% LoAs between −67 to 54 ms) for awECG lead I, II, and V6 tracings compared with sECG measurements [7]. Spaccarotella et al. similarly reported in adult patients good interobserver agreement, with a Cohen’s kappa coefficient of 0.619, and a measurement bias of ≤15 ms (with 95% LoAs between −44 to 73 ms) for awECG lead I, II, and V2 tracings [8].
There is paucity of paediatric data on the comparison of QTc interval between awECG and sECG tracings. Kobel et al. compared awECG lead I QT intervals, among other parameters, against the sECG QT intervals in children with and without congenital heart disease and reported correlation coefficients of 0.827 and 0.830 in children with structurally normal heart and those with congenital heart disease, respectively [14]. However, inspection of their Bland-Altman plot revealed that the measurement bias was as high as −150 ms for a patient with an average QT interval of about 580 ms. The present study systemically evaluated for the first time QTc intervals acquired from three different leads by placement of the Apple Watch in different body positions, measured using an electronic caliper, and obtained from children and adolescents with and without congenital LQTS. We showed excellent intra- and interobserver agreements in the measurement of QTc interval and feasibility of obtaining optimal awECG tracings in more than 95% of patients for each of the three lead positions. Importantly, we found the QTc measurements based on awECG leads II and V5 tracings had a bias of ≤11 ms (with 95% LoAs between –33 and 53 ms), which was similar to that reported in adults [7], [8]. On the other hand, measurements based on awECG lead I had the highest average bias of 21 ms. The usually higher amplitude of T wave and the more obvious T wave offset in awECG lead II and V5 tracings probably account for their smaller measurement bias, rendering them more useful for the evaluation of QTc interval. The similar finding of Strik et al. of higher T wave amplitude in left lateral praecordial lead compared with that in other leads [7] lends support to our proposition.
Few studies have focused on the detection of prolongation of QTc interval. In adult patients admitted to hospital for acute coronary syndrome and other cardiac conditions, a sensitivity of 69% and specificity of 88% in detecting prolongation of QTc interval (>460 ms) using awECG lead I, II, and V2 tracings have been reported [8]. The causes of prolongation of QTc interval are, however, unclear. In a study of sudden cardiac arrest-associated abnormalities in young adults, Nasarre et al. reported that the combination of awECG leads I, V1, V3, and V6 tracings has a sensitivity of 90% and a specificity of 100% in the diagnosis of prolonged QT intervals in 10 of their adult patients with LQTS, although details on the reproducibility and bias in QT and QTc measurements are lacking [18].
In children and adolescents, the present study shows a reassuringly high sensitivity and specificity (both >87%) in the detection of QTc prolongation based on the awECG lead V5 tracing in both males and females. The >87% specificity, while still relatively high but not approaching 100%, can be accounted by the tendency of the Apple Watch to overestimate the QTc interval. Given these findings, it can be speculated that extra resources would be required for assessment of false positive results if the smartwatch is to be used for the purpose of screening for prolongation of QTc interval. We have further explored using awECG-derived QTc interval to identify patients with congenital LQTS, clinically diagnosed based on Schwartz score [16]. Interestingly, prolongation of QTc interval based on awECG leads II and V5 tracings has sensitivity and specificity exceeding 85% in identifying congenital LQTS in our patient cohort.
Genotype-specific T wave morphologies are well described in patients with LQT1 (early-onset, broad-based T waves), LQT2 (asymmetric, low-amplitude, bifid or notched T waves), and LQT3 (long isoelectric ST segments with late-appearing normal morphology T waves) [19]. T wave analysis may be helpful in the diagnosis of concealed LQTS without prolongation of QTc interval and provide clues to the underlying genotypic abnormalities [19], [20]. The present study provides evidence of potential usefulness of using awECG tracings in defining the presence and the type of T wave abnormalities that could draw inferences to the underlying subtype of LQTS.
The findings of the present study have clinical and translational implications. First, the value of telemedicine and remote monitoring has been shown during the COVID-19 pandemic [21]. The validation in the present study of the measurement of QTc interval and assessment of T wave morphologies based on awECG tracings in children and adolescents paves the way for innovative approaches to the care of paediatric patients with cardiac arrhythmias. Second, it would have been ideal to be able to couple acquisition of smartwatch ECG tracings with automated measurement of QTc interval and interpretation of T wave morphologies utilizing artificial intelligence in one goal. Nonetheless, Mannhart et al. reported automated calculation of QTc interval was only successful in 56% of adult patients assessed using the Withings Scanwatch (Withings SA, Issy les Moulineaux, France) [22]. Further works are required to refine algorithms for automated measurement of smartwatch-derived QTc interval. Third, the Apple Heart Study has provided data to suggest the ability of an algorithm to identify atrial fibrillation in users whom it notifies of irregular pulses [23]. An algorithm based on awECG tracings for identification of prolonged QTc interval and features of congenital LQTS (prolonged QTc with abnormal T wave morphologies) may perhaps also be a real possibility in due course.
Several limitations to this study warrant comments. First, the number of patients with congenital LQTS is relatively small. Notwithstanding, the awECG tracings could still be demonstrated to strongly discriminate patients with from those without prolongation of QTc interval. Second, patients with congenital LQTS in our cohort were on medications, which may perhaps confound the identification of congenital LQTS but should not affect the comparison of sECG-QTc and awECG-derived QTc intervals. Third, the sECG and awECG tracings were performed sequentially rather than simultaneously, with possible variations in heart rate and QT interval. Nonetheless, as awECG was immediately performed after sECG, the discrepancy should be minimal.
In conclusion, awECG leads II and V5 tracings can be used for reproducible and accurate measurement of QTc interval, ascertainment of abnormal T wave morphologies, and detection of prolonged QTc interval in children and adolescents with congenital LQTS.
CRediT authorship contribution statement
Jennifer Yee-ming Li: Conceptualization, Methodology, Data curation, Formal analysis, Writing-original draft, Writing-review and editing. Sit-yee Kwok: Conceptualization, Methodology, Data curation, Formal analysis, Writing-review and editing. Sabrina Tsao: Methodology, Data curation, Formal analysis, Writing-review and editing. Charis Hoi-yan Chung: Data curation, Formal analysis, Writing-review and editing. Wilfred Hing-sang Wong: Data curation, Formal analysis, Supervision, Writing-review and editing. Yiu-fai Cheung: Conceptualization, Methodology, Resources, Software, Supervision, Validation, Writing-review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Nil.
Sources of funding
Nil.
Registration number
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101232.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- 1.Schwartz P.J., Crotti L., Insolia R. Long-QT syndrome: from genetics to management. Circ. Arrhythm. Electrophysiol. 2012;5(4):868–877. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz P.J., Stramba-Badiale M., Crotti L., Pedrazzini M., Besana A., Bosi G., Gabbarini F., Goulene K., Insolia R., Mannarino S., Mosca F., Nespoli L., Rimini A., Rosati E., Salice P., Spazzolini C. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120(18):1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwok S.Y., Liu A.PY., Chan C.YY., Lun K.S., Fung J.LF., Mak C.CY., Chung B.HY., Yung T.C. Clinical and genetic profile of congenital long QT syndrome in Hong Kong: a 20-year experience in paediatrics. Hong Kong Med. J. 2018 doi: 10.12809/hkmj187487. [DOI] [PubMed] [Google Scholar]
- 4.Hofman N., Wilde A.A.M., Kaab S., van Langen I.M., Tanck M.W.T., Mannens M.M.A.M., Hinterseer M., Beckmann B.-M., Tan H.L. Diagnostic criteria for congenital long QT syndrome in the era of molecular genetics: do we need a scoring system? Eur. Heart J. 2007;28(5):575–580. doi: 10.1093/eurheartj/ehl355. [DOI] [PubMed] [Google Scholar]
- 5.Quaglini S., Rognoni C., Spazzolini C., Priori S.G., Mannarino S., Schwartz P.J. Cost-effectiveness of neonatal ECG screening for the long QT syndrome. Eur. Heart J. 2006;27:1824–1832. doi: 10.1093/eurheartj/ehl115. [DOI] [PubMed] [Google Scholar]
- 6.ECG app marketing authorization letter, Food and Drug Administration. https://www.accessdata.fda.gov/cdrh_docs/pdf18/DEN180044.pdf. Published September 11, 2018. Accessed December 26, 2021.
- 7.Strik M., Caillol T., Ramirez F.D., Abu-Alrub S., Marchand H., Welte N., Ritter P., Haïssaguerre M., Ploux S., Bordachar P. Validating QT-Interval measurement using the Apple Watch ECG to enable remote monitoring during the COVID-19 pandemic. Circulation. 2020;142(4):416–418. doi: 10.1161/CIRCULATIONAHA.120.048253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaccarotella C.A.M., Migliarino S., Mongiardo A., Sabatino J., Santarpia G., De Rosa S., Curcio A., Indolfi C. Measurement of the QT interval using the Apple Watch. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-89199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samol A., Bischof K., Luani B., Pascut D., Wiemer M., Kaese S. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: A new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors (Basel) 2019;19:4377. doi: 10.3390/s19204377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaccarotella C.A.M., Polimeni A., Migliarino S., Principe E., Curcio A., Mongiardo A., Sorrentino S., De Rosa S., Indolfi C. Multichannel electrocardiograms obtained by a smartwatch for the diagnosis of ST-segment changes. JAMA Cardiol. 2020;5(10):1176. doi: 10.1001/jamacardio.2020.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Zande J., Strik M., Dubois R., Ploux S., Alrub S.A., Caillol T., Nasarre M., Donker D.W., Oppersma E., Bordachar P. Using a smartwatch to record precordial electrocardiograms: a validation study. Sensors (Basel) 2023;23(5):2555. doi: 10.3390/s23052555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waddell‐Smith K., Gow R.M., Skinner J.R. How to measure a QT interval. Med. J. Aust. 2017;207(3):107–110. doi: 10.5694/mja16.00442. [DOI] [PubMed] [Google Scholar]
- 13.Mönnig G., Eckardt L., Wedekind H., et al. Electrocardiographic risk stratification in families with congenital long QT syndrome. Eur. Heart J. 2006;27:2074–2080. doi: 10.1093/eurheartj/ehl159. [DOI] [PubMed] [Google Scholar]
- 14.Kobel M., Kalden P., Michaelis A., Markel F., Mensch S., Weidenbach M., Riede F.T., Löffelbein F., Bollmann A., Shamloo A.S., Dähnert I., Gebauer R.A., Paech C. Accuracy of the Apple Watch iECG in children with and without congenital heart disease. Pediatr. Cardiol. 2022;43(1):191–196. doi: 10.1007/s00246-021-02715-w. [DOI] [PubMed] [Google Scholar]
- 15.Paech C., Kobel M., Michaelis A., Gebauer R.A., Kalden P., Dähnert I., Thome U., Markel F., Rützel S. Accuracy of the Apple Watch single-lead ECG recordings in pre-term neonates. Cardiol. Young. 2022;32(10):1633–1637. doi: 10.1017/S1047951121004765. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz P.J., Ackerman M.J. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur. Heart J. 2013;34(40):3109–3116. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 17.Rautaharju P.M., Surawicz B., Gettes L.S., et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 18.Nasarre M., Strik M., Daniel Ramirez F., Buliard S., Marchand H., Abu-Alrub S., Ploux S., Haïssaguerre M., Bordachar P. Using a smartwatch electrocardiogram to detect abnormalities associated with sudden cardiac arrest in young adults. Europace. 2022;24(3):406–412. doi: 10.1093/europace/euab192. [DOI] [PubMed] [Google Scholar]
- 19.Porta-Sánchez A., Spillane D.R., Harris L., et al. T-Wave morphology analysis in congenital long QT syndrome discriminates patients from healthy individuals. JACC Clin Electrophysiol. 2017;3:374–381. doi: 10.1016/j.jacep.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Sugrue A., Noseworthy P.A., Kremen V., Bos J.M., Qiang B.o., Rohatgi R.K., Sapir Y., Attia Z.I., Brady P., Asirvatham S.J., Friedman P.A., Ackerman M.J. Identification of concealed and manifest long QT syndrome using a novel T wave analysis program. Circ. Arrhythm. Electrophysiol. 2016;9(7):e003830. doi: 10.1161/CIRCEP.115.003830. [DOI] [PubMed] [Google Scholar]
- 21.Behar J.A., Liu C., Kotzen K., Tsutsui K., Corino V.D.A., Singh J., Pimentel M.A.F., Warrick P., Zaunseder S., Andreotti F., Sebag D., Kopanitsa G., McSharry P.E., Karlen W., Karmakar C., Clifford G.D. Remote health diagnosis and monitoring in the time of COVID-19. Physiol. Meas. 2020;41(10):10TR01. doi: 10.1088/1361-6579/abba0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannhart D., Hennings E., Lischer M., Vernier C., Du Fay de Lavallaz J., Knecht S., Schaer B., Osswald S., Kühne M., Sticherling C., Badertscher P. Clinical validation of automated corrected QT-interval measurements from a single lead electrocardiogram using a novel smartwatch. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.906079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez M.V., Mahaffey K.W., Hedlin H., Rumsfeld J.S., Garcia A., Ferris T., Balasubramanian V., Russo A.M., Rajmane A., Cheung L., Hung G., Lee J., Kowey P., Talati N., Nag D., Gummidipundi S.E., Beatty A., Hills M.T., Desai S., Granger C.B., Desai M., Turakhia M.P. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.