Abstract
Introduction
Underreporting is a major limitation of the voluntary reporting system of adverse drug reactions (ADRs). A 2009 systematic review showed the knowledge and attitudes of health professionals were strongly related with underreporting of ADRs.
Objective
Our aim was to update our previous systematic review to determine factors (sociodemographic, knowledge and attitudes) associated with the underreporting of ADRs by healthcare professionals.
Methods
We searched the MEDLINE and EMBASE databases for studies published between 2007 and 2021 that met the following inclusion criteria: (1) published in English, French, Portuguese or Spanish; (2) involving health professionals; and (3) the goal was to evaluate factors associated with underreporting of ADRs through spontaneous reporting.
Results
Overall, 65 papers were included. While health professional sociodemographic characteristics did not influence underreporting, knowledge and attitudes continue to show a significant effect: (1) ignorance (only serious ADRs need to be reported) in 86.2%; (2) lethargy (procrastination, lack of interest, and other excuses) in 84.6%; (3) complacency (the belief that only well tolerated drugs are allowed on the market) in 46.2%; (4) diffidence (fear of appearing ridiculous for reporting merely suspected ADRs) in 44.6%; and (5) insecurity (it is nearly impossible to determine whether or not a drug is responsible for a specific adverse reaction) in 33.8%, and the absence of feedback in 9.2%. In this review, the non-obligation to reporting and confidentiality emerge as new reasons for underreporting.
Conclusions
Attitudes regarding the reporting of adverse reactions continue to be the main determinants of underreporting. Even though these are potentially modifiable factors through educational interventions, minimal changes have been observed since 2009.
Clinical Trials Registration
PROSPERO registration number CRD42021227944.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-023-01302-7.
Key Points
| Underreporting is the major limitation of the voluntary reporting system of adverse drug reactions (ADRs). |
| Our 2009 systematic review showed the knowledge and attitudes of health professionals were strongly related with underreporting of ADRs. |
| This present systematic review shows, nowadays, knowledge and attitudes continue to be the main determinants of underreporting, even though they are easily modifiable factors. |
Introduction
Adverse drug reactions (ADRs) are responsible for 10% of outpatient appointments [1, 2] and 3.5–10% of hospital admissions [1, 2], and are the fifth leading cause of death in hospitalized patients, in addition to prolonging stays and presenting a high economic impact [3, 4]. The spontaneous reporting of suspected ADRs by health professionals allows continuous determination of the benefit–risk ratio of a given drug and is one of the best methods to generate signals regarding unexpected events and rare ADRs [1, 5].
Underreporting is a major limitation of spontaneous notification systems, as it is estimated that only 6–10% of all ADRs are reported [6, 7]. On the one hand, this high underreporting rate prevents ADRs from being quantified in order to calculate their impact in terms of incidence and risk [6], and on the other hand, it delays the activation of warning signals, with the consequent repercussions on public health [5, 6, 8]. These delays in decisions to restrict a drug’s use or to withdraw it may result in many more patients being affected [5].
In 2009, our group carried out a systematic review on factors associated with the underreporting of ADRs [8], which showed that a high proportion of studies found that the main factors related to underreporting are the knowledge and attitudes of health professionals. We believe that an update of this review may be of interest for several reasons: (1) numerous studies have been published since 2006; (2) we wanted to check whether these studies have identified new factors related to underreporting; and (3) in our 2009 review, methodological problems had been identified (many studies do not specify the type of design, or have a low percentage of participation and response) and we consider that it would be of interest to assess whether the methodological quality has improved. In the current manuscript, we update our previous review [8] on the factors (socioprofessional characteristics, knowledges, attitudes) associated with underreporting of ADRs by health professionals.
Methods and Data Extraction
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020, 27-item checklist guideline, and its research protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (registry number CRD42021227944).
Bibliographic Search Method
A bibliographic search of studies published between January 2007 and February 2021 was performed in the MEDLINE (Pubmed) and EMBASE scientific databases. The search terms used were (‘attitude*’ OR ‘knowledge*’ OR ‘barrier*’ OR ‘facilitators*’) AND (‘adverse drug reaction reporting systems’ [MeSH] OR ‘drug related side effects and adverse reactions’ [MeSH]) AND ‘report*’.
Studies were required to meet the following inclusion criteria: (1) they were published in English, French, Portuguese or Spanish; (2) the study population was defined as healthcare professionals (doctors, pharmacists, nurses, administrators, residents, and other health professionals); (3) articles whose objective was to evaluate the factors associated with ADR underreporting; and (4) articles that address ADR reporting through spontaneous reporting.
Studies were excluded if they met any of the following criteria: (1) did not have an abstract and/or full text; (2) conference abstracts, thesis, comments, letters, abstracts, or editorials; (3) systematic reviews and/or meta-analyses; (4) articles on a specific pathology and/or treatment; (5) were carried out on patients and/or consumers; (6) did not focus on the study objective; (7) identified attitudes and knowledge but were not directly associated with the reasons for underreporting.
Quality Evaluation
An assessment of the quality and risk of bias of the articles was performed using the AXIS cross-sectional study assessment tool [9]. This tool contains 20 questions, 10 related to the methods, 5 related to the results,which indicates the importance of these sections in quality, 1 to the introduction, and 4 to the discussion (Table 1).
Table 1.
Reasons for ADR underreporting based on Inman´s ‘seven deadly sins’ and others identified in other systematic reviews and included studies
| Reasons | Explanation | No. of studies | |
|---|---|---|---|
| Total [N (%)] | 50% with more qualitya [N (%)] | ||
| Professional activities | |||
| Ambitionb | To publish personal case series | 2 (3.1) | 1 (3.1) |
| Financial reimbursementb | Belief that there should be financial incentives or a reward for reporting | 14 (21.5) | 8 (25.0) |
| Legal aspectsb | Fear of possible involvement in a lawsuit | 19 (29.2) | 9 (28.1) |
| Knowledge and attitudes | |||
| Fearb |
Fear of harming the patient Fear of damaging relationships and negative impact on the company that produced or marketed the drug Fear of confidentiality issues |
7 (10.8) | 2 (6.3) |
| Complacencyb | Conviction that only well tolerated drugs are on the market and that serious ADRs are well documented when the drug is marketed | 30 (46.2) | 14 (43.8) |
| Ignoranceb |
Lack of knowledge to recognize the ADR and its importance Lack of knowledge about the requirements needed to report, where to report, how to describe the notification, and how the information is further used; belief that only serious or unexpected ADRs should be reported |
56 (86.2) | 29 (90.6) |
| Indifferenceb | Belief that one case could not contribute to medical knowledge | 18 (27.7) | 11 (34.4) |
| Diffidenceb |
Lack of confidence and fear of appearing ridiculous for making a report of ADRs merely based on a suspicion Only reports if it is sure that it was drug-related |
29 (44.6) | 13 (40.6) |
| Insecurityc | The belief that it is nearly impossible to be certain that a particular drug was responsible for causing a specific adverse reaction | 22 (33.8) | 11 (34.4) |
| Unavailability of the reporting formc |
Do not have access to the report form Reporting forms are not available when needed |
22 (33.8) | 8 (25.0) |
| Excuses | |||
| Lethargyb |
Procrastination and postponing the notification Lack of time or motivation, effort, or interest to report Need for an easier method The report will generate extra work Forgetfulness |
55 (84.6) | 26 (81.3) |
| Othersb | Lack of feedback on the report submitted (Feedback) | 6 (9.2) | 2 (6.3) |
| Stopped taking the medicine | 38 (58.5) | 21 (65.6) | |
| ADR resolved | |||
| Lack of clinical training | |||
| Lack of communication | |||
| Lack of enough information about the patient for making a report (Information) | 13 (20.0) | 5 (15.6) | |
| Not their responsibility for reporting (Obligation) | 12 (18.5) | 8 (25.0) | |
|
Lack of confidence in the regulatory authority Reporting of ADR affects patient confidentiality issues (Confidentiality) |
6 (9.2) | 4 (12.5) | |
ADRs adverse drug reactions
aDefined by the number of AXIS criteria met by each paper
bProposed by Inman
cDescribe in the literature
Of the 20 questions, 7 evaluated the quality of reporting (1, 4, 10, 11, 12, 16 and 18), 7 evaluated the quality of the study design (2, 3, 5, 8, 17, 19 and 20), and 6 evaluated the possible introduction of biases in the study (6, 7, 9, 13, 14 and 15) [9].
Two reviewers (CC and PGA) independently conducted the critical assessment for each included study. In the case of disagreement, a third reviewer (CT, MTH, or AF) was responsible for resolving discrepancies.
Data Extraction
All articles retrieved were independently screened by two reviewers (CC and PGA), who further independently conducted the full-text analysis to assess suitability for the inclusion of each potentially eligible study. In the case of any divergent decisions, a third reviewer (CT, MTH, or AF) acted as referee to reach consensus.
The data extracted from the articles were entered into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA) and two tables were created. In the first table, the author (publication year), country, study population, workplace, sample size, survey distribution, survey scale and AXIS not fulfilled criteria were included (Online Resource Table S1).
The second table contained the author (publication year), response rate, personal and professional factors associated with ADR reporting, and reasons for not reporting the ADR (Online Resource Table S2). All the reasons cited have been extracted from the results of the studies and have been assigned to one of Inman’s ‘deadly sins’ [10–12]. In 1976, Inman proposed a theoretical model to explain the reasons for ADR underreporting by health professionals. This model was called Inman’s ‘seven deadly sins’ and included complacency diffidence, indifference, ignorance, ambition, financial reimbursement, legal aspects, fear, and feedback. The model has been modified and expanded, including insecurity, unavailability of notification form, and lethargy [10–12]. These reasons can be grouped into attitudes related to the professional activity (legal fears, economic interests, and ambition to publish), ADR-related knowledge and attitudes (complacency, diffidence, indifference, ignorance, and insecurity), and related to excuses (lethargy) [8].
The median of respondents who stated that this attitude determines their ADR reporting was calculated for each of the attitudes (Online Resource Table S3).
Sensitivity Analysis
In order to assess the impact of the quality of the articles included in the results of the review, a sensitivity analysis was performed. This subanalysis included articles whose AXIS score was above the median of the total studies.
Results
Article Selection
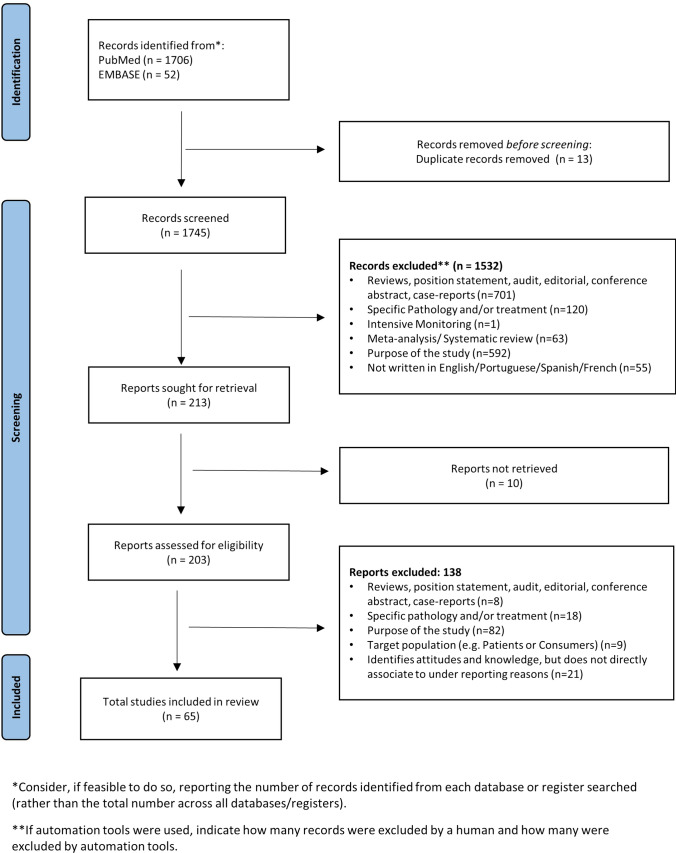
Using the chosen search strategy, 1758 articles were located—1706 in MEDLINE and 52 in EMBASE (see Fig. 1). After reading the abstracts, it was considered that 203 articles met the inclusion criteria, and a complete full-text analysis of these was carried out. After a full-text reading, 138 articles were excluded because they did not meet the inclusion criteria, and 65 articles were selected—57 from MEDLINE and 8 from EMBASE (Fig. 1)
Fig. 1.
Study identification process
Characteristics of the Selected Articles
More than half of the articles (45/65, 69.2%) were cross-sectional studies [13, 14, 16–19, 21–24, 26–39, 41, 42, 44, 45, 47–51, 54–56, 59–61, 64, 65, 69–71, 74], 21.5% did not indicate the design type used [20, 25, 52, 57, 58, 62, 63, 66–68, 72, 73, 75, 76], 4.6% were case-control studies [40, 46, 77] (during the same period, the cases had reported ADR and the controls had not), 3.1% (2/65) were mixed studies [15, 43] (only data from the quantitative part were used), and one (1.5%) was an intervention study [53] (pre-intervention data were extracted). Two studies were conducted before a pharmacovigilance system was in place [17, 31].
According to geographical distribution, we located studies carried out in 36 countries: 38.5% were carried out in Asia [19, 22, 28, 29, 31, 32, 35, 37–39, 44, 46, 47, 50–53, 56, 58, 59, 61, 63–65, 72], 27.7% in Africa [13–15, 17, 18, 23, 27, 30, 34, 42, 45, 48, 49, 65, 69–71, 74], 24.6% in Europe [20, 21, 24, 25, 33, 40, 43, 54, 55, 57, 60, 62, 66, 73, 75, 77], 7.7% in America [16, 36, 41, 67, 76], and one in Australia [27].
Regarding the setting where the study was performed, 43.1% took place in hospitals [14, 15, 17–19, 22, 24, 30, 32, 35, 41–44, 46–48, 53, 56, 59, 63, 64, 66, 68–70, 73, 74], 21.5% in pharmacies [20, 21, 26, 31, 33, 38, 39, 51, 61, 65, 72, 75–77], 9.2% did not mention the place [16, 25, 52, 55, 57, 62], 6.2% in hospitals and pharmacies [28, 50, 67, 71], and 6.2% in primary care [13, 23, 27, 29]. In 10 articles, the results of surveys carried out in two different health facilities are combined [28, 34, 36, 37, 40, 49, 50, 54, 67, 71].
Regarding the study population, pharmacists were the most studied [20, 21, 26, 28, 31, 33, 39, 46, 50–52, 61, 65, 67, 68, 72, 75–77], followed by doctors, pharmacists and nurses together [13, 14, 19, 22, 27, 30, 36, 41, 44, 45, 49, 56, 64], and lastly, doctors [15, 37, 48, 54, 55, 58, 60, 63, 66, 70, 73]. In 30 articles, the survey was conducted on two or more types of professionals [13, 14, 16–19, 22–25, 27, 29, 30, 32, 34–36, 38, 41, 42, 44, 45, 47, 49, 53, 56, 57, 62, 64, 71] and in one study the survey was conducted on health professionals and patients [16].
Sample Size and Response Rate
The sample size (see Online Resource Table S1) varied between 80 [23, 77] and 3351 [57]. A total of 41 articles presented a sample of more than 200 subjects [13–15, 19–22, 24, 26, 28–33, 35–43, 45–49, 51–53, 55–58, 60–62, 65–69, 71–75], 9 had more than 1000 subjects [19, 39, 40, 49, 57, 62, 67, 73], and 3 did not mention the sample size [16, 25, 44].
In the articles that mentioned response rate, this varied between 16.4% [67] and 100% [17, 22, 23, 34, 63, 76] (see Online Resource Table S2). More than half of the articles had response rates > 50% [13–15, 17–24, 28–35, 37, 38, 41–44, 46, 48–50, 54, 56, 58, 59, 61, 63–65, 68, 70–77], and six reached 100% [17, 22, 23, 34, 63, 76]; nine articles did not mention the response rate [16, 26, 27, 39, 45, 47, 62, 66, 69].
Survey Distribution and Scale
The survey was distributed personally in more than half of the articles [13–15, 17–20, 22–24, 27–31, 37–39, 41, 42, 44, 47–51, 53, 54, 56, 58, 59, 61, 62, 64, 65, 72, 74, 76, 77], and the remainder were sent by internet [16, 25, 26, 33, 35, 36, 39, 52, 57, 62] or post [21, 33, 40, 46, 55, 60, 67, 73], while 10.8% did not mention how the survey was distributed [32, 34, 43, 45, 63, 69, 71]. In four articles, two or more distribution types were combined [33, 35, 39, 62] (see Online Resource Table S1)
The most used scale (Online Resource Table S1) to answer the survey questions was the Likert scale [15, 17–19, 21–23, 26–28, 32, 36, 38, 39, 43, 46, 49, 51, 55–57, 65, 66, 75, 77], followed by multiple-choice and free text [14, 24, 30, 37, 41, 50], while one article used the visual analog scale [40]. It is noteworthy that almost half (28/65) of the articles did not mention the scale used [13, 16, 20, 25, 29, 31, 34, 35, 42, 44, 45, 47, 48, 52–54, 58–60, 62, 64, 69–74, 76]. In 19 articles, multiple scales were used [14, 18, 21, 24, 26–28, 30, 37, 41, 43, 46, 50, 51, 55, 57, 66, 75, 77].
Factors Identified as Influential
Each of the selected articles was evaluated to identify the factors said to influence ADR underreporting.
Personal and Professional Factors
Regarding the personal and professional factors that influence ADR reporting (see Online Resource Table S2), years of experience stands out, with 12.3% (8 articles), training and profession were cited in 10.8% of the articles (7/65), age and workplace in 9.2% (6/65), qualification in 7.7% (5/65), and sex in 6.2% (4/65).
Influence of Knowledge and Attitudes
Table 1, Online Resource Table S2, and Online Resource Table S3 show that the most reported reasons by professionals for not reporting ADRs were lethargy and ignorance, followed by other reasons, such as lack of confidence and complacency: (1) ignorance in 56 (86.2%) articles, with an average of respondents who stated that attitude determines their ADR reporting (respondents average of 37.8%); (2) lethargy is described in 55 (84.6%) articles (respondents average of 33.3%); (3) other reasons (lack of internet access, lack of employer support, patient management is more important) in 38 (58.5%) articles (respondents average of 16.3%); (4) complacency (respondents average of 28.6%) in 30 (46.2%) articles; (5) diffidence (respondents average of 26.9%) in 29 (44.6%) articles; (6) unavailability of the reporting form (respondents average of 37.4%) and insecurity in 22 (33.8%) articles (respondents average of 34.4%); (7) legal aspects in 19 (29.2%) articles (respondents average of 19.5%); (8) indifference in 18 (27.7%) articles (respondents average of 23.5%); (9) financial reimbursement in 14 (21.5%) articles (respondents average of 22.2%); (10) lack of information in 13 (20.0%) articles (respondents average of 23.3%); (11) obligation or duty to inform in 12 (18.5%) articles (respondents average of 20.9%); (12) fear in 7 (10.8%) articles (respondents average of 24.5%); (13) confidentiality of both the patient and the professional (respondents average of 13.4%) and feedback in 6 (9.2%) articles (respondents average of 29.4%); and (14) ambition in 2 (3.1%) articles (respondents average of 15.9%).
AXIS Tool
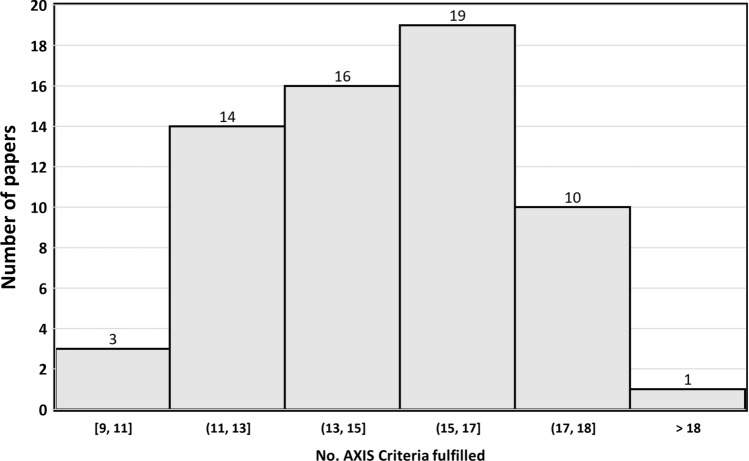
The results of AXIS tool evaluation (see Online Resource Table S1, and Fig. 2) indicated that 29.2% (19/65) of the studies met more than 17 AXIS criteria (see Table S1), almost half of the studies (29/65, 44.6%) met 14–16 criteria, and about one-quarter of the studies (17/65, 26.2%) met 13 or fewer criteria.
Fig. 2.
Distribution of papers by the number of AXIS criteria fulfilled
The criteria “Were the aims/objectives of the study clear?”, “Were the risk factor and outcome variables measured appropriate to the aim of the study”, and “Were the basic data adequately described?” were fulfilled in all articles.
In contrast, no articles met the criteria “If appropriate, was information about non-responders described?”, only 13 met the criteria “Were measures undertaken to address and categorize non-responders?”, and 34 met the criteria “Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation?”.
Sensitivity Analysis: Results for the Highest-Quality Studies
The median AXIS score was 16 criteria. Table 1 shows the most frequent criteria among the 32 articles with the highest degree of evidence. It is observed that ignorance (90.6%), lethargy (81.3%), complacency (43.8%), and difference (40.6%) were the most named attitudes on the part of health professionals, results very similar to the total of the studies.
Discussion
This review was based on more studies than our 2009 review (65 vs. 45) [8] and has confirmed and extended the previous findings: health professionals’ attitudes to ADRs, modeled through Inman’s ‘seven deadly sins’, continue to be the most important determinants of underreporting, and personal and professional characteristics continue to show little influence. In addition, the new information included in the current review allowed to associate the perception of absence of an obligation to notify and the lack of information and confidentiality with the underreporting. These results can help design interventions that improve ADR reporting, since the main reasons found in this review are potentially modifiable.
Sociodemographic and professional characteristics are only cited as factors associated with the reporting of ADRs in fewer than half of the articles included and yet attitudes and knowledge are cited in one way or another in almost all the articles. These factors can be grouped into four categories [8, 78]: (1) factors associated with ADR-related knowledge and attitudes (fear, complacency, insecurity, diffidence, indifference, and ignorance); (2) factors relating to the professional activity (financial incentives, legal aspects, and ambition to publish); (3) excuses made by professionals (lethargy, unavailability of the reporting form); and (4) there is another group of factors, such as the absence of an obligation to notify, lack of information or confidentiality, which emerge in this review as being associated with underreporting.
Adverse Drug Reaction-Related Knowledge and Attitudes
Inman’s knowledge/attitude related to ‘sins’ show associations with underreporting in a high proportion of studies. Thus, ignorance about pharmacovigilance, how to recognize ADRs, which types and how they should be reported, as well as the requirements needed and what has to be done with the submitted report, was the attitude that most conditioned underreporting in our review (52/65), which is consistent with other systematic reviews [8, 79, 80]. Related to ignorance is complacency, which is based on the conviction that adverse reactions of medication are known when the drug comes on to the market and that only well tolerated medications are marketed, and appears to be associated with reporting of a figure very similar to that of the previous revision of 2009. This seems to indicate that there have been no significant advances in the training of health professionals on ADR since ignorance and complacency are potentially modified through training on undergraduate[81, 82] and postgraduate pharmacovigilance education [81]. In this sense, educational sessions (workshops or lectures) [83–87] showed a positive impact on ADR reporting. Nonetheless, the increased rate of SR originated was shown to decrease over time [83, 84, 88]. Consequently, interventions should be carried out periodically and not only reinforce what has been learned but also provide information on possible updates that may have occurred. It could also be interesting to try nudge strategies, which have been shown to be effective in improving the behaviors of health professionals in other fields [89].
A diffidence attitude results in the lack of confidence and fear of appearing ridiculous (a sentiment of ‘foolish’) either by the diagnosis made or for reporting a suspicious ADR, hence the thought to only report if the health professional is totally sure the ADR was due to a given medicine. This reason can be related to the thought that it is impossible to have certainty and the concern that the report may be wrong (insecurity). Once again, we would like to emphasize the importance of education and practical training with constant contact with ADR cases to further improve confidence and certainty.
Factors Related to Professional Activity
Compared with the 2009 review, a lower proportion of legal aspects have been reported (29/45 vs. 19/65). However, in this review, a new factor related to legal aspects that had not been detected in the previous review has emerged: patient confidentiality, which appears in 9% of the articles. On the other hand, ‘Financial reimbursement’ is reported in a higher proportion (21% of articles) as a reason for not reporting an ADR compared with the previous review (11%). In fact, interventions based on economic incentives have been shown to be effective [90–92], which is consistent with the perception that reporting is not the health professionals professional duty, reported in 18% of articles.
Excuses
The third group encompasses attitudes related to excuses, such as lethargy (procrastination and postponing the notification, lack of time or motivation, effort or interest to report, need for an easier method, the report will generate extra work, and forgetfulness), which is the most frequent reason for not reporting (53/65; 81%). This can be combated by providing health professionals with training (emphasizing the sort time needed to report [84]), facilitating the notification process, or eliminating the barriers they have to notify ADRs [8]. Another frequent excuse is the unavailability of a yellow card/ADR form or a shipping address. In this sense, interventions based on the use of information systems (active or passive) and use of an electronic reporting tool has been seen to increase notification. Strategies such as web-based software, hyperlinks, telephone applications, online reporting form, and electronic health records are examples that have emerged with this advance [93–99].
Other Reasons
This was the third most cited reason by healthcare professionals and includes statements related to lack of feedback from the national regulatory authorities on the submitted form, lack of training, and lack of enough information about the patient to make the report. We believe this is understandable, i.e. the lack of feedback can discourage reporting as people expect to receive feedback to know if what they did was correct. Receiving personalized feedback on the reporting procedure has been described as a motive for reporting ADRs [21, 73, 100, 101]. The lack of information may, for example, be tackled by increasing the consultation time, sharing information from different databases, or even registering a telephone or email contact to clarify doubts at any time.
Methodology Discussion of the Included Studies
From the methodological point of view and compared with the previous review of 2009, a significant methodological improvement is observed in many aspects, fundamentally in terms of specification of the design (64/65 vs. 3/45 in the 2009 review) and sample size (41/65 articles have more than 200 subjects vs. 25/45 in the 2009 review). The percentage of responses is also higher among the articles included in this review (average of 75% vs. 64% in the 2009 review). Nevertheless, some AXIS factors, especially those linked to non-responders are still not sufficiently described. However sensitivity analysis to assess whether the results of the current review were influenced by the quality of the articles showed that the results for half of the studies that met more AXIS criteria were very similar to the overall review. Finally, the geographical distribution of our review focuses more on Asian and African countries, while the 2009 review focused on European countries. Despite this difference, the findings on the influence of attitudes are similar.
Limitations
This systematic review has several limitations. First, the great variety of methods used in the articles. The study population, the selected place/setting, the survey distribution, or the scale are very heterogeneous. In two articles, the study was carried out before the implementation of the pharmacovigilance system.
Second, it is common that when the study population includes various groups of health professionals, the results are not shown stratified by health professional type, which prevents us from detecting which factors are associated with each profession and makes comparison between studies difficult.
Third, regarding the reasons cited for underreporting, very few articles conducted the survey using Inman’s reasons as items. The definition of ‘sins’ was not exactly the same in all the articles and therefore the interpretations, both of the authors and those made in this systematic review, may differ from the reasons cited by Inman.
Finally, although an improvement in methodological quality is observed with respect to the 2009 review, great heterogeneity is observed in the fullfilment of the AXIS tool criteria.
Conclusion
The results of this review show that, as seen in the 2009 review, low ADR reporting is still associated with a series of attitudes (ignorance, lack of confidence, or complacency) and excuses that are potentially modifiable through training or by facilitating the notification process. This suggests that over the last decade there have been no significant advances in the training of health professionals on ADR reporting and that it is necessary for health systems and undergraduate (academy/training levels, or courses) centers to deepen the training of health professionals on the subject of drug safety. This would provide a greater capacity to activate early warnings to the pharmacovigilance systems and thus allow the health authorities to react more quickly to ensure that the least possible number of patients are affected.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Elena Lopez-Gonzalez for her commentaries, and Michael Benedict for reviewing and revising the English.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Declarations
Funding
This study has been funded in part by the Instituto de Salud Carlos III (ISCIII) through the project PI19/01006, co-financed by FEDER, European Union.
Conflict of interest
Patricia García-Abeijon, Catarina Costa, Margarita Taracido, Maria Teresa Herdeiro, Carla Torre, and Adolfo Figueiras have no conflicts of interest that are directly relevant to the contents of this article.
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
Conceptualization: AF, CT, MTH, MT. Article screening: PGA, CC. Writing—original draft: PGA, CC. Writing—review and editing: MT, MTH, CT, AF. Final approval: PGA, CC, MT, MTH, CT, AF. All authors read and approved the final version of the manuscript.
References
- 1.Montané E, Santesmases J. Adverse drug reaction. Med Clin (Bar). 2020;154(5):178–1842. doi: 10.1016/j.medcli.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–989. doi: 10.1345/aph.1P627. [DOI] [PubMed] [Google Scholar]
- 3.Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–453. doi: 10.1007/s40264-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483–485. doi: 10.1001/jama.284.4.483. [DOI] [PubMed] [Google Scholar]
- 5.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998 doi: 10.1016/S0149-2918(98)80007-6. [DOI] [PubMed] [Google Scholar]
- 6.Hazell L, Shakir SAW. Under-reporting of adverse drug reactions. A systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Herdeiro MT, Figueiras A, Polónia J, et al. Physicians’ attitudes and adverse drug reaction reporting. Drug Saf. 2005;28:825–833. doi: 10.2165/00002018-200528090-00007. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6(12):e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inman WHW, Weber JCT. The United Kingdom. In: Inman WHW, editor. Monitoring for drug safety. 2. Lancaster: MTP Press Ltd; 1986. pp. 13–47. [Google Scholar]
- 11.Inman WHW. Assessment of drug safety problems. In: Gent M, Shigmatsu I, editors. Epidemiological issues in reported drug-induced illnesses. Honolulu: McMaster University Library Press; 1976. pp. 17–24. [Google Scholar]
- 12.Inman WH. Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol. 1996;41(5):434–435. doi: 10.1111/j.1365-2125.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 13.Haines HM, Meyer JC, Summers RS, Godman BB. Knowledge, attitudes and practices of health care professionals towards adverse drug reaction reporting in public sector primary health care facilities in a South African district. Eur J Clin Pharmacol. 2020;76(7):991–1001. doi: 10.1007/s00228-020-02862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gidey K, Seifu M, Hailu BY, Asgedom SW, Niriayo YL. Healthcare professionals knowledge, attitude and practice of adverse drug reactions reporting in Ethiopia: a cross-sectional study. BMJ Open. 2020;10(2):e034553. doi: 10.1136/bmjopen-2019-034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadew SS, Beyene KG, Beza SW. Adverse drug reaction reporting practice and associated factors among medical doctors in government hospitals in Addis Ababa, Ethiopia. PLoS ONE. 2020;15(1):1–19. doi: 10.1371/journal.pone.0227712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melo JRR, Duarte EC, de Araújo FK, et al. Under-reporting of adverse drug reactions among healthcare professionals in Brazil: an estimate based on national pharmacovigilance survey. J Young Pharm. 2020;12(4):360–365. doi: 10.5530/jyp.2020.12.92. [DOI] [Google Scholar]
- 17.Braiki R, Douville F, Hasine AB, Souli I. Facteurs liés au signalement des évènements indésirables associés aux soins dans un hôpital Tunisien. Sante Publique. 2019;31(4):553–559. doi: 10.3917/spub.194.0553. [DOI] [PubMed] [Google Scholar]
- 18.Kassa Alemu B, Biru TT. Health care professionals' knowledge, attitude, and practice towards adverse drug reaction reporting and associated factors at selected public hospitals in Northeast Ethiopia: a cross-sectional study. Biomed Res Int. 2019; 8690546. [DOI] [PMC free article] [PubMed]
- 19.Le TT, Nguyen TTH, Nguyen C, Tran NH, Tran LA, Nguyen TB, Nguyen N, Nguyen HA. Factors associated with spontaneous adverse drug reaction reporting among healthcare professionals in Vietnam. J Clin Pharm Ther. 2020;45(1):122–127. doi: 10.1111/jcpt.13037. [DOI] [PubMed] [Google Scholar]
- 20.Kopciuch D, Zaprutko T, Paczkowska A, Ratajczak P, Zielińska-Tomczak Ł, Kus K, Nowakowska E. Safety of medicines-pharmacists' knowledge, practice, and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Pharmacoepidemiol Drug Saf. 2019;28(12):1543–1551. doi: 10.1002/pds.4792. [DOI] [PubMed] [Google Scholar]
- 21.Hughes ML, Weiss M. Adverse drug reaction reporting by community pharmacists—the barriers and facilitators. Pharmacoepidemiol Drug Saf. 2019;28(12):1552–1559. doi: 10.1002/pds.4800. [DOI] [PubMed] [Google Scholar]
- 22.Danekhu K, Shrestha S, Aryal S, Shankar PR. Health-care professionals’ knowledge and perception of adverse drug reaction reporting and pharmacovigilance in a tertiary care teaching hospital of Nepal. Hosp Pharm. 2021;56(3):178–186. doi: 10.1177/0018578719883796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adisa R, Omitogun TI. Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, southwestern Nigeria. BMC Health Serv Res. 2019;19(1):926. doi: 10.1186/s12913-019-4775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ergün Y, Ergün TB, Toker E, Ünal E, Akben M. Knowledge attitude and practice of Turkish health professionals towards pharmacovigilance in a university hospital. Int Health. 2019;11(3):177–184. doi: 10.1093/inthealth/ihy073. [DOI] [PubMed] [Google Scholar]
- 25.Thompson A, Randall C, Howard J, Barker C, Bowden D, Mooney P, et al. Nonmedical prescriber experiences of training and competence to report adverse drug reactions in the UK. J Clin Pharm Ther. 2019;44(1):78–83. doi: 10.1111/jcpt.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Curtain C, Bereznicki L, Zaidi STR. Community pharmacists' knowledge and perspectives of reporting adverse drug reactions in Australia: a cross-sectional survey. Int J Clin Pharm. 2018;40(4):878–889. doi: 10.1007/s11096-018-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seid MA, Kasahun AE, Mante BM, Gebremariam SN. Healthcare professionals' knowledge, attitude and practice towards adverse drug reaction (ADR) reporting at the health center level in Ethiopia. Int J Clin Pharm. 2018;40(4):895–902. doi: 10.1007/s11096-018-0682-0. [DOI] [PubMed] [Google Scholar]
- 28.Bahnassi A, Al-Harbi F. Syrian pharmacovigilance system: a survey of pharmacists' knowledge, attitudes and practices. East Mediterr Health J. 2018;24(6):569–578. doi: 10.26719/2018.24.6.569. [DOI] [PubMed] [Google Scholar]
- 29.Lemay J, Alsaleh FM, Al-Buresli L, Al-Mutairi M, Abahussain EA, Bayoud T. Reporting of Adverse Drug Reactions in Primary Care Settings in Kuwait: A Comparative Study of Physicians and Pharmacists. Med Princ Pract. 2018;27(1):30–38. doi: 10.1159/000487236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terblanche A, Meyer JC, Godman B, Summers RS. Knowledge, attitudes and perspective on adverse drug reaction reporting in a public sector hospital in South Africa: baseline analysis. Hosp Pract (1995). 2017;45(5):238–245. doi: 10.1080/21548331.2017.1381013. [DOI] [PubMed] [Google Scholar]
- 31.Hajj A, Hallit S, Ramia E, Salameh P, Order of Pharmacists Scientific Committee – Medication Safety Subcommittee Medication safety knowledge, attitudes and practices among community pharmacists in Lebanon. Curr Med Res Opin. 2018;34(1):149–156. doi: 10.1080/03007995.2017.1361916. [DOI] [PubMed] [Google Scholar]
- 32.Abu Hammour K, El-Dahiyat F, Abu FR. Health care professionals knowledge and perception of pharmacovigilance in a tertiary care teaching hospital in Amman, Jordan. J Eval Clin Pract. 2017;23(3):608–613. doi: 10.1111/jep.12683. [DOI] [PubMed] [Google Scholar]
- 33.Cheema E, Haseeb A, Khan TM, Sutcliffe P, Singer DR. Barriers to reporting of adverse drugs reactions: a cross sectional study among community pharmacists in United Kingdom. Pharm Pract. 2017;15(3):931. doi: 10.18549/PharmPract.2017.03.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurmesa LT, Dedefo MG. Factors affecting adverse drug reaction reporting of healthcare professionals and their knowledge, attitude, and practice towards ADR reporting in Nekemte Town, West Ethiopia. Biomed Res Int. 2016;2016:5728462. doi: 10.1155/2016/5728462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almandil NB. Healthcare professionals' awareness and knowledge of adverse drug reactions and pharmacovigilance. Saudi Med J. 2016;37(12):1359–1364. doi: 10.15537/smj.2016.12.17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stergiopoulos S, Brown CA, Felix T, Grampp G, Getz KA. A survey of adverse event reporting practices among US healthcare professionals. Drug Saf. 2016;39(11):1117–1127. doi: 10.1007/s40264-016-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peymani P, Tabrizi R, Afifi S, Namazi S, Heydari ST, Shirazi MK, et al. Knowledge, attitude and practice of General Practitioners towards adverse drug reaction reporting in South of Iran, Shiraz (Pharmacoepidemiology report) Int J Risk Saf Med. 2016;28(1):25–31. doi: 10.3233/JRS-160670. [DOI] [PubMed] [Google Scholar]
- 38.Amin MN, Khan TM, Dewan SM, Islam MS, Moghal MR, Ming LC. Cross-sectional study exploring barriers to adverse drug reactions reporting in community pharmacy settings in Dhaka, Bangladesh. BMJ Open. 2016;6(8):e010912. doi: 10.1136/bmjopen-2015-010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu YM, Lee E, Koo BS, Jeong KH, Choi KH, Kang LK, Lee MS, Choi KH, Oh JM, Shin WG. Predictive factors of spontaneous reporting of adverse drug reactions among community pharmacists. PLoS ONE. 2016;11(5):e0155517. doi: 10.1371/journal.pone.0155517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendes Marques JI, Polónia JM, Figueiras AG, Costa Santos CM, Herdeiro MT. Nurses' attitudes and spontaneous adverse drug reaction reporting: a case-control study in Portugal. J Nurs Manag. 2016;24(3):409–416. doi: 10.1111/jonm.12337. [DOI] [PubMed] [Google Scholar]
- 41.Cerruti L, Lebel D, Bussières JF. Perception de la pharmacovigilance par les pharmaciens hospitaliers québécois [Hospital pharmacists' perception of pharmacovigilance in Quebec] Ann Pharm Fr. 2016;74(2):137–145. doi: 10.1016/j.pharma.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Katusiime B, Semakula D, Lubinga SJ. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. Afr Health Sci. 2015;15(4):1308–1317. doi: 10.4314/ahs.v15i4.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Angelis A, Giusti A, Colaceci S, Vellone E, Alvaro R. Nurses' reporting of suspect adverse drug reactions: a mixed-methods study. Ann Ist Super Sanita. 2015;51(4):277–283. doi: 10.4415/ANN_15_04_06. [DOI] [PubMed] [Google Scholar]
- 44.Alshammari TM, Alamri KK, Ghawa YA, Alohali NF, Abualkol SA, Aljadhey HS. Knowledge and attitude of health-care professionals in hospitals towards pharmacovigilance in Saudi Arabia. Int J Clin Pharm. 2015;37(6):1104–1110. doi: 10.1007/s11096-015-0165-5. [DOI] [PubMed] [Google Scholar]
- 45.Nde F, Fah AB, Simo FA, Wouessidjewe D. State of knowledge of Cameroonian drug prescribers on pharmacovigilance. Pan Afr Med J. 2015;20:70. doi: 10.11604/pamj.2015.20.70.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Zhou Z, Yang S, Feng B, Zhao J, Liu H, Huang H, Fang Y. Factors that affect adverse drug reaction reporting among hospital pharmacists in Western China. Int J Clin Pharm. 2015;37(3):457–464. doi: 10.1007/s11096-015-0065-8. [DOI] [PubMed] [Google Scholar]
- 47.Tandon VR, Mahajan V, Khajuria V, Gillani Z. Under-reporting of adverse drug reactions: a challenge for pharmacovigilance in India. Indian J Pharmacol. 2015;47(1):65–71. doi: 10.4103/0253-7613.150344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabblah GT, Akweongo P, Darko D, Dodoo AN, Sulley AM. Adverse drug reaction reporting by doctors in a developing country: a case study from Ghana. Ghana Med J. 2014;48(4):189–193. doi: 10.4314/gmj.v48i4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open. 2014;4(11):e005869. doi: 10.1136/bmjopen-2014-005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afifi S, Maharloui N, Peymani P, Namazi S, Gharaei AG, Jahani P, Lankarani KB. Adverse drug reactions reporting: pharmacists' knowledge, attitude and practice in Shiraz. Iran. Int J Risk Saf Med. 2014;26(3):139–145. doi: 10.3233/JRS-140620. [DOI] [PubMed] [Google Scholar]
- 51.Elkalmi RM, Hassali MA, Ibrahim MI, Jamshed SQ, Al-Lela OQ. Community pharmacists' attitudes, perceptions, and barriers toward adverse drug reaction reporting in Malaysia: a quantitative insight. J Patient Saf. 2014;10(2):81–87. doi: 10.1097/PTS.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 52.Wilbur K. Pharmacovigilance in Qatar: a survey of pharmacists. East Mediterr Health J. 2013;19(11):930–935. doi: 10.26719/2013.19.11.930. [DOI] [PubMed] [Google Scholar]
- 53.Sanghavi DR, Dhande PP, Pandit VA. Perception of pharmacovigilance among doctors in a tertiary care hospital: influence of an interventional lecture. Int J Risk Saf Med. 2013;25(4):197–204. doi: 10.3233/JRS-130598. [DOI] [PubMed] [Google Scholar]
- 54.Stoynova V, Getov IN, Naseva EK, Lebanova HV, Grigorov EE. Physicians' knowledge and attitude towards adverse event reporting system and result to intervention-randomized nested trial among Bulgarian physicians. Med Glas (Zenica). 2013;10(2):365–372. [PubMed] [Google Scholar]
- 55.Yip J, Radford DR, Brown D. How do UK dentists deal with adverse drug reaction reporting? Br Dent J. 2013;214(8):E22. doi: 10.1038/sj.bdj.2013.426. [DOI] [PubMed] [Google Scholar]
- 56.Santosh KC, Tragulpiankit P, Gorsanan S, Edwards IR. Attitudes among healthcare professionals to the reporting of adverse drug reactions in Nepal. BMC Pharmacol Toxicol. 2013;14:16. doi: 10.1186/2050-6511-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart D, MacLure K, Paudyal V, Hughes C, Courtenay M, McLay J. Non-medical prescribers and pharmacovigilance: participation, competence and future needs. Int J Clin Pharm. 2013;35(2):268–274. doi: 10.1007/s11096-012-9739-7. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal R, Daher AM, Mohd IN. Knowledge, practices and attitudes towards adverse drug reaction reporting by private practitioners from Klang Valley in Malaysia. Malays J Med Sci. 2013;20(2):52–61. [PMC free article] [PubMed] [Google Scholar]
- 59.Pimpalkhute SA, Jaiswal KM, Sontakke SD, Bajait CS, Gaikwad A. Evaluation of awareness about pharmacovigilance and adverse drug reaction monitoring in resident doctors of a tertiary care teaching hospital. Indian J Med Sci. 2012;66(3–4):55–61. doi: 10.4103/0019-5359.110902. [DOI] [PubMed] [Google Scholar]
- 60.Biagi C, Montanaro N, Buccellato E, Roberto G, Vaccheri A, Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia-Romagna region) Eur J Clin Pharmacol. 2013;69(2):237–244. doi: 10.1007/s00228-012-1321-7. [DOI] [PubMed] [Google Scholar]
- 61.Prakasam A, Nidamanuri A, Kumar S. Knowledge, perception and practice of pharmacovigilance among community pharmacists in South India. Pharm Pract (Internet) 2012;10(4):222–226. doi: 10.4321/S1886-36552012000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giofrè C, Scicchitano F, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety in Calabria (Italy): 2012 adverse events analysis. J Pharmacol Pharmacother. 2013;4(Suppl 1):S55–60. doi: 10.4103/0976-500X.120963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chopra D, Wardhan N, Rehan HS. Knowledge, attitude and practices associated with adverse drug reaction reporting amongst doctors in a teaching hospital. Int J Risk Saf Med. 2011;23(4):227–232. doi: 10.3233/JRS-2011-0543. [DOI] [PubMed] [Google Scholar]
- 64.Palaian S, Ibrahim MI, Mishra P. Health professionals' knowledge, attitude and practices towards pharmacovigilance in Nepal. Pharm Pract (Granada) 2011;9(4):228–235. doi: 10.4321/S1886-36552011000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oreagba IA, Ogunleye OJ, Olayemi SO. The knowledge, perceptions and practice of pharmacovigilance amongst community pharmacists in Lagos state, South West Nigeria. Pharmacoepidemiol Drug Saf. 2011;20(1):30–35. doi: 10.1002/pds.2021. [DOI] [PubMed] [Google Scholar]
- 66.Moumtzoglou A. Factors that prevent physicians reporting adverse events. Int J Health Care Qual Assur. 2010;23(1):51–58. doi: 10.1108/09526861011010677. [DOI] [PubMed] [Google Scholar]
- 67.Gavaza P, Brown CM, Khoza S. Texas pharmacists' opinions on reporting serious adverse drug events to the Food and Drug Administration: a qualitative study. Pharm World Sci. 2010;32(5):651–657. doi: 10.1007/s11096-010-9420-y. [DOI] [PubMed] [Google Scholar]
- 68.Su C, Ji H, Su Y. Hospital pharmacists' knowledge and opinions regarding adverse drug reaction reporting in Northern China. Pharmacoepidemiol Drug Saf. 2010;19(3):217–222. doi: 10.1002/pds.1792. [DOI] [PubMed] [Google Scholar]
- 69.Ohaju-Obodo JO, Iribhogbe OI. Extent of pharmacovigilance among resident doctors in Edo and Lagos states of Nigeria. Pharmacoepidemiol Drug Saf. 2010;19(2):191–195. doi: 10.1002/pds.1724. [DOI] [PubMed] [Google Scholar]
- 70.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elnour AA, Ahmed AD, Yousif MA, Shehab A. Awareness and reporting of adverse drug reactions among health care professionals in Sudan. Jt Comm J Qual Patient Saf. 2009;35(6):324–329. doi: 10.1016/s1553-7250(09)35046-1. [DOI] [PubMed] [Google Scholar]
- 72.Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31(2):183–187. doi: 10.1007/s11096-008-9276-6. [DOI] [PubMed] [Google Scholar]
- 73.Ekman E, Bäckström M. Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol. 2009;65(1):43–46. doi: 10.1007/s00228-008-0564-9. [DOI] [PubMed] [Google Scholar]
- 74.Okezie EO, Olufunmilayo F. Adverse drug reactions reporting by physicians in Ibadan, Nigeria. Pharmacoepidemiol Drug Saf. 2008;17(5):517–522. doi: 10.1002/pds.1597. [DOI] [PubMed] [Google Scholar]
- 75.Toklu HZ, Uysal MK. The knowledge and attitude of the Turkish community pharmacists toward pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 2008;30(5):556–562. doi: 10.1007/s11096-008-9209-4. [DOI] [PubMed] [Google Scholar]
- 76.Gossell-Williams M, Adebayo SA. The Pharmwatch Programme: challenges to engaging the community pharmacists in Jamaica. Pharm Pract. 2008;6(4):187–190. doi: 10.4321/s1886-36552008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30(11):1073–1082. doi: 10.2165/00002018-200730110-00006. [DOI] [PubMed] [Google Scholar]
- 78.Herdeiro MT, Polonia J, Gestal-Otero JJ, Figueiras A. Factors that influence spontaneous reporting of adverse drug reactions: a model centralized in the medical professional. J Eval Clin Pract. 2004;10(4):483–489. doi: 10.1111/j.1365-2753.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 79.Varallo FR, Guimarães SOP, Abjaude SAR, Mastroianni PC. Causes for the underreporting of adverse drug events by health professionals: A systematic review. Rev da Esc Enferm. 2014;48(4):739–747. doi: 10.1590/S0080-623420140000400023. [DOI] [PubMed] [Google Scholar]
- 80.Hailu AD, Mohammed SA. Adverse drug reaction reporting in Ethiopia: systematic review. Biomed Res Int. 2020 doi: 10.1155/2020/8569314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herrera CR. Undergraduate and postgraduate pharmacovigilance education: a proposal for appropriate curriculum content. Br J Clin Pharmacol. 2020;86(4):779–790. doi: 10.1111/bcp.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tripathi RK, Jalgaonkar SV, Sarkate PV, Rege NN. Implementation of a module to promote competency in adverse drug reaction reporting in undergraduate medical students. Indian J Pharmacol. 2016;48(Suppl 1):S69–S73. doi: 10.4103/0253-7613.193314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shchory MP, Goldstein LH, Arcavi L, Shihmanter R, Berkovitch M, Levy A. Increasing adverse drug reaction reporting-how can we do better? PLoS ONE. 2020;15:1–15. doi: 10.1371/journal.pone.0235591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Figueiras A, Herdeiro MT, Polónia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296(9):1086–1093. doi: 10.1001/jama.296.9.1086. [DOI] [PubMed] [Google Scholar]
- 85.Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polnia J, Falco A, Figueiras A. Workshop-and telephone-based interventions to improve adverse drug reaction reporting: a cluster-randomized trial in Portugal. Drug Saf. 2012;35(8):655–665. doi: 10.2165/11599750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 86.Shrestha S, Sharma S, Bhasima R, Kunwor P, Adhikari B, Sapkota B. Impact of an educational intervention on pharmacovigilance knowledge and attitudes among health professionals in a Nepal cancer hospital. BMC Med Educ. 2020;20(1):1–10. doi: 10.1186/s12909-020-02084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shrestha S, Shrestha R, Abidi A, et al. Workshop on adverse drug reaction reporting, pharmacovigilance and its implementation in cancer hospital in Nepal: an event report. Adv Med Educ Pract. 2020;11:9–14. doi: 10.2147/AMEP.S225208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richards D, Toop L, Graham P. Do clinical practice education groups result in sustained change in GP prescribing? Fam Pr. 2003;20(2):199–206. doi: 10.1093/fampra/20.2.199. [DOI] [PubMed] [Google Scholar]
- 89.Yoong SL, Hall A, Stacey F, Grady A, Sutherland R, Wyse R, et al. Nudge strategies to improve healthcare providers' implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci. 2020;15(1):50. doi: 10.1186/s13012-020-01011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feely J, Moriarty S, O’Connor P. Pharmacy-coordinated program that encourages physician reporting of adverse drug reactions. Am J Hosp Pharm. 1990;47(6):1327–1333. [PubMed] [Google Scholar]
- 91.Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reactions by using a fee. BMJ (Clinical Res ed). 1990;300(6716):22–23. doi: 10.1136/bmj.300.6716.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bäckström M, Mjörndal T. A small economic inducement to stimulate increased reporting of adverse drug reactions—a way of dealing with an old problem? Eur J Clin Pharmacol. 2006;62(5):381–385. doi: 10.1007/s00228-005-0072-0. [DOI] [PubMed] [Google Scholar]
- 93.Ribeiro-Vaz I, Santos C, Da Costa-Pereira A, Cruz-Correia R. Promoting spontaneous adverse drug reaction reporting in hospitals using a hyperlink to the online reporting form: an ecological study in Portugal. Drug Saf. 2012;35(5):387–394. doi: 10.2165/11597190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 94.Ribeiro-Vaz I, Silva AM, Costa Santos C, Cruz-Correia R. How to promote adverse drug reaction reports using information systems—a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2016;16(1):1–10. doi: 10.1186/s12911-016-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maguire A, Douglas I, Smeeth L, Thompson M. Secondary use of electronic health record data: spontaneous triggered adverse drug event reporting. Pharmacoepidemiol Drug Saf. 2010;19(12):1211–1215. doi: 10.1002/pds.2027. [DOI] [PubMed] [Google Scholar]
- 96.Li R, Zaidi STR, Chen T, Castelino R. Effectiveness of interventions to improve adverse drug reaction reporting by healthcare professionals over the last decade: a systematic review. Pharmacoepidemiol Drug Saf. 2020;29(1):1–8. doi: 10.1002/pds.4906. [DOI] [PubMed] [Google Scholar]
- 97.Lander AR, Blicher TM, Jimenez-solem E, Jespersen M, Kampmann JP, Christensen HR. Introducing an adverse drug event manager. Eur J Hosp Pharm Sci Pract. 2013;20(2):78–81. doi: 10.1136/ejhpharm-2012-000171. [DOI] [Google Scholar]
- 98.Johansson M, Brunlöf G, Edward C, Wallerstedt SM. Effects of e-mails containing ADR information and a current case report on ADR reporting rate and quality of reports. Eur J Clin Pharmacol. 2009;65(5):511–514. doi: 10.1007/s00228-008-0603-6. [DOI] [PubMed] [Google Scholar]
- 99.Abadie D, Chebane L, Bert M, Durrieu G, Montastruc J. Online reporting of adverse drug reactions: a study from a French Regional Pharmacovigilance Center. Therapie. 2014;69(5):395–400. doi: 10.2515/therapie/2014035. [DOI] [PubMed] [Google Scholar]
- 100.Oosterhuis I, Van Hunsel FPAM, Van Puijenbroek EP. Expectations for feedback in adverse drug reporting by healthcare professionals in the Netherlands. Drug Saf. 2012;35(3):221–232. doi: 10.2165/11594910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 101.Aldryhim AY, Alomair A, Alqhtani M, et al. Factors that facilitate reporting of adverse drug reactions by pharmacists in Saudi Arabia. Expert Opin Drug Saf. 2019;18(8):745–752. doi: 10.1080/14740338.2019.1632287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.