Abstract
Neuronal plasticity is critical for the maintenance and modulation of brain activity. Emerging evidence indicates that glial cells actively shape neuroplasticity, allowing for highly flexible regulation of synaptic transmission, neuronal excitability, and network synchronization. Astrocytes regulate synaptogenesis, stabilize synaptic connectivity, and preserve the balance between excitation and inhibition in neuronal networks. Microglia, the brain-resident immune cells, continuously monitor and sculpt synapses, allowing for the remodeling of brain circuits. Glia-mediated neuroplasticity is driven by neuronal activity, controlled by a plethora of feedback signaling mechanisms and crucially involves extracellular matrix remodeling in the central nervous system. This review summarizes the key findings considering neurotransmission regulation and metabolic support by astrocyte-neuronal networks, and synaptic remodeling mediated by microglia. Novel data indicate that astrocytes and microglia are pivotal for controlling brain function, indicating the necessity to rethink neurocentric neuroplasticity views.
Keywords: Neuroplasticity, Astrocyte, Microglia, Neuron-glia interactions, Inflammation, Extracellular matrix
Introduction
Neuroplasticity is a key to understanding brain development, learning, and homeostatic regulation in the central nervous system (CNS). In a broad sense, the term “neuroplasticity” refers to the ability of nervous tissue to change during normal functioning or in pathology. The mechanisms of neuronal plasticity include modulation of synaptic strength (i.e., synaptic plasticity), structural remodeling, and adjustment of intrinsic neuronal properties such as excitability or firing rate. Although neuroplasticity is traditionally associated with neuron-based pathways, recent experimental data emphasize the role of regulatory mechanisms involving glial cells and the brain extracellular matrix (ECM). In the mature brain, neurogenesis and axonal sprouting are inhibited by the ECM [1], but gliogenesis remains active [2]. Ample evidence indicates that glia (meaning “glue” in Greek) are vividly interacting brain cells that communicate via gap junctions and cytokines in health and disease [3]. The four major glial cell populations in the CNS are NG2-glia, oligodendrocytes, astrocytes, and microglia. NG2-glia is essential for the renewal of glial cells (for review, see [4]), and oligodendrocytes are required for myelin formation (for review, see [5]). Neurons and glial cells collectively shape the brain ECM [6], which is an important mediator of intercellular signaling in the extracellular space [7]. ECM is mainly composed of polysaccharides and proteoglycans that act as extracellular scaffolds and provide a highly regulated environment for intercellular communication by regulating the diffusion of metabolites and signaling molecules [8]. ECM components regulate the hydrodynamics of the extracellular space, compartmentalize cell surfaces, and bind signaling mediators. The conventional classification of ECM in the brain parenchyma includes synaptic, interstitial, and condensed matrices of perineuronal nets [9, 10]. The different neuroplasticity-regulating properties of these matrices may vary based on their molecular composition and localization. While astrocytes produce a major part of ECM components, microglia remodel the ECM during neuroinflammation and physiological immune surveillance in the brain. In this review, we highlight the critical role of astrocytes, microglia, and ECM in the regulation of neuronal activity and plasticity at different organizational levels—from single synapses to networks.
Astrocytic cradle of the synapse
Astrocytes received their name in the late nineteenth century because of the stellate appearance that was revealed by Camillo Golgi and Santiago Ramón y Cajal using the silver-chromate and gold chloride-sublimate techniques, correspondingly [11]. Today, immunohistochemical labeling of glial acidic fibrillary protein (GFAP), which remains one of the most widely used astrocytic marker proteins, shows similar star-like morphology. However, dye-filling techniques and genetic labeling of astrocytic membrane proteins reveal a dense pattern of highly ramified branches [12]. Therefore, the ground truth is that astrocytes are rather dandelion like than stellate. The thin astrocytic processes enwrap presynaptic terminals and dendritic spines of excitatory synapses [13], creating the spongiform microdomains [14] that provide the structural background for local astrocyte-synapse communication. Emphasizing the intimate proximity between the astrocyte and the synapse, astrocytic perisynaptic processes are called astrocytic cradles [15]. The essential role of astrocytic cradles for controlling synaptic transmission is reflected by the tripartite synapse concept [16] and summarized in Fig. 1.
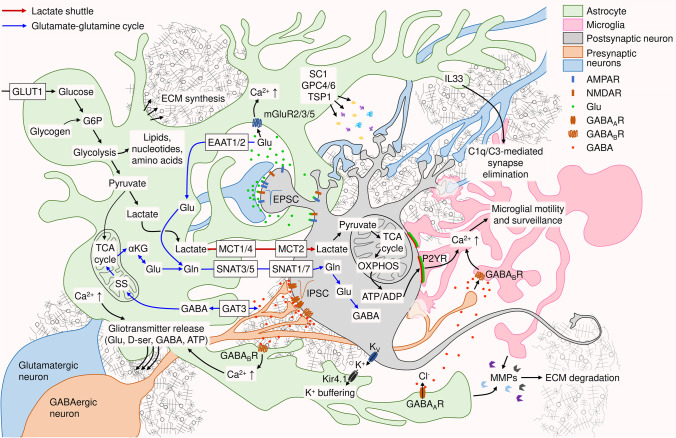
Fig. 1.
Graphical summary of neuroplasticity mechanisms mediated by glia. Intimate interactions between astrocytes, microglia, and ECM allow for dynamic control of neuronal activity and synaptic transmission. While astrocytes regulate neuronal function by providing metabolic support, modulating neurotransmission, and producing ECM, microglia can sculpt neuronal synapses in an activity-dependent manner. Abbreviations: αKG, α-ketoglutarate; ATP/ADP, adenosine tri- and diphosphate; AMPAR, AMPA receptor; D-ser, D-serine; EAAT, excitatory amino acid transporter; ECM, extracellular matrix; EPSC, excitatory postsynaptic current; IPSC, inhibitory postsynaptic current; G6P, glucose-6-phosphate; GABAAR, ionotropic GABA receptor A; GABABR, metabotropic GABA receptor B; GAT3, GABA transporter 3; Glu, glutamate; Gln, glutamine; GLUT1, glucose transporter 1; IL33, interleukin 33; mGluR, metabotropic glutamate receptor; MMPs, matrix metalloproteases; MCT, monocarboxylate transporter; NMDAR, NMDA receptor; OXPHOS, oxidative phosphorylation; P2YR, purinergic P2Y receptor; SNAT, sodium-coupled neutral amino acid transporter; SS, succinic semialdehyde; TCA cycle, tricarboxylic acid cycle (Krebs cycle)
In tripartite synapses, astrocytes enhance the adhesion between pre- and postsynaptic membranes. While the key synaptic adhesion molecules of the neurexin-neuroligin complex are predominantly synthesized by neurons, astrocytes produce ephrins, integrins, and cadherins, and promote the expression of other cell adhesion molecules of the immunoglobulin superfamily (IgCAMs) including SynCAMs and NCAMs to further stabilize the synapse. The adhesion molecules released by astrocytes promote the formation and stabilize synapses, which makes them essential for maintaining synaptic transmission [17]. Astrocytes also produce glypicans, the heparan sulfate proteoglycans that induce the formation of functional synapses and modulate synaptic plasticity by promoting glutamate receptor clustering [18]. In addition, astrocytes produce other ECM proteins such as thrombospondins and hevin, which have been shown to regulate synapse formation and stability. For a more exhaustive review of cell adhesion in the tripartite synapse, see [19]. The synaptic ECM components produced by astrocytes have long lifetimes [20, 21] and actively modulate synaptic activity [22]. The implications of ECM in synaptic activity regulation have led to the emergence of the more recent tetrapartite synapse concept [23].
On a functional level, astrocytic cradles dynamically control neurotransmitter concentration. Perisynaptic astrocytic membranes create diffusion barriers that limit the spillover of neurotransmitters [24] and express excitatory amino acid transporters (EAATs). EAAT1 (also known as GLAST1 and SLC1A3) and EAAT2 (also known as GLT1 and SLC1A2) mediate rapid reuptake of glutamate, thereby shaping postsynaptic current responses [25]. The expression and uptake capacity of these transporters depend on neuronal activity [26, 27] and are regulated by astrocytic Ca/calmodulin-dependent kinase CaMKII [28, 29]. Interestingly, EAATs 1 and 2 can be synthesized locally in the astrocytic processes [30], which allows for the fast upregulation of glutamate reuptake if necessary.
Inhibitory synapses are predominantly established directly on neuronal somas or dendritic shafts and rarely localize to dendritic spines [31]. To the best of our knowledge, there is no direct evidence of astrocytic cradle formation around inhibitory synapses. However, astrocytes actively sequester GABA from the perisynaptic space via the high-affinity GABA transporter GAT3 [32], providing a reuptake mechanism similar to those for glutamate. Thus, neurotransmitter uptake by astrocytes regulates both excitatory and inhibitory signaling in neuronal networks.
Gliotransmission and neuronal activity regulation
Astrocytes regulate neuronal activity not only by removing neurotransmitters from the extracellular space but also by releasing them in an activity-dependent manner. To describe this mechanism, the term “gliotransmission” has been coined, and the neurotransmitters released from astrocytes are commonly called gliotransmitters [33]. Astrocytes express membrane receptors for the vast majority of mammalian neurotransmitters [34], and, although they do not generate neuron-like action potentials, astrocytes respond to neurotransmitter application and neuronal stimulation (e.g., after sensory stimuli) with transient elevations of intracellular Ca2+ concentrations [35, 36]. Analyzing Ca2+ signals, also called Ca2+ events, is currently the key method for understanding the physiology of astrocyte-neuronal interactions [37, 38]. Depending on the strength of neuron-to-astrocyte stimulation, Ca2+ events may localize to the peripheral astrocytic processes (microdomain activity) or spread over the entire cell and its neighbors (global events or waves). While the microdomain Ca2+ activity can be triggered by membrane transporters and ion channels [39], the major Ca2+ events are mediated by inositol triphosphate (IP3) signaling and store-operated Ca2+ release [40]. In the seminal works [41, 42], the Ca2+ waves were detected in cultured astrocytes after norepinephrine (NE) application or after prolonged electrical stimulation. More recent in vivo experiments identified the critical importance of astrocytic calcium signaling for neuronal activity regulation [43–45].
Ca2+ events trigger the release of gliotransmitters, which regulate synapse activity locally, heterosynaptically, or globally by potentiating or inhibiting synaptic transmission and neuronal excitability (for review, see [33, 46]). Interestingly, astrocytes use similar molecular machinery for the vesicular release of gliotransmitters [47, 48]. In excitatory synapses, the release of astrocytic glutamate and D-serine mediates synaptic potentiation [48–50] and stimulates the barrage firing of inhibitory interneurons [51]. On the other hand, ATP/adenosine release from astrocytes stimulated by both excitatory and inhibitory activity induces heterosynaptic suppression of glutamatergic synapses [43, 52]. In inhibitory synapses, GABA stimulates astrocytic GABAB receptors, induces long-lasting Ca2+ oscillations [53], and stimulates excitatory neurotransmission via glutamate release [54]. Conversely, GAT3 transporter activity following GABAergic stimulation induces astrocytic ATP release and inhibitory synapse potentiation [55]. Gliotransmission therefore can act as a jack of all trades in neuronal activity regulation. However, the selection mechanisms determining astrocytic responses to neuronal stimulation remain largely unknown.
The described mechanisms of neuron-glia interactions provide flexible modulation of neuroplasticity and indicate a high capability of brain networks for self-regulation. Due to technical difficulties, most experimental studies that we reviewed here were conducted on the sub-cellular and cellular levels, making it yet challenging to conclude how the key neuroplasticity mechanisms mediated by glia integrate on the functional network level. Emerging evidence indicates that astrocytic signaling is essential for neuronal synchronization and inhibitory network refinement [56, 57]. Astrocytes can modulate the efficacy of both excitatory and inhibitory synapses, thereby extending the dynamic range of neuroplasticity and increasing the computational power of local circuits [57] by tuning the excitation-inhibition balance. Recently, it was shown that astrocytes encode spatial information, and the expected reward location can be decoded from their activity in an awake mouse brain [58]. Based on the available data, we propose that multicellular interactions in neuron-glial networks promote ranged propagation of inhibition and excitation and support excitation-inhibition balance in local neuronal networks (Fig. 2).
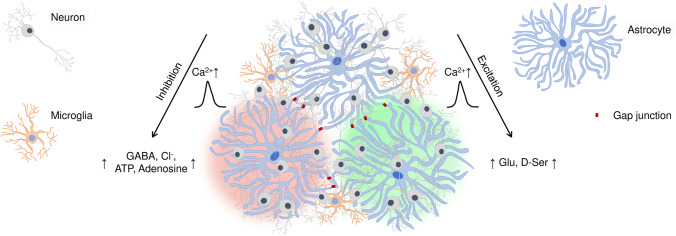
Fig. 2.
Propagation of excitatory and inhibitory signals in neuron-glial networks. On the mesoscale level of organization, inhibition-excitation balance in local brain networks can be orchestrated by calcium signals propagating through adjacent astrocytes
Although single astrocytes occupy distinct territories and establish non-intersecting islands of synaptic regulation [59], they are functionally connected into a syncytium-like network by gap junctions [60]. Astrocytic coupling via connexin 43 and 30 channels allows for intercellular Ca2+ signaling and metabolic coupling, which is necessary for preventing epileptiform activity [61, 62].
Through gap junctions, the traveling Ca2+ events may transfer excitatory or inhibitory stimuli from the territory of one astrocyte to another. Thereby, astrocytic networks can regulate neuronal synchronization on a slower time scale than provided by synaptic neurotransmission. Neurotransmitter release from the astrocytes is less area restricted than synaptic release and can therefore affect extrasynaptic receptors of multiple neurons within the astrocytic territory [56], thereby regulating the excitation-inhibition balance without remodeling synaptic connectivity. Tonic inhibition by astrocytic GABA release [63, 64] and the efflux of chloride via astrocytic GABAA channels [65] efficiently modulates network inhibition. Similarly, excitatory gliotransmission and stimulation of extrasynaptic AMPA and NMDA receptors [33, 46] can contribute to spreading excitation.
Potassium buffering in astrocytes and perineuronal nets
While the control over neurotransmitter concentrations regulates the amplitude, frequency, and dynamics of postsynaptic responses, neuronal excitability, and firing patterns depend on the astrocytic capacity for buffering K+ ions. Extracellular K+ concentration changes the equilibrium potential for this ion, thereby affecting the repolarization and hyperpolarization phases of the action potential. In absence of astrocytes, accumulation of extracellular K+ during neuronal activity will depolarize the neuronal membrane, inducing tonic spike firing and eventually blocking neuronal activity and conductivity. To prevent these detrimental effects, astrocytes buffer up to 80% of the released K+ via Kir4.1 channels [66]. Ki4.1-mediated K+ buffering works in concert with astrocytic glutamate transporters and Na+/K+-ATPases [67], thereby maintaining ionic homeostasis in the extracellular space.
In a subpopulation of fast-spiking interneurons, the interstitial brain ECM condensates into peculiar structures known as perineuronal nets [9]. Neuronal activity consolidates the diffusely expressed polyanionic macromolecules into densely packed lattice-shaped layers that can sequester K+ ions extracellularly [68, 69]. The reduced synthesis of hyaluronan, which is one of the largest anionic polysaccharides in the extracellular space, causes altered neuronal activity and seizures [8]. Therefore, both astrocytic Kir4.1 channels and polyanionic ECM are critical for K+ buffering and neuronal activity maintenance.
Metabolic regulation of neuronal activity
Neurons are arguably the most energy-demanding cells of the body that do not maintain their own consumption of metabolites. Neuronal metabolism is dominated by mitochondrial oxidative phosphorylation (OXPHOS), which has the highest ATP production efficiency among known metabolic pathways [70]. Glycolysis is strongly downregulated in mature neurons, making them dependent on the import of lactate via the astrocyte-neuron lactate shuttle pathway (Fig. 1). In contrast, astrocytes predominantly use glycolysis that supports the biosynthesis of lipids, nucleotides, and amino acids, and produce ample amounts of lactate [71]. Under normal conditions, astrocytic glycolysis is fueled by glucose uptake from the blood via glucose transporter GLUT1. Astrocytic glycolysis is pivotal for brain function, and, in case of altered glucose supply from the blood (e.g., in stroke), astrocytes can temporarily sustain glycolysis by deriving glucose-6-phosphate (G6P) from glycogen granules in their cytoplasm [72]. At the last step of glycolysis, astrocytes convert most of the produced pyruvate into lactate, which is released into the extracellular space by the monocarboxylate transporters MCT1 and MCT4. Neurons import extracellular lactate using MCT2 and convert it back to pyruvate for fueling the mitochondrial tricarboxylic acid (TCA) cycle and OXPHOS. Thereby, astrocyte-neuron lactate proves the key source of energy for the maintenance of neuronal activity.
In postsynaptic neurons, lactate potentiates NMDAR-mediated currents [73] by increasing NADH/NAD+ ratio. NMDAR potentiation by lactate depends on the redox sensitivity of the NR1 subunit [74] and is required for long-term memory formation [75]. Therefore, the transfer of lactate by the astrocyte-neuron lactate shuttle is an essential metabolic mechanism of neuroplasticity.
Neurotransmitters released during neuronal activity provide another crucial substrate for astrocytic metabolism. Following the uptake of glutamate via EAATs, glutamine synthase (GS) catalyzes the condensation of glutamate and ammonia to form glutamine. The importance of this reaction is evidenced by the ample expression of GS in astrocytes. With minor limitations, GS can be considered an astrocytic marker protein [76]. Astrocytic glutamine can be transferred to neurons by several transporter systems (for review, see [77]), of which sodium-coupled neutral amino acid transporters SNAT3 and SNAT5 (also known as SN1 and SN2, correspondingly) localize on the astrocytic membranes, and SNAT1 (also known as SAT1) and SNAT7 are predominantly neuronal [78]. In neurons, glutamine is converted back to glutamate by phosphate-activated glutaminase (PAG), thereby creating the astrocyte-neuronal glutamate-glutamine cycle [79], as shown in Fig. 1. GABA enters the glutamate-glutamine cycle via the TCA cycle through conversion to succinic semialdehyde (SS) and succinate in astrocytic mitochondria [80]. Glutamate is then synthesized from α-ketoglutarate and converted to glutamine by GS. In inhibitory neurons, astrocytic glutamine is one of the main precursors of GABA synthesis [81].
Glutamine transport supports synaptic plasticity by providing the essential substrate for neurotransmitter synthesis in both glutamate- and GABAergic neurons [82]. Genetic disruption of SNAT1 hampered neuronal GABA synthesis and impaired neurotransmission, plasticity, and cortical processing [83] in mice. Inhibition of GS results in reduced synaptic efficacy and altered long-term potentiation (LTP) [84]. Conclusively, the astrocyte-neuronal glutamate-glutamine cycle is critical for neurotransmission and neuroplasticity regulation.
Remodeling synaptic connectivity in the adult brain
Neuronal networks generate complex activity patterns that require not only the adjustment of synaptic strengths but also the dynamic remodeling of synaptic connectivity. Essential connectivity is established in the juvenile brain before the end of the critical plasticity period [85], which is signified by the maturation of excitatory and inhibitory neuronal networks [86] and the enrichment of plasticity-restricting components of the ECM [87]. Removing and establishing synapses in the mature CNS is mediated by microglia, the immune cells of the brain, and requires their coordinated interaction with astrocytes and ECM.
In the adult brain, both interstitial matrix and perineuronal nets contain axon-repelling chondroitin sulfate glycosaminoglycans (CSPGs) and semaphorins, which restrict new synapse formation [88, 89]. Thereby, CSPG-rich zones such as perineuronal nets compartmentalize neuronal surfaces and create permissive and restrictive microdomains that allow for highly precise targeting of the newly formed synapses. At the same time, ECM integrity is essential for maintaining inhibitory control in neuronal networks, and the depletion of ECM triggers excessive synchronization of neuronal activity [90]. Therefore, retaining excitation-inhibition balance during synaptic reorganization requires restricted ECM degradation that is confined by the area of remodeling. This locality can be achieved through the controlled release of matrix metalloproteases (MMPs) by glial cells [91] and highly accurate synapse elimination by microglia [92, 93].
Microglia are brain-resident macrophages [94] that continually survey the microenvironment [95] and rapidly detect tissue damage [96] or neuronal activity changes [97]. Neuronal activity promotes microglial surveillance via norepinephrine [98] and GABA signaling [99]. Microglial processes establish intercellular junction contacts with neuronal bodies [97]. Within these contacts, microglial purinergic receptors are co-clustered with neuronal potassium channels, creating a specialized nano-architecture optimized for purinergic cell-to-cell communication. Since the activity of a neuron highly correlates with mitochondrial ATP production, the microglia-neuron junctions allow for the rapid stimulation of microglia via ATP/ADP signaling. In stimulated microglia, Ca2+ signaling attunes to neuronal activity [100]. Neuronal interleukin 33 (IL33) regulates microglial activity under physiological conditions and promotes the phagocytosis of ECM components by microglia [101]. Neuronal activity regulates microglial phagocytosis [92], but, to the best of our knowledge, evidence for activity-dependent ECM remodeling by microglia is lacking currently. Although further studies are required to decipher the role of microglia-ECM interactions in synaptic remodeling, the available data suggests that microglia can locally remodel ECM, eliminate synapses, and promote synaptic plasticity depending on neuronal activity. Of note, inhibitory synapses are preferentially sculpted by GABA-receptive microglia involving GABABR-mediated signaling [102].
The specificity of activity-dependent synapse elimination is ensured by the phagocytic complement components C1q and C3 in microglia [92] and by exposure of phosphatidyl serine and pentraxins on presynaptic membranes [103, 104]. While the expression of microglial C1q/C3 can be induced by neuronal and astrocytic IL33 release [101, 105], the regulatory mechanisms of presynaptic expression of pentraxins and phosphatidyl serine remain to be understood.
As key mediators of synaptic remodeling, microglia cells contribute to the maintenance of excitation-inhibition balance on the network level. The patrolling function of microglia is regulated by extracellular chemokines including ATP [106, 107] and fractalkine [108, 109], which allow these cells to travel significant distances in the brain parenchyma [95, 110]. Due to the high mobility, a single stimulated microglia cell can potentially remodel multiple synapses and contribute to ranged modulation of excitation and inhibition. Microglia shape the developing neural circuits by engulfing excessive immature synapses via the complement receptor 3(CR3)/C3-dependent phagocytosis [92], a mechanism that is essential for normal synaptogenesis during brain development [111]. Interestingly, a similar mechanism contributes to pathological synapse loss due to upregulated microglial phagocytosis in Alzheimer’s disease [112]. In a healthy brain, microglia can also shape synaptic connectivity using complement-independent mechanisms including trogocytosis [93] and TWEAK signaling [113].
While the possibility of establishing new synapses in mature neuronal networks can be attributed to the local ECM degradation and synapse elimination by microglia, the capability of establishing new synapses is defined by the regulatory molecules produced by astrocytes [114]. Thrombospondin 1 (TSP1) induces the formation of structurally complete but functionally silent synapses. The establishment of active synapses requires the enrollment of glypicans 4 and 6 (GPC4/6) and hevin (also known as secreted protein acidic and rich in cysteine (SPARC) like 1, or SP1). The synaptogenic function of hevin is antagonized by SPARC [115], which is locally synthesized in astrocytic processes [30], allowing for the dynamic control of new synapse formation.
Collectively, the available evidence suggests that synaptic remodeling is likely orchestrated in three steps: local ECM degradation, synapse elimination by microglia, and astrocyte-mediated synapse formation. To support this hypothesis, further research is needed.
Neuroinflammation and neuroplasticity
The mechanisms of homeostatic brain activity regulation that we reviewed here can be severely altered by inflammatory signaling in multiple neurological diseases. For example, in stroke, neuroinflammation contributes to both damage and remodeling of brain tissue [116]. In both acute and chronic stroke phases, peripheral blood immune cells invade the ischemic brain parenchyma [117, 118], and preclinical studies indicate that immunomodulatory treatments can promote neurological recovery post stroke [119]. After the onset of cerebral ischemia, the release of alarmins [120] and cytokines triggers the pro-inflammatory microglia phenotype [121], which, in turn, can promote the neurotoxic phenotype in reactive astrocytes [122]. A recent study [123] has demonstrated that the neurotoxicity of ischemic astrocytes involves metabolic switching mediated by STAT3 activation. Post-stroke reactive gliosis alters interstitial ECM composition in the brain, which includes the upregulation of short-chain hyaluronan [124] and tenascin-C [125]. As an endogenous ligand of toll-like receptor 4 and inflammatory regulator, tenascin-C regulates morphological alterations in reactive glia [125, 126]. Post-stroke ECM remodeling also involves perineuronal nets [127, 128], and their transient degradation may support neurological recovery.
Neuroinflammation induces similar alterations in reactive glia and ECM in multiple neurodegenerative diseases including Alzheimer’s, Huntington’s, and multiple sclerosis. In Alzheimer’s disease, the release of amyloid-b induces microglial reprogramming and activation [129], which causes synaptic degeneration by complement-mediated microglial phagocytosis [112]. Activated microglia upregulate multiple ECM-degrading enzymes during neuroinflammation [130]. Most likely, the release of extracellular proteases from reactive microglia contributes to the decomposition of perineuronal nets in Alzheimer’s and Huntington’s diseases [131, 132]. Multiple studies using the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis demonstrated that the prolonged reactivity in microglia and astrocytes induces neurodegeneration [133, 134]. A recent study demonstrated that ECM composition in EAE altered due to the compromised glycosaminoglycan metabolism [135], showing that multiple sclerosis progression is associated with the reduced expression of 4-sulfated chondroitin sulfates and the increased synthesis of hyaluronic acid.
The lack of an in-depth mechanistic understanding of ECM-glia interactions during neuroinflammation hinders the development of novel therapies for neurodegenerative diseases. However, we believe that the ongoing studies in the field will propose promising translational approaches in the future.
Concluding remarks
Interactions between neurons, glia, and ECM allow for highly flexible modulation of neuronal plasticity. Ample evidence indicates that neuroplasticity is not restricted to neurons, and, in certain cases, can be dominated by glia. With multiple questions that remain open, future research on glia-mediated neuroplasticity will extend the current understanding of brain activity regulation.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by German Research Foundation (DFG) projects 389030878, 405358801, 428817542, and 505462125 to D.M.H., and 467228103 to E.D.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Egor Dzyubenko, Email: egor.dzyubenko@uk-essen.de.
Dirk M. Hermann, Email: dirk.hermann@uk-essen.de
References
- 1.Fawcett JW. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog Brain Res. 2015;218:213–226. doi: 10.1016/bs.pbr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Rusznak Z, Henskens W, Schofield E, Kim WS, Fu Y. Adult neurogenesis and gliogenesis: possible mechanisms for neurorestoration. Exp Neurobiol. 2016;25(3):103–112. doi: 10.5607/en.2016.25.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol. 2016;4:71. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63(8):1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8(2):a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzyubenko E, Gottschling C, Faissner A. Neuron-glia interactions in neural plasticity: contributions of neural extracellular matrix and perineuronal nets. Neural Plast. 2016;2016:5214961. doi: 10.1155/2016/5214961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson C, Hrabětová S. Brain extracellular space: the final frontier of neuroscience. Biophys J. 2017;113(10):2133–2142. doi: 10.1016/j.bpj.2017.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y. Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci. 2014;34(18):6164–6176. doi: 10.1523/JNEUROSCI.3458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20(8):451–465. doi: 10.1038/s41583-019-0196-3. [DOI] [PubMed] [Google Scholar]
- 10.Frischknecht R, Seidenbecher CI. The crosstalk of hyaluronan-based extracellular matrix and synapses. Neuron Glia Biol. 2008;4(3):249–257. doi: 10.1017/S1740925X09990226. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard JA, Binder DK. History of Astrocytes. In: Hubbard JA, Binder DK, editors. Astrocytes and epilepsy. San Diego: Academic Press; 2016. pp. 1–38. [Google Scholar]
- 12.Lanjakornsiripan D, Pior BJ, Kawaguchi D, Furutachi S, Tahara T, Katsuyama Y, Suzuki Y, Fukazawa Y, Gotoh Y. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun. 2018;9(1):1623. doi: 10.1038/s41467-018-03940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrilov N, Golyagina I, Brazhe A, Scimemi A, Turlapov V, Semyanov A. Astrocytic coverage of dendritic spines, dendritic shafts, and axonal boutons in hippocampal neuropil. Front Cell Neurosci. 2018;12:248. doi: 10.3389/fncel.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arizono M, Inavalli V, Panatier A, Pfeiffer T, Angibaud J, Levet F, Ter Veer MJT, Stobart J, Bellocchio L, Mikoshiba K, Marsicano G, Weber B, Oliet SHR, Nagerl UV. Structural basis of astrocytic Ca(2+) signals at tripartite synapses. Nat Commun. 2020;11(1):1906. doi: 10.1038/s41467-020-15648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8(3):206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486(7403):410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillen AEJ, Burbach JPH, Hol EM. Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog Neurobiol. 2018;165-167:66–86. doi: 10.1016/j.pneurobio.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Fornasiero EF, Mandad S, Wildhagen H, Alevra M, Rammner B, Keihani S, Opazo F, Urban I, Ischebeck T, Sakib MS, Fard MK, Kirli K, Centeno TP, Vidal RO, Rahman RU, Benito E, Fischer A, Dennerlein S, Rehling P, Feussner I, Bonn S, Simons M, Urlaub H, Rizzoli SO. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat Commun. 2018;9(1):4230. doi: 10.1038/s41467-018-06519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dankovich TM, Rizzoli SO. The synaptic extracellular matrix: long-lived, stable, and still remarkably dynamic. Front Synaptic Neurosci. 2022;14:854956. doi: 10.3389/fnsyn.2022.854956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11(11):735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- 23.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21(2):353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sykova E. Glial diffusion barriers during aging and pathological states. Prog Brain Res. 2001;132:339–363. doi: 10.1016/S0079-6123(01)32087-3. [DOI] [PubMed] [Google Scholar]
- 25.Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci. 2015;18(2):219–226. doi: 10.1038/nn.3901. [DOI] [PubMed] [Google Scholar]
- 26.Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE, Dailey ME. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia. 2012;60(2):175–188. doi: 10.1002/glia.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poitry-Yamate CL, Vutskits L, Rauen T. Neuronal-induced and glutamate-dependent activation of glial glutamate transporter function. J Neurochem. 2002;82(4):987–997. doi: 10.1046/j.1471-4159.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 28.Ashpole NM, Chawla AR, Martin MP, Brustovetsky T, Brustovetsky N, Hudmon A. Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP *. J Biol Chem. 2013;288(20):14599–14611. doi: 10.1074/jbc.M113.466235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underhill SM, Wheeler DS, Amara SG. Differential regulation of two isoforms of the glial glutamate transporter EAAT2 by DLG1 and CaMKII. J Neurosci. 2015;35(13):5260–5270. doi: 10.1523/JNEUROSCI.4365-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakers K, Lake AM, Khazanchi R, Ouwenga R, Vasek MJ, Dani A, Dougherty JD. Astrocytes locally translate transcripts in their peripheral processes. Proc Natl Acad Sci U S A. 2017;114(19):E3830–E3838. doi: 10.1073/pnas.1617782114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boivin JR, Nedivi E. Functional implications of inhibitory synapse placement on signal processing in pyramidal neuron dendrites. Curr Opin Neurobiol. 2018;51:16–22. doi: 10.1016/j.conb.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci. 1996;16(19):6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51(4):439–455. doi: 10.1016/S0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 35.Covelo A, Araque A (2018) Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife 7:e32237. 10.7554/eLife.32237 [DOI] [PMC free article] [PubMed]
- 36.Schipke CG, Haas B, Kettenmann H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex. Cereb Cortex. 2008;18(10):2450–2459. doi: 10.1093/cercor/bhn009. [DOI] [PubMed] [Google Scholar]
- 37.Dzyubenko E, Prazuch W, Pillath-Eilers M, Polanska J, Hermann DM. Analysing intercellular communication in astrocytic networks using “astral”. Front Cell Neurosci. 2021;15:689268. doi: 10.3389/fncel.2021.689268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, DelRosso NV, Vaidyanathan TV, Cahill MK, Reitman ME, Pittolo S, Mi X, Yu G, Poskanzer KE. Accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology. Nat Neurosci. 2019;22(11):1936–1944. doi: 10.1038/s41593-019-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rungta RL, Bernier L-P, Dissing-Olesen L, Groten CJ, LeDue JM, Ko R, Drissler S, MacVicar BA. Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia. 2016;64(12):2093–2103. doi: 10.1002/glia.23042. [DOI] [PubMed] [Google Scholar]
- 40.Verkhratsky A, Rodriguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol. 2012;353(1-2):45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 41.Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15(8):5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263(5154):1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 43.Boddum K, Jensen TP, Magloire V, Kristiansen U, Rusakov DA, Pavlov I, Walker MC. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat Commun. 2016;7:13572. doi: 10.1038/ncomms13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun. 2014;5(1):3262. doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A. 2016;113(19):E2675–E2684. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6(8):626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 47.Malarkey EB, Parpura V (2008)Mechanisms of glutamate release from astrocytes. Neurochem Int 52(1-2):142-54 [DOI] [PMC free article] [PubMed]
- 48.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317(5841):1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 49.Neame S, Safory H, Radzishevsky I, Touitou A, Marchesani F, Marchetti M, Kellner S, Berlin S, Foltyn VN, Engelender S, Billard JM, Wolosker H. The NMDA receptor activation by d-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc Natl Acad Sci U S A. 2019;116(41):20736–20742. doi: 10.1073/pnas.1909458116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100(25):15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deemyad T, Luthi J, Spruston N. Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat Commun. 2018;9(1):4336. doi: 10.1038/s41467-018-06338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40(5):971–982. doi: 10.1016/S0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 53.Mariotti L, Losi G, Sessolo M, Marcon I, Carmignoto G. The inhibitory neurotransmitter GABA evokes long-lasting Ca(2+) oscillations in cortical astrocytes. Glia. 2016;64(3):363–373. doi: 10.1002/glia.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perea G, Gomez R, Mederos S, Covelo A, Ballesteros JJ, Schlosser L, Hernandez-Vivanco A, Martin-Fernandez M, Quintana R, Rayan A, Diez A, Fuenzalida M, Agarwal A, Bergles DE, Bettler B, Manahan-Vaughan D, Martin ED, Kirchhoff F, Araque A (2016) Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife 5:e20362. 10.7554/eLife.20362 [DOI] [PMC free article] [PubMed]
- 55.Matos M, Bosson A, Riebe I, Reynell C, Vallee J, Laplante I, Panatier A, Robitaille R, Lacaille JC. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cells. Nat Commun. 2018;9(1):4254. doi: 10.1038/s41467-018-06731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43(5):729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Mederos S, Perea G. GABAergic-astrocyte signaling: a refinement of inhibitory brain networks. Glia. 2019;67(10):1842–1851. doi: 10.1002/glia.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doron A, Rubin A, Benmelech-Chovav A, Benaim N, Carmi T, Refaeli R, Novick N, Kreisel T, Ziv Y, Goshen I. Hippocampal astrocytes encode reward location. Nature. 2022;609(7928):772–778. doi: 10.1038/s41586-022-05146-6. [DOI] [PubMed] [Google Scholar]
- 59.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27(24):6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anders S, Minge D, Griemsmann S, Herde MK, Steinhauser C, Henneberger C. Spatial properties of astrocyte gap junction coupling in the rat hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20130600. doi: 10.1098/rstb.2013.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bedner P, Dupper A, Huttmann K, Muller J, Herde MK, Dublin P, Deshpande T, Schramm J, Haussler U, Haas CA, Henneberger C, Theis M, Steinhauser C. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain. 2015;138(Pt 5):1208–1222. doi: 10.1093/brain/awv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 63.Pandit S, Neupane C, Woo J, Sharma R, Nam MH, Lee GS, Yi MH, Shin N, Kim DW, Cho H, Jeon BH, Kim HW, Lee CJ, Park JB. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia. 2020;68(5):1065–1080. doi: 10.1002/glia.23762. [DOI] [PubMed] [Google Scholar]
- 64.Woo J, Min JO, Kang DS, Kim YS, Jung GH, Park HJ, Kim S, An H, Kwon J, Kim J, Shim I, Kim HG, Lee CJ, Yoon BE. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc Natl Acad Sci U S A. 2018;115(19):5004–5009. doi: 10.1073/pnas.1721187115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A. Cl(-) homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses. J Physiol. 2013;591(16):3901–3917. doi: 10.1113/jphysiol.2013.257162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107(3):589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larsen BR, Stoica A, MacAulay N. Managing brain extracellular K(+) during neuronal activity: the physiological role of the Na(+)/K(+)-ATPase subunit isoforms. Front Physiol. 2016;7:141. doi: 10.3389/fphys.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8(3):183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 69.Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67(5):570–588. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 70.Bauernfeind AL, Barks SK, Duka T, Grossman LI, Hof PR, Sherwood CC. Aerobic glycolysis in the primate brain: reconsidering the implications for growth and maintenance. Brain Struct Funct. 2014;219(4):1149–1167. doi: 10.1007/s00429-013-0662-z. [DOI] [PubMed] [Google Scholar]
- 71.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 72.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111(33):12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi Y, Chen HV, Lipton SA. Three pairs of cysteine residues mediate both redox and zn2+ modulation of the nmda receptor. J Neurosci. 2001;21(2):392–400. doi: 10.1523/JNEUROSCI.21-02-00392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anlauf E, Derouiche A. Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front Endocrinol (Lausanne) 2013;4:144. doi: 10.3389/fendo.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leke R, Schousboe A. The glutamine transporters and their role in the glutamate/GABA-glutamine cycle. Adv Neurobiol. 2016;13:223–257. doi: 10.1007/978-3-319-45096-4_8. [DOI] [PubMed] [Google Scholar]
- 78.Hagglund MG, Sreedharan S, Nilsson VC, Shaik JH, Almkvist IM, Backlin S, Wrange O, Fredriksson R. Identification of SLC38A7 (SNAT7) protein as a glutamine transporter expressed in neurons. J Biol Chem. 2011;286(23):20500–20511. doi: 10.1074/jbc.M110.162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen JV, Jakobsen E, Westi EW, Lie MEK, Voss CM, Aldana BI, Schousboe A, Wellendorph P, Bak LK, Pinborg LH, Waagepetersen HS. Extensive astrocyte metabolism of gamma-aminobutyric acid (GABA) sustains glutamine synthesis in the mammalian cerebral cortex. Glia. 2020;68(12):2601–2612. doi: 10.1002/glia.23872. [DOI] [PubMed] [Google Scholar]
- 81.Sonnewald U, Westergaard N, Schousboe A, Svendsen JS, Unsgard G, Petersen SB. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem Int. 1993;22(1):19–29. doi: 10.1016/0197-0186(93)90064-C. [DOI] [PubMed] [Google Scholar]
- 82.Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 2013;4:102. doi: 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qureshi T, Sorensen C, Berghuis P, Jensen V, Dobszay MB, Farkas T, Dalen KT, Guo C, Hassel B, Utheim TP, Hvalby O, Hafting T, Harkany T, Fyhn M, Chaudhry FA. The glutamine transporter Slc38a1 regulates GABAergic neurotransmission and synaptic plasticity. Cereb Cortex. 2019;29(12):5166–5179. doi: 10.1093/cercor/bhz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ivens S, Caliskan G, Papageorgiou I, Cesetti T, Malich A, Kann O, Heinemann U, Stork O, Albrecht A. Persistent increase in ventral hippocampal long-term potentiation by juvenile stress: a role for astrocytic glutamine synthetase. Glia. 2019;67(12):2279–2293. doi: 10.1002/glia.23683. [DOI] [PubMed] [Google Scholar]
- 85.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 86.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 87.Gundelfinger ED, Frischknecht R, Choquet D, Heine M. Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci. 2010;31(12):2156–2165. doi: 10.1111/j.1460-9568.2010.07253.x. [DOI] [PubMed] [Google Scholar]
- 88.Carulli D, Foscarin S, Faralli A, Pajaj E, Rossi F. Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci. 2013;57:10–22. doi: 10.1016/j.mcn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Pearson CS, Mencio CP, Barber AC, Martin KR, Geller HM (2018) Identification of a critical sulfation in chondroitin that inhibits axonal regeneration. Elife 7:e37139. 10.7554/eLife.37139 [DOI] [PMC free article] [PubMed]
- 90.Dzyubenko E, Fleischer M, Manrique-Castano D, Borbor M, Kleinschnitz C, Faissner A, Hermann DM. Inhibitory control in neuronal networks relies on the extracellular matrix integrity. Cell Mol Life Sci. 2021;78(14):5647–5663. doi: 10.1007/s00018-021-03861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13(11):743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9(1):1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bennett ML, Bennett FC. The influence of environment and origin on brain resident macrophages and implications for therapy. Nat Neurosci. 2020;23(2):157–166. doi: 10.1038/s41593-019-0545-6. [DOI] [PubMed] [Google Scholar]
- 95.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 96.Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, Csaszar E, Fekete R, West BL, Katona G, Rozsa B, Denes A. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cserep C, Posfai B, Lenart N, Fekete R, Laszlo ZI, Lele Z, Orsolits B, Molnar G, Heindl S, Schwarcz AD, Ujvari K, Kornyei Z, Toth K, Szabadits E, Sperlagh B, Baranyi M, Csiba L, Hortobagyi T, Magloczky Z, Martinecz B, Szabo G, Erdelyi F, Szipocs R, Tamkun MM, Gesierich B, Duering M, Katona I, Liesz A, Tamas G, Denes A. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367(6477):528–537. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 98.Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, Umpierre AD, Zhu J, Bosco DB, Dong H, Wu LJ. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci. 2019;22(11):1771–1781. doi: 10.1038/s41593-019-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Logiacco F, Xia P, Georgiev SV, Franconi C, Chang YJ, Ugursu B, Sporbert A, Kuhn R, Kettenmann H, Semtner M. Microglia sense neuronal activity via GABA in the early postnatal hippocampus. Cell Rep. 2021;37(13):110128. doi: 10.1016/j.celrep.2021.110128. [DOI] [PubMed] [Google Scholar]
- 100.Umpierre AD, Bystrom LL, Ying Y, Liu YU, Worrell G, Wu LJ (2020) Microglial calcium signaling is attuned to neuronal activity in awake mice. Elife 7:9:e56502. 10.7554/eLife.56502 [DOI] [PMC free article] [PubMed]
- 101.Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, Taloma SE, Barron JJ, Molofsky AB, Kheirbek MA, Molofsky AV. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182(2):388–403.e15. doi: 10.1016/j.cell.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Favuzzi E, Huang S, Saldi GA, Binan L, Ibrahim LA, Fernandez-Otero M, Cao Y, Zeine A, Sefah A, Zheng K, Xu Q, Khlestova E, Farhi SL, Bonneau R, Datta SR, Stevens B, Fishell G. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell. 2021;184(15):4048–4063 e32. doi: 10.1016/j.cell.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kovacs RA, Vadaszi H, Bulyaki E, Torok G, Toth V, Matyas D, Kun J, Hunyadi-Gulyas E, Fedor FZ, Csincsi A, Medzihradszky K, Homolya L, Juhasz G, Kekesi KA, Jozsi M, Gyorffy BA, Kardos J. Identification of neuronal pentraxins as synaptic binding partners of C1q and the involvement of NP1 in synaptic pruning in adult mice. Front Immunol. 2020;11:599771. doi: 10.3389/fimmu.2020.599771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scott-Hewitt N, Perrucci F, Morini R, Erreni M, Mahoney M, Witkowska A, Carey A, Faggiani E, Schuetz LT, Mason S, Tamborini M, Bizzotto M, Passoni L, Filipello F, Jahn R, Stevens B, Matteoli M. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020;39(16):e105380. doi: 10.15252/embj.2020105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, Akil O, Joshita S, Barres BA, Paz JT, Molofsky AB, Molofsky AV. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359(6381):1269–1273. doi: 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dou Y, Wu H-J, Li H-Q, Qin S, Wang Y-E, Li J, Lou H-F, Chen Z, Li X-M, Luo Q-M, Duan S. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012;22(6):1022–1033. doi: 10.1038/cr.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan W-B, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 108.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci. 1998;95(18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37(4):314–327. doi: 10.1002/glia.10037. [DOI] [PubMed] [Google Scholar]
- 110.Eyo UB, Wu LJ. Microglia: lifelong patrolling immune cells of the brain. Prog Neurobiol. 2019;179:101614. doi: 10.1016/j.pneurobio.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 112.Dejanovic B, Huntley MA, De Mazière A, Meilandt WJ, Wu T, Srinivasan K, Jiang Z, Gandham V, Friedman BA, Ngu H, Foreman O, Carano RAD, Chih B, Klumperman J, Bakalarski C, Hanson JE, Sheng M. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron. 2018;100(6):1322–1336.e7. doi: 10.1016/j.neuron.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 113.Cheadle L, Rivera SA, Phelps JS, Ennis KA, Stevens B, Burkly LC, Lee W-CA, Greenberg ME. Sensory experience engages microglia to shape neural connectivity through a non-phagocytic mechanism. Neuron. 2020;108(3):451–468.e9. doi: 10.1016/j.neuron.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Allen NJ, Eroglu C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96(3):697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A. 2011;108(32):E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dzyubenko E, Manrique-Castano D, Kleinschnitz C, Faissner A, Hermann DM. Role of immune responses for extracellular matrix remodeling in the ischemic brain. Ther Adv Neurol Disord. 2018;11:1756286418818092. doi: 10.1177/1756286418818092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Enzmann G, Mysiorek C, Gorina R, Cheng YJ, Ghavampour S, Hannocks MJ, Prinz V, Dirnagl U, Endres M, Prinz M, Beschorner R, Harter PN, Mittelbronn M, Engelhardt B, Sorokin L. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol. 2013;125(3):395–412. doi: 10.1007/s00401-012-1076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neumann J, Riek-Burchardt M, Herz J, Doeppner TR, Konig R, Hutten H, Etemire E, Mann L, Klingberg A, Fischer T, Gortler MW, Heinze HJ, Reichardt P, Schraven B, Hermann DM, Reymann KG, Gunzer M. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129(2):259–277. doi: 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- 119.Malone K, Amu S, Moore AC, Waeber C. Immunomodulatory therapeutic strategies in stroke. Front Pharmacol. 2019;10:630. doi: 10.3389/fphar.2019.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28(5):927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 121.Sun Y, Hei M, Fang Z, Tang Z, Wang B, Hu N. High-mobility group box 1 contributes to cerebral cortex injury in a neonatal hypoxic-ischemic rat model by regulating the phenotypic polarization of microglia. Front Cell Neurosci. 2019;13:506. doi: 10.3389/fncel.2019.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borbor M, Yin D, Brockmeier U, Wang C, Doeckel M, Pillath-Eilers M, Kaltwasser B, Hermann DM, Dzyubenko E. Neurotoxicity of ischemic astrocytes involves STAT3-mediated metabolic switching and depends on glycogen usage, Glia n/a(n/a) 2023. [DOI] [PubMed] [Google Scholar]
- 124.Al'Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, Kumar S, Kumar P, Mitsios N, Slevin M. Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain. 2006;129(Pt 8):2158–2176. doi: 10.1093/brain/awl139. [DOI] [PubMed] [Google Scholar]
- 125.Dzyubenko E, Manrique-Castano D, Pillath-Eilers M, Vasileiadou P, Reinhard J, Faissner A, Hermann DM. Tenascin-C restricts reactive astrogliosis in the ischemic brain. Matrix Biol. 2022;110:1–15. doi: 10.1016/j.matbio.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 126.Manrique-Castano D, Dzyubenko E, Borbor M, Vasileiadou P, Kleinschnitz C, Roll L, Faissner A, Hermann DM. Tenascin-C preserves microglia surveillance and restricts leukocyte and, more specifically, T cell infiltration of the ischemic brain. Brain Behav Immun. 2021;91:639–648. doi: 10.1016/j.bbi.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 127.Dzyubenko E, Manrique-Castano D, Kleinschnitz C, Faissner A, Hermann DM. Topological remodeling of cortical perineuronal nets in focal cerebral ischemia and mild hypoperfusion. Matrix Biol. 2018;74:121–132. doi: 10.1016/j.matbio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 128.Hartig W, Mages B, Aleithe S, Nitzsche B, Altmann S, Barthel H, Krueger M, Michalski D. Damaged neocortical perineuronal nets due to experimental focal cerebral ischemia in mice, rats and sheep. Front Integr Neurosci. 2017;11(15):15. doi: 10.3389/fnint.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, Mook-Jung I. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 2019;30(3):493–507 e6. doi: 10.1016/j.cmet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 130.Lively S, Schlichter LC. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J Neuroinflammation. 2013;10:75. doi: 10.1186/1742-2094-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crapser JD, Ochaba J, Soni N, Reidling JC, Thompson LM, Green KN. Microglial depletion prevents extracellular matrix changes and striatal volume reduction in a model of Huntington’s disease. Brain. 2020;143(1):266–288. doi: 10.1093/brain/awz363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crapser JD, Spangenberg EE, Barahona RA, Arreola MA, Hohsfield LA, Green KN. Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine. 2020;58:102919. doi: 10.1016/j.ebiom.2020.102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutierrez-Vazquez C, Hewson P, Staszewski O, Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, Quintana FJ. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wheeler MA, Jaronen M, Covacu R, Zandee SEJ, Scalisi G, Rothhammer V, Tjon EC, Chao CC, Kenison JE, Blain M, Rao VTS, Hewson P, Barroso A, Gutierrez-Vazquez C, Prat A, Antel JP, Hauser R, Quintana FJ. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell. 2019;176(3):581–596.e18. doi: 10.1016/j.cell.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Silva RV, Biskup K, Zabala-Jouvin JK, Batzdorf CS, Stellmach C, Morr AS, Sack I, Ludwig A, Blanchard V, Infante-Duarte C. Brain inflammation induces alterations in glycosaminoglycan metabolism and subsequent changes in CS-4S and hyaluronic acid. Int J Biol Macromol. 2023;230:123214. doi: 10.1016/j.ijbiomac.2023.123214. [DOI] [PubMed] [Google Scholar]