Abstract
Immune checkpoint inhibitors (ICIs) are safe and efficacious treatments for advanced primary liver cancer (PLC). The efficacy of different ICIs in the treatment of liver cancer remains unclear. The purpose of this study was to explore whether there is a difference in the efficacy and safety of various programmed cell death protein 1 (PD-1) inhibitors in combination with lenvatinib in the treatment of unresectable PLC. Patients with PLC treated with lenvatinib in combination with PD-1 inhibitors (camrelizumab, tislelizumab, sintilimab, or pembrolizumab) between January 2018 and December 2021 were retrospectively enrolled. Tumor response, adverse events, and grades were evaluated. Kaplan–Meier analysis and log-rank test were used to compare the overall survival and progression-free survival of patients treated with different PD-1 inhibitors. Cox regression analysis was used for univariate and multivariate analyses to identify clinical variables related to treatment efficacy. This study included a total of 176 patients who received a combination of lenvatinib and PD-1 inhibitors. Of these, 103 patients received camrelizumab, 44 received tislelizumab, 20 received sintilimab, and 9 received pembrolizumab. There was no significant difference in the pairwise comparison of camrelizumab, tislelizumab, sintilimab, and pembrolizumab using Kaplan–Meier survival analysis. Adverse events occurred in 40 (22.7%) patients (grade ≥ 3, 2.3%). The incidence of grade 3 adverse events among the four PD-1 inhibitor groups was below 5%. Camrelizumab, tislelizumab, sintilimab, and pembrolizumab are viable options for patients with unresectable PLC. These PD-1 inhibitors in combination with lenvatinib showed good safety profiles. The results guide selecting treatment for patients with unresectable PLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-023-00708-0.
Keywords: Primary liver cancer, Immune checkpoint inhibitors, Anti-angiogenesis drug, Combination therapy
Introduction
Primary liver cancer (PLC) is a global health concern. It was the sixth most frequently diagnosed cancer and the third leading cause of cancer-related deaths worldwide in 2020, with approximately 906 000 new cases and 830 000 deaths [1]. Liver cancer is associated with a 5-year survival rate of just 18%, making it one of the deadliest cancers. Patients from Asian countries, such as China, have worse outcomes, with 5-year survival rates as low as 12% [2]. Early-stage liver cancers may be cured with surgical resection, ablation, or liver transplantation. Unfortunately, more than 70% of patients with liver cancer are in the middle and late disease stages when they are first diagnosed [3, 4]. Patients with advanced disease lose the opportunity for surgery; such patients can only receive palliative treatment, such as systematic treatment, and their prognosis is poor [5].
In 2007, the FDA approved the multi-targeted tyrosine kinase inhibitor sorafenib, which greatly changed the treatment landscape of hepatocellular carcinoma (HCC) [6]. Lenvatinib is another multi-targeted tyrosine kinase inhibitor (TKI) that can be taken orally and is as effective as sorafenib in inhibiting tumor angiogenesis and growth. As a result, lenvatinib therapy has been included as the second recommended first-line targeted molecular therapy in the 2019 National Comprehensive Cancer Network (NCCN) guidelines [7]. Several successful programs have been completed, leading to the regulatory approval of cabozantinib and ramucirumab, as well as the breakthrough combination therapy of atezolizumab and bevacizumab [8–10]. Despite the promising preliminary results of anti-angiogenic agent and immune checkpoint inhibitor (ICI) combination therapy in advanced HCC, it still encounters various challenges, including the absence of reliable biomarkers to determine treatment response [11–16].
Immune checkpoint inhibitors have been evaluated in clinical trials for the treatment of HCC, but results from single-agent ICI trials have been disappointing. However, immune-based combination therapies have shown more promising results. The combination of atezolizumab and bevacizumab marks a significant advancement in the treatment of HCC and represents a new milestone in the field of HCC treatment [10]. The phase III LEAP-002 study, which compared the combination of lenvatinib and pembrolizumab with the combination of lenvatinib and placebo as first-line treatment for advanced unresectable hepatocellular carcinoma, did not meet the primary endpoints of overall survival (OS) and progression-free survival (PFS) [17]. A result from a large-scale real-world study showed that there was no significant difference in OS between the combination of lenvatinib and pembrolizumab and the combination of lenvatinib with other programmed cell death protein 1 (PD-1) inhibitors [18].
Several studies on the combination of lenvatinib and ICIs have been conducted, and preliminary results show that these combinations are well tolerated [19–22]. Lenvatinib combined with ICIs has shown good antitumor efficacy and safety in patients with liver cancer. Previous studies have explored the efficacy and safety of ICIs combined with lenvatinib and reported their efficacy and adverse event (AE) rates [19, 21, 23–25]. Many clinical trials are still ongoing to investigate the effectiveness of combining lenvatinib with various ICIs [26]. There are significant differences in the drugs used, patients involved, study designs, and phases of research among these clinical trials, and the clinical outcomes are inconsistent. There is still a lack of clinical trials comparing two different ICIs, so it is still unclear what differences in efficacy and safety exist among different ICIs.
Additionally, PD-1 inhibitors developed by different manufacturers vary in composition and molecular structure. Camrelizumab is a humanized monoclonal antibody that binds to the PD-1 receptor [27, 28]. Tislelizumab is another monoclonal antibody that specifically binds to PD-1 with high affinity and specificity [29]. Sintilimab is a recombinant humanized IgG4 monoclonal antibody that targets PD-1 [30]. Pembrolizumab is a humanized IgG4 monoclonal antibody that targets PD-1 [31]. The pharmacokinetic behavior of anti PD-1 antibodies from different companies and the differences in their affinity for the human neonatal Fc receptor (hFcRn) may result in variations in drug efficacy. Furthermore, different PD-1 inhibitors have distinct mechanisms of action and target inhibition efficiency, which may lead to differences in therapeutic effects [27, 28].
To date, there is currently a lack of research exploring whether there are differences in the efficacy and safety of different ICIs combined with lenvatinib, and which combination regimen can bring better benefits to patients. Hence, we aimed to investigate whether there are differences in the efficacy and safety of various PD-1 inhibitors in combination with lenvatinib for the treatment of unresectable PLC.
Methods
Study population and data collection
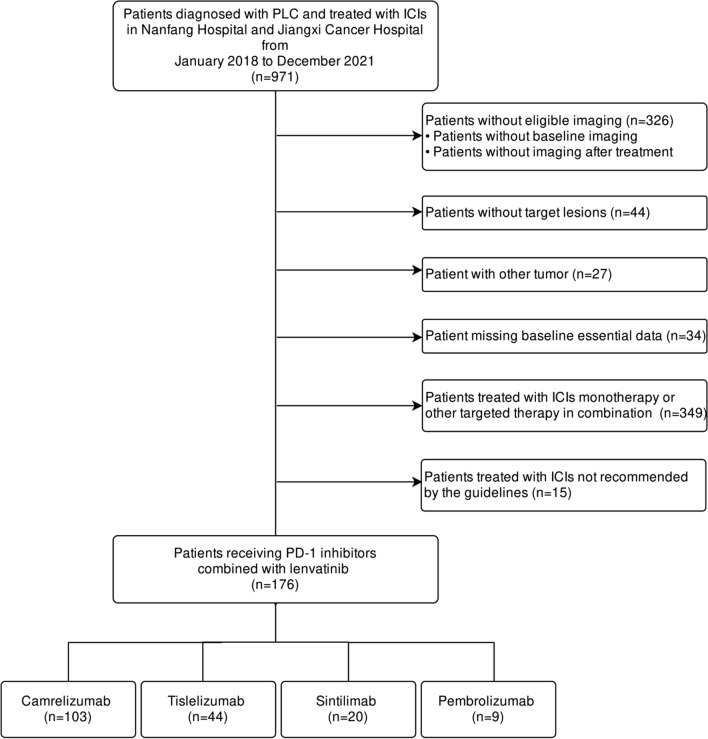
Between January 2018 and December 2021, patients who were histologically or clinically diagnosed with PLC according to the criteria of the NCCN Clinical Practice Guidelines [32], and treated with lenvatinib in combination with an ICI at the Southern Hospital and Jiangxi Cancer Hospital, were retrospectively enrolled. The flowchart of the patient selection process is shown in Fig. 1. The inclusion criteria were as follows: (1) patients diagnosed with PLC and treated with ICIs in combination with lenvatinib and (2) patients with Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2 points. The exclusion criteria were as follows: (1) patients who had not undergone the required imaging examination before and after treatment; (2) patients without measurable target lesions; (3) patients with tumors other than PLC; (4) patients without baseline data; (5) patients treated with ICI monotherapy; and (6) patients treated with ICIs not recommended by the guidelines. Before treatment with ICIs, the following data of eligible patients were collected: age; sex; cirrhosis presence; hepatitis B virus infection; ECOG PS; Child–Pugh class; Barcelona Clinic Liver Cancer stage (BCLC); portal vein tumor thrombus (PVTT); lines of systemic treatment; type of ICI; tumor metastasis presence; ascites; splenomegaly; routine blood test data including neutrophils and lymphocyte counts, neutrophil to lymphocyte ratio (NLR), hemoglobin (HGB) level; and other laboratory tests. Portal vein tumor thrombus was classified into one of four types [33, 34]. Four PD-1 inhibitors (camrelizumab, tislelizumab, sintilimab, and pembrolizumab) were included in this study.
Fig. 1.
Study flowchart. PLC primary liver cancer, ICI immune checkpoint inhibitor
Assessment and endpoints
An assessment of treatment response was scheduled at weeks 6–8 using magnetic resonance imaging (MRI) or dynamic computed tomography (CT). Splenomegaly was also diagnosed using CT imaging or MRI. Tumor response, including the objective response rate (ORR) and disease control rate (DCR), was evaluated according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) [35]. The treatment responses were evaluated and classified by a radiologist.
Adverse events and their grades were recorded according to the Common Terminology Criteria for Adverse Events (CTCAE; version 5.0). The primary endpoint was overall survival (OS) and the secondary endpoint was progression-free survival (PFS). Overall survival was defined as the period from the start of PD-1 inhibitor treatment to death. Progression-free survival was defined as the time from the first use of PD-1 inhibitors to disease progression or death.
Statistical analysis
Descriptive statistics were used to summarise the baseline characteristics, tumor response, and AE data. Kaplan–Meier analysis and log-rank test were used to compare the OS and PFS of patients treated with different PD-1 inhibitors. Cox regression analysis was used for univariate and multivariate analyses to identify clinical variables related to efficacy. All tests were bilateral, and statistical significance was set at P < 0.05. In the pairwise comparison of Kaplan–Meier survival analysis, Bonferroni correction was used, and the difference was considered statistically significant when the P value was less than 0.0083. SPSS version 26 software (IBM Corp., Chicago, Illinois, USA) and R (version 4.1.0) were used for all statistical analyses.
Results
Patient characteristics
One hundred and seventy-six patients who received lenvatinib in combination with PD-1 inhibitors were included in this study. The baseline patient characteristics are summarised in Table 1. Of these, 103 patients received camrelizumab, 44 received tislelizumab, 20 received sintilimab, and 9 received pembrolizumab. Fifty-one patients (29.0%) were aged 60 years or older. One hundred and fifty-three patients (86.9%) were treated with PD-1 inhibitors as the first-line treatment. Most patients had BCLC stage B or C disease, accounting for 19.3% and 80.1%, respectively. One hundred and twenty-seven patients had intrahepatic metastasis, 41 had lung metastasis, and 58 had lymph node metastasis. Eighty-seven patients had extrahepatic metastasis.
Table 1.
Patient and disease characteristics (N = 176)
| Patient characteristic | No | Percent of cohort |
|---|---|---|
| Age | ||
| < 60 | 125 | 71.0 |
| ≥ 60 | 51 | 29.0 |
| Sex | ||
| Male | 159 | 90.3 |
| Female | 17 | 9.7 |
| Number of lesions | ||
| Single | 25 | 14.2 |
| Multiple | 151 | 85.8 |
| Number of treatment lines | ||
| 1 | 153 | 86.9 |
| 2 | 20 | 11.4 |
| ≥ 3 | 3 | 1.7 |
| Immunotherapy | ||
| Camrelizumab | 103 | 58.5 |
| Tislelizumab | 44 | 25.0 |
| Sintilimab | 20 | 11.4 |
| Pembrolizumab | 9 | 5.1 |
| ECOG PS | ||
| 0 | 106 | 60.2 |
| ≥ 1 | 70 | 39.8 |
| BCLC stage | ||
| A | 1 | 0.6 |
| B | 34 | 19.3 |
| C | 141 | 80.1 |
| Child–Pugh score | ||
| A | 132 | 75.0 |
| B | 43 | 24.4 |
| C | 1 | 0.6 |
| HBV infection | ||
| No | 17 | 9.7 |
| Yes | 159 | 90.3 |
| Antiviral therapy before treatment | ||
| No | 34 | 19.3 |
| Yes | 142 | 80.7 |
| Liver metastasis | ||
| No | 49 | 27.8 |
| Yes | 127 | 72.2 |
| Lung metastasis | ||
| No | 135 | 76.7 |
| Yes | 41 | 23.3 |
| Lymph node metastasis | ||
| No | 118 | 67.0 |
| Yes | 58 | 33.0 |
| Extrahepatic metastasis | ||
| No | 89 | 50.6 |
| Yes | 87 | 49.4 |
| PVTT | ||
| No | 86 | 48.9 |
| Yes | 90 | 51.1 |
| Ascites | ||
| No | 123 | 69.9 |
| Yes | 53 | 30.1 |
| NLR | ||
| < 3 | 96 | 54.5 |
| ≥ 3 | 80 | 45.5 |
| HGB | ||
| ≥ 120 g/L | 116 | 65.9 |
| < 120 g/L | 60 | 34.1 |
ECOG PS Eastern Cooperative Oncology Group performance status, BCLC Barcelona Clinic Liver Cancer, HBV Hepatitis B virus, PVTT Portal vein tumour thrombus, NLR neutrophil to lymphocyte ratio, HGB haemoglobin
Tumor response and safety
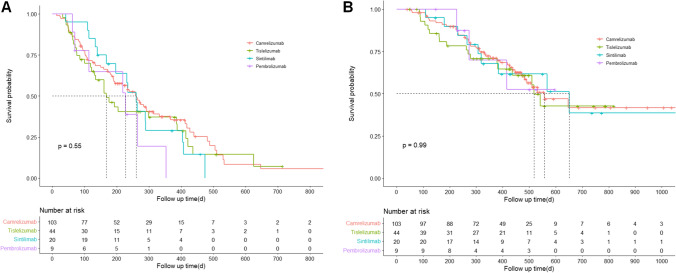
The median follow-up duration was 481 days (95% confidence interval (CI): 454–517 days). At the time of data cut-off, 85 patients were still alive and 21 were lost to follow-up. The median OS of all patients was 557 days (95% CI: 439–675 days, mean 658.5 days) and the median PFS was 234 days (95% CI: 186–282 days, mean 286.0 days). The ORR was 20.5% and the DCR was 79.0% for all patients. Thirty-seven patients (21.0%) had progressive diseases as the best response (Table 2). The ORR in the patients treated with camrelizumab, tislelizumab, sintilimab, and pembrolizumab was 23.3%, 15.9%, 20.0%, and 11.1%, respectively. The DCR was 80.6%, 75.0%, 80.0%, and 77.8% in patients receiving camrelizumab, tislelizumab, sintilimab, and pembrolizumab, respectively. The median PFS of the patients receiving camrelizumab, tislelizumab, sintilimab, and pembrolizumab was 260 days (95% CI: 212–308 days), 168 days (95% CI: 113–224 days), 260 days (95% CI: 218–302 days), and 227 days (95% CI: 79–375 days), respectively. The median OS of the patients receiving camrelizumab, tislelizumab, and sintilimab was 557 days (95% CI: 410–704 days), 518 days (95% CI: 470–566 days), and 651 days (95% CI: 312–990 days), respectively. Similar 1-year OS rates were observed among the four groups: 71.2% for camrelizumab, 70.8% for tislelizumab, 67.9% for sintilimab, and 70.0% for pembrolizumab. The Kaplan–Meier survival curves for OS and PFS are shown in Fig. 2. There was no significant difference in the pairwise comparison of patients treated with the different PD-1 inhibitors using Kaplan–Meier survival analysis (P > 0.0083) (Table 3). In our study, 156 patients were diagnosed with HCC, 20 with intrahepatic cholangiocarcinoma (ICC), or with HCC-ICC. Another survival analysis was performed on 156 patients diagnosed with HCC. The results of Kaplan–Meier survival analysis and paired comparisons between groups are shown in Fig. S1 and Table S1. No significant difference in the pairwise comparison of patients treated with the different PD-1 inhibitors (P > 0.0083).
Table 2.
Response outcomes
| Outcome | Total (n = 176) | Camrelizumb (n = 103) | Tislelizumab (n = 44) | Sintilimab (n = 20) | Pembrolizumab (n = 9) |
|---|---|---|---|---|---|
| Best response | |||||
| Partial response | 36 (20.5) | 24 (23.3) | 7 (15.9) | 4 (20.0) | 1 (11.1) |
| Stable disease | 103 (58.5) | 59 (57.3) | 26 (59.1) | 12 (60.0) | 6 (66.7) |
| Progressive disease | 37 (21.0) | 20(19.4) | 11 (25.0) | 4 (20.0) | 2 (22.2) |
| Overall response rate | 20.5% | 23.3% | 15.9% | 20.0% | 11.1% |
| Disease control rate | 79.0% | 80.6% | 75.0% | 80.0% | 77.8% |
| Median progression-free survival, day | 234 | 260 | 168 | 260 | 227 |
| Median overall survival, day | 577 | 557 | 518 | 651 | - |
| 1-year overall survival rate | 70.7% | 71.2% | 70.8% | 67.9% | 70.0% |
| 2-year overall survival rate | 41.4% | 41.8% | 42.7% | 38.6% | - |
Fig. 2.
Kaplan–Meier analysis. Progression-free survival (A) and overall survival (B) in patients receiving different PD-1 inhibitors combined with lenvatinib
Table 3.
Pairwise comparison of Kaplan–Meier survival analysis of different PD-1 inhibitors
| Immunotherapy | Camrelizumab | Tislelizumab | Sintilimab | Pembrolizuma b |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Chi-square | P | Chi-square | P | Chi-square | P | Chi-square | P | ||
| OS | Camrelizumab | 0.111 | 0.739 | < 0.001 | 0.997 | 0.001 | 0.975 | ||
| Tislelizumab | 0.111 | 0.739 | 0.082 | 0.774 | 0.086 | 0.770 | |||
| Sintilimab | < 0.001 | 0.997 | 0.082 | 0.774 | 0.005 | 0.941 | |||
| Pembrolizumab | 0.001 | 0.975 | 0.086 | 0.77 | 0.005 | 0.941 | |||
| PFS | Camrelizumab | 0.630 | 0.427 | 0.439 | 0.507 | 1.667 | 0.197 | ||
| Tislelizumab | 0.630 | 0.427 | 0.045 | 0.832 | 0.387 | 0.534 | |||
| Sintilimab | 0.439 | 0.507 | 0.045 | 0.832 | 1.497 | 0.221 | |||
| Pembrolizumab | 1.667 | 0.197 | 0.387 | 0.534 | 1.497 | 0.221 | |||
OS overall survival, PFS progression-free survival
Adverse events occurred in 45 (23.8%) patients (grade ≥ 3, 2.1%). Among them, endocrine AEs were the most frequent (≥ 6.4% of patients) (Table 4). The incidence of AEs in the camrelizumab, tislelizumab, sintilimab, and pembrolizumab groups was 22.3%, 25.0%, 25.0%, and 11.1%, respectively. Grade 3 or higher AEs occurred in patients treated with camrelizumab (2 cases) and tislelizumab (2 cases). One patient who received lenvatinib in combination with tislelizumab stopped PD-1 inhibitor therapy because of grade 4 pneumonia and grade 4 anemia. Two patients who received lenvatinib in combination with camrelizumab stopped immunotherapy owing to adverse cardiac events. No deaths due to fatal AEs were reported.
Table 4.
Adverse events
| Total (%) | Camrelizumab | Tislelizumab | Sintilimab | Pembrolizumab | |
|---|---|---|---|---|---|
| Any grade | 40 (22.7) | 23 (22.3) | 11 (25.0) | 5 (25.0) | 1 (11.1) |
| Grade ≥ 3 | 4 (2.3) | 2 (1.9) | 2 (4.5) | 0 (0) | 0 (0) |
| Skin | 10 (5.7) | 7 (6.8) | 2 (4.5) | 1 (5.0) | 0 (0) |
| Endocrine | 9 (5.1) | 5 (4.9) | 1 (2.3) | 3 (15.0) | 0 (0) |
| Liver | 4 (2.3) | 1 (1.0) | 2 (4.5) | 0 (0) | 1 (11.1) |
| Stomach and intestines | 4 (2.3) | 2 (1.9) | 2 (4.5) | 0 (0) | 0 (0) |
| Lung | 1 (0.6) | 0 (0) | 1 (2.3) | 0 (0) | 0 (0) |
| Kidney | 2 (1.1) | 2 (1.9) | 0 (0) | 0 (0) | 0 (0) |
| Blood | 7 (4.0) | 5 (4.9) | 2 (4.5) | 0 (0) | 0 (0) |
| Heart | 10 (5.7) | 4 (3.9) | 5 (11.4) | 1 (5.0) | 0 (0) |
Univariate and multivariate analyses
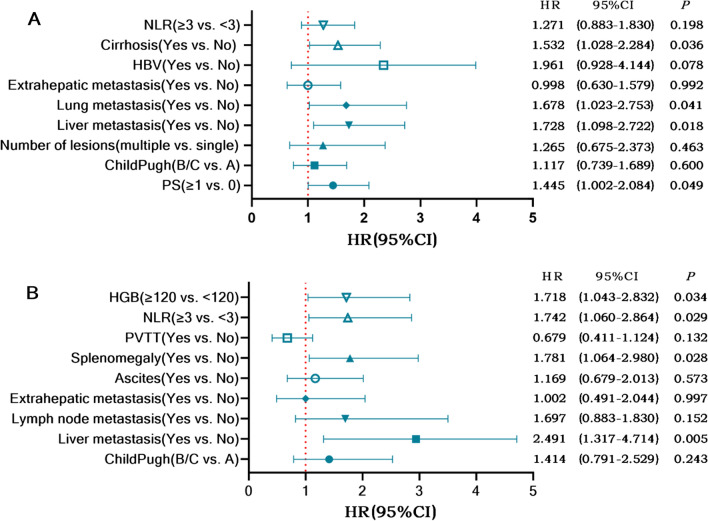
In the univariate analysis of PFS, we found that different PD-1 inhibitors were not risk factors for PFS. The multivariate analysis suggested that ECOG PS, cirrhosis, intrahepatic metastasis, and pulmonary metastasis were independent risk factors for PFS (Table S2). The univariate analysis demonstrated that different PD-1 inhibitors were not risk factors for OS. Child–Pugh class, intrahepatic metastasis, lymph node metastasis, extrahepatic metastasis, ascites, splenomegaly, NLR, HGB, and PVTT were included in the multivariate analysis, and the results showed that intrahepatic metastasis, splenomegaly, NLR, and HGB were independent risk factors for OS (Table S3). Forest plots of the multivariate COX regression analysis for PFS and OS are shown in Fig. 3.
Fig. 3.
Forest plot of multivariate COX regression analysis for progression-free survival (A) and for overall survival (B)
Discussion
In this real-world study, we explored the efficacy and safety of different PD-1 inhibitors in combination with lenvatinib for the treatment of unresectable PLC. There was no significant difference in the efficacy of camrelizumab, tislelizumab, sintilimab, or pembrolizumab combined with lenvatinib. All AEs associated with the PD-1 inhibitors were manageable. Intrahepatic metastasis, splenomegaly, NLR, and HGB were independent risk factors for disease prognosis under treatment with PD-1 inhibitors in combination with lenvatinib.
In clinical trials, the combination of antiangiogenic drugs and ICIs has achieved significant clinical efficacy, with higher ORR and better survival results than monotherapy [10, 19, 36–38]. The combination of ICIs and targeted therapy has good efficacy and safety and will become an indispensable therapeutic option for liver cancer in the future. Future studies should focus on finding a better combination of ICI inhibitors and targeted therapy. Most previous studies have explored the differences between combination therapy and monotherapy, and very few studies have evaluated the difference in efficacy among different ICIs combined with lenvatinib. A study found that there was no significant difference in OS and PFS between using combination of lenvatinib and pembrolizumab versus using combination of lenvatinib and other anti-PD-1. In our study, we further compared the efficacy of four PD-1 inhibitors. A study involving 29 patients showed that lenvatinib combined with nivolumab and lenvatinib combined with pembrolizumab was associated with an ORR of 37.5% and 7.7%, respectively. Kaplan–Meier survival estimates indicated that there was no difference between the groups in terms of OS and PFS [39]. Our study included a larger sample and more types of PD-1 inhibitors, and our results also revealed that there was no significant difference in OS and PFS among various PD-1 inhibitors used in combination with lenvatinib. In addition, the current study included a real-world cohort of patients with unresectable PLC who are typically excluded from prospective clinical trials. Therefore, our results guide the treatment of such patients.
A meta-analysis comparing the efficacy and safety of nivolumab and pembrolizumab in patients with non-small cell lung cancer showed no significant difference in efficacy [40]. Torasawa et al. reported that the efficacy and safety of nivolumab and pembrolizumab in subsequent treatment lines were not significantly different in patients with advanced non-small cell lung cancer [41]. However, patients with PLC were not included in their study. Compared to these studies, our study included a large number of patients with PLC.
In terms of safety, high-grade hepatic toxicity was observed in a previous study, which caused four deaths. However, there was no significant difference in the frequency of AEs between nivolumab and pembrolizumab [39]. Another study showed that the incidence of all-grade AEs was 27%, and the incidence of grade 3 or higher AEs was 6% in patients with advanced hepatocellular carcinoma who received ICI monotherapy [42]. It has been reported that serious AEs after ICI and tyrosine kinase inhibitor combination treatment occur in up to 67% of patients [19]. Although 22.7% of the patients experienced AEs related to PD-1 inhibitors in the current study, all AEs were manageable; there were no fatal AEs, and the four PD-1 inhibitors were well tolerated by the patients. Compared to the aforementioned results, no new or unexpected AEs occurred in our cohort [19, 37, 43].
Our previous study results suggest that lymph node metastasis and splenomegaly is an independent prognostic factor for PLC in immunotherapy [44–46]. A meta-analysis provided strong or highly suggestive evidence that NLR is associated with cancer prognosis [47]. A prospective study showed that a normal pre-treatment HGB level is a favorable prognostic marker in patients with advanced tumors receiving ICI treatment [48]. Our results are similar to those of previous studies.
The present study had some limitations. First, it was a retrospective study that includes a wide range of different patient populations and the study was limited to an Asian population with a relatively small sample size. Owing to the heterogeneity of the study population and the different combination therapies, the results must be interpreted with caution. Second, the number of patients receiving pembrolizumab and sintilimab was relatively small, which reduced the quality of the conclusions. In addition, the median follow-up time of this study was not long enough, and a longer follow-up time is required to obtain more meaningful median OS results in the cohort. Finally, the research results need to be verified in a larger population and prospective studies.
Conclusions
Our results suggest that camrelizumab, tislelizumab, sintilimab, and pembrolizumab are viable options for patients with unresectable PLC. PD-1 inhibitors combined with lenvatinib have good safety, and this real-world study guides selecting treatment for patients with PLC.
Supplementary Information
Acknowledgements
We would like to thank all data collectors for supporting this study. We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- AE
Adverse event
- BCLC
Barcelona Clinic Liver Cancer
- CT
Computed tomography
- DCR
Disease control rate
- ICI
Immune checkpoint inhibitor
- MRI
Magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- ORR
Objective response rate
- OS
Overall survival
- PFS
Progression-free survival
- PLC
Primary liver cancer
- PVTT
Portal vein tumor thrombus
Author contributions
Conception and design: QML, QCS, YJ, JZH, HBZ; administrative support: HC, LSX; provision of study materials or patients: LL, HZD; collection and assembly of data: CH, RNL, LZ, JRW; data analysis and interpretation: QML, QCS, YJ, JZH, XW; manuscript writing: all authors; final approval of manuscript: all authors.
Funding
This work was supported by the National Nature Science Foundation of China (Grant Nos. 81773008 and 81972897), Guangzhou Science and Technology Project (Grant No. 202201011183), China Postdoctoral Science Foundation (Grant No. 2021M701629), the Scientific Research Project of Guangdong Provincial Administration of Traditional Chinese Medicine (Grant No. 20202128), and Guangdong Natural Science Foundation (Grant No. 2022A1515110656).
Data availability
Data are available from the authors upon reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement for informed consent from the patients was waived because of the retrospective nature of the study. The study design was approved by the Medical Ethics Committee of Nanfang Hospital, Southern Medical University (approval number: NFEC-2021-048).
Consent for publication
The requirement for written informed consent was waived because of the retrospective nature of the study.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi-mei Li, Qing-can Sun, Yan Jian, Jing-zhe He and Hong-bo Zhu contributed equally to this work.
Contributor Information
Han-zhi Dong, Email: ndzhlyy1394@ncu.edu.cn.
Lu-shan Xiao, Email: 15622178423@163.com.
Hao Cui, Email: haocui2021@163.com.
References:
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16:333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 12.Viscardi G, Tralongo AC, Massari F, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi: 10.1016/j.ejca.2022.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo A, Ricci AD, Di Federico A, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol. 2021;11:803133. doi: 10.3389/fonc.2021.803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31:361–369. doi: 10.1080/13543784.2022.2009455. [DOI] [PubMed] [Google Scholar]
- 15.Hong C, Dong HZ, Li RN, et al. Predictive value of the hepatic immune predictive index for patients with primary liver cancer treated with immune checkpoint inhibitors. Dig Dis. 2022 doi: 10.1159/000527574. [DOI] [PubMed] [Google Scholar]
- 16.Xiao LS, Li RN, Cui H, et al. Use of computed tomography-derived body composition to determine the prognosis of patients with primary liver cancer treated with immune checkpoint inhibitors: a retrospective cohort study. BMC Cancer. 2022;22:737. doi: 10.1186/s12885-022-09823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn RS, Kudo M, Merle P, et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2022;33:S1401. doi: 10.1016/j.annonc.2022.08.031. [DOI] [Google Scholar]
- 18.Yang X, Chen B, Wang Y, et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023 doi: 10.1007/s12072-022-10480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol. 2021;7:113–123. doi: 10.1001/jamaoncol.2020.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Chang N, Shi L, et al. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: a retrospective, real-world study. Heliyon. 2022;8:e9538. doi: 10.1016/j.heliyon.2022.e09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 24.Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi: 10.1016/j.ejca.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Wu WC, Lin TY, Chen MH, et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;40:789–797. doi: 10.1007/s10637-022-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203–222. doi: 10.1038/s41575-022-00704-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang SJ, Dougan SK, Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. 2023 doi: 10.1016/j.trecan.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okiyama N, Tanaka R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int. 2022;71:169–178. doi: 10.1016/j.alit.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Ren Z, Ducreux M, Abou-Alfa GK, et al. Tislelizumab in patients with previously treated advanced hepatocellular carcinoma (RATIONALE-208): a multicenter, non-randomized, open-label, phase 2 trial. Liver Cancer. 2023;12:72–84. doi: 10.1159/000527175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Wang C, Cao L, Li S. Treatment of advanced intrahepatic cholangiocarcinoma with sintilimab combined with tegafur-gimeracil-oteracil potassium capsules (S-1): a case report. Ann Palliat Med. 2020;9:497–503. doi: 10.21037/apm.2020.03.14. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Li T, Bai Y, et al. Efficacy and safety of pembrolizumab versus sintilimab treatment in patients with advanced squamous lung cancer: a real-world study in China. Front Oncol. 2023;13:1147903. doi: 10.3389/fonc.2023.1147903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson AB, Dangelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 33.Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surg Today. 2014;44:219–226. doi: 10.1007/s00595-013-0585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikai I, Yamamoto Y, Yamamoto N, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12:65–75. doi: 10.1016/S1055-3207(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 35.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Luo XY, Wu KM, He XX. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets. J Exp Clin Cancer Res. 2021;40:172. doi: 10.1186/s13046-021-01968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label. Phase II Trial Clin Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 38.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, Xu L, Ma T, et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol. 2021;11:751159. doi: 10.3389/fonc.2021.751159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng TR, Tsai FP, Wu TW. Indirect comparison between pembrolizumab and nivolumab for the treatment of non-small cell lung cancer: a meta-analysis of randomized clinical trials. Int Immunopharmacol. 2017;49:85–94. doi: 10.1016/j.intimp.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Torasawa M, Yoshida T, Yagishita S, et al. Nivolumab versus pembrolizumab in previously-treated advanced non-small cell lung cancer patients: a propensity-matched real-world analysis. Lung Cancer. 2022;167:49–57. doi: 10.1016/j.lungcan.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Qin S, Zuo Z. Immune-related myocarditis in two patients receiving camrelizumab therapy and document analysis. J Oncol Pharm Pract. 2022;28:1350–1356. doi: 10.1177/10781552211027339. [DOI] [PubMed] [Google Scholar]
- 44.Xiao LS, Hu CY, Cui H, et al. Splenomegaly in predicting the survival of patients with advanced primary liver cancer treated with immune checkpoint inhibitors. Cancer Med. 2022 doi: 10.1002/cam4.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao LS, Li QM, Hu CY, et al. Lung metastasis and lymph node metastasis are risk factors for hyperprogressive disease in primary liver cancer patients treated with immune checkpoint inhibitors. Ann Palliat Med. 2021;10:11244–11254. doi: 10.21037/apm-21-2023. [DOI] [PubMed] [Google Scholar]
- 46.Xiao L, Liao Y, Wang J, et al. Efficacy and safety of immune checkpoint inhibitors in elderly patients with primary liver cancer: a retrospective, multicenter, real-world cohort study. Cancer Immunol Immunother. 2023 doi: 10.1007/s00262-023-03417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360. doi: 10.1186/s12916-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Zhang F, Yuan F, et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol. 2020;12:431382207. doi: 10.1177/1758835920970049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors upon reasonable request.
Not applicable.