Abstract
STUDY QUESTION
Are dietary phytochemicals associated with the risk of teratozoospermia?
SUMMARY ANSWER
Dietary intake of carotene, including total carotene, α-carotene, β-carotene as well as retinol equivalent, and lutein + zeaxanthin, were inversely correlated with the risk of teratozoospermia.
WHAT IS KNOWN ALREADY
Phytochemicals are natural plant derived bioactive compounds, which have been reported to be potentially associated with male reproductive health. To date, no study has investigated the association between phytochemical intake and the risk of teratozoospermia.
STUDY DESIGN, SIZE, DURATION
This hospital-based case–control study, which included 146 newly diagnosed teratozoospermia cases and 581 controls with normozoospermia from infertile couples, was conducted in a hospital-based infertility clinic in China, from June 2020 to December 2020.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Dietary information was collected using a validated semi-quantitative 110-item food frequency questionnaire. Unconditional logistic regression was applied to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between phytochemical (i.e. phytosterol, carotene, flavonoid, isoflavone, anthocyanidin, lutein + zeaxanthin, and resveratrol) intake and the risk of teratozoospermia.
MAIN RESULTS AND THE ROLE OF CHANCE
We observed a decreased risk of teratozoospermia for the highest compared with the lowest tertile consumption of total carotene (OR = 0.40, 95% CI = 0.21–0.77), α-carotene (OR = 0.53, 95% CI = 0.30–0.93), β-carotene (OR = 0.47, 95% CI = 0.25–0.88), retinol equivalent (OR = 0.47, 95% CI = 0.24–0.90), and lutein + zeaxanthin (OR = 0.35, 95% CI = 0.19–0.66), with all of the associations showing evident linear trends (all P trend <0.05). In addition, significant dose–response associations were observed between campestanol and α-carotene consumption and the risk of teratozoospermia. Moreover, there was a significant multiplicative interaction between BMI and lutein + zeaxanthin intake (P interaction <0.05).
LIMITATIONS, REASONS FOR CAUTION
The cases and controls were not a random sample of the entire target population, which could lead to admission rate bias. Nevertheless, the controls were enrolled from the same infertility clinic, which could reduce the bias caused by selection and increase the comparability. Furthermore, our study only included a Chinese population, therefore caution is required regarding generalization of our findings to other populations.
WIDER IMPLICATIONS OF THE FINDINGS
Dietary phytochemicals, namely carotene, lutein, and zeaxanthin, might exert a positive effect on teratozoospermia. These phytochemicals are common in the daily diet and dietary supplements, and thus may provide a preventive intervention for teratozoospermia.
STUDY FUNDING/COMPETING INTERESTS
This study was funded by Natural Science Foundation of Liaoning Province (No. 2022-MS-219 to X.B.W.), Outstanding Scientific Fund of Shengjing Hospital (No. M1150 to Q.J.W.), Clinical Research Cultivation Project of Shengjing Hospital (No. M0071 to B.C.P.), and JieBangGuaShuai Project of Liaoning Province (No. 2021JH1/1040050 to Y.H.Z.). All authors declared that there was no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: association, case–control study, China, diet, nutrient, phytochemical, sperm quality, teratozoospermia
WHAT DOES THIS MEAN FOR PATIENTS?
Teratozoospermia, a condition which is characterized by the majority of sperm having an abnormal structure, is a common cause of male infertility yet, at present, there is no effective treatment. Available evidence indicates that several physiological, environmental, and genetic factors may cause teratozoospermia, but these factors are difficult to modify. Therefore, the identification of factors that could be changed is important for the prevention of teratozoospermia. Previous studies also report that diet, as a potentially modifiable factor, is correlated to sperm morphology.
Phytochemicals are a group of naturally bioactive compounds that exist in multiple plant foods, and they may have effects on sperm quality. However, based on only a few studies, current evidence for an association between dietary phytochemical intake and risk of teratozoospermia is inconclusive. Hence, we performed a large case–control study where we compared 146 men with teratozoospermia to 581 healthy controls with normal sperm.
Our results showed that dietary intake of phytochemicals, specifically carotene and lutein + zeaxanthin, was linked to a decreased risk of teratozoospermia. These findings shed some light on the effect of phytochemicals on the development of teratozoospermia. If further studies, also in non-Chinese populations, are carried out and confirm our results, the possibility may then exist to reduce sperm abnormalities in this condition through diet.
Introduction
Teratozoospermia is a common cause of male infertility, and around 50% of male infertility involves morphological sperm defects at some level (Inhorn and Patrizio, 2015; Datta et al., 2016; Tüttelmann et al., 2018). Teratozoospermia is defined as the percentage of normal sperm below the lower limit of the reference value (<4% normal morphology) according to the World Health Organization (WHO) (World Health Organization, 2010). Available evidence has shown that the etiology of teratozoospermia is associated with varicoceles, harmful environmental factors, infection in the reproductive organs, and genetic heterogeneity (Coutton et al., 2015; Beurois et al., 2020; Jiao et al., 2021; Fan et al., 2022). However, these factors are difficult to modify. Moreover, to date, there is no clear and effective treatment for teratozoospermia. Therefore, it is essential to seek effective interventions for the prevention of teratozoospermia. Previous studies have shown that diet, as a potentially modifiable factor, was correlated with sperm morphology (Afeiche et al., 2014a,b; Yörüsün et al., 2020; Soubry et al., 2021).
Phytochemicals are a group of naturally occurring non-nutritive bioactive compounds, which are abundant in vegetables, fruits, whole grains, and other plant foods (Manach et al., 2004). Although hundreds of phytochemicals have been discovered, phytosterol, carotene, flavonoid, isoflavone, anthocyanidin, lutein, zeaxanthin, and resveratrol are the main phytochemicals in the daily diet of Chinese populations (Yang et al., 2018). Available evidence has suggested that several phytochemicals are associated with better sperm quality (Mínguez-Alarcón et al., 2012; De Cosmi et al., 2021; Talebi et al., 2022). For example, α-carotene intake was associated with a higher sperm concentration (SC) and total sperm count (TSC), and β-carotene intake was positively related to TSC (De Cosmi et al., 2021). Similarly, a cross-sectional study from Spain showed a positive association between dietary β-carotene intake and total motile sperm count (Mínguez-Alarcón et al., 2012). Moreover, β-carotene intake was associated with lower sperm DNA fragmentation index, and lutein intake was positively correlated to TSC (Rahimlou et al., 2019). In addition, these phytochemicals might affect the etiology of teratozoospermia through several biological mechanisms, including reducing oxidative stress as well as anti-inflammatory and anti-apoptotic activity (el-Demerdash et al., 2004; Alahmar, 2018; Zhang et al., 2022).
Compared with SC and motility, morphological defects may be the single most important factor reflecting the actual fertilization capacity of sperm (Guzick et al., 2001; Oborna et al., 2009; Skowronek et al., 2012; Hosseinzadeh Colagar et al., 2013). Although there may be a positive association between phytochemical consumption and sperm quality, the evidence for an association between phytochemical intake and risk of teratozoospermia is inconclusive. Therefore, to fill the gap in epidemiological evidence, we carried out a hospital-based case–control study to explore the topic and provide additional evidence as a basis for future research.
Materials and methods
Study design and population
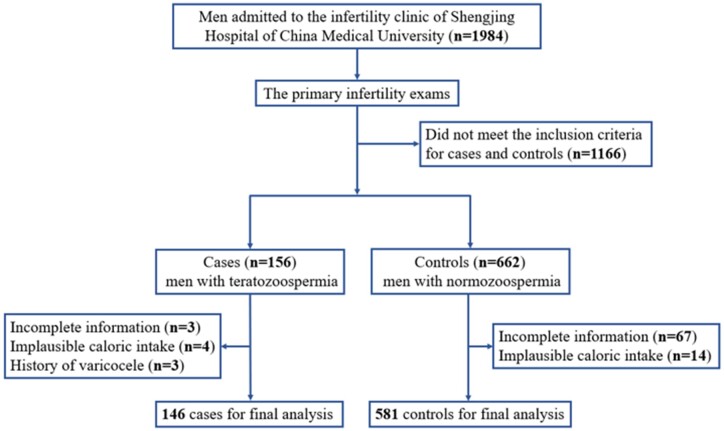
The participants in this case–control study were enrolled from the infertility clinic at Shengjing Hospital of China Medical University, Shenyang, China, between June 2020 and December 2020. Briefly, 1984 men admitted to the infertility clinic were enrolled for the present study. After the primary infertility examinations, participants were separated into two groups, on the basis of the WHO laboratory manual for the examination and processing of human semen (World Health Organization, 2010): cases (n = 156), who were men with teratozoospermia (<4% normal morphology), and controls (n = 662), who were men with normozoospermia from infertile couples (≥15 × 106 of sperm/ml, ≥32% progressive motility, ≥40% total motility, and ≥4% normal morphology). All participants were required to complete a structured and self-administered health status questionnaire through face-to-face interviews by trained professional personnel. We further excluded participants with incomplete information (n = 70), an implausible total caloric intake (<800 or >4200 kcal/day) (n = 18), and history of varicocele (n = 3) (Cui et al., 2022; Liu et al., 2022). Finally, 146 cases and 581 controls were eligible for the final analysis (Fig. 1). The study was approved by the Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China (2017PS190K). All participants provided written informed consent prior to participation.
Figure 1.
Flow diagram for selection of the case–control study populations in an analysis of phytochemical consumption and risk of teratozoospermia in Chinese men.
Semen collection and analysis
After 3–7 days abstinence, all participants were required to collect semen samples into a sterilized plastic tube through masturbation in a dedicated semen collection room. Condoms and lubricants were forbidden in the process. Semen samples were liquefied in a 37°C-water bath for 45–60 min before analysis. Ejaculate volume was directly measured, and the semen pH was evaluated using the standard pH test strips. SC, TSC, total motility, and the percentage of each motile grade of sperm were measured using a computer-assisted sperm analysis (CASA) system (WLJY 9000, Beijing Weili New Century Science & Tech. Dev. Co. Ltd, Beijing, China).
Sperm smears were stained by the Papanicolaou method (Chantziantoniou et al., 2017). Specifically, 5–10 µl semen was dropped on one end of the slide according to the SC, and another slide was used to spread the sample. Two smears were prepared for each specimen, marked with a pencil on the frosted part of the slide, and disinfected with pasteurization immediately after air drying. Sperm morphology was observed by bright field microscopy at 1000× magnification under oil, where the acrosome region of the sperm head is light blue, the posterior area of the acrosome is dark blue, the middle segment is red, and the tail is blue. Two hundred intact sperm, with head and tail, were counted to determine the percentage of normal morphological sperm, according to the WHO laboratory manual (World Health Organization, 2021). Each semen sample was measured twice in succession by two trained technicians.
External quality control was conducted by experienced technicians through a national quality control program on semen analysis, which was organized by the Society of Reproductive Medicine, Chinese Medical Association (Li et al., 2022). The test items included SC, TSC, total motility, and sperm morphology. We tested the control samples from the Central Laboratory for the above characteristics and sent the average value back for evaluation and monitoring. Overall, the median coefficients of variation for SC, TSC, total motility, and sperm morphology were 25.6%, 18.9%, 13.3%, and 36.8%, respectively.
Data collection
General demographic characteristics, including age, education, annual household income, smoking status, alcohol drinking, and dietary change, were collected through the self-administered questionnaires. Smoking was defined as smoking at least one cigarette per day for more than 6 months, and alcohol drinking was defined as drinking alcohol at least once a week for more than 6 months. Dietary change described the participants who had deliberately changed their eating habits recently, with four possible responses: 3 years ago, 1–2 years ago, this year, and no change. Additionally, anthropometric parameters, including height and weight, were measured using a standard protocol. BMI was calculated as weight in kilograms divided by height in squared meters from these measurements. The metabolic equivalent task (MET) of each activity was subsequently multiplied by the frequency and duration of physical activity to calculate total physical activity in MET hours per week (MET/hours/week) (Ainsworth et al., 2011; Du et al., 2013).
Dietary assessment
Dietary data were assessed through a validated 110-item semi-quantitative food frequency questionnaire (FFQ) administered by well trained and skilled personnel at baseline. The FFQ in the present study was based on the FFQ of the northeast cohort study in China (Cui et al., 2023), which was designed to evaluate the frequency and portion size of dietary intake and supplement use over the past 12 months. The validity and reliability of the FFQ have been verified in our previous studies (Liu et al., 2021; Wang et al., 2021). For most food items, the Spearman correlation coefficients and intraclass correlation coefficients for reproducibility were above 0.5, and the correlation coefficients were 0.3–0.7 between the FFQ and weighed diet records (Liu et al., 2022; Cui et al., 2023). All participants were asked to report their usual intake frequency of each food item with seven response options: almost never, two to three times per month, one time per week, two to three times per week, four to six times per week, one to two times per day, and more than two times per day. The consumption of each food item was calculated by multiplying fitted portion sizes (g/time) with the frequency of each food item consumed per day (Zhang et al., 2021). Then, the nutrient intake was calculated by multiplying the amount of each food item with its nutrient content and linking the information to the Chinese food composition table (Yang et al., 2018), and subsequently summing nutrient contributions across all food items (Hu et al., 2018). In the current study, the following phytochemicals were analyzed: phytosterol (including total phytosterol, campestanol, β-sitostanol, campesterol, stigmasterol, and β-sitosterol), carotene (including total carotene, α-carotene, β-carotene, and retinol equivalent), flavonoid (including total flavonoid, quercetin, myricetin, luteolin, kaempferol, and apigenin), isoflavone (including total isoflavone, daidzein, glycitein, and genistein), anthocyanidin (including total anthocyanidin, delphinidin, cyanidin, and peonidin), lutein + zeaxanthin, and resveratrol (including resveratrol and polydatin).
Statistical analysis
The Kolmogorov–Smirnov test was performed to evaluate the normality of the distribution for continuous variables. Descriptive statistics for sociodemographic, diet, and sperm characteristics between the case and control groups were examined using Student’s t test or Kruskal–Wallis test for continuous variables and Chi-square test for categorical variables. Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR), while categorical variables were expressed as a number with percentage. Phytochemical intakes were categorized into tertiles based on the consumptions of the control group, and the lowest tertile of intake served as the reference category. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for the associations of phytochemicals intake with risk of teratozoospermia through unconditional logistic regression analyses. The linear trend cross increasing tertiles was tested by using the median value of each tertile as a continuous variable in respective logistic regression models. Moreover, we tested the nonlinear associations between phytochemicals intake and the risk of teratozoospermia. The nonlinear associations were modeled by penalized cubic splines with three equally spaced knots (5th, 50th, and 95th percentiles), which have better estimation accuracy in nonlinear data and are not heavily affected by the number and location of knots (Govindarajulu et al., 2009).
Three incremental models were selected to assess the associations. In Model 1, we adjusted for age (<32 or ≥32, years) and total energy intake (continuous, kcal/day). To account for lifestyle factors and clinical characteristics, Model 2 was further adjusted for BMI (continuous, kg/m2), alcohol drinking (yes or no), smoking status (yes or no), dietary change (yes or no), annual household income (<50, 50 to <100, or ≥ 100, thousand yuan), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), physical activity (continuous, MET/hours/week), and abstinence time (continuous, days). Considering that there may be interactions among different types of phytochemicals, Model 3 was mutually adjusted for different types of phytochemical intake included in the study. Covariates for the final models were determined based on the correlation with phytochemical, clinical significance, and previous studies (Wang et al., 2021; Cui et al., 2022; Zhao et al., 2022).
Several subgroup analyses were carried out to evaluate the effect modification according to age (<32 compared with ≥32 years), BMI (<25 compared with ≥25 kg/m2), physical activity (≤127.57 compared with >127.57 MET/hours/week), alcohol drinking (yes compared with no), and smoking status (yes compared with no). The category of physical activity was determined by the median of the control group. The likelihood-ratio tests were performed to assess potential interactions between phytochemical intake and these stratified variables. The residual method and nutrient-density model are two commonly used methods in energy adjustment, which are based on different algorithms (Willett et al., 1997; Tomova et al., 2022). However, there is little or no explanation regarding which approach is more suitable (Hu et al., 1999; Ahmadi-Abhari et al., 2014). Therefore, we implemented sensitivity analyses that adjusted energy intake using both methods to test the robustness of primary findings. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA), and two-sided P values <0.05 were considered statistically significant.
Results
The general characteristics of cases and controls are presented in Table 1. Cases with teratozoospermia had a significantly lower abstinence time, SC, TSC, progressive motility, total motility, and proportion of normal sperm morphology. Tables 2, 3, 4, and 5 and Supplementary Tables S1, S2, and S3 show the associations between phytochemical intake and risk of teratozoospermia. We found the highest tertile of total carotene (OR = 0.40, 95% CI = 0.21–0.77), α-carotene (OR = 0.53, 95% CI = 0.30–0.93), β-carotene (OR = 0.47, 95% CI = 0.25–0.88), retinol equivalent (OR = 0.47, 95% CI = 0.24–0.90), and lutein + zeaxanthin (OR = 0.35, 95% CI = 0.19–0.66) intakes was significantly correlated to the decreased risk of teratozoospermia, with an evident linear trend (all P trend <0.05) (Tables 3 and 5). No significant association was observed between other phytochemicals and the risk of teratozoospermia. Moreover, our findings indicated that curvilinear relationships exist between campestanol as well as α-carotene intake and the risk of teratozoospermia (all P non-linear <0.05), while other phytochemicals showed no significant curvilinear relationship (Supplementary Figs S1, S2, S3, S4, S5, S6, and S7).
Table 1.
General characteristics of the participants in a study of phytochemical consumption and risk of teratozoospermia in Chinese men.
| Characteristics | Case | Control | P value * |
|---|---|---|---|
| No. of participants | 146 | 581 | |
| Age (years), median (IQR) | 33.00 (29.00, 35.00) | 32.00 (29.00, 34.00) | 0.10 |
| BMI (kg/m2), median (IQR) | 25.95 (23.46, 29.40) | 25.95 (23.36, 28.73) | 0.44 |
| Ever smoking (n, %) | 78 (53.42) | 307 (52.84) | 0.90 |
| Ever alcohol drinking (n, %) | 58 (39.73) | 250 (43.03) | 0.47 |
| Ever dietary change (n, %) | 39 (26.71) | 115 (19.79) | 0.07 |
| Education level (n, %) | 0.35 | ||
| Junior secondary or below | 28 (19.18) | 141 (24.27) | |
| Senior high school/technical secondary school | 25 (17.12) | 82 (14.11) | |
| Junior college/university or above | 93 (63.70) | 358 (61.62) | |
| Annual family income (thousand yuan), (n, %) | 0.68 | ||
| <50 | 25 (17.12) | 94 (16.18) | |
| 50 to <100 | 51 (34.93) | 226 (38.90) | |
| ≥100 | 70 (47.95) | 261 (44.92) | |
| Physical activity (MET/hours/week), median (IQR) | 155.44 (99.40, 239.73) | 127.57 (98.35, 226.67) | 0.08 |
| Abstinence time (days), median (IQR) | 4.00 (3.00, 4.00) | 4.00 (3.00, 5.00) | <0.05 |
| Semen parameters, median (IQR) | |||
| Ejaculate volume (ml) | 3.20 (2.20, 4.00) | 3.20 (2.50, 4.00) | 0.21 |
| Sperm concentration (106/ml) | 54.40 (33.71, 79.84) | 62.26 (42.63, 87.59) | <0.05 |
| Total sperm count (106/ml) | 159.04 (93.20, 271.46) | 211.18 (131.00, 297.53) | <0.05 |
| Progressive motility (%) | 41.13 (36.13, 48.80) | 43.06 (37.85, 50.40) | <0.05 |
| Total motility (%) | 50.00 (44.54, 59.22) | 53.65 (46.44, 62.23) | <0.05 |
| Normal sperm morphology (%) | 2.00 (1.00, 3.00) | 6.00 (4.00, 8.00) | <0.05 |
| Dietary intake, median (IQR) | |||
| Vegetables (g/day) | 174.69 (121.61, 262.50) | 158.63 (102.00, 243.66) | 0.07 |
| Fruits (g/day) | 124.17 (56.50, 235.69) | 110.90 (55.56, 207.06) | 0.37 |
| Legumes and legume products (g/day) | 61.78 (40.18, 106.88) | 58.57 (40.02, 130.04) | 0.99 |
| Total energy (kcal/day) | 1657.24 (1374.24, 1997.89) | 1683.75 (1400.51, 2048.60) | 0.69 |
| Total phytosterola (mg/day) | 42.31 (30.11, 69.44) | 40.98 (27.47,62.03) | 0.20 |
| Total caroteneb (µg/day) | 1184.80 (778.75, 1727.56) | 1036.83 (663.89, 1694.16) | 0.06 |
| Total flavonoidc (mg/day) | 42.50 (24.94, 74.03) | 38.93 (21.66, 65.26) | 0.19 |
| Total isoflavoned (mg/day) | 11.04 (7.24, 23.67) | 11.20 (7.25, 24.76) | 0.80 |
| Total anthocyanidine (mg/day) | 4.40 (2.84, 7.54) | 4.28 (2.51, 6.76) | 0.20 |
| Lutein + zeaxanthin (µg/day) | 801.35 (600.99, 1238.60) | 677.34 (522.99, 1120.53) | 0.82 |
| Resveratrol (mg/day) | 77.02 (40.67, 174.24) | 76.61 (40.39, 164.73) | 0.45 |
IQR, interquartile range; MET, metabolic equivalent task.
Continuous variables are shown as mean with SD, and categorical variables are expressed as number with percentages.
P values were determined with Kruskal–Wallis test for continuous variables and Chi-square test for categorical variables. All statistical tests are two sided.
Total phytosterol included campestanol, β-sitostanol, campesterol, stigmasterol, and β-sitosterol.
Total carotene included α-carotene and β-carotene.
Total flavonoid included quercetin, myricetin, luteolin, kaempferol, and apigenin.
Total isoflavone included daidzein, glycitein, and genistein.
Total anthocyanidin included delphinidin, cyanidin, and peonidin.
Table 2.
Adjusted odds ratios and 95% CIs for teratozoospermia by intake of phytosterol.
| Variables | Cases (N = 146) | Controls (N = 581) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|---|
| Total phytosterol (mg/day) | |||||
| T1 (<32.13) | 44 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (32.13 to <52.86) | 45 | 193 | 0.92 (0.57–1.49) | 0.99 (0.61–1.62) | 1.02 (0.61–1.71) |
| T3 (≥52.86) | 57 | 195 | 0.68 (0.40–1.15) | 0.72 (0.42–1.25) | 0.75 (0.36–1.53) |
| P for trend* | 0.13 | 0.20 | 0.38 | ||
| Campestanol (mg/day) | |||||
| T1 (<0.23) | 33 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (0.23 to <0.46) | 60 | 193 | 0.54 (0.33–0.87) | 0.55 (0.33–0.89) | 0.53 (0.32–0.87) |
| T3 (≥0.46) | 53 | 195 | 0.59 (0.35–1.00) | 0.61 (0.35–1.05) | 0.53 (0.26–1.09) |
| P for trend* | 0.23 | 0.29 | 0.23 | ||
| β-Sitostanol (mg/day) | |||||
| T1 (<1.47) | 40 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (1.47 to <2.52) | 51 | 193 | 0.75 (0.47–1.21) | 0.77 (0.47–1.25) | 0.77 (0.47–1.27) |
| T3 (≥ 2.52) | 55 | 195 | 0.65 (0.38–1.11) | 0.69 (0.40–1.20) | 0.70 (0.35–1.43) |
| P for trend* | 0.14 | 0.24 | 0.37 | ||
| Campesterol (mg/day) | |||||
| T1 (<3.97) | 45 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (3.97 to <6.95) | 50 | 193 | 0.89 (0.56–1.41) | 0.94 (0.59–1.51) | 1.01 (0.61–1.66) |
| T3 (≥6.95) | 51 | 195 | 0.84 (0.49–1.45) | 0.93 (0.53–1.64) | 1.16 (0.55–2.48) |
| P for trend* | 0.55 | 0.83 | 0.69 | ||
| Stigmasterol (mg/day) | |||||
| T1 (<4.11) | 40 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (4.11 to <7.07) | 58 | 193 | 0.68 (0.42–1.07) | 0.69 (0.43–1.11) | 0.76 (0.46–1.24) |
| T3 (≥7.07) | 48 | 195 | 0.80 (0.46–1.39) | 0.88 (0.50–1.55) | 1.12 (0.52–2.41) |
| P for trend* | 0.57 | 0.81 | 0.73 | ||
| β-Sitosterol (mg/day) | |||||
| T1 (<22.01) | 41 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (22.01 to <36.95) | 51 | 193 | 0.76 (0.47–1.22) | 0.80 (0.50–1.30) | 0.85 (0.51–1.41) |
| T3 (≥36.95) | 54 | 195 | 0.67 (0.39–1.16) | 0.72 (0.41–1.27) | 0.81 (0.39–1.67) |
| P for trend* | 0.18 | 0.29 | 0.60 |
Ref, reference; T, tertile.
Total phytosterol is the sum of campestanol, β-sitostanol, campesterol, stigmasterol, and β-sitosterol.
Model 1: adjusted for age (<32 or ≥32, years) and total energy intake (continuous, kcal/day).
Model 2: same as Model 1 and further adjusted for BMI (continuous, kg/m2), alcohol drinking (yes or no), smoking status (yes or no), dietary change (yes or no), household income (<50, 50 to <100, or ≥100, thousand yuan), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), physical activity (continuous, MET/hours/week), and abstinence time (continuous, days). MET: metabolic equivalent task.
Model 3: same as Model 2 and further adjusted for total carotene (continuous, µg/day), total flavonoid (continuous, mg/day), total isoflavone (continuous, mg/day), total anthocyanidin (continuous, mg/day), lutein + zeaxanthin (continuous, µg/day), and resveratrol (continuous, mg/day).
P-value for linear trend calculated from category median values.
Table 3.
Adjusted odds ratios and 95% CIs for teratozoospermia by intake of carotene.
| Variables | Cases (N = 146) | Controls (N = 581) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|---|
| Total carotene (µg/day) | |||||
| T1 (<746.40) | 32 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (746.40 to <1373.44) | 55 | 193 | 0.56 (0.34–0.90) | 0.55 (0.33–0.90) | 0.54 (0.32–0.89) |
| T3 (≥1373.44) | 59 | 195 | 0.48 (0.28–0.81) | 0.50 (0.29–0.85) | 0.40 (0.21–0.77) |
| P for trend* | <0.05 | <0.05 | <0.05 | ||
| α-Carotene (µg/day) | |||||
| T1 (<76.17) | 39 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (76.17 to <121.98) | 44 | 193 | 0.87 (0.54–1.41) | 0.89 (0.54–1.45) | 0.85 (0.52–1.40) |
| T3 (≥121.98) | 63 | 195 | 0.57 (0.35–0.92) | 0.56 (0.33–0.92) | 0.53 (0.30–0.93) |
| P for trend* | <0.05 | <0.05 | <0.05 | ||
| β-Carotene (µg/day) | |||||
| T1 (<685.70) | 36 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (685.70 to <1218.33) | 50 | 193 | 0.68 (0.42–1.10) | 0.69 (0.42–1.12) | 0.67 (0.41–1.11) |
| T3 (≥1218.33) | 60 | 195 | 0.54 (0.32–0.89) | 0.57 (0.34–0.96) | 0.47 (0.25–0.88) |
| P for trend* | <0.05 | 0.05 | <0.05 | ||
| Retinol equivalent (µgRE/day) | |||||
| T1 (<119.08) | 33 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (119.08 to <217.07) | 56 | 193 | 0.56 (0.34–0.90) | 0.57 (0.34–0.92) | 0.56 (0.34–0.92) |
| T3 (≥217.07) | 57 | 195 | 0.52 (0.31–0.87) | 0.55 (0.32–0.94) | 0.47 (0.24–0.90) |
| P for trend* | <0.05 | 0.09 | <0.05 |
Ref, reference; T, tertile.
Total carotene is the sum of α-carotene and β-carotene.
Model 1: adjusted for age (<32 or ≥32, years) and total energy intake (continuous, kcal/day).
Model 2: same as Model 1 and further adjusted for BMI (continuous, kg/m2), alcohol drinking (yes or no), smoking status (yes or no), dietary change (yes or no), household income (<50, 50 to <100, or ≥100, thousand yuan), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), physical activity (continuous, MET/hours/week), and abstinence time (continuous, days). MET: metabolic equivalent task.
Model 3: same as Model 2 and further adjusted for total phytosterol (continuous, mg/day), total flavonoid (continuous, mg/day), total isoflavone (continuous, mg/day), total anthocyanidin (continuous, mg/day), lutein + zeaxanthin (continuous, µg/day), and resveratrol (continuous, mg/day).
P-value for linear trend calculated from category median values.
Table 4.
Adjusted odds ratios and 95% CIs for teratozoospermia by intake of anthocyanidin.
| Variables | Cases (N = 146) | Controls (N = 581) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|---|
| Total anthocyanidin (mg/d) | |||||
| T1 (<3.05) | 39 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (3.05 to <5.68) | 58 | 193 | 0.66 (0.41–1.04) | 0.68 (0.42–1.09) | 0.70 (0.43–1.14) |
| T3 (≥5.68) | 49 | 195 | 0.75 (0.43–1.28) | 0.74 (0.42–1.31) | 0.92 (0.48–1.76) |
| P for trend* | 0.37 | 0.38 | 0.90 | ||
| Delphinidin (mg/day) | |||||
| T1 (<0.42) | 41 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (0.42 to <1.30) | 54 | 193 | 0.75 (0.47–1.18) | 0.80 (0.50–1.28) | 0.83 (0.51–1.34) |
| T3 (≥1.30) | 51 | 195 | 0.78 (0.47–1.28) | 0.80 (0.48–1.32) | 0.88 (0.51–1.52) |
| P for trend* | 0.47 | 0.49 | 0.78 | ||
| Cyanidin (mg/day) | |||||
| T1 (<1.76) | 39 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (1.76 to <3.24) | 55 | 193 | 0.70 (0.44–1.11) | 0.77 (0.48–1.23) | 0.82 (0.51–1.34) |
| T3 (≥3.24) | 52 | 195 | 0.70 (0.41–1.17) | 0.74 (0.43–1.28) | 0.95 (0.50–1.80) |
| P for trend* | 0.22 | 0.33 | 0.93 | ||
| Peonidin (mg/day) | |||||
| T1 (<0.40) | 39 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (0.40 to <0.97) | 58 | 192 | 0.65 (0.41–1.03) | 0.60 (0.37–0.97) | 0.60 (0.37–0.96) |
| T3 (≥0.97) | 49 | 196 | 0.77 (0.46–1.29) | 0.74 (0.43–1.26) | 0.86 (0.48–1.56) |
| P for trend* | 0.28 | 0.23 | 0.43 |
Ref, reference; T, tertile.
Total anthocyanidin is the sum of delphinidin, cyanidin, and peonidin.
Model 1: adjusted for age (<32 or ≥32, years) and total energy intake (continuous, kcal/day).
Model 2: same as Model 1 and further adjusted for BMI (continuous, kg/m2), alcohol drinking (yes or no), smoking status (yes or no), dietary change (yes or no), household income (<50, 50 to <100, or ≥100, thousand yuan), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), physical activity (continuous, MET/hours/week), and abstinence time (continuous, days). MET: metabolic equivalent task.
Model 3: same as Model 2 and further adjusted for total phytosterol (continuous, mg/day), total carotene (continuous, µg/day), total flavonoid (continuous, mg/day), total isoflavone (continuous, mg/day), lutein + zeaxanthin (continuous, µg/day), and resveratrol (continuous, mg/day).
P-value for linear trend calculated from category median values.
Table 5.
Adjusted odds ratios and 95% CIs for teratozoospermia by intake of lutein + zeaxanthin.
| Variables | Cases (N = 146) | Controls (N = 581) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|---|
| Lutein + zeaxanthin (µg/day) | |||||
| T1 (<579.98) | 31 | 193 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (579.98 to <953.77) | 49 | 193 | 0.61 (0.37–0.99) | 0.62 (0.37–1.04) | 0.59 (0.35–0.99) |
| T3 (≥953.77) | 66 | 195 | 0.42 (0.25–0.69) | 0.44 (0.26–0.75) | 0.35 (0.19–0.66) |
| P for trend* | <0.05 | <0.05 | <0.05 |
Ref, reference; T, tertile.
Model 1: adjusted for age (<32 or ≥32, years) and total energy intake (continuous, kcal/day).
Model 2: same as Model 1 and further adjusted for BMI (continuous, kg/m2), alcohol drinking (yes or no), smoking status (yes or no), dietary change (yes or no), household income (<50, 50 to <100, or ≥100, thousand yuan), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), physical activity (continuous, MET/hours/week), and abstinence time (continuous, days). MET: metabolic equivalent task.
Model 3: same as Model 2 and further adjusted for total phytosterol (continuous, mg/day), total carotene (continuous, µg/day), total flavonoid (continuous, mg/day), total isoflavone (continuous, mg/day), total anthocyanidin (continuous, mg/day), and resveratrol (continuous, mg/day).
P-value for linear trend calculated from category median values.
The associations between phytochemical intake and the risk of teratozoospermia across different subgroups were consistent with the primary findings except for total anthocyanidin and total isoflavone (Supplementary Figs S8 and S9). Total anthocyanidin intake was significantly correlated to a decreased risk of teratozoospermia in the subgroup of patients with BMI <25 kg/m2 (Supplementary Fig. S8). Moreover, total isoflavone intake was significantly positively associated with the risk of teratozoospermia in the subgroup of patients with BMI ≥25 kg/m2, low physical activity, and no smoking (Supplementary Fig. S9). Of note, we observed significant multiplicative interaction between BMI and lutein + zeaxanthin consumption on the risk of teratozoospermia.
In the sensitivity analyses that adjusted for energy intake with the residual method and nutrient-density method (Supplementary Tables S4, S5, S6, S7, S8, S9, and S10), we found that the correlation between total carotene, β-carotene, and retinol equivalent intake and the risk of teratozoospermia remained significant. However, no association was observed between α-carotene and lutein + zeaxanthin intake and the risk of teratozoospermia. Interestingly, after adjusting for energy intake with the residual method, we found total phytosterol and β-sitosterol intakes were associated with a decreased risk of teratozoospermia (Supplementary Table S4). Similarly, after adjusting energy intake with the nutrient-density method, β-sitosterol, and campestanol intakes were inversely correlated to the risk of teratozoospermia (Supplementary Table S4).
Discussion
In this hospital-based case–control study, we first investigated the associations between intake of a variety of phytochemicals and the risk of teratozoospermia. We found that higher total carotene, α-carotene, β-carotene, retinol equivalent, as well as lutein + zeaxanthin consumptions were significantly associated with a decreased risk of teratozoospermia. It was interesting to note that we observed a significant interaction on the multiplicative scale between BMI and lutein + zeaxanthin intake on the risk of teratozoospermia. Moreover, significant curvilinear associations were observed between campestanol as well as α-carotene intake and the risk of teratozoospermia.
Although there is a lack of literature on the association between phytochemical intake and the risk of teratozoospermia, several prior studies have provided data on phytochemical consumption and sperm quality. For instance, Mínguez-Alarcón et al. (2012) have presented findings from 215 young university men, in which β-carotene was positively associated with total motile sperm count, while null associations were observed between α-carotene as well as lutein + zeaxanthin and sperm quality. In addition, De Cosmi et al. (2021) performed an investigation among 323 men in an Italian fertility clinic, and found a high level of α-carotene intake was positively associated with SC and TSC, and high β-carotene consumption was inversely associated with the risk of low TSC; however, no significant association between lutein intake and sperm quality was observed. Furthermore, evidence from a cross-sectional study with 175 infertile Iranian men indicated high β-carotene intake was associated with a lower sperm DNA fragmentation index and high lutein intake was associated with better TSC, whereas no significant association was found between α-carotene and sperm quality (Rahimlou et al., 2019). Overall, these studies indicated that high α-carotene, β-carotene, lutein, and zeaxanthin intake might be correlated with better sperm quality, and further investigation is warranted to further demonstrate their association with risk of teratozoospermia.
Interestingly, higher total isoflavone intake was positively associated with the risk of teratozoospermia in some subgroup analyses but not the main analyses. Nevertheless, owing to the limited participants in some subgroups, we could not fully eliminate the possibility of accidental findings. Although no previous literature has investigated this topic, numerous studies have provided evidence on isoflavone intake and sperm quality. For example, Xia et al. (2013) performed a case–control study with 608 infertile men and 469 fertile men, and the results suggested that daidzein was correlated to lower SC, TSC, and sperm motility. A similar pattern was observed between genistein and SC, TSC, as well as sperm motility (Xia et al., 2013). On the contrary, Povey et al. (2020) found that consumption of daidzein and genistein was associated with favorable sperm motile count. Moreover, Chavarro et al. (2008) assessed 99 male partners of subfertile couples, and found a null association between dietary isoflavone consumption and sperm quality. Given the limited evidence on isoflavone and the risk of teratozoospermia, further studies are warranted to validate our results.
There are several underlying biological mechanisms that might help to explain the findings of our study. Evidence has suggested that overproduction of seminal reactive oxygen species may be directly related to the occurrence of abnormal spermatozoa and the incidence of teratozoospermia (Agarwal et al., 2014). β-Carotene could inhibit the propagation of radical initiated lipid peroxidation and reduce the production of reactive oxygen species, which would reduce oxidative stress (el-Demerdash et al., 2004; Schmid et al., 2012), thereby leading to an improvement of sperm quality (Alahmar, 2018). In addition, lutein decreased malondialdehyde and 8-hydroxydeoxyguanosine levels and increased glutathione peroxidase concentration through activating nuclear factor erythroid2-related factor 2 (Nrf2) signaling, which improved the oxidative stress level and maintained the normal testicular structure and sex hormone levels (Aladaileh et al., 2019). Thus, carotene and lutein may play a protective role in sperm morphology through reducing oxidative stress.
Another possibility is that these phytochemicals may reduce the risk of teratozoospermia via anti-apoptosis. Previous studies have reported that poor sperm morphology was associated with higher rates of apoptosis-related enzyme (caspase) expression (Ammar et al., 2020). β-Carotene might exert anti-apoptotic effects and inhibit germ cell apoptosis, thereby reducing reproductive damage and improving sperm quality (Vardi et al., 2009; Orazizadeh et al., 2014). Moreover, lutein could downregulate nuclear factor kappa-B (NF-κB) and the pro-apoptosis biomarkers (Bax, Cyt c, and caspase-3) and upregulate the expression of Nrf2, heme oxygenase-1, and the anti-apoptotic molecule Bcl-2, which protected against reproductive injury through exerting anti-apoptotic properties (Li et al., 2016). However, research on the mechanisms associated with these phytochemicals and the risk of teratozoospermia is still limited. Current studies suggest that carotenes and the sum of lutein and zeaxanthin have a protective effect against teratozoospermia, but they need to be further verified in animal studies before they could be used in humans. Once the outcomes of these further studies are known, randomized controlled trials could then be designed, with further studies to help identify the biological mechanisms involved in men.
Several strengths of our study are worth mentioning. First, this was the first study to focus on the associations between diverse phytochemicals intake and the risk of teratozoospermia. Second, a relatively large study population with a high participant rate was achieved, which could provide more reliable results and reduce selection bias. Third, we rigorously adjusted for potential confounding factors, which strengthened the credibility of our findings. Fourth, we performed multifaceted subgroup analyses and considered the interactions of phytochemicals with several key influential factors to further strength the reliability of our primary results.
There are several limitations in our study that should be noted. First, owing to the design of the case–control study, recall bias is inevitable. However, dietary information was collected by well trained and skilled personnel through face-to-face interviews, which might alleviate this concern. Second, dietary intake was collected through a semi-quantitative FFQ, which might lead to incorrect estimates of various nutrient intakes. However, the FFQ used in our study was verified by previous studies, with high validity and reliability (Liu et al., 2022; Cui et al., 2023). Third, the cases and controls of the present study were not a random sample of the entire target population, which could lead to admission rate bias. Nonetheless, the controls recruited in the current study were from the same infertility clinic in Shengjing hospital, which could reduce the bias caused by selection as much as possible and increase the comparability of cases and controls. Fourth, although many confounders were considered, the impact of unknown or unmeasured factors might not be fully eliminated in any of observational studies. Finally, in the present study, we restricted our analysis to the Chinese population. Therefore, we must be cautious if generalizing our findings to other populations.
Conclusion
Taken together, our study demonstrated that higher consumptions of total carotene, α-carotene, β-carotene, retinol equivalent, and lutein + zeaxanthin were inversely associated with the risk of teratozoospermia. Well-designed prospective cohort studies and randomized controlled clinical trials are needed to confirm our findings.
Supplementary Material
Acknowledgements
We thank the research team for their daily efforts in material collection and manuscript writing.
Contributor Information
Jun-Qi Zhao, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China; Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China; Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China.
Jia-Le Lv, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China; Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China; Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China.
Xiao-Bin Wang, Center for Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China.
Yi-Fan Wei, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China; Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China; Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China.
Ren-Hao Guo, Center for Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China.
Xu Leng, Center for Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China.
Qiang Du, Center for Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China.
Dong-Hui Huang, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China; Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China; Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China.
Qi-Jun Wu, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China; Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China; Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China; Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China; NHC Key Laboratory of Advanced Reproductive Medicine and Fertility (China Medical University), National Health Commission, Shenyang, China.
Bo-Chen Pan, Center for Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China.
Yu-Hong Zhao, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China; Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China; Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China.
Data availability
The data that support the findings of our study is available from the corresponding author upon reasonable request.
Authors’ roles
J.-Q.Z., J.-L.L., X.-B.W., Q.-J.W., B.-C.P., and Y.-H.Z. conceived the study. Q.-J.W., B.-C.P., and Y.-H.Z. contributed to the design. X.-B.W., R.-H.G., X.L., and Q.D. collected the data. X.-B.W. and R.-H.G. cleaned the data and checked the discrepancy. J.-Q.Z., Y.-F.W., D.-H.H., and Q.-J.W. analyzed the data. J.-Q.Z., J.-L.L., X.-B.W., Y.-F.W., Q.-J.W., and Y.-H.Z. drafted the article and revised it critically for important intellectual content. Q.-.J.W., B.-C.P., and Y.-H.Z. agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors interpreted the data, read the manuscript, and approved the final vision. J.-Q.Z. and J.-L.L. contributed equally to this work.
Funding
This work was supported by the Natural Science Foundation of Liaoning Province (No. 2022-MS-219 to X.B.W.), Outstanding Scientific Fund of Shengjing Hospital (No. M1150 to Q.J.W.), Clinical Research Cultivation Project of Shengjing hospital (No. M0071 to B.C.P.), and the JieBangGuaShuai Project of Liaoning Province (No. 2021JH1/1040050 to Y.H.Z.).
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE.. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 2014a;101:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE.. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr 2014b;144:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Tvrda E, Sharma R.. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol 2014;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi-Abhari S, Luben RN, Powell N, Bhaniani A, Chowdhury R, Wareham NJ, Forouhi NG, Khaw KT.. Dietary intake of carbohydrates and risk of type 2 diabetes: the European Prospective Investigation into Cancer-Norfolk study. Br J Nutr 2014;111:342–352. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS.. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- Aladaileh SH, Hussein OE, Abukhalil MH, Saghir SAM, Bin-Jumah M, Alfwuaires MA, Germoush MO, Almaiman AA, Mahmoud AM.. Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel, Switzerland) 2019;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar AT. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med 2018;45:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar O, Mehdi M, Muratori M.. Teratozoospermia: its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology 2020;8:1095–1106. [DOI] [PubMed] [Google Scholar]
- Beurois J, Cazin C, Kherraf ZE, Martinez G, Celse T, Touré A, Arnoult C, Ray PF, Coutton C.. Genetics of teratozoospermia: back to the head. Best Pract Res Clin Endocrinol Metab 2020;34:101473. [DOI] [PubMed] [Google Scholar]
- Chantziantoniou N, Donnelly AD, Mukherjee M, Boon ME, Austin RM.. Inception and development of the Papanicolaou stain method. Acta Cytol 2017;61:266–280. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Sadio SM, Hauser R.. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod (Oxford, England) 2008;23:2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF.. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update 2015;21:455–485. [DOI] [PubMed] [Google Scholar]
- Cui Q, Wang HH, Wu QJ, Wang XB, Guo RH, Leng X, Tan XL, Du Q, Pan BC.. Diet quality scores and asthenoteratozoospermia risk: finding from a hospital-based case-control study in China. Front Nutr 2022;9:859143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Xia Y, Liu YS, Sun YF, Ye K, Li WJ, Wu QJ, Chang Q, Zhao YH.. Validity and reproducibility of a FFQ for assessing dietary intake among residents of northeast China: northeast cohort study of China. Br J Nutr 2023;129:1252–1265. [DOI] [PubMed] [Google Scholar]
- Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, Glasier A, Sonnenberg P, Field N, Mercer CH. et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum Reprod (Oxford, England) 2016;31:2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cosmi V, Parazzini F, Agostoni C, Noli S, Cipriani S, La Vecchia I, Ferrari S, Esposito G, Bravi F, Ricci E.. Antioxidant vitamins and carotenoids intake and the association with poor semen quality: a cross-sectional analysis of men referring to an Italian fertility clinic. Front Nutr 2021;8:737077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du HD, Bennett D, Li LM, Whitlock G, Guo Y, Collins R, Chen JS, Bian Z, Hong LS, Feng SX. et al. ; China Kadoorie Biobank Collaborative Group. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr 2013;97:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH.. Role of alpha-tocopherol and beta-carotene in ameliorating the fenvalerate-induced changes in oxidative stress, hemato-biochemical parameters, and semen quality of male rats. J Environ Sci Health B 2004;39:443–459. [DOI] [PubMed] [Google Scholar]
- Fan Y, Huang CH, Chen J, Chen YY, Wang Y, Yan ZG, Yu WN, Wu HB, Yang Y, Nie LT. et al. Mutations in CCIN cause teratozoospermia and male infertility. Sci Bull (Beijing) 2022;67:2112–2123. [DOI] [PubMed] [Google Scholar]
- Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA.. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat 2009;5:Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA. et al. ; National Cooperative Reproductive Medicine Network. Sperm morphology, motility, and concentration in fertile and infertile men. New Engl J Med 2001;345:1388–1393. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh Colagar A, Karimi F, Jorsaraei SG.. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno-teratospermic men. Iran Red Crescent Med J 2013;15:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC.. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–540. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ding M, Yuan C, Wu K, Smith-Warner SA, Hu FB, Chan AT, Meyerhardt JA, Ogino S, Fuchs CS. et al. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology 2018;154:916–926.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn MC, Patrizio P.. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–426. [DOI] [PubMed] [Google Scholar]
- Jiao SY, Yang YH, Chen SR.. Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update 2021;27:154–189. [DOI] [PubMed] [Google Scholar]
- Li SG, Xu SZ, Niu Q, Ding YS, Pang LJ, Ma RL, Jing MX, Wang K, Ma XM, Feng GL. et al. Lutein alleviates arsenic-induced reproductive toxicity in male mice via Nrf2 signaling. Hum Exp Toxicol 2016;35:491–500. [DOI] [PubMed] [Google Scholar]
- Li XY, Wang XB, Wu QJ, Guo RH, Leng X, Du Q, Pan BC, Zhao YH.. Short total sleep duration and poor sleep quality might be associated with asthenozoospermia risk: a case-control study. Front Physiol 2022;13:959009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FH, Wang XB, Wen ZY, Wang HY, Zhang M, Zhang S, Jiang YT, Zhang JY, Sun H, Pan BC. et al. Dietary inflammatory index and risk of asthenozoospermia: a hospital-based case-controlled study in China. Front Nutr 2021;8:706869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YS, Zhang YX, Wang XB, Wu QJ, Liu FH, Pan BC, Zhao YH.. Associations between meat and vegetable intake, cooking methods, and asthenozoospermia: a hospital-based case-control study in China. Nutrients 2022;14:1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L.. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–747. [DOI] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Sarabia-Cos L, Vivero-Salmerón G, Vioque J, Navarrete-Muñoz EM, Torres-Cantero AM.. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod 2012;27:2807–2814. [DOI] [PubMed] [Google Scholar]
- Oborna I, Fingerova H, Novotny J, Brezinova J, Svobodova M, Aziz N.. Reactive oxygen species in human semen in relation to leukocyte contamination. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2009;153:53–57. [DOI] [PubMed] [Google Scholar]
- Orazizadeh M, Khorsandi L, Absalan F, Hashemitabar M, Daneshi E.. Effect of beta-carotene on titanium oxide nanoparticles-induced testicular toxicity in mice. J Assist Reprod Genet 2014;31:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povey AC, Clyma JA, McNamee R, Moore HD, Baillie H, Pacey AA, Cade JE, Cherry NM; Participating Centres of Chaps-UK. Phytoestrogen intake and other dietary risk factors for low motile sperm count and poor sperm morphology. Andrology 2020;8:1805–1814. [DOI] [PubMed] [Google Scholar]
- Rahimlou M, Sohaei S, Nasr-Esfahani M, Nouri M.. Dietary antioxidant intake in relation to semen quality parameters in infertile men: a cross-sectional study. Clin Nutr Res 2019;8:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid TE, Eskenazi B, Marchetti F, Young S, Weldon RH, Baumgartner A, Anderson D, Wyrobek AJ.. Micronutrients intake is associated with improved sperm DNA quality in older men. Fertil Steril 2012;98:1130–1137.e1. [DOI] [PubMed] [Google Scholar]
- Skowronek F, Casanova G, Alciaturi J, Capurro A, Cantu L, Montes JM, Sapiro R.. DNA sperm damage correlates with nuclear ultrastructural sperm defects in teratozoospermic men. Andrologia 2012;44:59–65. [DOI] [PubMed] [Google Scholar]
- Soubry A, Murphy SK, Vansant G, He Y, Price TM, Hoyo C.. Opposing epigenetic signatures in human sperm by intake of fast food versus healthy food. Front Endocrinol (Lausanne) 2021;12:625204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi S, Arab A, Sorraya N.. The association between dietary antioxidants and semen parameters: a cross-sectional study among iranian infertile men. Biol Trace Elem Res 2022;200:3957–3964. [DOI] [PubMed] [Google Scholar]
- Tomova GD, Arnold KF, Gilthorpe MS, Tennant PWG.. Adjustment for energy intake in nutritional research: a causal inference perspective. Am J Clin Nutr 2022;115:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüttelmann F, Ruckert C, Röpke A.. Disorders of spermatogenesis: perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med Genet 2018;30:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A.. Antiapoptotic and antioxidant effects of beta-carotene against methotrexate-induced testicular injury. Fertil Steril 2009;92:2028–2033. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2021. [Google Scholar]
- Wang XB, Wu QJ, Guo RH, Leng X, Du Q, Zhao YH, Pan BC.. Dairy product consumption and oligo-astheno-teratozoospermia risk: a hospital-based case-control study in China. Front Nutr 2021;8:742375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Wu QJ, Liu FH, Zhang S, Wang HY, Guo RH, Leng X, Du Q, Zhao YH, Pan BC.. The association between dairy product consumption and asthenozoospermia risk: a hospital-based case-control study. Front Nutr 2021;8:714291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Howe GR, Kushi LH.. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–1228S; Discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- Xia YK, Chen MJ, Zhu PF, Lu CC, Fu GB, Zhou XJ, Chen DZ, Wang HH, Hang B, Wang SL. et al. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ Int 2013;59:161–167. [DOI] [PubMed] [Google Scholar]
- Yang YX, Wang YG, He M, Pan XC, Wang Z.. China Food Composition, Standard Edition. Beijing: Peking University Medical Press, 2018. [Google Scholar]
- Yörüsün T, Akdevelioğlu Y, Karabacak RO, Bozkurt N, Şanlıer N, Yeşil S, Gümüşlü S.. Nutritional factors related to male fertility: Turkish sample. Afr J Reprod Health 2020;24:85–95. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Xia Y, Chang Q, Gao SY, Zhao YH.. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ Int 2021;147:106347. [DOI] [PubMed] [Google Scholar]
- Zhang YB, Ding HY, Xu L, Zhao SL, Hu SN, Ma AG, Ma Y.. Lutein can alleviate oxidative stress, inflammation, and apoptosis induced by excessive alcohol to ameliorate reproductive damage in male rats. Nutrients 2022;14:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JQ, Hao YY, Gong TT, Wei YF, Zheng G, Du ZD, Zou BJ, Yan S, Liu FH, Gao S. et al. Phytosterol intake and overall survival in newly diagnosed ovarian cancer patients: an ambispective cohort study. Front Nutr 2022;9:974367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of our study is available from the corresponding author upon reasonable request.