Abstract
Liver disease is a serious health problem affecting people worldwide at an alarming rate. The present study aimed to investigate the protective effects of Ganoderma lucidum against CCl4-induced liver toxicity in rats. The experimental Long Evans rats were divided into five groups, of which four groups were treated with carbon tetrachloride (CCl4). Among the CCl4 treated groups, one of the groups was treated with silymarin and two of them with ethanolic extract of G. lucidum at 100 and 200 mg/Kg body weight. The oxidative stress parameters and endogenous antioxidant enzyme concentrations were assessed by biochemical tests. Liver enzymes ALT, AST, and ALP were determined spectrophotometrically. Histopathological examinations were carried out to assess hepatic tissue damage and fibrosis. Reverse transcription PCR (RT-PCR) was performed to determine the expression of IL-1β, IL-6, IL-10, TNF-α, and TGF-β genes. Gas Chromatography-Mass Spectroscopy (GC–MS) analysis revealed that G. lucidum is rich in several phytochemicals including 6-Octadecanoic acid (55.81%), l-( +)-Ascorbic acid 2,6-dihexadecanoate (18.72%), Cis-11-Eicosenamide (5.76%), and Octadecanoic acid (5.26%). Treatment with the G. lucidum extract reduced the elevated ALT, AST, ALP levels, and cellular oxidative stress markers and increased the endogenous antioxidant levels. Histopathology observations revealed that the inflammation, infiltration of immune cells, and aberration of collagen fibers in the hepatocytes were altered by the G. lucidum treatment. The increased expression of inflammatory cytokines TNF-α, TGF-β, IL-1 β, and IL-6 were markedly suppressed by G. lucidum extract treatment. G. lucidum also prevented the suppression of protective IL-10 expression by CCl4. This study strongly suggests that G. lucidum extract possesses significant hepatoprotective activity as evidenced by reduced oxidative stress and inflammation mediated by suppression in inflammatory cytokine expression and increased protective IL-10 cytokine expression.
Subject terms: Biochemistry, Biological techniques, Drug discovery, Medical research, Drug development, Experimental models of disease, Preclinical research
Introduction
Oxidation is an essential step of cellular metabolism that leads to the generation of free radicals and reactive oxygen species (ROS) both physiologically and pathologically. These highly reactive radicals may accelerate the oxidation of biomolecules in the cells and tissues of living organisms leading to damage and death. Uncontrolled generation of free radicals is involved in the progression of many diseases such as liver cirrhosis, atherosclerosis, arthritis, especially rheumatoid arthritis, cancer, and many degenerative processes, including aging1. Endogenous oxidative enzymes like superoxide dismutase (SOD), glutathione peroxidase, and catalase (CAT) capture the free radicals and convert them into less reactive ones, thereby protecting against oxidative damage2. Along with endogenous compounds, some exogenous chemicals such as α-tocopherol, ascorbic acid, carotenoids, and polyphenol compounds also play essential roles in scavenging the free radicals, breaking down the free radicals chain reaction, and neutralizing the reactive oxygen species3,4. Oxidative damage by pathological or age-related causes cannot be overcome easily when there is an imbalance in the endogenous protective compounds in the body. In this situation, an intake of antioxidants containing supplements and foods becomes essential5.
The liver is the primary organ for metabolizing nutrients, drugs, and xenobiotics mainly by a family of enzymes called cytochrome P-4506. Carbon tetrachloride (CCl4) is a well-known exogenous hepatotoxic agent. Free radical generation during the hepatic metabolism of CCl4 by cytochrome P-450 leads to lipid peroxidation, covalent bonding of radicals with the structural proteins, nuclear DNA and mitochondrial DNA, and aberration of DNA. CCl4 activates the resident macrophages of the liver known as Kupffer cells that produce and secrete chemoattractants and neutrophil activators which causes the recruitment of neutrophils into that site. Neutrophils release reactive oxygen species and cause inflammatory changes in the hepatocytes leading to hepatotoxicity7. Endogenous and exogenous antioxidants can capture the free radicals and prevent hepatocellular damage8.
Besides nutritionally beneficial primary compounds such as protein, vitamins and minerals, mushrooms also contain many secondary constituents, including phenolic compounds such as alkaloids, terpenes, and steroids. Mushrooms help in lowering blood pressure, blood glucose and cholesterol levels, and are found useful in the treatment of diabetes, atherosclerosis, hypercholesterolemia, heart diseases, osteoporosis and peptic ulcer9–11. Hepatoprotective, anti-inflammatory, antibacterial, antiviral, immunomodulatory, and anticancer properties of various types of mushrooms have been reported previously11–13. Approximately 10,000 different species of mushrooms have been identified14–17. People in many Asian countries take the fruit body of G. lucidum to treat coronary diseases, arteriosclerosis, hepatitis, arthritis, nephritis, bronchitis, asthma, hypertension, cancer, and gastric ulcer18. The varied ethnopharmacological uses of G. lucidum have inspired researchers to analyze the chemical constituents in its extract.
Gas chromatography-mass spectrometry (GC–MS) is one of the highly reliable techniques to identify the bioactive compounds such as phenolics, terpenoids, long chain and branched chain hydrocarbons, alcohols, alkaloids, organic acids and esters19. However, for better evaluation of chemical profile of compounds and investigation of triterpenoid patterns present in G. lucidum, multiple methods have been adopted such as RP-HPLC, HPLC–UV-ESI–MS, NMR and FT-IR19–21. Triterpenoids and polysaccharides are the two important pharmacologically active principles found in the fruiting bodies of G. lucidum. Polysaccharides obtained from G. lucidum are known to exhibit a wide range of pharmacological effects such as antioxidant, immunomodulatory, neuroprotective, antidiabetic, anti-inflammatory and anticancer activities22,23. Pure β-glucans, heterofucans, heteromannans and their complexes with peptides have been isolated from G. lucidum fruiting bodies and the pure glucose polymer and the polymer built of β-D-glucose and α-D-galactose have shown antioxidant activities24. Protection against reactive oxygen species (ROS) and liver damage has been demonstrated by crude polysaccharides extracted from G. lucidum22,23. The effects of total triterpenoid extracts from mushrooms were studied in experimental liver injury models induced by hepatotoxic agents such as ethanol, carbon tetrachloride and D-galactosamine in mice13. Several studies have reported the effectiveness of triterpenoids against hepatotoxicity, tumor, hypertension, hypercholesterolemia, and platelet aggregation18. Mushrooms are also rich in phenolics and flavonoids which contribute towards the antioxidant effects together with other health benefits12,13. Investigation of antioxidant activity through multiple in vitro models have demonstrated significant free radical scavenging activities of G. lucidum extracts10 which could help in improving oxidative stress-associated diseases such as diabetes, degenerative disorders, stroke and liver diseases. Against this background, the present study aims to evaluate the potential of G. lucidum extract in ameliorating carbon tetrachloride-induced hepatotoxicity and associated oxidative stress in Long Evans rats.
Results
Gas chromatography-mass spectroscopy (GC–MS) analysis
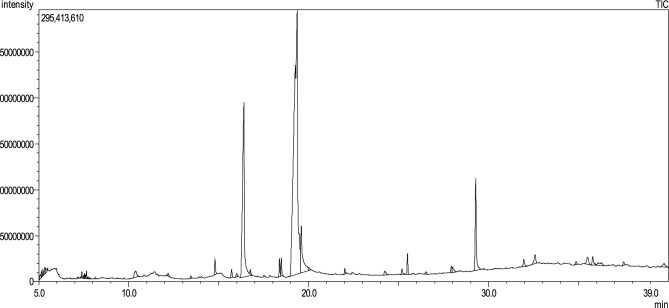
We analyzed the ethanolic extract of G. lucidum powder using a triple-quad GC–MS/MS system as shown in Fig. 1. Thirty-seven phytochemicals were identified in the chromatogram which are tabulated along with retention time (Ret Time), molecular formula, molecular weight and area (%) (Table 1). The most abundant compound was 6-Octadecanoic acid with a peak area of 55.81%. Other notable compounds were l-( +)-Ascorbic acid 2,6-dihexadecanoate (18.72%), Cis-11-Eicosenamide (5.76%), and Octadecanoic acid (5.26%).
Figure 1.
GC chromatogram of the ethanolic extract of G. lucidum powder.
Table 1.
Compounds identified by GC–MS/MS analysis of the ethanolic extract of G. lucidum powder.
| S/N | Ret. time | Area (%) | Chemical name | Nature | Molecular weight | Molecular formula |
|---|---|---|---|---|---|---|
| 1. | 5.085 | 0.27 | Benzene, propyl- | Alkylbenzene | 120.19 | C9H12 |
| 2. | 5.153 | 0.21 | Benzene, 1-ethyl-3-methyl- | Alkylbenzene | 120.19 | C9H12 |
| 3. | 5.334 | 0.36 | Hexanoic acid | Fatty acid | 116.16 | C6H12O2 |
| 4. | 5.405 | 0.26 | 1-Pentene, 5-chloro-4-(chloromethyl)- | Alkene | 153.05 | C6H10Cl2 |
| 5. | 7.485 | 0.1 | Nonanoic acid | Fatty acid | 158.24 | C9H18O2 |
| 6. | 7.532 | 0.15 | 2-Octene-1-ol, 3,7-dimethyl- | Alcohol | 156.26 | C10H20O |
| 7. | 7.607 | 0.09 | Acetic acid, 2,2’-(3,6-dimethoxy-1,4-dioxane-2,5-diylidene)bis-,dimethyl ester | Dimethyl ester | 288.25100 | C12H16O8 |
| 8. | 7.654 | 0.24 | Cyclohexanol, 5-methyl-2(1-methylethyl)-,(a.alpha.,2.beta.,5alpha)-(. + / − .)- | Cyclohexanols | 156.26 | C10H20O |
| 9. | 7.789 | 0.11 | D-Arabino-Hexose, 2-deoxy-,diethyl mercaptal | Mercaptals | 270.4 | C10H22O4S2 |
| 10. | 10.381 | 0.79 | 1,3-Propandiol, 2-(hydroxymethyl)-2-nitro- | Polyols | 151.11 | C4H9NO5 |
| 11. | 12.198 | 0.15 | Ethyl .alpha-d-glucopyranoside | O-glycosyl compounds | 208.21 | C8H16O6 |
| 12. | 13.542 | 0.12 | Tetradecanoic acid | Fatty acid | 228.37 | C14H28O2 |
| 13. | 14.793 | 0.76 | Pentadecanoic acid | Fatty acid | 242.40 | C15H30O2 |
| 14. | 15.722 | 0.46 | Triacontanoic acid | Fatty acid | 452.8 | C30H60O2 |
| 15. | 16.003 | 0.23 | Palmitoleic acid | Fatty acids, Monounsaturated | 254.41 | C16H30O2 |
| 16. | 16.406 | 18.72 | l-( +)-Ascorbic acid 2,6-dihexadecanoate | Fatty acid esters | 652.9 | C38H68O8 |
| 17. | 16.759 | 0.2 | Hexadecanoic acid, ethyl ester | Fatty acid ethyl ester | 284.5 | C18H36O2 |
| 18. | 18.379 | 0.81 | Methyl 10-trans, 12-cis-octadecadienoate | Fatty acid methyl ester | 294.5 | C19H34O2 |
| 19. | 18.483 | 0.81 | 9-Octadecanoic acid, methyl ester, (E)- | fatty acid methyl ester | 296.48 | C19H36O2 |
| 20. | 19.363 | 55.81 | 6-Octadecanoic acid | Fatty acids | 282.46 | C18H34O2 |
| 21. | 19.589 | 5.26 | Octadecanoic acid | Fatty acid | 284.47 | C18H36O2 |
| 22. | 20.03 | 0.17 | n-Pentadecanol | Long-chain fatty alcohol | 228.41 | C15H32O |
| 23. | 24.243 | 0.39 | Carbamic acid, 2-(dimethylamino)ethyl ester | Quaternary ammonium compounds | 132.16 | C5H12N2O2 |
| 24. | 25.183 | 0.38 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 1-monoacylglycerols | 330.50 | C19H38O4 |
| 25. | 25.494 | 0.99 | Bis(2-ethylhexyl) phthalate | Phthalate ester | 390.55 | C24H38O4 |
| 26. | 26.526 | 0.16 | Lanosterol | Tetracyclic triterpenoid | 426.71 | C30H50O |
| 27. | 27.928 | 0.39 | 6,9-Octadecadienoic acid, methyl ester | Fatty acid methyl ester | 294.50 | C19H34O2 |
| 28. | 28 | 0.49 | 2,3-Dihydroxypropyl elaidate | Monoacylglycerol | 356.50 | C21H40O4 |
| 29. | 29.286 | 5.76 | Cis-11-Eicosenamide | Primary fatty amide | 309.50 | C20H39NO |
| 30. | 31.962 | 0.55 | Ergosta-5,7,9(11),22-tetraen3-ol,(3.beta.,22E)- | Phytosterol | 394.63 | C28H42O |
| 31. | 32.581 | 0.85 | 20.xi.-Lanosta-7,9(11)-dien-21-oic acid, 16.alpha.-hydroxy-24-methylene-3-oxo- | Lanostane triterpene | 482.69 | C31H46O4 |
| 32. | 34.861 | 0.24 | Ergosta-5,7,9(11)-tetraen-3-ol,(3.beta.,22E)- | Phytosterol | 394.63 | C28H42O |
| 33. | 35.504 | 0.81 | Ergosterol | Phytosterol | 396.64 | C28H44O |
| 34. | 35.788 | 0.68 | Ergosta-5,7-dien-3-ol,(3.beta.)- | Phytosterol | 398.66 | C28H46O |
| 35. | 36.087 | 0.76 | Rhodopin | Carotenoid | 554.88 | C40H58O |
| 36. | 37.505 | 0.29 | gamma.-Sitosterol | Phytosterols | 414.70 | C29H50O |
| 37. | 39.754 | 0.5 | Retinol | Retinoids | 286.45 | C20H30O |
Effect of G. lucidum extract on the body weight
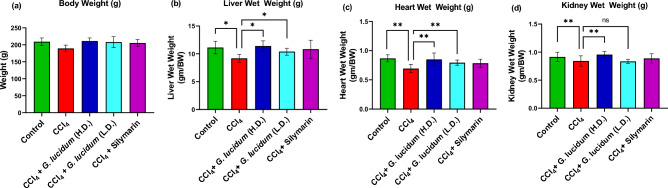
All the rats were weighed routinely throughout the experimental period. As shown in Fig. 2a, no statistically significant difference in body weights was noticed among different experimental groups. This shows that carbon tetrachloride, silymarin, or the sample extract did not adversely affect the body weight of the experimental animals.
Figure 2.
Effect of G. lucidum extract supplementation on the body weight (a) and the organ wet weight (b–d) in different groups. Statistical analysis was carried out by one‐way ANOVA followed by Newman–Keul’s post hoc test. Statistical significance was considered at *p ≤ 0.05 and **p ≤ 0.01, ns not significant. HD high dose (200 mg/kg), LD low dose (100 mg/kg).
Effect of G. lucidum extract on organ wet weight
Statistically significant differences in the liver, heart, and kidney weights of rats have been observed between different groups of experimental animals (p ≤ 0.05). The disease group (CCl4 treated rats) showed the lowest organ weights among all the groups. As shown in Fig. 2b, the control group, the disease-treatment (high dose) group, and the standard silymarin group showed similar liver weight. The carbon tetrachloride group had a significant reduction in liver weight. The rats of the disease group (CCl4 treated group) also showed a significant decrease in heart (Fig. 2c) weight compared to the control and disease treatment groups. As shown in Fig. 2d, a significant decrease in kidney weight was obseved in disease group compared to the control and high dose-disease treatment groups. However, low dose extract treatment did not show any significant differnce in kidney weight as compared to the disease group.
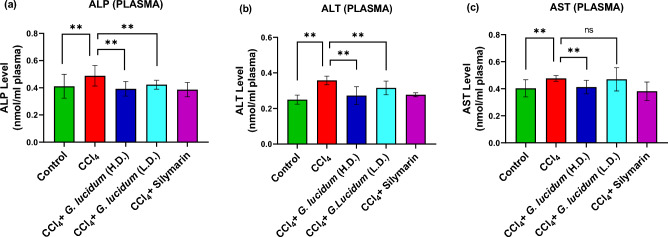
Effect of G. lucidum extract on ALP, ALT, and AST activities
A significantly high plasma level of ALP and ALT was observed in the disease group (CCl4 treated group) compared to the other groups (p ≤ 0.01) (Fig. 3). Lower levels of ALP and ALT were observed with both low and high dose treatment groups compared to the disease group (Fig. 3a,b). The high dose treatment group exhibited a lower AST level (p ≤ 0.01) whereas low dose treatment did not, compared to the disease group (Fig. 3c).
Figure 3.
Effect of G. lucidum extract supplementation on ALP (a), ALT (b), and AST (c) enzyme activities in plasma of CCl4 administered rats. Statistical analysis was carried out by one‐way ANOVA followed by Newman–Keul’s post hoc test. Statistical significance was considered at *p ≤ 0.05 and **p ≤ 0.01, ns not significant. HD high dose (200 mg/kg), LD low dose (100 mg/kg).
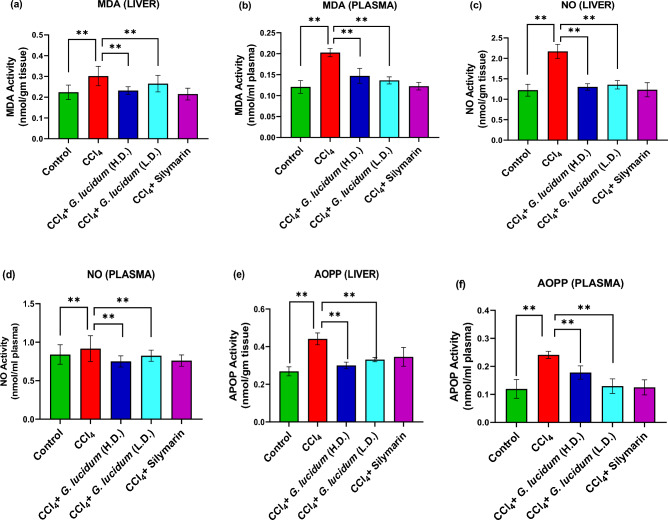
Effect of G. lucidum extract on oxidative stress parameters
Significant elevation in malondialdehyde (MDA) level was observed in the disease group compared to the control (p ≤ 0.01) (Fig. 4a,b). In contrast, the liver and plasma samples from the high dose treatment group showed a significant decrease in MDA level compared to the disease group (p ≤ 0.01). MDA level was also significantly reduced in the standard group (silymarin treatment) compared to the disease group, and the reduction was more than in both extract treatment groups. A higher concentration of MDA was observed in plasma than in liver homogenate.
Figure 4.
Effect G. lucidum powder extract on MDA (a, b), NO (c, d), and AOPP (e, f) activities in liver and plasma of CCl4 administered rats. Statistical significance was considered at *p ≤ 0.05, **p ≤ 0.01. HD high dose (200 mg/kg), LD low dose (100 mg/kg).
The level of NO in the liver homogenate (Fig. 4c) of the disease group was much higher than the control, standard, and extract treatment groups. At the same time, it was almost identical among the control, standard, low dose, and high dose treatment groups. As shown in Fig. 4d, the highest level of NO was observed in plasma (Fig. 4d) of the disease group whereas, high dose treatment and standard treatment groups showed much lower levels of plasma NO (p ≤ 0.01).
The Fig. 4e shows that ethanolic extract of G. lucidum (200 mg/kg) significantly (p ≤ 0.01) diminished the level of AOPP in the liver as compared to the disease group. A similar change was observed in the standard group treated with silymarin and the low-dose treatment group. However, high dose treatment showed better effect compared to the low dose treatment. On the other hand, low dose treatment was found to be more effective in reducing plasma AOPP levels (Fig. 4f) compared to the high dose treatment.
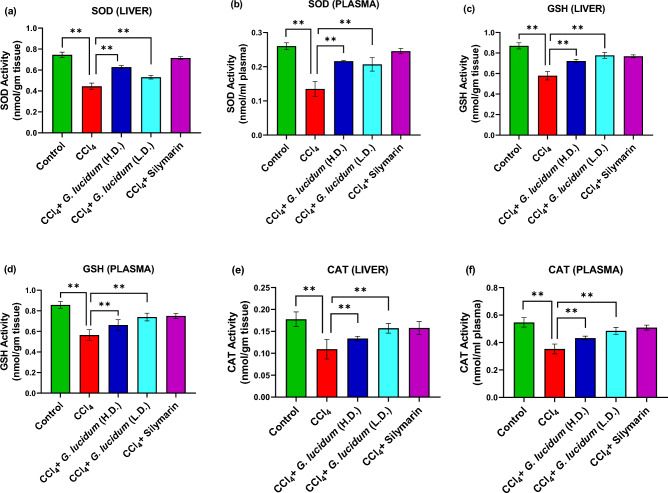
Effect of G. lucidum extract on antioxidant enzymes activities
Extract and standard treatment groups showed increased SOD, GSH, and CAT levels in both liver homogenate and plasma samples compared to the disease group (Fig. 5).The increase in SOD level was higher in the standard group (silymarin treatment) than in the extract treatment groups (Fig. 5a,b). As shown in (Fig. 5a), the higher dose of G. lucidum extract increased the SOD level more than the low dose treatment in liver homogenate.
Figure 5.
Effect of G. lucidum extract supplementation on SOD (a, b), GSH (c, d) and CAT (e, f) activities in liver and plasma of CCl4 administered rats. Statistical significance was considered at *p ≤ 0.05, **p ≤ 0.01. HD high dose (200 mg/kg), LD low dose (100 mg/kg).
GSH level was found to be higher in the standard and the extract treatment groups, both in liver homogenate and plasma (Fig. 5c,d) as compared to the disease group. The low dose and standard treatment groups showed similar levels of GSH (Fig. 5c,d). A higher CAT level was observed in the standard and low dose treatment group than in the high dose treatment group in both liver homogenate and plasma (Fig. 5e,f).
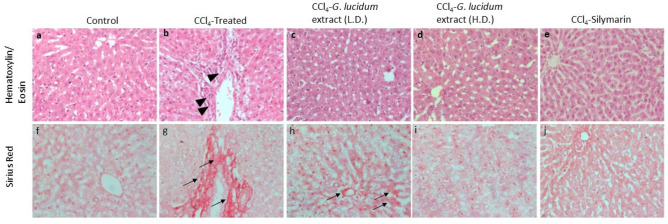
Histopathological observation
The hematoxylin/eosin staining of the liver tissue revealed regular internal cellular morphological structure (Fig. 6a). No abnormalities, infiltration of immune cells (e.g., mononuclear cells), and tissue necrosis were observed in the internal structure of the control group (Fig. 6a). Infiltration and necrosis were observed in the disease group (CCl4 treated group) (Fig. 6b, arrowheads), which was altered in the low dose (Fig. 6c) and high dose- (Fig. 6d) mushroom extract-treated groups. The standard silymarin-treated rats showed regular tissue architecture (Fig. 6e).
Figure 6.
Histopathological observations showing the effects of CCl4-induced hepatotoxicity and the hepatoprotective effect of G. lucidum extract (Low dose: 100 mg/kg and High dose: 200 mg/kg). The upper panel (a–e) shows hematoxylin/eosin staining and the lower panel (f–j) shows Sirius Red staining. Arrowheads indicate infiltrating cells (b) and arrows indicate collagen deposition (g, h).
The Sirius red staining of the liver tissues showed normal distribution of collagen fibers in the control group (Fig. 6f). Intense collagen and fatty infiltration distribution were observed in the CCl4 treated disease group indicating significant fibrosis (Fig. 6g, arrows). The low dose mushroom-treated group exhibited slightly higher collagen deposition (Fig. 6h, arrows) than the control. However, the collagen distribution was nearly normal with high dose mushroom or silymarin treatment (Fig. 6i,j respectively). Quantification data is provided in Figure S1.
Gene expression analysis
Prominent expression of the housekeeping gene GAPDH was observed in the control, disease, and disease-treatment (High dose) group (Fig. 7, Figure S2). The administration of CCl4 or mushroom extract did not affect GAPDH gene expression and acted as an internal control. The expression of IL-1β, an inflammatory cytokine, was observed in carbon tetrachloride treated groups, which was suppressed by the extract treatment. The expression of IL-6 was also observed in the disease group that the extract treatment suppressed. IL-10 is an inflammation protector. Administration of carbon tetrachloride suppressed this hepatoprotective IL-10 gene expression, whereas a higher level of IL-10 gene expression was observed in the extract group. This indicates that supplementation with G. lucidum extract can protect against inflammation. Higher expression of inflammatory cytokine TNF-α was observed in the disease group compared to the control and treatment groups. The results support that Ganoderma extract has an ameliorative effect on hepatotoxicity as exhibited by lower expression of TNF-α. CCl4-induced hepatotoxicity increased TGF-β expression, which is responsible for liver fibrosis. In this study, the disease group showed TGF-β expression, which was reduced by treatment with the extract (High dose).
Figure 7.
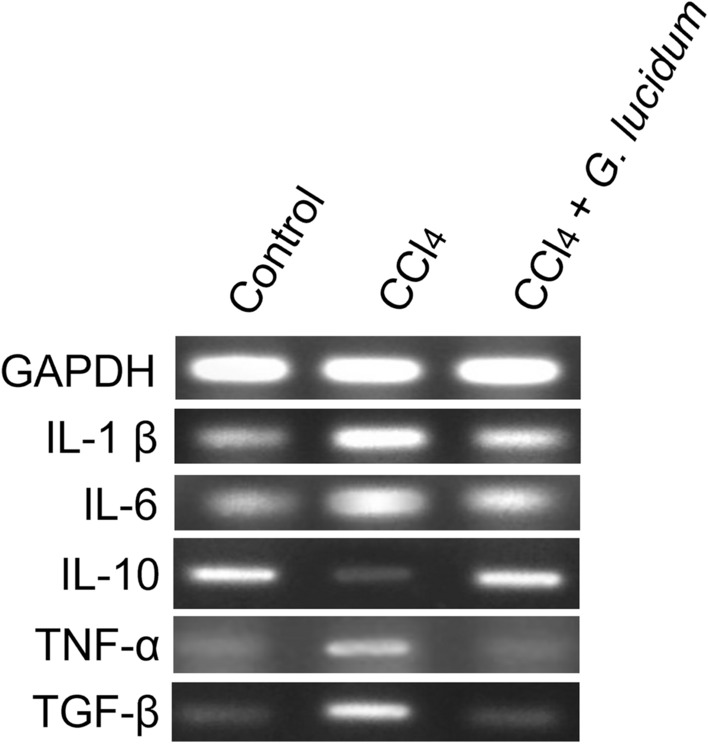
Expression of a housekeeping gene (GAPDH) and cytokine genes (IL-1β, IL-6, Il-10, TNF-α, TGF-β) in liver tissues collected from different groups.
Discussion
While nutritional benefits of G. lucidum have been known for centuries, a recent focus is the exploration of medicinal applications of the extract and isolated phytochemicals. Phytochemical analysis using GCMS revealed that G. lucidum ethanolic extract contains bioactive compounds (Table 1) with pharmacological effects such as antioxidant, anti-inflammatory, anticancer, antimicrobial, and hepatoprotective effects. l-( +)-Ascorbic acid 2,6-dihexadecanoate (18.72%) which was identified as one of the major constituents is known to be a potent antioxidant with hepatoprotective25 effects. Triterpenoids, which were found in the mushroom extract are reported to provide protection against liver injury induced by toxic chemicals such as CCl4, thioacetamide and alcohol13. Triterpenoids are emerging as a unique group of phytochemicals with multifunctional activities like anti-adipogensis, anti-inflammatory, analgesic, antipyretic, cardiotonic, sedative and hepatoprotective effects9,26. Ergosterols present in the mushroom extract as evidenced by GCMS analysis, have been shown to exert anti-tumor, anti-angiogenic21 and protective effects against liver fibrosis 11. Several studies have demonstrated the hepatoprotective and antioxidant effects of retinoid27,28. 9-Octadecenoic acid and its esters have been shown to exhibit antiandrogenic, 5-alpha reductase-inhibiting, cancer-preventive, anti-inflammatory, antioxidant and hypocholesterolemic activity12,19,29. Hexadecanoic acid detected in the mushroom extract has also been reported to have antioxidant, hypocholesterolemic, antibacterial and anticancer activities12,19,29. Furthermore, 6-Octadecenoic acid, a major constituent (55.81%), detected in the G. lucidum has been shown to possess anti-cancer and antimicrobial activities19.
In this study, the hepatoprotective effects of G. lucidum extract were explored in a rat model of carbon tetrachloride-induced hepatic damage. The effects were compared with the standard silymarin. Administration of carbon tetrachloride significantly reduced the internal organs’ weight, whereas co-administration of extract and CCl4, or silymarin and CCl4 didn’t show any significant reduction in the organs’ weights (Fig. 2). This is in accordance with previous studies30,31.
CCl4 administration raised hepatic AOPP, MDA, and NO levels and increased lipid peroxidation leading to antioxidant defense failure and hepatic damage32. ROS are often generated at physiological and pathological levels, either due to cellular metabolism or in response to an insult to the body's homeostasis. Excess free radicals cause oxidative stress indicators like MDA, AOPP, and NO to rise33. Increased ROS level degrades membrane phospholipids and decreases antioxidant levels in the affected tissues34. In this study, CCl4-induced hepatic damage raised the level of stress biomarkers which included MDA, AOPP, and NO. In contrast, extracts and standard treatment groups exhibited decreased stress biomarkers. Oxidative stress triggers the mineralocorticoid receptor activation35, leading to reduced levels of SOD, GSH, and CAT enzymes. These enzymes are part of the body’s first-line defense against oxidative stress and scavenge the ROS to sustain the physiological condition.
Protective effect of G. lucidum extracts has been demonstrated against hepatotoxicants such as ethanol, cyclophosphamide, benzo[a]pyrene and CCl4 in experimental animals at varying concentrations13,36–39. Lin et al. (1995)37 reported 66% hepatoprotection by aqueous extract of G. lucidum at 100 mg/kg dose whereas, methanolic extract of G. lucidum at 500 mg/ kg dose has been shown to prevent benzo[a]pyrene induced hepatic damage in rats40. Ameliorative effect of G. lucidum on carbon tetrachloride-induced liver fibrosis in rats has been demonstrated previously at a dose of 600 mg/kg38 and 2000 mg/kg crude extract36. However, in this study ethanolic extract of locally grown G. lucidum showed significant hepatoprotection in rats at 200 mg/kg dose. The findings of this study indicate that the fruiting bodies of G. lucidum occurring in Bangladesh, could present promising candidate for the development of effective liver protective agents.
In this study, the disease group exhibited a decrease in the levels of SOD, CAT, and GSH compared to the control, which was significantly (p ≤ 0.01) protected with the ethanolic extract of G. lucidum (EEGL) supplementation. Sirius-red and Hematoxylin/ Eosin staining revealed that these biochemical alterations led to histopathologic changes (Fig. 6). In the liver tissue of diseased (CCl4-treated) rats, we found altered hepatic architecture, fibrosis, and a buildup of darkly stained nuclei. The oxidative state, hepatic enzyme activity, and histological alterations were apparently undisturbed when EEGL or silymarin was given concurrently (Fig. 6). Similar results were reported by previous studies with plant extracts30,41,42.
Any pathological damage to the hepatocytes releases the liver marker enzymes into the blood circulation, e.g., Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Alkaline Phosphate (ALP), etc. The level of ALT, AST, ALP, bilirubin, and low-density lipoprotein (LDH) in the blood rises as the hepatocytes are destroyed. The results obtained from the biochemical analysis in our study are in line with the previous reports43–45 that showed an increase in the ALT, AST, ALP, lactate dehydrogenase concentration, and a decrease in the concentration of glutathione peroxidase, SOD, and CAT in CCl4-induced hepatotoxicity in male Wistar rats. In the current study, the mushroom extract provided noticeable protection against the elevation of liver marker levels in test animals.
For a better understanding of the efficacy of G. lucidum extract, expression analysis of some housekeeping, inflammatory, and liver regeneration associated genes has been carried out. The housekeeping gene GAPDH expression showed no change in any group, which indicates that the Ganoderma extract or CCl4 did not affect the expression of the housekeeping gene. Eltahir et al.46 reported an increase in the expression of several inflammatory cytokine genes associated with CCl4 administration in rats. Interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α) mRNA expression, necrosis factor-kappa B (NF-κB) proteins level, TGF-β, COX-2 expression has also been observed in the CCl4-induced hepatic injury in rats46. In our study, increased expression of inflammatory cytokines TNF-α, TGF-β, IL-1 β, and IL-6 due to CCl4 was suppressed by the ethanolic extract of G. lucidum. Additionally, the CCl4-mediated decrease in expression of the inflammatory protector IL-10 gene was restored by the extract treatment. Our findings demonstrate that G. lucidum is effective in preventing the elevated level of liver markers thereby ameliorating oxidative stress in hepatic injury. At a molecular level, it showed a reduction in expression of the studied inflammatory gene and an increase in the hepatoprotective genes.
Our findings demonstrate that G. lucidum could be used as a promising hepatoprotective agent and in the treatment of hepatic fibrosis. GCMS analysis suggests that presence of hepatoprotective compounds such as ascorbic acid, triterpenoids, ergosterols and retinol together with numerous other bioactive molecules may contribute towards hepatoprotective effect of mushroom. This study also provides valuable insights into mechanisms that underlie the hepatoprotective effect of G. lucidum, supposedly mediated through attenuation of lipid peroxidation, restored activities of antioxidant enzymes, downregulation of inflammatory cytokines genes and upregulation of the protective genes like IL-10. Phytochemical diversity of secondary metabolites indicates potential of G. lucidum for the development of newer drugs. Further investigations with isolated pure compounds are desirable to elucidate the precise therapeutic potential of G. lucidum for clinical applications.
Conclusions
In our study, CCl4 reliably induced hepatotoxicity and oxidative stress in Long Evans rats. The disease group displayed elevated MDA, AOPP, and NO levels and attenuated SOD, CAT, and GSH levels. Interestingly, supplementation with the G. lucidum extract or the standard antioxidant silymarin efficiently protected the experimental rats from oxidative stress-induced pathological changes. These findings were further supported by gene expression analysis and histopathological studies. Thus, it would be interesting to explore the antioxidant and hepatoprotective properties of the isolated phytochemicals from G. lucidum in future studies.
Materials and methods
Chemicals
Carbon tetrachloride (CCl4) was purchased from Qualigens Fine Chemicals, India. Silybin R (Silymarin, reference drug) capsules were obtained from Square Pharmaceuticals Ltd., Bangladesh. Kits used in the determination of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were purchased from Human Diagnostics, Germany. GoScriptTM Reverse Transcription System (Promega) was used to perform the reverse transcription process. All other chemicals and solvents were of the analytical grade and obtained from Sigma or Merck.
Extract preparation
Dried G. lucidum powder was obtained from whole G. lucidum (Figure S3) as described previously10 and extracted with ethanol. Extraction was carried out approximately for seven days, accompanied by occasional shaking and stirring. The mixture was filtered first through a piece of clean, white cotton material and finally through Whatman No-1 filter paper. The extract was evaporated to dryness under reduced pressure by a rotary vacuum evaporator followed by overnight incubation at room temperature to ensure complete evaporation of residual solvents. The dried extract was stored at 4 °C in a refrigerator until used for analysis39.
Gas chromatography-mass spectroscopy (GC–MS) analysis
Phytochemicals of the dried G. lucidum powder extract were analyzed using a GC–MS/MS system (GCMS-TQ8040, Shimadzu). Chromatographic separation was carried out in a capillary column (Rxi-Sms, 30 m × 0.25 mm × 0.25 μm) using helium (99.99% pure, flow rate: 1.2 ml/min) as the carrier gas. The column oven temperature was 75 °C and the injection temperature was 250 °C. MS conditions were as follows: ion source temperature 230 °C; interface temperature 250 °C; detector gain 1.13 kV + 0.2 kV; end time 35 min; Acq. mode Q3 Scan; event time 0.5 s; scan speed 2000; m/z range 50–1000, injection volume 0.5 μl (10 μg/ml).
Experimental animals
Thirty Long Evans rats (average weight 190 ± 10 g), 10–12 weeks old, collected from the Animal House of the Department of Pharmaceutical Sciences, North South University, were used in this study. The experimental inclusion criteria of the test animals were specific parameters such as body weight, fitness, and health. The animals were kept in individual cages under standard conditions (12 h light/dark cycle; 23–26 °C) with free access to standard diet and water. The overall study protocol complied with the standards for animal care and experimentation of Institutional Animal Care and Use Committee (IACUC) of North South University in accordance with the guidelines from the Council for International Organization of Medical Sciences and The International Council for Laboratory Animal Science (CIOMS/ICLAS, 2012) and the Nuffield Council on Bioethics (NCB). The study was reported in accordance with ARRIVE guidelines47.
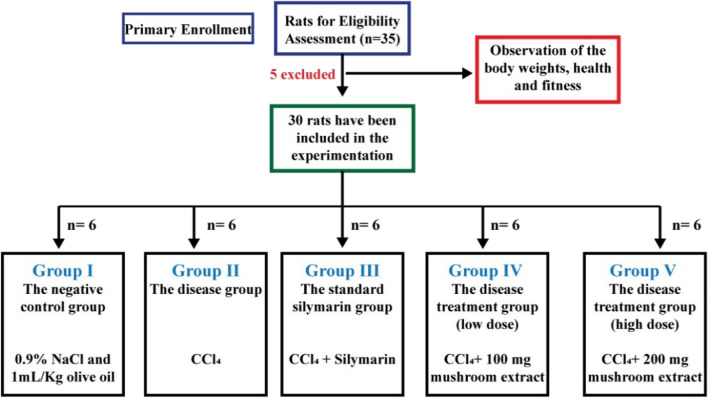
Study design and treatment
Thirty male Long Evans rats selected for the experiment were divided into five experimental groups, each consisting of six rats. The treatment period was two weeks for all the groups, and all the substances were administered intragastrically. The groups and their specifications have been mentioned in Table 2.
Table 2.
Study groups and the specifications of each group.
| Group name | Group type | Treatment specification |
|---|---|---|
| Group I | Negative control | 1 mL/Kg body weight of saline (0.9% Sodium chloride solution) and 1 mL/kg olive oil twice a week |
| Group II | Disease group | CCl4 at a dose of 1 mg/Kg body weight dissolved in olive oil (1:3 ratio) twice a week |
| Group III | Standard group | CCl4 at a dose of 1 mg/Kg body weight dissolved in olive oil (1:3 ratio) twice a week + silymarin 50 mg/Kg body weight daily |
| Group IV | Disease-treatment group (low dose) | CCl4 at a dose of 1 mg/Kg body weight dissolved in olive oil (1:3 ratio) twice a week + mushroom extract at a dose of 100 mg/Kg body weight daily |
| Group V | Disease-treatment group (high dose) | CCl4: olive oil (1:3 ratio) at a dose of 1 mL/Kg body weight twice a week + mushroom extract at a dose of 200 mg/Kg body weight daily |
The inclusion and exclusion of the test animals and the overall experimentation groups, along with the test criteria of each group, have been shown diagrammatically in Fig. 8.
Figure 8.
The inclusion and exclusion of the test animals and the experimentation groups.
The test agents were administered intragastrically using a feeding needle and a 10 mL syringe to measure the volume accurately. The rats' body weight, food, and water intake were checked daily. After two weeks of treatment, the animals of all groups were sacrificed after anesthetization with intraperitoneal ketamine hydrochloride injection. 3 mL blood samples from each animal were collected in citrate buffer tubes and centrifuged at 2000 rpm for 15 min at 4 °C for serum separation. Plasma was transferred in pre-labeled eppendorf tubes and stored at − 20 °C for further analysis. The liver and other organs such as the heart, spleen, and kidney were harvested. For histopathological analysis, parts of each rat liver were stored in the 10% formalin (pH 7.4). For gene expression analysis in hepatocytes, specimens from the liver were cut into pieces and stored at − 20°C30.
Body weight and organ weight determination
The rats of all groups were weighed regularly, and the average weight of each group was determined to assess the impact of treatments and test chemicals on the body weight. After dissection, the weight of collected organs such as liver, kidney, heart, and spleen was recorded, and variation among each group was analyzed.
Biochemical parameters
Hepatotoxicity assessment
Quantitative determination of the liver function marker enzymes; alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) in plasma was carried out with a spectrophotometer using commercial diagnostic kits according to the manufacturer’s protocol.
Estimation of lipid peroxidation
Lipid peroxidation and oxidative stress in plasma and liver tissue were determined colorimetrically by measuring thiobarbituric acid reactive substances (TBARS). The sample (tissue homogenate/blood plasma) was treated with phosphate buffer solution (PBS), glacial acidic acid (AA), and thiobarbituric acid (TBA 0.67%) and boiled for 30 min. For homogenates, the ratio of PBS, AA, and TBA was 4:5:5, whereas, for plasma, it was 1:2:2. This was followed by measuring the absorbance of the cooled and clear supernatant at 490 nm wavelength48. The extent of lipid peroxidation was expressed as MDA equivalents.
Estimation of nitric oxide (NO)
This assay is based on the fact that nitric oxide present in the sample solution undergoes diazotization with sulfanilamide followed by coupling with N-(1-naphthyl) ethylenediamine hydrochloride (NED). Briefly, 20 µL tissue homogenate or 50 µL plasma samples were taken in a 96-well plate. This was followed by mixing with 0.33% (w/v) sulphanilamide and 0.1% (w/v) N-(1-naphthyl) ethylenediamine hydrochloride in phosphate buffer solution. Then absorbance was measured at 405 nm wavelength. NO level was determined using a standard curve made by serial dilution of 1 mM sodium nitrite stock solution49.
Determination of advanced oxidation protein products (AOPP)
The tissue homogenate/plasma sample was taken with phosphate buffer solution (PBS) and mixed with 0.45% (w/v) potassium iodide and glacial acidic acid. The mixture was left for 2 min at room temperature to complete the reaction. Absorbance was recorded at 405 nm wavelength50.
Reduced glutathione assay (GSH)
The tissue homogenate or plasma sample was mixed with phosphate buffer solution (1:9). This was followed by adding 100 µL 5, 5-dithiobis-2-nitrobenzoic acid (DTNB) and incubating for 1 h at 4 °C. Absorbance was measured at 450 nm wavelength51.
Catalase assay (CAT)
Tissue homogenate or plasma sample was taken in a 96-well culture plate, and phosphate buffer was added. This was followed by adding the appropriate concentrations of hydrogen peroxide solution to the mixture. Absorbance was recorded at 450 nm wavelength three times at 3 min intervals52.
Determination of superoxide dismutase (SOD)
The tissue homogenate/plasma sample (10 µL) was added to a 90 µL phosphate buffer solution. This was followed by adding 100 µL adrenaline and immediately measuring absorbance at 490 nm wavelength. A control reaction consisting of all the ingredients except the tissue/plasma was analyzed simultaneously53. SOD activity was determined in terms of inhibiting autooxidation of adrenaline to adrenochrome.
Gene expression profile
RNA isolation and purification
Total RNA was isolated from frozen liver tissue samples using Trizol reagent54. RNase-free reagents were used throughout the process, and the samples were treated with DNase I to avoid contamination of genomic DNA. Isolated RNA was dissolved in RNAase-free water and stored at − 20 °C. The yield and integrity of isolated RNA were evaluated by measuring absorbance at 260, 280, and 310 nm and agarose gel (1%) electrophoresis.
Reverse transcription and PCR analysis
RNA samples were reverse-transcribed to first-strand complementary DNA (cDNA) using GoScriptTM Reverse Transcription System (Promega) according to the manufacturer’s instructions. For semi-quantitative analysis of specific mRNA, 4 µL of cDNA template was mixed with 10 µL of PCR master mix, 4 µL nuclease-free water, and 1 µL of (100 nmol/mL) forward and reverse primers (Table S1) in PCR tubes and the mix was put into the TaKaRa PCR Thermal Cycler (TP650, TaKaRa, Japan). The following conditions were used: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at the corresponding temperature for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 7 min. Finally, the gene expression was analyzed by agarose gel electrophoresis.
Histopathological studies
Liver tissues excised from different groups of animals were fixed in neutral buffered formalin (10%), dehydrated in graded alcohol, and embedded in paraffin. Microtome Sects. (0.5 µ) were prepared and stained with hematoxylin/eosin and Sirius red stains. This was followed by observation under a light microscope (Olympus DP12, Japan) at 20 × magnification to assess histopathological changes30.
Statistical analysis
All data were presented as the Mean ± Standard Error of Mean (SEM) (n = 6). The results were subjected to analysis of variance (ANOVA) by using Graph pad Prism 8. Values were considered statistically significant at p ≤ 0.05.
Ethical approval
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of North South University (IACUC Id: 2020/OR-NSU/IACUC-No.1105) for studies involving animals.
Supplementary Information
Author contributions
F.T.J.: Investigation, writing—original draft preparation. S.H.: investigation, writing—original draft preparation. P.J.: Conceptualization, formal analysis, writing—review and editing, supervision, funding acquisition. A.T.B.: investigation, writing—original draft preparation. T.E.: Formal analysis, visualization. R.A.: Formal analysis, writing—review and editing. S.M.S.: Formal analysis, writing—review and editing. A.K.B.: Writing—original draft preparation, writing—review and editing. H.M.R.: Conceptualization, formal analysis, writing—review and editing, visualization, supervision, All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by an internal grant (to Preeti Jain) from North South University (CTRG Id: CTRG-20-SHLS-35).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fatima Tuj Johra and Sukria Hossain.
Contributor Information
Preeti Jain, Email: preti.jain@northsouth.edu.
Hasan Mahmud Reza, Email: hasan.reza@northsouth.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35228-y.
References
- 1.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Oxford University Press; 2015. [Google Scholar]
- 2.Zulaikhah S. The role of antioxidant to prevent free radicals in the body. Sains Med. 2017;8:39. [Google Scholar]
- 3.Kebede M, Emire S. Application of antioxidants in food processing industry: options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. 2019;5:38–49. doi: 10.17140/AFTNSOJ-5-155. [DOI] [Google Scholar]
- 4.Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, β-carotene and β-tocopherol in Aloe vera. Oxid. Med. Cell. Longev. 2009;2:99–106. doi: 10.4161/oxim.2.2.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruskovska T, Maksimova V, Milenkovic D. Polyphenols in human nutrition: from the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020;123:241–254. doi: 10.1017/S0007114519002733. [DOI] [PubMed] [Google Scholar]
- 6.Almazroo OA, Miah MK, Venkataramanan R. Drug metabolism in the liver. Clin. Liver Dis. 2017;21:1–20. doi: 10.1016/j.cld.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Bruccoleri A, et al. Induction of early-immediate genes by tumor necrosis factor α contribute to liver repair following chemical-induced hepatotoxicity. Hepatology. 1997;25:133–141. doi: 10.1002/hep.510250125. [DOI] [PubMed] [Google Scholar]
- 8.Berger ML, Bhatt H, Combes B, Estabrook RW. CCl4- induced toxicity in isolated hepatocytes: The importance of direct solvent injury. Hepatology. 1986;6:36–45. doi: 10.1002/hep.1840060108. [DOI] [PubMed] [Google Scholar]
- 9.Galappaththi MCA, et al. A review of Ganoderma Triterpenoids and their bioactivities. Biomolecules. 2023;13:24. doi: 10.3390/biom13010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bristy AT, et al. Evaluation of total phenolic content, HPLC analysis, and antioxidant potential of three local varieties of mushroom: A comparative study. Int. J. Food Sci. 2022;2022:e3834936. doi: 10.1155/2022/3834936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Y, et al. Ergosterol is the active compound of cultured mycelium Cordyceps sinensis on Antiliver fibrosis. Evid. Complement. Alternat. Med. 2014;2014:e537234. doi: 10.1155/2014/537234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesan K, Jayachandran M, Xu B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2018;58:1165–1229. doi: 10.1080/10408398.2016.1244154. [DOI] [PubMed] [Google Scholar]
- 13.Soares AA, et al. Hepatoprotective effects of mushrooms. Molecules. 2013;18:7609–7630. doi: 10.3390/molecules18077609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricus blazei Murrill: Review of literature and pharmaco-toxicological problems. Evid. Complement. Alternat. Med. 2008;5:3–15. doi: 10.1093/ecam/nem007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kües U, Liu Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 2000;54:141–152. doi: 10.1007/s002530000396. [DOI] [PubMed] [Google Scholar]
- 16.Manzi P, Aguzzi A, Pizzoferrato L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001;73:321–325. doi: 10.1016/S0308-8146(00)00304-6. [DOI] [Google Scholar]
- 17.Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SMN, Somasundaram R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006;54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- 18.Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- 19.Ralte L, Khiangte L, Thangjam NM, Kumar A, Singh YT. GC–MS and molecular docking analyses of phytochemicals from the underutilized plant, Parkia timoriana revealed candidate anti-cancerous and anti-inflammatory agents. Sci. Rep. 2022;12:3395. doi: 10.1038/s41598-022-07320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keypour S, Rafati H, Riahi H, Mirzajani F, Moradali MF. Qualitative analysis of ganoderic acids in Ganoderma lucidum from Iran and China by RP-HPLC and electrospray ionisation-mass spectrometry (ESI-MS) Food Chem. 2010;119:1704–1708. doi: 10.1016/j.foodchem.2009.09.058. [DOI] [Google Scholar]
- 21.Chen S, et al. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Front. Chem. 2017;5:85. doi: 10.3389/fchem.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seweryn E, Ziala A, Gamian A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients. 2021;13:2725. doi: 10.3390/nu13082725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susilo RJK, et al. Hepatoprotective effect of crude polysaccharides extracted from Ganoderma lucidum against carbon tetrachloride-induced liver injury in mice. Vet. World. 2019;12:1987–1991. doi: 10.14202/vetworld.2019.1987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Wang H, Pang X, Yao W, Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010;46:451–457. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Abdulrazzaq AM, et al. Hepatoprotective actions of ascorbic acid, alpha lipoic acid and silymarin or their combination against acetaminophen-induced hepatotoxicity in rats. Med. Mex. 2019;55:181. doi: 10.3390/medicina55050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishayee A, Ahmed S, Brankov N, Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. Lanmark. 2011;16:980–996. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uboh FE, Ekaidem IS, Ebong PE, Umoh IB. The hepatoprotective effect of vitamin A against gasoline vapor toxicity in rats. Gastroenterol. Res. 2009;2:162–167. doi: 10.4021/gr2009.06.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melis M, Tang X-H, Trasino SE, Gudas LJ. Retinoids in the pathogenesis and treatment of liver diseases. Nutrients. 2022;14:1456. doi: 10.3390/nu14071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah MD, D’Souza UJA, Iqbal M. The potential protective effect of Commelina nudiflora L. against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, mediated by suppression of oxidative stress and inflammation. Environ. Health Prev. Med. 2017;22:66. doi: 10.1186/s12199-017-0673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamia SS, et al. Coenzyme Q10 and silymarin reduce CCl4-induced oxidative stress and liver and kidney injury in ovariectomized rats—Implications for protective therapy in chronic liver and kidney diseases. Pathophysiology. 2021;28:50–63. doi: 10.3390/pathophysiology28010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meharie BG, Amare GG, Belayneh YM. Evaluation of hepatoprotective activity of the crude extract and solvent fractions of Clutia abyssinica (Euphorbiaceae) leaf against CCl4-induced hepatotoxicity in mice. J. Exp. Pharmacol. 2020;12:137–150. doi: 10.2147/JEP.S248677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cemek M, et al. Protective potential of royal jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem. Toxicol. 2010;48:2827–2832. doi: 10.1016/j.fct.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Singal PK, Beamish RE, Dhalla NS. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. In: Spitzer JJ, editor. Myocardial Injury. London: Springer US; 1983. [DOI] [PubMed] [Google Scholar]
- 34.Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol. Ther. 2001;89:187–206. doi: 10.1016/S0163-7258(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, et al. Changes in glucocorticoid and mineralocorticoid receptors of liver and kidney cytosols after pathologic stress and its regulation in rats. Crit. Care Med. 2002;30:623–627. doi: 10.1097/00003246-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Lin J-M, Lin C-C, Chiu H-F, Yang J-J, Lee S-G. Evaluation of the anti-inflammatory and liver-protective effects of Anoectochilus formosanus, Ganoderma lucidum and Gynostemma pentaphyllum in rats. Am. J. Chin. Med. 1993;21:59–69. doi: 10.1142/S0192415X9300008X. [DOI] [PubMed] [Google Scholar]
- 37.Lin J-M, Lin C-C, Chen M-F, Ujiie T, Takada A. Radical scavenger and antihepatotoxic activity of Ganoderma formosanum, Ganoderma lucidum and Ganoderma neo-japonicum. J. Ethnopharmacol. 1995;47:33–41. doi: 10.1016/0378-8741(95)01251-8. [DOI] [PubMed] [Google Scholar]
- 38.Lin W-C, Lin W-L. Ameliorative effect of Ganoderma lucidum on carbon tetrachloride-induced liver fibrosis in rats. World J. Gastroenterol. WJG. 2006;12:265–270. doi: 10.3748/wjg.v12.i2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dabdoub B, Mohammed R, Abdulhadi H. Ganoderma lucidum attenuates and prevents CCL4-induced hepatic and renal damage in sprague -dawley rats. Syst. Rev. Pharm. 2020;30136:98565. doi: 10.13140/RG.2.2.30136.98565. [DOI] [Google Scholar]
- 40.Lakshmi B, Ajith TA, Jose N, Janardhanan KK. Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo [a] pyrene. J. Ethnopharmacol. 2006;107:297–303. doi: 10.1016/j.jep.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Alsahli MA, et al. 6-Gingerol, a major ingredient of ginger attenuates Diethylnitrosamine-induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediat. Inflamm. 2021;2021:e6661937. doi: 10.1155/2021/6661937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, et al. Protection effect of Kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS ONE. 2014;9:e88498. doi: 10.1371/journal.pone.0088498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta RK, et al. Hepatoprotective effect of Solanum xanthocarpum fruit extract against CCl4 induced acute liver toxicity in experimental animals. Asian Pac. J. Trop. Med. 2011;4:964–968. doi: 10.1016/S1995-7645(11)60227-7. [DOI] [PubMed] [Google Scholar]
- 44.Mistry S, Dutt KR, Jena J. Protective effect of Sida cordata leaf extract against CCl4 induced acute liver toxicity in rats. Asian Pac. J. Trop. Med. 2013;6:280–284. doi: 10.1016/S1995-7645(13)60057-7. [DOI] [PubMed] [Google Scholar]
- 45.Shahjahan M, Vani G, Devi CS. Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. J. Med. Food. 2005;8:261–265. doi: 10.1089/jmf.2005.8.261. [DOI] [PubMed] [Google Scholar]
- 46.Eltahir HM, et al. Antioxidant, anti-inflammatory and anti-fibrotic effects of Boswellia serrate gum resin in CCl4-induced hepatotoxicity. Exp. Ther. Med. 2020;19:1313–1321. doi: 10.3892/etm.2019.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.du Sert NP, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:e3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 49.Iwakiri Y, Kim MY. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015;36:524–536. doi: 10.1016/j.tips.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witko-Sarsat V, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 51.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 52.Mueller S, Riedel H-D, Stremmel W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal. Biochem. 1997;245:55–60. doi: 10.1006/abio.1996.9939. [DOI] [PubMed] [Google Scholar]
- 53.Ma L, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31:99–105. doi: 10.1016/j.biomaterials.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 54.Chomczynski P, Mackey K. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.