Abstract
Fragile X syndrome is caused by expansion of a d(CGG) trinucleotide repeat sequence in the 5′ untranslated region of the first exon of the FMR1 gene. Repeat expansion is thought to be instigated by formation of d(CGG)n secondary structures. Stable FMR1 d(CGG)n runs in normal individuals consist of 6–52 d(CGG) repeats that are interrupted every 9–11 triplets by a single d(AGG) trinucleotide. By contrast, individuals having fragile X syndrome premutation or full mutation present >54–200 or >200–2000 monotonous d(CGG) repeats, respectively. Here we show that the presence of interspersed d(AGG) triplets diminished in vitro formation of bimolecular tetrahelical structures of d(CGG)18 oligomers. Tetraplex structures formed by d(CGG)n oligomers containing d(AGG) interspersions had lower thermal stability. In addition, tetraplex structures of d(CGG)18 oligomers interspersed by d(AGG) triplets were unwound by human Werner syndrome DNA helicase at rates and to an extent that exceeded the unwinding of tetraplex form consisting of monotonous d(CGG)18. Diminished formation and stability of tetraplex structures of d(AGG)-containing FMR1 d(CGG)2–50 tracts might restrict their expansion in normal individuals.
INTRODUCTION
Fragile X syndrome is the most common inherited cause of mental retardation, affecting one male in 1500–4000 and one female in 2500–8000 (1). The responsible mutation constitutes a substantial expansion of a d(CGG) trinucleotide repeat in the 5′ untranslated region of the first exon of the housekeeping gene FMR1 (2). The location of this mutation coincides with a Xq27.3 folate-sensitive fragile site that characterizes cells of afflicted males (3). Normal subjects carry 6–52 d(CGG) repeats that are arrayed in d(CGG)7–13 blocks separated by a single d(AGG) triplet with an average periodicity of 10 repeats (4–6). Phenotypically unaffected premutation carriers have 54–200 copies of d(CGG) trinucleotide that are either entirely uninterrupted by d(AGG) triplets or have long runs of monotonous d(CGG) repeats at their 3′ end (4–6). A full mutation, leading to clinical presentation of the syndrome, involves expansion of the monotonous d(CGG) repeats to >200–2000 copies (7–11). Subsequent to d(CGG) expansion in maternal oocytes or early in development (12), the repeat sequence (13–16) and an adjacent CpG island (16) become hypermethylated. Full expansion and hypermethylation of d(CGG) result in loss of FMR1 expression (16–18) and in delayed replication of FMR1 and of a chromosomal domain extending hundreds of kilobases 5′ and 3′ to the expanded trinucleotide tract (19,20).
Several lines of evidence indicate that formation of hairpin and/or tetraplex structures by the d(CGG)n tract are early events leading to the expansion of the repeat sequence and to its hypermethylation. When incubated in vitro under physiological-like conditions, d(CGG)n oligomers readily form hairpin (21–25) and tetraplex (26–28) structures. It has been suggested that in vivo realignment of d(CGG)n hairpin or higher order secondary structure during DNA replication might engender slippage of DNA polymerase, leading to deletion or expansion of the repeat sequence (29,30). Also, evidence was presented showing that d(CGG)n hairpins promote hypermethylation of this repeat sequence in fragile X syndrome cells (26). Stable secondary structures of the expanded d(CGG) repeat sequence might also block transcription and replication of FMR1 as demonstrated by the in vitro (31,32) and in vivo (30) arrest of the progression of DNA polymerases along d(CGG)n template stretches.
The presence of d(AGG) triplets interspersed within the d(CGG)n tract distinguishes stable d(CGG) repeats in FMR1 alleles of normal individuals from the expanded monotonous repeats of fragile X syndrome premutation or full mutation (4–6). Here we inquired whether interspersed d(AGG) trinucleotides affect the formation and stability of d(CGG)n tetraplex structures. We show that bimolecular tetraplex structures of d(AGG)-containing d(CGG)n tracts are formed at a reduced efficiency and exhibit diminished thermal stability. Further, interruption of d(CGG)n tracts by d(AGG) trinucleotides accelerates and increases the extent of unwinding of tetraplex d(CGG)n structures by human Werner syndrome DNA helicase.
MATERIALS AND METHODS
Isotopically 5′-labeled [γ-32P]ATP (~3000 Ci/mmol) was provided by Amersham and New England Nuclear. Bacteriophage T4 polynucleotide kinase was a product of Promega. Filter paper, DE-81 and 3, was purchased from Whatman. Sigma supplied acryl/bisacrylamide (19:1 or 30:1.2). Amresco provided N,N,N′,N′-tetramethylenediamine. Bromophenol blue and xylene cyanol FF were purchased from IBI.
DNA oligonucleotides
DNA oligomers, listed in Table 1, were supplied by Operon Technologies. The oligonucleotides were purified by electrophoresis through a denaturing gel of 12% polyacrylamide (acrylamide:bisacrylamide, 19:1), 8 M urea as described (33), except that salt and acrylamide residues were removed by twice precipitating the DNA with ethanol.
Table 1. DNA oligomers used in this study.
| Oligomer designation | Nucleotide sequence |
|---|---|
| d(CGG)7 | 5′-d(CGG)7-3′ |
| d(CGG)18 | 5′-d(CGG)18-3′ |
| d(A/CGG)18 1:5 | 5′-d[CGGAGG(CGG)4AGG(CGG)4AGG(CGG)4AGGCGG]-3′ |
| tail d(CGG)18 | 5′-d[(CGG)18CGTGGACTC]-3′ |
| tail d(A/CGG)18 1:5 | 5′-d(CGGAGG(CGG)4AGG(CGG)4AGG(CGG)4AGGCGGCGTGGACTC)-3′ |
| tail d(A/CGG)18 1:7 | 5′-d(CGGAGG(CGG)6AGG(CGG)6AGG(CGG)2CGTGGACTC)-3′ |
| tail d(A/CGG)18 1:10 | 5′-d(CGGAGG(CGG)9AGG(CGG)6CGTGGACTC)-3′ |
Interspersed d(AGG) trinucleotides are underlined. A 3′ non-d(CGG) tail is italicized.
Preparation of tetraplex DNA oligomers
Purified DNA oligomers that were 5′ end-labeled with 32P in a T4 polynucleotide kinase catalyzed reaction (34) were maintained in their single-stranded conformation by being stored as a solution of 0.25 µM DNA in water and boiled immediately prior to use. Bimolecular quadruplex structures of 32P-5′-labeled d(CGG)n oligomers with or without interspersed d(AGG) triplets were formed by incubating at 55°C for 20–24 h a single oligomer or an indicated mixture of two oligomers (~50 µM total DNA concentration) in 10 µl TE buffer (10 mM Tris–HCl buffer, pH 8.0, 1 mM EDTA) containing 500 mM NaCl. The reaction was terminated by rapidly cooling and diluting the mixtures 10-fold in 25 mM Tris–HCl buffer pH 8.0, 1 mM EDTA, 0.5 mM DTT and 20% glycerol. To resolve slowly migrating tetraplex DNA complexes from remaining single-stranded oligomers, DNA was electrophoresed under 80–100 V and at 4°C through a non-denaturing 8–12% polyacrylamide gel (acrylamide:bisacrylamide, 30:1.2) in 0.5× TBE buffer (1.25 mM EDTA in 45 mM Tris–borate buffer, pH 8.3) containing 50 mM NaCl. Concentrations of labeled quadruplex forms of the various oligomers were deduced from measurements of the specific radioactivity of source oligomers and of the radioactivity of the electrophoretically-separated tetraplex structures.
Werner DNA helicase activity assay
DNA helicase reaction mixtures contained in a final volume of 10 µl: 40 mM Tris–HCl buffer, pH 7.4, 4 mM MgCl2, 20 mM KCl, 5 mM DTT, 1 mM ATP, 10 µg BSA and 300 fmol 32P-5′-labeled tetraplex DNA. After adding specified amounts of recombinant Werner DNA helicase (WRN) purified to homogeneity (35), the reaction mixtures were incubated at 37°C for indicated time periods. Reactions were terminated by rapidly cooling the samples and adding 2 µl of a solution of 40% glycerol, 50 mM EDTA, 2% SDS, 3% bromophenol blue and 3% xylene cyanol. Displaced DNA single-strands were resolved from remaining tetraplex DNA by electrophoresis at 4°C and under 80–120 V through a non-denaturing 8–12% polyacrylamide gel in 0.5× TBE buffer, 10 mM NaCl. Gels that were dried on Whatman 3 filter paper were exposed to autoradiographic film to visualize resolved DNA bands. Amounts of tetraplex DNA and of displaced single-strands were quantified by exposing gels dried on Whatman DE81 filter paper to phosphorimager plate (Fuji).
RESULTS
Tracts of d(CGG)n with or without interspersed d(AGG) triplets form bimolecular tetraplex structures
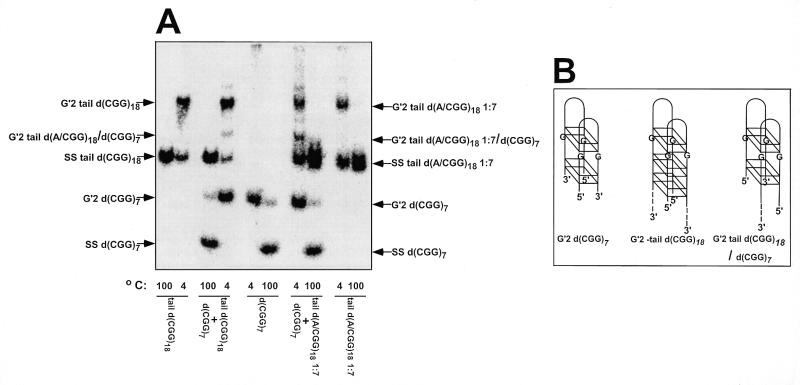
To study the effect of interspersed d(AGG) triplets on structural transformations of d(CGG)n runs, we first inquired whether d(AGG)-containing d(CGG)n oligonucleotides formed bimolecular tetraplex structures. 32P-5′-labeled d(CGG)7, tail d(CGG)18 and tail d(A/CGG)18 1:7 [the 1:7 ratio refers to the frequency of interspersed d(AGG) triplets within the d(CGG)n run; see Table 1 for the nucleotide sequence of all oligomers used] were incubated at 55°C for 20 h in the presence of 500 mM NaCl. As seen in Figure 1, all three oligomers formed electrophoretically-retarded DNA structures that could be heat denatured into their rapidly migrating single-strand constituents. Similar complexes were also formed by d(CGG)18 and d(A/CGG)18 1:5 under the described incubation conditions. Additionally, complexes of d(CGG)18 and d(A/CGG)18 1:5 or of their 3′-tailed counterparts did not form in the absence of Na+ ions (results not shown). The above results indicated that the presence of the non-d(CGG) unpaired 3′-tail did not affect complex formation. A non-d(CGG) 3′ single-strand tail was required for the unwinding of tetraplex DNA by WRN helicase (36). Since WRN was used at a later stage of this study (vide infra), tailed oligomers were used throughout this work. The observed alkali ion-dependence of complex formation suggested that complexes of d(CGG)18 and d(A/CGG)18 1:5 with or without an unpaired tail represented tetraplex structures of the oligomers (26–28). To determine the stoichiometry of the observed retarded DNA structures, 32P-5′-labeled d(CGG)7 was incubated at 55°C in the presence of Na+ with equimolar amounts of tail d(CGG)18 or tail d(A/CGG)18 1:7. As shown in Figure 1, in addition to retarded complexes characteristic of each oligomer, the DNA mixtures yielded third hybrid species. As previously described (28,36,37), generation of three retarded bands in mixtures of d(CGG)7 with tail d(CGG)18 or tail d(A/CGG)18 1:7 indicated that the slowly migrating complexes of tail d(CGG)18 with or without interspersed d(AAG) triplets represented G′2 bimolecular DNA structures. That these DNA complexes were tetrahelices was indicated by the obligatory requirement for alkali cations for their formation and stability (results not shown; 26,36). More specifically, we have shown that similar complexes of d(CGG)7 displayed circular dichroism (CD) spectrum characteristic of antiparallel tetraplex structures (37). By probing the guanine N7 position with dimethylsulfate we also showed that these structures were maintained by non-Watson–Crick guanine·guanine hydrogen bonds (26). The antiparallel tetraplex structure of oligo d(CGG)n was also demonstrated by NMR analysis (28). The indistinguishable electrophoretic behavior, stoichiometry and obligatory requirement for alkali ions of G′2 tetraplex tail d(CGG)18 and of the retarded complex of tail d(A/CGG) 1:7 suggested that the latter also represented a G′2 tetraplex structure.
Figure 1.
Stoichiometry of tetraplex structures of tail d(CGG)18 and tail d(A/CGG)18 1:7. Tetrahelical DNA structures were formed at 55°C for 20 h in mixtures that contained in a final volume of 10 µl of TE buffer, 500 mM NaCl and 55 µM of 32P-5′-labeled d(CGG)7, tail d(CGG)18 or tail d(A/CGG)18 or mixtures of 25 µM d(CGG)7 with 25 µM tail d(CGG)18 or tail d(A/CGG)18 1:7. The reaction mixtures were diluted 10-fold in 25 mM Tris–HCl buffer pH 8.0, 1 mM EDTA, 0.5 mM DTT, 20% glycerol and electrophoretically-retarded DNA complexes were resolved from rapidly migrating single-stranded oligomers by electrophoresis at 4°C through a non-denaturing 10% polyacrylamide gel containing 50 mM NaCl in 0.5× TBE buffer. Control DNA samples were denatured at 100°C for 10 min prior to electrophoresis to view the migration of single-stranded oligomers. (A) Electrophoretic separation of tetraplex and single-stranded DNA. Arrows mark the positions of bands of single-stranded and tetraplex DNA complexes. Under the reaction conditions employed, 57 and 28% of tail d(CGG)18 or tail d(A/CGG)18, respectively, were accumulated in tetraplex form. Note hybrid complex bands in samples containing 1:1 mixtures of 32P-5′-labeled d(CGG)7 with tail d(CGG)18 or tail d(A/CGG)18 1:7. (B) Scheme of tetraplex structures of d(CGG)7, tail d(CGG)18 and their hybrid. Based on the findings in (A) and on previously described results (36,37), the bimolecular tetrahelices are depicted as dimers of two hairpins bonded by guanine quartets. Shown are G′2 bimolecular tetraplexes without or with a non-d(CGG) single-stranded tail at their 3′ end (dashed lines). Two pairs of stacked guanine quartets are schematically drawn for G′2 d(CGG)7 tetraplex and three in G′2 tail d(CGG)18 to mark the difference in the number of d(CGG) repeats in the two oligomers. The two hairpins are aligned against each other in one of several possible orientations.
Interspersed d(AGG) triplets diminish the formation of G′2 tetraplex d(CGG)n structures and decrease their stability
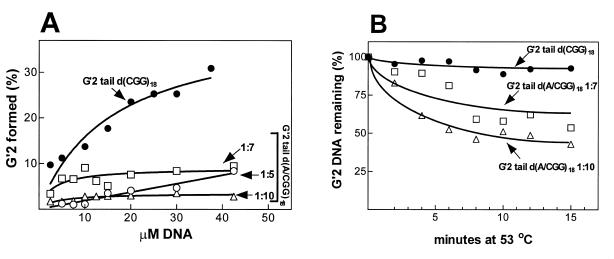
We first studied the effect of interspersed d(AGG) triplets on the extent of formation of G′2 tetraplex structures of d(CGG)n. Increasing concentrations of 5′-32P-labeled tail d(CGG)18 and of oligomers that contained interspersed triplets at d(AGG) to d(CGG) frequencies of 1:5, 1:7 or 1:10, were incubated at 55°C and in the presence of 500 mM NaCl. Formed tetraplex DNA complexes were resolved electrophoretically from remaining single-strands and their proportions were determined by phosphorimager analysis. As seen in Figure 2A, G′2 tail d(CGG)18 tetraplex was progressively accumulated in a second-order reaction as previously demonstrated for shorter d(CGG)n tracts (26,35,36). However, whereas up to ~30% of ~35 µM tail d(CGG)18 accumulated in a G′2 tetraplex form, only 2.5–8% of >40 mM of oligomers that contained interspersed d(AGG) triplets were found in G′2 tetraplex forms (Fig. 2A).
Figure 2.
Interspersed d(AGG) trinucleotides diminish the formation and stability of G′2 tetraplex forms of 3′-tail d(CGG)18 oligomers. (A) Extent of formation of tetraplex structures by monotonous and d(AGG)-containing d(CGG)n oligomers. Increasing concentrations of 5′-32P-labeled oligomers: tail d(CGG)1 8; tail d(A/CGG)18 1:5; tail d(A/CGG)18 1:7; and tail d(A/CGG)18 1:10 were incubated at 55°C for 20 h in the presence of 500 mM NaCl. The reaction mixtures were diluted and electrophoresed through a non-denaturing 8% polyacrylamide gel as detailed in Figure 1. Proportions of rapidly migrating single-stranded DNA and retarded tetraplex DNA were quantified by phosphorimaging. (B) Kinetics of heat denaturation of monotonous and d(AGG)-containing d(CGG)18 oligomers. G′2 tetraplex DNA structures of the indicated 5′-32P-labeled oligomers were formed as in (A), suspended in 50 mM NaCl and incubated at 53°C for the indicated periods of time. The rapidly cooled DNA samples were resolved at 4°C by non-denaturing electrophoresis through a 8% polyacrylamide gel and amounts of remaining G′2 tetraplex DNA structures were quantified by phosphorimaging analysis as in (A).
To compare the thermal stabilities of G′2 tetraplex structures of monotonous and d(AGG)-containing d(CGG)18 oligomers, they were heated at 53°C for increasing time periods and the amounts of denatured tetraplex structures were quantified by phosphorimager analysis. As seen in Figure 2B, whereas G′2 tail d(CGG)18 remained largely intact after up to 15 min at 53°C, ~35 and 50% of G′2 tail d(A/CGG)18 1:7 and 1:10, respectively, were denatured under the same conditions. The greater thermal lability of d(AGG)-containing d(CGG)18 tetraplex structures was confirmed by direct measurements of their melting temperatures. Whereas the Tm value of G′2 tail d(CGG)18 was 57°C, the Tm of G′2 tail d(A/CGG)18 1:7 was 48.5°C (Fig. 3). Similarly, G′2 tail d(A/CGG)18 1:10 was found to have a Tm value of 48°C (results not shown). Notably, formation or denaturation of the tetraplex DNA structures were critically affected by the ionic strength of the reaction mixture. Thus, whereas the presence of 500 mM NaCl allowed formation at 55°C of tetraplex structures of the oligomers (Figs 1 and 2), these tetrahelical structures were denatured in the presence of 50 mM NaCl at 48–57°C (Fig. 3).
Figure 3.
Interspersed d(AGG) trinucleotides lower the melting temperature of G′2 tetraplex d(CGG)18. Duplicate samples of bimolecular tetraplex structures of 5′-32P-labeled tail d(CGG)18 and tail d(A/CGG)18 1:7 were formed as described in Figure 1 and suspended at 30 µM each in TE buffer containing 50 mM NaCl. Tetraplexes of tail d(CGG)18 and tail d(C/AGG)18 1:7 constituted 33.5 and 14.5%, respectively, of the total DNA input. Aliquots of the DNA solutions were incubated for 10 min at the indicated increasing temperatures. Tetraplex DNA was resolved from denatured DNA single-strands by electrophoresis through a non-denaturing 8% polyacrylamide gel and proportions of the remaining tetraplex structures were quantified by phosphorimaging analysis. Values shown are averages of two independent determinations at each temperature.
Interspersed d(AGG) triplets enhance unwinding of G′2 tetraplex d(CGG)18 by WRN helicase
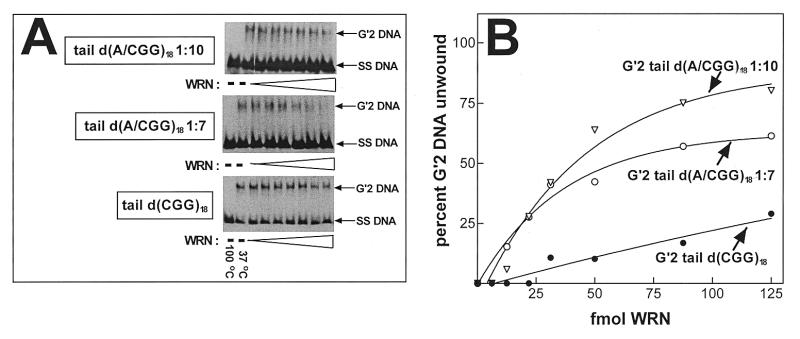
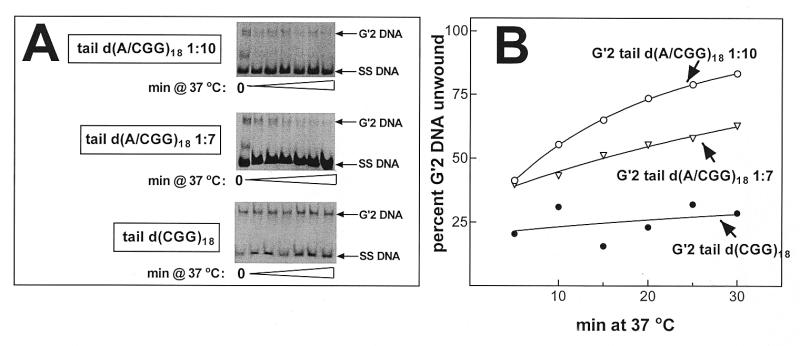
We have shown recently that human WRN DNA helicase unwinds bimolecular G′2 tetraplex forms of d(CGG)7 and that this activity required unpaired non-d(CGG) tails at the 3′ or 5′ end of the oligomer (36). We inquired, therefore, whether the decreased heat stability of d(AGG)-containing tetraplex structures of d(CGG)18 is also reflected in increased unwinding by WRN helicase. Similar amounts of 5′-32P-labeled G′2 tail d(CGG)18, G′2 tail d(A/CGG)18 1:7 or G′2 tail d(A/CGG)18 1:10 were incubated under helicase reaction conditions with increasing amounts of WRN. Destabilization of the tetraplex DNA structures was assessed by non-denaturing gel electrophoresis DNA strand displacement analysis (see Materials and Methods). As seen in Figure 4A, whereas G′2 tail d(CGG)18 was only minimally unwound by WRN under the helicase assay conditions, large proportions of d(A/CGG)18 1:7 or G′2 d(A/CGG)18 1:10 were destabilized by the enzyme. Quantitative results of a typical experiment, shown in Figure 4B, indicated that whereas G′2 tail d(CGG)18 was unwound to a maximum extent of 25%, up to ~60 and ~80% of G′2 tetraplex forms of tail d(A/CGG)18 1:7 or tail d(A/CGG)18 1:10, respectively, became destabilized. Kinetic analysis confirmed that the presence of interspersed d(AGG) triplets accelerated the unwinding of G′2 tetraplex d(CGG)18 by WRN. Reaction mixtures, each containing 300 fmol 5′-32P-labeled tail d(CGG)18, tail d(A/CGG)18 1:7 or tail d(A/CGG)18 1:10, were incubated for increasing time periods with 75 fmol WRN under helicase assay conditions. Electrophoretic resolution of intact and unwound tetraplex DNA shown in Figure 5A indicated that whereas G′2 tail d(CGG)18 was unwound to a limited extent over the duration of WRN action, substantial proportions of G′2 tail d(A/CGG)18 1:7 or G′2 tail d(A/CGG)18 1:10 were dissociated into their single-strand constituents. Quantitative analysis of the results of a typical experiment, presented in Figure 5B, indicated that the initial rates of WRN-catalyzed unwinding of G′2 tail d(A/CGG)18 1:7 or G′2 tail d(A/CGG)18 1:10 were higher than for tail d(CGG)18. Also, by 30 min, WRN destabilized ~60 and ~85% of G′2 tail d(A/CGG)18 1:7 and G′2 tail d(A/CGG)18 1:10, respectively, whereas only ~25% of G′2 tail d(CGG)18 became unwound.
Figure 4.
Interspersed d(AGG) triplets increase unwinding of G′2 tetraplex d(CGG)n by WRN helicase. The indicated G′2 tetraplex d(CGG)18 structures, with or without interspersed d(AGG) trinucleotides, were generated as in Figure 1 and 300 fmol aliquots of each G′2 DNA species were incubated at 37°C for 15 min under helicase assay conditions (see Materials and Methods). Electrophoretic resolution of tetraplex and single-stranded DNA and their quantification were conducted as in Figure 2. (A) Representative electrophoregrams of WRN-resolved G′2 tetraplex structures of tail d(CGG)18, tail d(A/CGG)18 1:7 and tail d(A/CGG)18 1:10. Migration of the single-stranded oligomers is viewed in control DNA samples that were heated at 100°C for 10 min to denature the tetraplex structures. (B) Titration of WRN activity of tetraplex DNA unwinding. Amounts of WRN-resolved G′2 DNA were quantified by phosphorimaging analysis. Zero amounts of unwound G′2 tetraplex DNA were determined in samples that were incubated at 37°C for 15 min without WRN.
Figure 5.
Interspersed d(AGG) triplets accelerate the unwinding of G′2 tetraplex d(CGG)n by WRN helicase. The indicated G′2 tetraplex d(CGG)18 structures were generated as in Figure 1 and 300 fmol aliquots of each G′2 DNA species were incubated with 75 fmol WRN at 37°C for increasing time periods under helicase assay conditions (see Materials and Methods). Electrophoretic resolution of tetraplex and single-stranded DNA and their quantification were conducted as in Figure 2. (A) Representative electrophoregrams of the time course of WRN-catalyzed unwinding of G′2 tetraplex structures of tail d(CGG)18, tail d(A/CGG)18 1:7 and tail d(A/CGG)18 1:10. (B) Kinetics of tetraplex DNA unwinding by WRN. Amounts of WRN-resolved G′2 DNA were quantified by phosphorimaging analysis.
DISCUSSION
Although the expansion of the FMR1 d(CGG)n tract from fragile X premutation to full mutation has been studied extensively, much remains unknown about the transition from normal FMR1 to premutated alleles. Formation of secondary structures of the trinucleotide repeat sequence and slippage of DNA polymerase, both proposed to be responsible for allelic variation in microsatellites, have also been implicated in the expansion of the FMR1 d(CGG)n tract. Alleles that are more liable to mutate than others are drawn from specific haplotype background (38) or from a few founder chromosomes (39,40). Studies indicated that these alleles are distinguished by loss of interspersed d(AGG) trinucleotides toward the 3′ end of the FMR1 d(CGG)n repeat (4–6,41–43). Hence, loss of intervening d(AGG) triplets and the resulting formation of long blocks of monotonous d(CGG) repeats was suggested to be a precursor to expansion of the repeat sequence.
The d(CGG) trinucleotide repeat sequence displays a propensity to fold into hairpin structures (21–25) which readily dimerize to generate G′2 bimolecular tetraplex formations (26–28,32,36,37). Either or both d(CGG)n hairpin and tetraplex structures may promote DNA polymerase slippage and expansion of the repeat sequence (31,32). The stability of FMR1 alleles that contain d(AGG) triplets interspersed within the d(CGG)6–52 tract suggested that the interrupting d(AGG) trinucleotide might affect the formation or stability of secondary structures of d(CGG)n. In this paper we provided evidence to show that interspersion of d(AGG) within d(CGG)18 run reduced the extent of formation of G′2 tetraplex structures of the repeat sequence (Fig. 2), and significantly diminished their thermal stability (Figs 3 and 4). Our comparison of tetraplex formation and stability included d(CGG)18 oligomers that contained d(AGG) triplets interspersed at frequencies that exceeded or equalled the average physiological prevalence of d(AGG) to d(CGG) of 1:10 (4–6). Interestingly, the yield (Fig. 2) and thermal stability (Fig. 3) were the lowest for the G′2 tetraplex structure of tail d(A/CGG) 1:10. This could be due to the different placement of the intervening d(AGG) triplets in tail d(A/CGG)18 1:7 and tail d(A/CGG)18 1:10 (Table 1). Essentially, however, interruption of the FMR1 d(CGG)n sequence by d(AGG) triplets at an average physiological frequency of d(AGG) to d(CGG) of 1:10 might produce tetraplex structures of shorter lifetimes. This, in turn, should limit polymerase slippage and minimize expansion of the trinucleotide repeat sequence.
The observed diminished formation and stability of tetrahelical hairpin dimers of d(A/CGG)18 is in line with the reported decreased stability of hairpin structures of d(AGG)n-containing d(CGG)n stretches (21). Interspersed d(AGG) triplets were also reported to reduce the formation and complexity of slipped strand structures of annealed genomic d(CGG)n stretches (44). Usdin (45) reported that similarly to d(CGG)20 oligomers, tracts of d(AGG)20 formed stable unimolecular tetraplex structures. However, d(AGG)20 failed to form hairpin structures of a stability comparable to that of hairpin d(CGG)20 (45). These results, as well as the reduced stability of hairpins of d(AGG)-containing d(CGG)n tracts (21), suggest that d(AGG) triplets interfere with the formation and stability of d(CGG)n hairpin structures. A reduced propensity of d(AGG)-containing d(CGG)n tracts to fold into hairpins and diminished stability of hairpins formed might be the cause for the reduced formation and stability of G′2 tetrahelical dimers of d(AGG)-containing d(CGG)18 hairpins that we report here.
Tetraplex d(CGG)n was shown to be readily unwound by human WRN DNA helicase in an ATP and Mg2+-requiring reaction (36) and to be destabilized in ATP and Mg2+-independent fashion by two hnRNP-related murine proteins, qTBP42 and uqTBP25 (37). It was suggested that protein-mediated destabilization of tetraplex d(CGG)n might guard against polymerase slippage and thus minimize expansion of the repeat sequence (37). Evidence that we presented in Figures 4 and 5 showed that tetraplex structures of d(AGG)-containing d(CGG)18 tracts were unwound by WRN at a higher rate and to a greater extent than a tetrahelical formation of monotonous d(CGG)18. Thus, in addition to their lower rate of formation and reduced inherent stability, tetraplex structures of d(CGG)n tracts interrupted by interspersed d(AGG) triplets may also be unwound more efficiently by cellular proteins. The observed accelerated in vitro unwinding of these tetraplex DNA forms by WRN might be a model for their unwinding in vivo by as yet unidentified protein(s).
In summary, the observed retarded formation of tetraplex structures by d(CGG)n tracts that contain interspersed d(AGG) triplets, their lower inherent stability and their enhanced unwinding by WRN helicase, suggest that intervening d(AGG) triplets might reduce the probability of d(CGG)n tetraplex formation in vivo. This curtailed capacity to form DNA secondary structures may constitute the basis for the observed genetic stability of the d(AGG)-containing d(CGG) repeat tracts in normal subjects.
Acknowledgments
ACKNOWLEDGEMENTS
This work was initiated in the laboratory of Dr Lawrence A. Loeb at the University of Washington, Seattle. M.F. wishes to thank L.A.L. for his long standing support and friendship. The work was supported by grants to M.F. from the Israel Science Foundation, the US–Israel Binational Fund, the Conquer Fragile X Inc. Foundation and by a Technion V.P.R. Fund, Charles Krown Research Fund and by NCI grant P01-CA-47852 to L.A.L.
REFERENCES
- 1.Murray J., Cuckle,H., Taylor,G. and Hewison,J. (1997) Health Technol. Assess., 1, 1–71. [PubMed] [Google Scholar]
- 2.Verkerk A.J.M.H., Pieretti,M., Sutcliffe,J.S., Fu,Y.-H., Kuhl,D.P.A., Pizzuti,A., Reiner,O., Richards,S., Victoria,M.F., Zhang,F., Eussen,B.E., van Ommen,G.-J.B., Blonden,L.A.J., Riggins,G.J., Chastain,J.L., Kunst,C.B., Galjaard,H., Caskey,C.T., Nelson,D.L., Oostra,B.A. and Warren,S.T. (1991) Cell, 65, 905–914. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland G.R. (1977) Science, 197, 265–266. [DOI] [PubMed] [Google Scholar]
- 4.Kunst C.B. and Warren,S.T. (1994) Cell, 77, 853–861. [DOI] [PubMed] [Google Scholar]
- 5.Snow K., Tester,D.J., Kruckeberg,K.E., Schaid,D.J. and Thibodeau,S.N. (1994) Hum. Mol. Genet., 3, 1543–1551. [DOI] [PubMed] [Google Scholar]
- 6.Hirst M.C., Grewal,P.K. and Davies,K.E. (1994) Hum. Mol. Genet., 3, 1553–1560. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y.-H., Kuhl,D.P.A., Pizzuti,A., Pierreti,M., Sutcliffe,J.S., Richards,S., Verkerk,A.J.M.H., Holden,J.J.A., Fenwick,R.G.,Jr, Warren,S.T., Oostra,B.A., Nelson,D.L. and Caskey,C.T. (1991) Cell, 67, 1047–1058. [DOI] [PubMed] [Google Scholar]
- 8.Kremer E.J., Pritchard,M., Lynch,M., Yu,S., Holman,K., Baker,E., Warren,S.T., Schlessinger,D., Sutherland,G.R. and Richards,R.I. (1991) Science, 252, 1711–1714. [DOI] [PubMed] [Google Scholar]
- 9.Oberlé I., Rousseau,F., Heitz,D., Kretz,C., Devys,D., Hanauer,A., Boue,J., Bertheas,M.F. and Mandel,J.L. (1991) Science, 252, 1097–1102. [DOI] [PubMed] [Google Scholar]
- 10.Nakahori Y., Knight,S.J.L., Holland,J., Schwartz,C., Roche,A., Tarleton,J., Wong,S., Flint,T.J., Froster-Iskenius,U., Bentley,D., Davies,K.E. and Hirst,M.C. (1991) Nucleic Acids Res., 19, 4355–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu S., Pritchard,M., Kremer,E., Lynch,M., Nancarrow,J., Baker,E., Holman,K., Mulley,J.C., Warren,S.T., Schlessinger,D., Sutherland,G.R. and Richards,R.I. (1991) Science, 252, 1179–1181. [DOI] [PubMed] [Google Scholar]
- 12.Malter H.E., Iber,J.C., de Graff,E., Tarleton,J.C., Leisti,J., Warren,S.T. and Oostra,B.A. (1997) Nature Genet., 15, 165–169. [DOI] [PubMed] [Google Scholar]
- 13.Bell M.V., Hirst,M.C., Nakahori,Y., MacKinnon,R.N., Roche,A., Flint,T.J., Jacobs,P.A., Tommerup,N., Tranebjaerg,L., Froster-Iskenius,U., Kerr,B., Turner,G., Lindenbaum,R.H., Winter,R., Pembrey,M., Thibodeau,S. and Davies,K.E. (1991) Cell, 64, 861–866. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich A., Kioschis,P., Monaco,A.P., Gross,B., Korn,B., Williams,S.V., Sheer,D., Heitz,D., Oberlé,I., Toniolo,D., Warren,S., Lehrach,H. and Poustka,A. (1991) Nucleic Acids Res., 19, 2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heitz D., Rousseau,R., Devys,D., Saccone,S., Abderrahim,H., LePaslier,D., Cohen,D., Vincent,A., Toniolo,D., Della,V.G., Johnson,S., Schlessinger,D., Oberlé,I. and Mandel,J.L. (1991) Science, 251, 1236–1239. [DOI] [PubMed] [Google Scholar]
- 16.Hansen R.S., Gartler,S.M., Scott,C.R., Chen,S.-H. and Laird,C.D. (1992) Hum. Mol. Genet., 1, 571–578. [DOI] [PubMed] [Google Scholar]
- 17.Pieretti M., Zhang,F., Fu,Y.-H., Warren,S.T., Oostra,B.A., Caskey,C.T. and Nelson,D.L. (1991) Cell, 66, 817–822. [DOI] [PubMed] [Google Scholar]
- 18.Sutcliffe J.S., Nelson,D.L., Zhang,F., Pieretti,M., Caskey,C.T., Saxe,D. and Warren,S.T. (1992) Hum. Mol. Genet., 1, 397–400. [DOI] [PubMed] [Google Scholar]
- 19.Hansen R.S., Canfield,T.K., Lamb,M.M., Gartler,S.M. and Laird,C.D. (1993) Cell, 73, 1403–1409. [DOI] [PubMed] [Google Scholar]
- 20.Hansen R.S., Canfield,T.K., Fjeld,A.D., Mumm,S., Laird,C.D. and Gartler,S.M. (1997) Proc. Natl Acad. Sci. USA, 94, 4587–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gacy A.M., Goellner,G., Juranic,N., Macura,S. and McMurray,C.T. (1995) Cell, 81, 533–540. [DOI] [PubMed] [Google Scholar]
- 22.Chen X., Santhana Mariappan,S.V., Catasti,P., Ratliff,R., Moyzis,R.K., Laayoun,A., Smith,S., Bradbury,E.M. and Gupta,G. (1995) Proc. Natl Acad. Sci. USA, 92, 5199–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitas M., Yu,A., Dill,J. and Haworth,I.S. (1995) Biochemistry, 34, 12803–12811. [DOI] [PubMed] [Google Scholar]
- 24.Nadel Y., Weisman-Shomer,P. and Fry,M. (1995) J. Biol. Chem., 270, 28970–28978. [DOI] [PubMed] [Google Scholar]
- 25.Santhana Mariappan S.V., Catasti,P., Chen,X., Ratliff,R., Moyzis,R.K., Bradbury,E.M. and Gupta,G. (1996) Nucleic Acids Res., 24, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fry M. and Loeb,L.A. (1994) Proc. Natl Acad. Sci. USA, 91, 4950–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F.-M. (1995) J. Biol. Chem., 270, 23090–23096. [DOI] [PubMed] [Google Scholar]
- 28.Kettani A., Kumar,R.A. and Patel,D.J. (1995) J. Mol. Biol., 254, 638–656. [DOI] [PubMed] [Google Scholar]
- 29.Samadashwily G.M., Raca,G. and Mirkin,S.M. (1997) Nature Genet., 17, 298–303. [DOI] [PubMed] [Google Scholar]
- 30.Wells R.D. (1996) J. Biol. Chem., 271, 2875–2878. [DOI] [PubMed] [Google Scholar]
- 31.Kang S., Ohshima,K., Shimizu,M., Amirhaeri,S. and Wells,R.D. (1995) J. Biol. Chem., 270, 27014–27021. [DOI] [PubMed] [Google Scholar]
- 32.Usdin K. and Woodford,K.J. (1995) Nucleic Acids Res., 23, 4202–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fry M., Perrino,F.W., Levy,A. and Loeb,L.A. (1988) Nucleic Acids Res., 16, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Shen J.-C., Gray,M.D., Kamath-Loeb,A.S., Oshima,J., Fry,M. and Loeb,L.A. (1998) J. Biol. Chem., 273, 34139–34144. [DOI] [PubMed] [Google Scholar]
- 36.Fry M. and Loeb,L.A. (1999) J. Biol. Chem., 274, 12797–12803. [DOI] [PubMed] [Google Scholar]
- 37.Weisman-Shomer P., Naot,Y. and Fry,M. (2000) J. Biol. Chem., 275, 2231–2238. [DOI] [PubMed] [Google Scholar]
- 38.Kolehmainen K. (1994) Am. J. Med. Genet., 51, 428–435. [DOI] [PubMed] [Google Scholar]
- 39.Richards R.I., Holman,K., Friend,K., Kremer,E., Hillen,D., Staples,A., Brown,W.T., Goonewardena,P., Tarleton,J., Schwartz,C. and Sutherland,G.R. (1992) Nature Genet., 1, 257–260. [DOI] [PubMed] [Google Scholar]
- 40.Oudet C., von Koskull,H., Nordstrom,A.M., Peippo,M. and Mandel,J.L. (1993) Eur. J. Hum. Genet., 1, 181–189. [DOI] [PubMed] [Google Scholar]
- 41.Eichler E.E., Holden,J.A., Popovich,B.W., Reiss,A.L., Snow,K., Thibodeau,S.N., Richards,C.S., Ward,P.A. and Nelson,D. (1994) Nature Genet., 8, 88–94. [DOI] [PubMed] [Google Scholar]
- 42.Zhong N., Weihong,Y., Dobkin,C. and Brown,W.T. (1995) Am. J. Hum. Genet., 57, 351–361. [PMC free article] [PubMed] [Google Scholar]
- 43.Hirst M.C., Arinami,T. and Laird,C.D. (1997) Hum. Genet., 101, 214–218. [DOI] [PubMed] [Google Scholar]
- 44.Pearson C.E., Eichler,E.E,. Lorenzetti,D., Kramer,S.F., Zoghbi,H.Y., Nelson,D.L. and Sinden,R.R. (1998) Biochemistry, 37, 2701–2708. [DOI] [PubMed] [Google Scholar]
- 45.Usdin K. (1998) Nucleic Acids Res., 26, 4078–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]