Abstract
Diffuse large B-cell lymphoma featuring overexpression of MYC and BCL2 (double expressor lymphoma, DEL) is associated with poor outcomes. Existing evidence suggesting improved outcomes for DEL with the use of more intensive regimens than R-CHOP is restricted to younger patients and based on limited evidence from low patient numbers. We retrospectively evaluated the impact of intensive frontline regimens versus R-CHOP in a multicenter analysis across 7 academic medical centers in the United States. We collected 90 cases of DEL, forty-six out of 90 patients (51%) received R-CHOP and 44/90 (49%) received an intensive regimen, which was predominantly DA-EPOCH-R. Treatment cohorts were evenly balanced for demographics and disease characteristics, though the intensive group had a higher lactate dehydrogenase (LDH, 326 vs 230 U/L p = 0.06) and presence of B-symptoms (50% vs 22%, p = 0.01) compared to the R-CHOP cohort. There was no difference in PFS (median 53 vs 38 months, p= 0.49) or OS (67 vs not reached months, p = 0.14) between the R-CHOP and intensive therapy cohorts, respectively. On multivariate analysis, intensive therapy was associated with a hazard ratio of 2.35 (95% CI 0.74 – 7.41), though this was not statistically significant. Additionally, a subgroup analysis of intermediate high-risk lymphoma defined by IPI ≥3 did not identify a difference in survival outcomes between regimens. We conclude that in our multi-center cohort there is no evidence supporting the use of intensive regimens over R-CHOP, suggesting that R-CHOP remains the standard of care for treating DEL.
Keywords: double expressor lymphoma, initial therapy, retrospective clinical analysis
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents a spectrum of aggressive B-cell lymphomas with varying presentations and rates of cure based on genetic, histologic, and molecular characteristics.(1–3) To improve risk stratification and ultimately outcomes, many prognostic biomarkers have been identified, including co-expression of MYC and BCL2 proteins.(4, 5) Cases featuring dual-expression of MYC and BCL2 proteins, termed “double expressor lymphoma” (DEL), have been associated with inferior outcomes when compared to DLBCL cases lacking these features. (4, 6) (7, 8) (5) Green et. al. also demonstrated that DEL may be quite common, encompassing upwards of approximately 30% of newly diagnosed DLBCL.(4)
The inferior outcomes observed for DEL have prompted many providers to use more intensive regimens including dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) over standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemoimmunotherapy. Data to support the use of intensive regimens is limited to retrospective reports featuring limited case numbers or observing a benefit only in a subset of DEL cases. For example, a small retrospective analysis of ten Japanese patients confirmed the poor prognosis associated with DEL but did not observe a benefit for dose intensified chemoimmunotherapy in DEL cases.(9) Alternatively, a study of Danish patients with DHL or DEL identified improved outcomes when treated with rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (R-CHOEP) compared to R-CHOP but was restricted to younger (age ≤60 years) patients, featured small numbers (43 patients) within the DEL subsets, and did not exclude cases of DHL.(8) A separate multi-center study of Italian patients with DEL suggested that use DA-EPOCH-R improved overall survival (OS) and progression-free survival (PFS) in younger patients aged 65 years or less based on a post-hoc sub-group analysis.(10)
Unfortunately, intensive regimens are more toxic. For example, a large randomized controlled trial of DA-EPOCH-R versus R-CHOP for newly diagnosed DLBCL concluded that DA-EPOCH-R did not improve PFS or OS and was associated with much higher rates of grade 3–4 hematologic and non-hematologic toxicities.(11) Sub-set analysis for DEL within this cohort did not identify a survival advantage for DA-EPOCH-R, although this was limited by a small number of cases (n=42) in the DEL subset.
In light of the limited available data regarding optimal therapy for DEL, we performed a multi-center analysis of DEL patients treated in American institutions according to intensive regimens (i.e. DA-EPOCH-R) versus R-CHOP in the frontline setting.
Methods:
Cases of newly diagnosed DEL treated from 2013-2016 were reviewed from 7 US academic cancer centers. The study was approved by each center’s Institutional Review Board prior to data collection and sharing. DEL cases were defined by a hematopathologist at each academic center, with histologic confirmation of DLBCL with concurrent MYC and BCL2 co-expression without corresponding double gene rearrangement (i.e. cases of DHL were excluded). Centralized pathology review was not performed. Cases of primary mediastinal B-cell lymphoma were excluded. Cases missing required information on gene rearrangement to determine double-hit status were excluded. Cases of histologic transformation from prior indolent lymphoma were included. BCL6, MYC, and BCL2 overexpression were defined by an immunohistochemical (IHC) cell stain score of ≥30, 40, and 50%, respectively as recommended per World Health Organization (WHO) criteria.(2, 4, 7, 12) Triple-expressor lymphoma was defined as a subset of DEL cases featuring overexpression of MYC, BCL2, and BCL6 according to above definitions. Exclusion criteria, in additional to exclusion of DHL or THL, included HIV-positive status, CNS involvement of lymphoma at diagnosis, use of a non-anthracycline based regimen as initial therapy, and diagnosis of post-transplant lymphoproliferative disorder.
Definitions and Endpoints
The intensive chemotherapy cohort included patients who received any of the following regimens: DA-EPOCH-R; rituximab, hyper-cyclophosphamide, vincristine, doxorubicin, dexamethasone (R-hyper-CVAD); rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosphamide, etoposide, cytarabine (R-CODOX-M/IVAC); and rituximab ifosphamide, carboplatin, etoposide (R-ICE). To be included in the R-CHOP cohort, patients needed to receive all five medications in the regimen, but subjects requiring dose modifications were not excluded. If a patient received up to 2 cycles of R-CHOP but then proceeded to more intensive therapy when IHC testing was confirmed, they were counted as receiving intensive therapy as long as the intensive therapy was used for the majority of induction cycles. Patients who received 1 cycle of intensive therapy and de-escalated to R-CHOP after no more than2 cycles were scored as R-CHOP.
The primary objective was to evaluate the impact of frontline intensive chemotherapy (defined below) versus standard R-CHOP on PFS and OS. Demographic, clinical, and disease characteristics were also measured to estimate their impact on disease outcomes in univariate and multivariate analysis. Responses were classified according to standard criteria per each institution, typically aligning with Lugano criteria.(13)
Statistical Analysis
Categorical variables were compared using chi-square tests or Fisher’s exact tests for cells containing less than 5 subjects. For numeric data, we assessed statistical significance using ANOVA or Kruskal-Wallis for non-normally distributed data. OS was defined by the date of initiation of treatment to date of last follow-up or death from any cause. PFS was defined as time from treatment initiation to disease progression or death. Subjects who were alive or in remission were right-censored at the last follow up date. Event-free survival at 24 months (EFS24) was defined as time from treatment initiation to disease relapse, progression, or death by 24 months using a definitions previously described.(14) We used Kaplan-Meier survival curves to graphically display survival probabilities and log-rank tests for survival assessment. Non-relapse mortality and relapse were analyzed using competing risk methods: Gray’s nonparametric estimator for the cumulative incidence function and the associated tests. Death without evidence of relapse was considered a competing event for the incidence of relapse. Similarly, disease relapse was considered a competing event for the incidence of non-relapse mortality. Univariate and multivariate cox-proportional hazards models were used to assess the effect of covariates. The primary exposure variable was treatment type (intensive induction vs R-CHOP). Other covariates of interest include age, race, prior indolent lymphoma, international prognostic index score (IPI) score, and bone marrow involvement. All statistical tests were two-sided, and 5% (p ≤ 0.5) was set as the level of significance. Statistical analysis was done in R 3.6.3, including the “survival” and “survminer” packages.
Results:
Baseline demographics and Disease Characteristics
We collected 112 cases of DEL from all institutions and after excluding those with missing data, typically for MYC, BCL2, or BCL6 rearrangement, 90 cases were included in the analysis. Patients were stratified based on the induction therapy, where 46/90 (51%) and 44/90 (49%) were in the R-CHOP and intensive therapy cohort, respectively. Seven cases were initially treated with R-CHOP for up to 2 cycles before receiving an intensive regimen, typically once double-expresser status was determined. Intensive therapy was predominantly DA-EPOCH-R (39/44, 89%) with other regimens being R-CODOX-M/IVAC (n=1), R-hyper-CVAD (n=1), R-ICE (n=1), and 2 cases defined as “other” which included one use of R-DHAP (rituximab, cytarabine, cisplatin, dexamethasone) and one use of ProMACE-CytaBOM (cyclophosphamide, doxorubicin, etoposide, cytarabine, bleomycin, vincristine, methotrexate, prednisone). Baseline demographics and disease characteristics are displayed in Table 1. The median follow-up of our cohort was 2.7 years. Patient characteristics were similar between the two cohorts, including age (66 for both treatment groups) and gender (50% vs 43% female for R-CHOP vs intensive therapy). The intensive therapy cohort featured more cases of worse ECOG performance status (ECOG PS 2-4, 22% vs 34%, R-CHOP vs intensive) though this was not statistically significant (p = 0.32). Central nervous system (CNS) prophylaxis rates differed between the R-CHOP and intensive groups, with 11/46 (24%) and 26/44 (59%) receiving some form of CNS prophylaxis (p = 0.002), respectively. Disease characteristics were generally similar between the treatment cohorts. The intensive therapy cohort had a higher median serum lactate dehydrogenase (LDH) (230 vs 326 U/L, p = 0.06) and more frequent presence of B-symptoms (50% vs 22%, p = 0.01). Other variables including differences in IPI scores and percentage of GCB classification (61% vs 43%, p = 0.16, Table 1) were not significantly different. Ann-Arbor or Lugano stage and single-gene rearrangements of MYC, BCL2, and BCL6 were similar between treatment cohorts (Table 1).
Table 1:
Baseline Demographics and Disease Characteristics by Treatment Group
| Total N=90 | R-CHOP N = 46 | Intensive Therapy N = 44 | P-value | |
|---|---|---|---|---|
| Median Followup (Year, IQR) | 2.7 (1.5-3.7) | 3.0 (1.98-3.71) | 2.4 (1.2-3.4) | 0.041 |
| Expressor Status (%) | ||||
| DEL | 30 (33) | 15 (33) | 15 (34) | 1 |
| TEL | 60 (67) | 31 (67) | 28 (66) | |
| Age (range) | 66 (57-73) | 66 (55-74) | 66 (57-70) | 0.6 |
| Gender (%) | ||||
| Male | 48 (53) | 23 (50) | 25 (57) | 0.66 |
| Female | 42 (47) | 23 (50) | 19 (43) | |
| Race/Ethnicity (%) | 0.46 | |||
| White (non-Hispanic) | 65 (72) | 32 (70) | 33 (75) | |
| Black | 3 (3) | 1 (2) | 2 (5) | |
| Asian | 4 (4) | 2 (4) | 2 (5) | |
| Hispanic | 4 (4) | 1 (2) | 3 (7) | |
| Other/Unknown | 14 (16) | 10 (22) | 4 (9) | |
| ECOG Performance Status (%) | ||||
| 0-1 | 61 (72) | 32 (78) | 29 (66) | 0.32 |
| 2-4 | 24 (28) | 9 (22) | 15 (34) | |
| Ann-Arbor Stage (%) | ||||
| I | 9 (10) | 6 (13) | 3 (7.0) | 0.81 |
| II | 14 (16) | 7 (15) | 7 (16) | |
| III | 12 (13) | 6 (13) | 6 (14) | |
| IV | 55 (61) | 27 (59) | 28 (64) | |
| Prior Indolent Lymphoma (%) | ||||
| No | 76 (84) | 41 (89) | 35 (80) | 0.25 |
| Yes | 14 (16) | 5 (11) | 9 (20) | |
| B symptoms (%) | ||||
| No | 54 (64) | 32 (78) | 22 (50) | 0.01 |
| Yes | 31 (36) | 9 (22) | 22 (50) | |
| IPI Score (%) | ||||
| 0-1 | 17 (21) | 9 (24) | 8 (19) | 0.56 |
| 2 | 19 (24) | 11 (29) | 8 (19) | |
| 3 | 23 (28) | 9 (24) | 14 (33) | |
| 4-5 | 22 (27) | 9 (24) | 13 (30) | |
| LDH (U/L), Median (IQR) | 299 (197-479) | 230 (185-413) | 326 (216-598) | 0.06 |
| Cell of origin (%) | ||||
| GCB | 42 (48) | 26 (57) | 16 (39) | 0.16 |
| Non-GCB | 45 (52) | 20 (43) | 25 (61) | |
| Myc Rearrangement (%) | ||||
| No | 79 (88) | 39 (85) | 40 (91) | 0.52 |
| Yes | 11 (12) | 7 (15) | 4 (9) | |
| BCL6 Rearrangement (%) | ||||
| No | 78 (88) | 41 (91) | 37 (84) | 0.50 |
| Yes | 11 (12) | 4 (9) | 7 (16) | |
| BCL2 Rearrangement (%) | ||||
| No | 76 (85) | 38 (84) | 38 (86) | 1.00 |
| Yes | 13 (15) | 7 (16) | 6 (14) | |
| CNS Prophylaxis Used (%) | ||||
| No | 52 (58) | 34 (76) | 18 (41) | <0.01 |
| Yes | 37 (42) | 11 (24) | 26 (59) |
DEL: double-expressor lymphoma, TEL: triple-expressor lymphoma, defined as overexpression of MYC, BCL2, and BCL6. Intensive chemotherapy = dose-adjusted EPOCH-R, hyper-CVAD…) Statistically significant p-values are bolded. IQR: interquartile range, SD: standard deviation, ECOG: Eastern Cooperative Oncology Group, IPI: international prognostic index, LDH: lactate dehydrogenase, GCB: germinal center B-cell, CNS: central nervous system, R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
Disease Response
For the entire DEL cohort, complete responses (CR) were achieved in 72% (n=65), whereas 10% achieved partial response (PR, n=9) and approximately 17% demonstrated stable disease (n=1) or progressive disease (n=14). One patient receiving R-CHOP was hospitalized shortly after cycle 1 with an exacerbation of chronic obstructive pulmonary disease and died, his response was not evaluable but was included in PFS/OS analysis. Response to therapy differed between treatment groups (Figure 1) although this was overall not statistically significant (p = 0.07). A CR was achieved in 74% versus 71% (p = 0.76) of subjects for R-CHOP and intensive therapy, respectively. Additionally, a higher but not statistically significant percentage of subjects had a partial response (15% vs 5%, p = 0.16) in the R-CHOP group versus intensive therapy. A larger percentage of patients in the intensive cohort experienced no response or disease progression (9% vs 25%, p = 0.05) compared to the R-CHOP cohort. We attempted to report on subsequent lines of therapy including the use of autologous transplant, but were limited due to missing data, which precluded meaningful analysis.
Figure 1: Response Rates According to Induction Therapy.
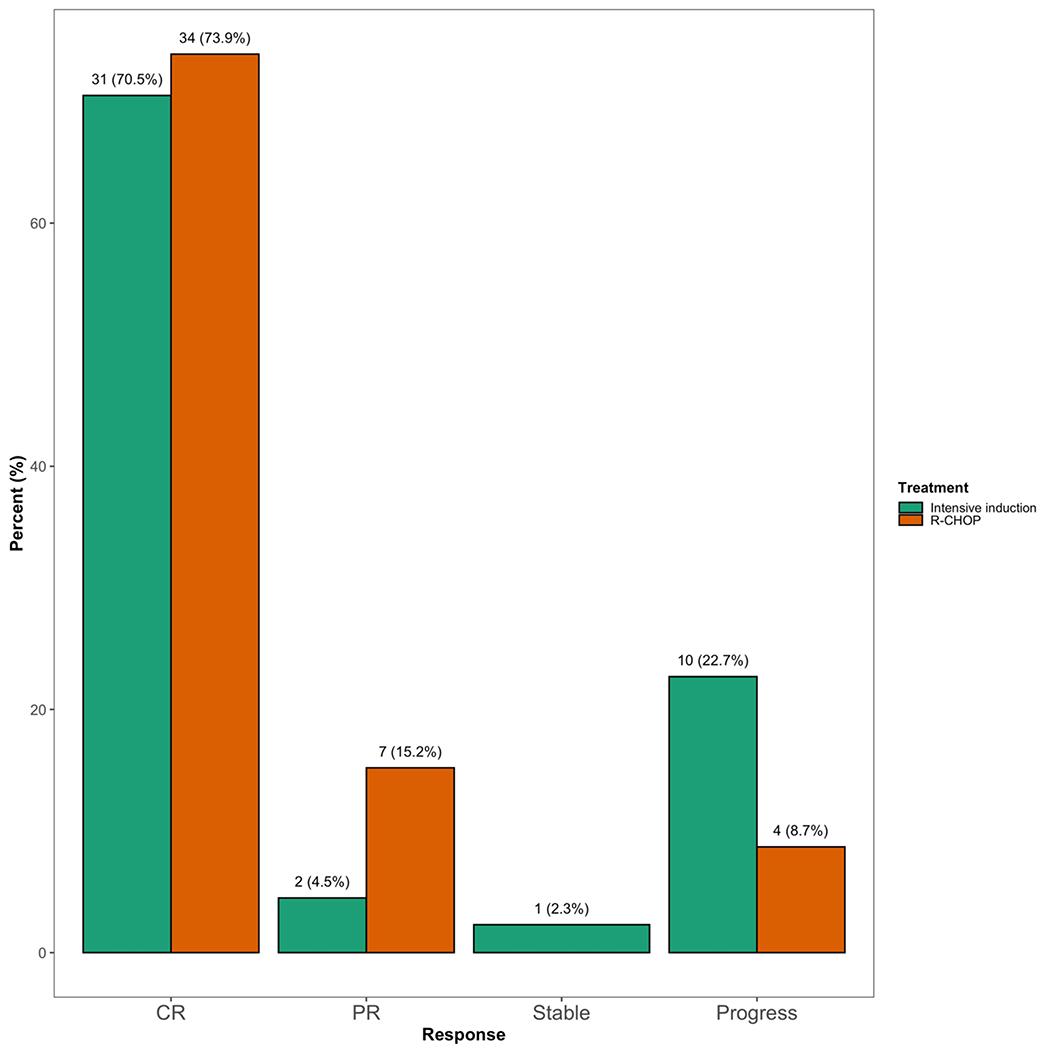
Percentages based on number evaluable per group (R-CHOP – red color, intensive- green color). CR: complete response, PR: partial response, Stable: stable disease, Progress: progressive disease.
Relapse and Survival Outcomes
The median follow-up of our cohort was 2.7 years. Survival outcomes are depicted in Figure 2. By treatment cohort, 17/46 (37%) in the R-CHOP group and 18/44 (41%) in the intensive-therapy cohort experienced disease relapse or progression. There were 4/35 (11%) relapses occurring 2 years or later after completing initial therapy, 1/17 relapses (6%) in the R-CHOP cohort and 3/18 (17%) in the intensive therapy cohort. Regarding CNS relapse, 8 subjects (10%) out of 82 evaluable (with data on CNS recurrence) experienced a CNS relapse, 5/8 (63%) of which had received CNS prophylaxis. By treatment cohort, 5/41 (12%) and 3/41 (7%) patients suffered a CNS relapse in the R-CHOP and intensive cohorts, respectively. Using death as a competing event, the cumulative incidence of relapse was compared between the treatment assignment (R-CHOP vs intensive) and was not statistically significant (Figure 3, p = 0.58). There were a total of 28 deaths out of 90 subjects (31%), where 12/46 (26%) and 16/44 (36%) subjects died in the R-CHOP and intensive therapy cohorts, respectively. Median PFS (Figure 2A) was 53 versus 38 months (R-CHOP vs intensive, p = 0.49). Median OS was 67 months versus not reached (R-CHOP vs intensive, p = 0.14). When separately comparing DA-EPOCH-R to R-CHOP (Figure 2A–2B), we observed no significant difference in PFS (53 vs 38 months, R-CHOP vs DA-EPOCH-R, p = 0.64) or OS (67 months vs not reached, R-CHOP vs DA-EPOCH-R, p = 0.27). There was also no difference in the cumulative incidence of non-relapse mortality according to treatment assignment (R-CHOP vs intensive, Figure 3, p = 0.85). EFS24 was similar between R-CHOP and intensive cohorts (EFS24 0.59 vs 0.56, R-CHOP vs intensive, p = 0.7). Additionally, the impact of expression of MYC, BCL2, and BCL6 (ie, triple-expression) on outcomes compared to double-expression of MYC and BCL2 was explored, where no statistically significant differences in PFS or OS were noted (Figures 2C and 2D). As we observed a larger fraction of high-intermediate and high-risk lymphoma (defined by IPI >3) in the intensive-therapy cohort as well as factors associated with higher IPI (i.e. larger percentage of ECOG PS 2-4 and advanced stage, higher LDH) , we performed a subgroup analysis of only subjects with intermediate-high and high-risk IPI ≥3, which also demonstrated similar outcomes between treatment assignment (Figure 2E and 2F).
Figure 2: Survival Analysis by Treatment and Lymphoma Sub-group.
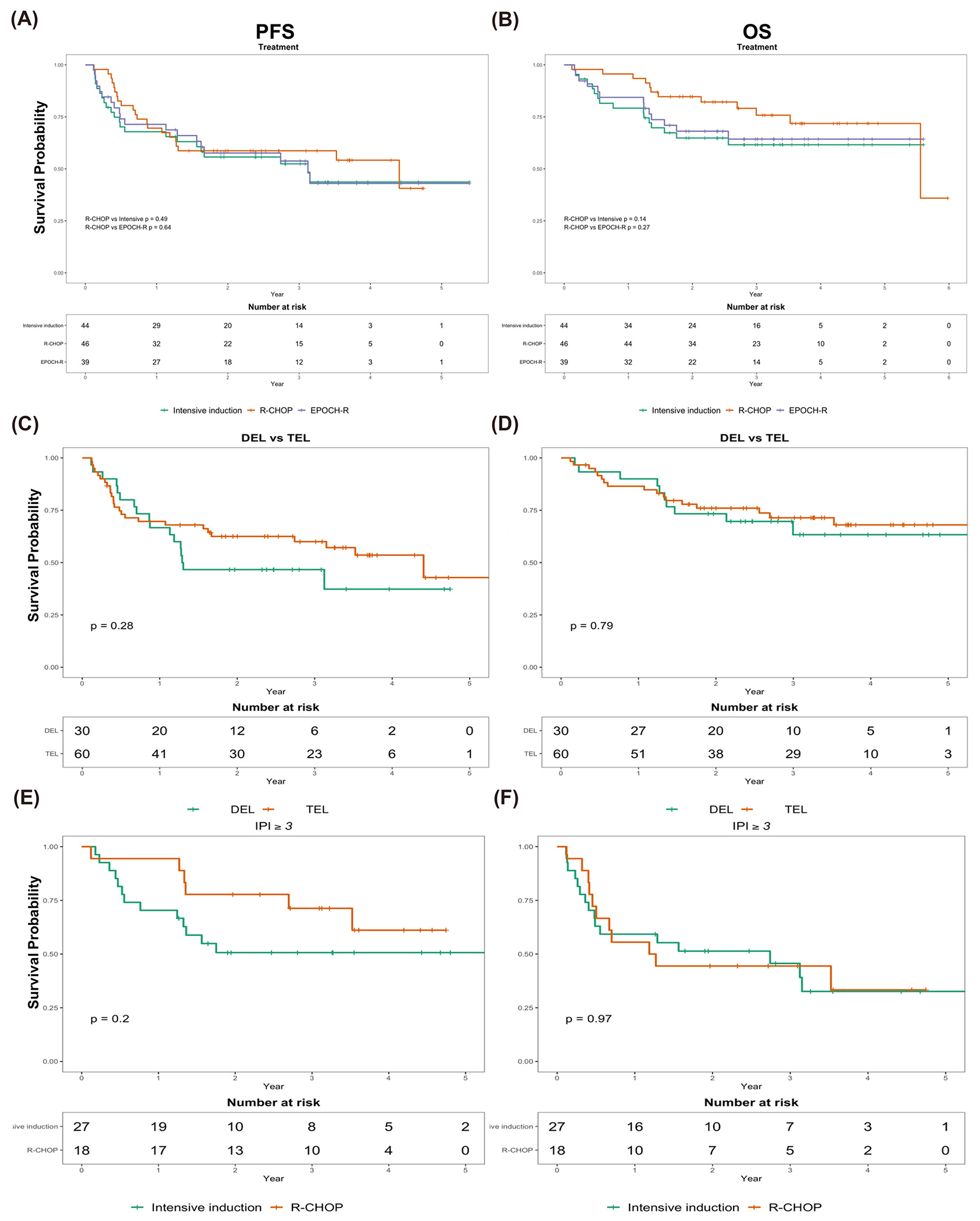
A: PFS by treatment assignment (green = intensive, red = R-CHOP, blue = DA-EPOCH-R). B) OS by treatment. C) PFS by expression status (red = TEL, green = DEL). D) OS by expression status (red= TEL, green = DEL). E: PFS for intermediate-high and high risk subgroup defined by IPI ≥3 F: OS for intermediate-high and high-risk subgroup defined by IPI ≥ 3. PFS: progression-free survival, OS: overall survival, IPI: international prognostic index, DEL: double expressor lymphoma, TEL: triple expressor lymphoma, defined as overexpression of MYC, BCL2, and BCL6.
Figure 3: Cumulative Incidence of Relapse and Non-Relapse Mortality by Treatment.
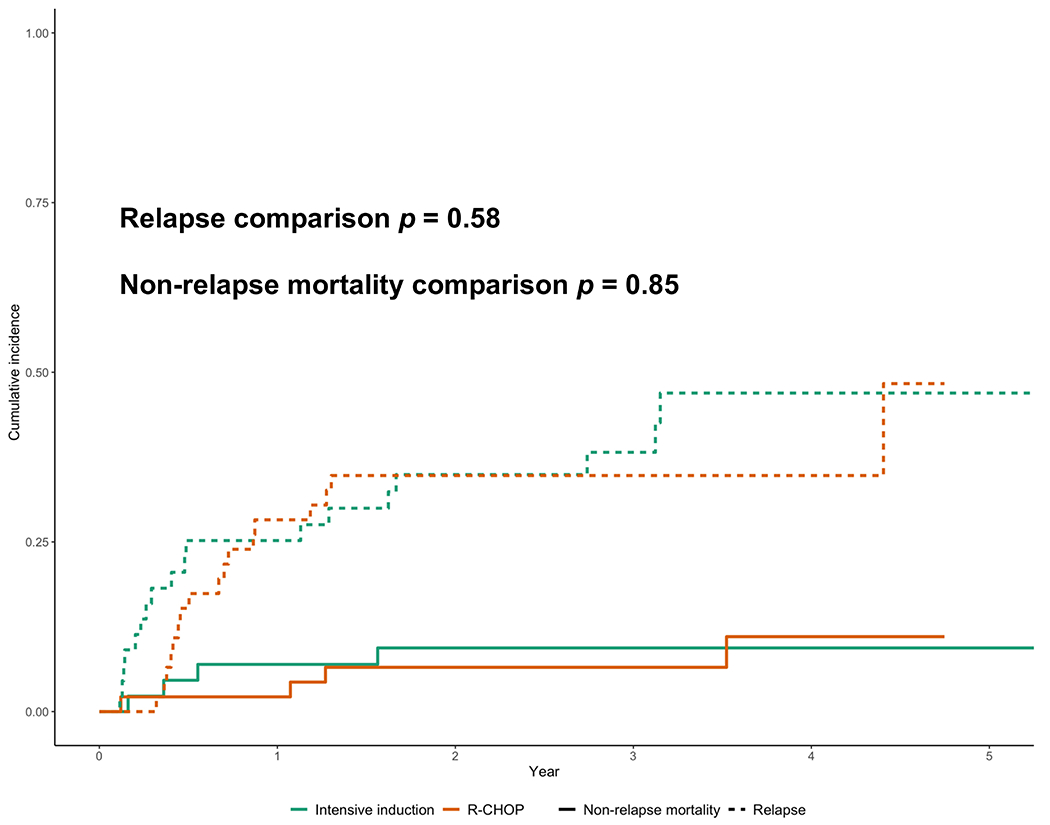
Green lines: intensive therapy, red lines: R-CHOP. Dashed lines depict incidence of relapse and solid lines depict non-relapse mortality.
Finally, to assess the impact of frontline treatment as well as other clinical covariates on OS, we performed a multivariate analysis (Table 2). All covariates described in Table 1 were tested for their impact on survival outcomes in univariate analysis; those covariates that met or approximated statistical significance are depicted in Table 2. We also included age, given prior reports suggesting a benefit for intensive therapy in those aged ≤65 years.(8, 10) Race (black) (HR 5.1, p = 0.009, 95% confidence interval (CI) 1.51-16.90), prior indolent lymphoma (HR 3.5, p = 0.003, 95% CI 1.54-8.09), and IPI score ≥ 3 (HR 4.4, p = 0.007, 95% CI 1.50-13.0) were significantly associated with reduced survival on univariate analysis. Bone marrow involvement was associated with a non-statistically significant adverse hazard ratio on univariate analysis (HR 2.1, p = 0.1, 95% CI 0.87-5.23). In univariate analysis, therapy assignment (intensive vs R-CHOP) was associated with reduced overall survival but was not statistically significant (HR 1.8, p = 0.15, 95% CI 0.82-3.70). These covariates were subsequently tested in the multivariate model. Only race (black) (HR 7.1, p = 0.02, 95% CI 1.33-37.30) and prior indolent lymphoma (HR 3.3, p = 0.035, 95% CI 1.09-10.10) remained significantly associated with adverse outcomes on multivariate analysis. Importantly, our cohort featured predominantly white subjects, with few cases of black or non-white race and thus may have introduced bias. Multivariate analysis demonstrated that use of intensive therapy compared to R-CHOP was associated with a HR of 2.4 (p = 0.15, 95% CI 0.74-7.41) for OS and was not statistically significant.
Table 2:
Univariate and Multivariate Factor Analysis for OS
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.01 | 0.98 – 1.04 | 0.48 | |||
| Chemotherapy | ||||||
| R-CHOP | Ref | |||||
| Intensive Therapy | 1.75 | 0.82 – 3.70 | 0.15 | 2.35 | 0.74 – 7.41 | 0.15 |
| Race | ||||||
| White | Ref | |||||
| Black | 5.05 | 1.51 – 16.9 | 0.009 | 7.05 | 1.33 – 37.3 | 0.022 |
| Prior Indolent Lymphoma | ||||||
| Absent | Ref | |||||
| Present | 3.53 | 1.54 – 8.09 | 0.003 | 3.32 | 1.09– 10.1 | 0.035 |
| IPI | ||||||
| 0-2 | Ref | |||||
| 3-5 | 4.41 | 1.50 – 13.0 | 0.007 | 1.62 | 0.45 – 5.87 | 0.5 |
| Bone Marrow Involvement | ||||||
| Absent | Ref | |||||
| Present | 2.13 | 0.87 – 5.23 | 0.10 | 1.64 | 0.56 –4.75 | 0.4 |
Bold items are statistically significant with p ≤ 0.05. OS: overall survival, HR: hazard ratio, CI: confidence interval, R-CHOP: rituximab, cyclophosphamide, hydroxydaunorubicin, Oncovin, prednisone, IPI: International Prognostic Index
Discussion
We conducted a multi-center retrospective review of over 90 subjects with DEL in 7 American academic centers. Approximately 50% of patients with DEL in our cohort were treated with a frontline intensive regimen instead of R-CHOP, suggesting that employment of intensive regimens is more widespread than predicted. Intensive therapy failed to improve outcomes even after adjusting for some of the known adverse prognostic factors for DLBCL, including IPI score. Age has been identified as an important factor, where those 65 years and younger benefit from intensive therapy, yet was not found to have an impact on survival outcomes in this cohort (HR 1, p = 0.23).(8, 10) Our multivariate regression model also identified a striking adverse hazard ratio for black race (HR 7.05, p = 0.02), though we suggest caution in interpreting this finding given the very low number of black patients within our cohort. Nevertheless, considering the increasingly recognized health disparities that black patients may face when receiving oncologic care, these findings are certainly worthy of more detailed investigation to determine causes including treatment patterns, disease biology, socioeconomic factors, or other causes.
We observed a high-rate of CNS relapse in our DEL cohort (8.8%). Furthermore, we saw a lower rate of CNS prophylaxis for the R-CHOP cohort (24% vs 59%, p = 0.002) and a relatively higher rate of CNS relapse when compared to intensive regimens (12% vs 7%). These findings are supportive of previously reported results demonstrating the higher risk of CNS relapse in DEL and may provide further evidence for clinicians to consider CNS prophylaxis for DEL patients, despite not requiring a more intensive systemic regimen than R-CHOP.(15)
Our study has several important strengths and weaknesses. To our knowledge, our cohort of 90 subjects with DEL is among the largest studied to address this question. The multi-center design improves our ability to generalize these findings to other academic centers and limits the impact of unevaluated center-specific practices that may confound outcomes. We also provide the first known data for a North American patient cohort, where treatment approaches may differ in subtle fashions between those treated in European centers. Finally, our median follow-up of 2.7 years is another strength of our analysis, especially as relapses after 2 years were rare (4/35, 11%, Figure 2) and EFS24 is an important surrogate for overall survival for DLBCL.(14)
The main weakness of our study remains its retrospective methodology, which precludes our ability to definitively determine if DA-EPOCH-R or similarly intensive approaches improve survival compared to R-CHOP. Furthermore, as the intensive therapy cohort tended to feature more adverse prognostic traits than the R-CHOP cohort, it may be that we failed to identify significant differences between cohorts because the intensive therapy cohort was confounded by the presence of higher-risk disease. Response rates were classified per each academic center, however, we acknowledge that specific data on how response (i.e. by PET/CT or CT) was assessed was not captured and therefore may add a degree of uncertainty to response data. We also do not have data on how the EPOCH-R regimen was dose adjusted, which may effect the regimen’s efficacy. We acknowledge that these are limitations of our report, but important observations lend support to our conclusions. First, we performed a sub-group analysis featuring only intermediate-high risk and high-risk disease using cases of only IPI ≥ 3 and observed no significant differences between treatment groups (figure 2E and 2F). Additionally, multivariate regression adjusting for several factors pertaining to adverse prognosis (IPI, prior indolent lymphoma, high LDH, etc) still did not observe a benefit for intensive therapies and displayed a larger hazard ratio for intensive therapy compared to univariate analysis, though this was not statistically significant.
Another limitation of our study includes the lack of centralized pathology review. All of our cases were obtained from large tertiary-care centers, and individually reviewed by academic hematopathologists at those institutions. Each center followed strict inclusion criteria for overexpression of MYC and BCL2 to be included as a DEL case according to WHO definitions and prior reports.(2, 4, 7, 12)
Finally, given the potential risk for toxicity associated with the use of intensive regimens, we would have ideally captured the toxicity of both regimens as another comparative measure. Although we did not capture and therefore cannot address the specific toxicities encountered by our cohort, evidence consistently suggests that regimens like DA-EPOCH-R are clearly more toxic than R-CHOP.(11, 16)
Conclusion
Overall, given the potential toxicity of intensive regimens over R-CHOP, we feel the burden of proof must lie with the more toxic therapy before recommending such an approach. Although it is understood that patients with DEL may have worse outcomes compared to those without, our findings do not support that more intensive regimens can actually improve on this adverse prognosis. We conclude that in our cohort of DEL patients, the use of intensive regimens did not improve survival. Our findings suggest that R-CHOP remain the standard approach to treating patients with DEL and highlight the need for randomized trials of novel therapeutics to improve outcomes in this high-risk population.
Acknowledgements:
This work was supported by an NIH NHLBI T32 training grant (5T32HL007899‐20) to Christopher R. D’Angelo.
Conflict of Interest Statement:
The authors have no relevant conflicts of interest to disclose. Outside the submitted work, Dr. Epperla serves on the speaker’s bureau for Verastem and Beigene, is on the advisory board for Karyopharm, and receives an honorarium from Genzyme. Dr. Karmali serves on the speaker’s bureau for Kite/Gilead, AstraZeneca, Beigene, is on the advisory board for Kite/Gilead, BMS/Celgene/Juno, Karyopharm, Janssen, Morphosys, and receives research support from Kite/Gilead, BMS/Celgene/Juno, and Takeda.
Footnotes
Ethical Statement: This research was conducted in accordance with all university ethical guidelines. IRB approval was obtained from each participating institution prior to conducting research investigations.
References
- 1.Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31(2):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378(15):1396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–7. [DOI] [PubMed] [Google Scholar]
- 5.Staiger AM, Ziepert M, Horn H, Scott DW, Barth TFE, Bernd HW, et al. Clinical Impact of the Cell-of-Origin Classification and the MYC/ BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35(22):2515–26. [DOI] [PubMed] [Google Scholar]
- 6.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–31; quiz 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen MO, Gang AO, Brown P, Pedersen M, Knudsen H, Nielsen SL, et al. Real world data on young patients with high-risk diffuse large B-cell lymphoma treated with R-CHOP or R-CHOEP - MYC, BCL2 and BCL6 as prognostic biomarkers. PLoS One. 2017;12(10):e0186983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi H, Miura K, Nakagawa M, Sugitani M, Amano Y, Kurita D, et al. Negative impact of concurrent overexpression of MYC and BCL2 in patients with advanced diffuse large B-cell lymphoma treated with dose-intensified immunochemotherapy. Leuk Lymphoma. 2016;57(12):2784–90. [DOI] [PubMed] [Google Scholar]
- 10.Dodero A, Guidetti A, Tucci A, Barretta F, Novo M, Devizzi L, et al. Dose-adjusted EPOCH plus rituximab improves the clinical outcome of young patients affected by double expressor diffuse large B-cell lymphoma. Leukemia. 2019;33(4):1047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett NL, Wilson WH, Jung SH, Hsi ED, Maurer MJ, Pederson LD, et al. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage KJ, Slack GW, Mottok A, Sehn LH, Villa D, Kansara R, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182–8. [DOI] [PubMed] [Google Scholar]
- 16.Shah NN, Szabo A, Huntington SF, Epperla N, Reddy N, Ganguly S, et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol. 2018;180(4):534–44. [DOI] [PubMed] [Google Scholar]


