Abstract
This review aims to collate information about the analytical methodologies, bioanalytical methodologies, pharmaceutical formulations, solid-state studies, and the current and future market scenario for a relatively new class of drugs, Roxadustat. Roxadustat is a hypoxia-inducible factor propyl hydroxylase inhibitor that significantly increases blood hemoglobin via the action of transcriptional activator HIF. As the molecule has a promising role in stimulating erythropoiesis, it is considered an ideal therapeutic agent for patients with anemia. In the current review, an attempt has been made to compile the pharmacological, pharmacokinetic, and pharmacodynamic characteristics of Roxadustat and systematically present product development data. This drug has several polymorphs of cocrystal, co-former, and salt, which have been explained in detail in the current work. The comprehensive review summarizes all the chromatographic methods and is presented in table form. This review has extensively covered Liquid chromatography-tandem mass spectrometry methods used to analyze Roxadustat in the biological matrix. The literature needs more data on forced degradation study, impurity profiling, gas chromatography, analytical methods for assay, dissolution, and different formulation aspects of Roxadustat.
Keywords: Roxadustat, Solid-state study, Polymorphism, Co-former, Cocrystal, Formulation, High-performance liquid chromatography, Bioanalysis, Mass spectrometry, LC-MS
Graphical abstract
Abbreviations
- LC-MS
Liquid chromatography-mass spectrometry
- API
Active pharmaceutical ingredient
- CKD
Chronic kidney disease
- EPO
Erythropoietin
- NDD
Non-dialysis dependent
- DD
Dialysis-dependent
- HIF-PHD
Hypoxia-Inducible-Factor-Prolyl-Hydroxylase
- CDSCO
Central Drugs Standard Control Organisation
- DMFs
Drug master file
- DMSO
Dimethyl sulfoxide
- DSC
Differential Scanning Calorimetry
- WADA
World Anti-Doping Agency
- QuEChERS
Quick Easy Cheap Effective Rugged Safe
1. Introduction
Anemia is a clinical condition where the patient lacks adequate healthy red blood cells to carry acceptable oxygen levels to the body tissues [1]. Anemia can be a result of chronic kidney disease (CKD) due to impairment of multiple biological processes like irregular iron metabolism, inflammation, dysfunction of cascade pathway of synthesis of red blood cells, oxidative stress, erythropoietin (EPO) deficiency, blood loss, infection, and inadequate supply of nutrients [2]. Comparatively, EPO deficiency is the primary cause of anemia. EPO synthesis occurs in peritubular interstitial fibroblast cells located in the kidney. In CKD, renal EPO-producing cells get dysfunctional and transdifferentiate into myofibroblasts which leads to a decrease in the synthesis of EPO, thereby resulting in the progressive development of anemia. Roxadustat is a hypoxia-inducible prolyl hydroxylase inhibitor with a proven promising erythropoiesis-stimulating ability. It causes an increase in blood oxygen-carrying capacity in patients with chronic kidney disease with anemia [[3], [4], [5]]. Generally, the drug is administered orally (50 mg/dose) thrice a week to anemic patients. The dose can vary according to the patient’s condition, but it should not exceed 3.0 mg/kg. However, Roxadustat is currently used under the brand Evrezo in Chile, South Korea, and parts of China and Japan for treating anemia in CKD non-dialysis-dependent (NDD) and dialysis-dependent (DD) adult patients. The European Medicines Agency has accepted the application for the sale of Evrezo in European countries. Furthermore, AstraZeneca, FibroGen, and other pharmaceutical companies are aggressively trying to commercialize Roxadustat in Europe, Turkey, Russia, the Commonwealth of the Independent States, the Middle East, and South Africa [6].
There exist reports stating the misuse of Roxadustat by sports personnel owning to its erythropoietic properties apart from its use for the treatment of anemia [7]. Subsequently, a suitable quantitative and qualitative analysis method is required to identify Roxadustat and its metabolites in biological fluids. Furthermore, developing an analytical methodology for identifying impurities is necessary for every new drug candidate. Inclusive impurity profiling and regulatory requirements are essential for understanding the safety and efficacy of pharmaceutical products. Similarly, forced degradation studies are necessary to access information on drug stability under various degradation conditions. The current review presented a comprehensive overview of the status of analytical methods, pharmaceutical formulations, solid-state studies, and market scenarios related to Roxadustat. In addition, the review encompassed all the physicochemical properties of Roxadustat, which would play a pivotal role in formulation establishment.
The prevailing article further emphasized that many polymorphs, cocrystals, salts, and co-formers are listed along with the analytical results. In addition to their clinical applications, pharmacokinetic and pharmacodynamic parameters have also been considered. An overview of all the analytical methodologies, such as Chromatography and LC-MS, has been provided. To the best of our knowledge, only a few articles were published on bioanalytical methods for analyzing Roxadustat from biological matrices such as urine and plasma using chromatography. There are very few articles for the analysis of API and formulation related to Roxadustat. Further, various in vitro studies were reported in the literature involving liver microsomes, human hepatocytes, equine liver microsomes, Cunninghamella elegans, and S9. To give a clear perspective on the market and regulatory status of Roxadustat, we have covered all the current patent-related information.
2. Global regulatory and intellectual property status
Roxadustat, a Hypoxia-Inducible-Factor-Prolyl-Hydroxylase (HIF-PHD) enzyme inhibitor, was developed by FibroGen in collaboration with Astellas and AstraZeneca. FibroGen holds several patents on this drug in the US and some in China. This molecule was first approved for anemic treatment in chronic kidney disease patients in China in December 2018 [8]. Japanese authorities approved its use in 2019 for treating anemic patients with chronic kidney disease who are on a dialysis cycle and in 2020 for patients without dialysis [9]. In April 2021, CDSCO granted permission to import and market Roxadustat in India to treat anemia. The European Medicines Agency approved the use of the Roxadustat drug in August 2021 [10].
3. Market status
8 API suppliers for Roxadustat are listed on the international platform, with 2 US DMFs filed. The reference price of the drug in USD/kg is $13,200. Dr. Reddy’s Laboratories Ltd. is one of the API suppliers with US DMFs filed. Other API suppliers include Suzhou Biosyntech, Visit Pharmaceuticals, Mylan Inc., Sichuan Renan Pharmaceuticals, Hangzhou Longshine Biotech Co., Ltd., and Teva API Ltd [11].
4. Physicochemical properties
The physicochemical properties of Roxadustat are summarized in Table 1. The drug is a white to pale green solid and exhibits solubilities in organic solvents like DMSO (30 mg/mL) and DMF (50 mg/mL), and water (0.1 mg/mL). Roxadustat is an N-acyl glycine analog of 4-hydroxy-1-1methy-7-phenoxyisoquinoline-3-carboxylic acid. This molecule possesses relatively high permeability with low solubility. This active pharmaceutical ingredient has both acidic and basic functionalities. Therefore, Roxadustat ionization can be controlled by both acidic as well as basic solutions. The ionized form of the molecule shows much higher water solubility; however, the neutral state is lipophilic with increased membrane permeability [11]. A hydrophobic pocket in the enzyme Hypoxia-inducible factor- PHI(Propyl hydroxylase) is essential for the binding of Roxadustat [12]. Roxadustat exhibits more than one crystalline form having different physical properties. The crystalline forms of a drug can also show different stability behaviors and bioavailability.
Table 1.
Physicochemical properties of Roxadustat.
| Chemical Structure of Roxadustat | 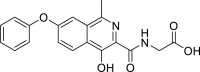 |
|---|---|
| IUPAC name | (4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carbonyl) glycine 2-[(4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carbonyl) amino] acetic acid |
| Appearance | White to Pale Green Solid |
| Chemical Formula | C19H16N2O5 |
| Molecular Weight | 352.3460 |
| m/z: | 352.1059 (100.0%), 353.1093 (20.5%), 354.1126 (2.0%), 354.1102 (1.0%) |
| CAS No. | 808,118-40-3 |
| Melting range | 199–215 °C |
| Solubility | Slightly in water, 30 mg/mL DMSO, Ethanol |
| LogP | 3.13, 1.85 |
| pKa (Strongest Acidic) | 2.75 |
| pKa (Strongest Basic) | 3.84 |
| Index of Refraction | 1.674 |
5. Solid-state characterization
The solid-state properties of a compound are crucial in the field of pharmaceutical development. A drug molecule or compound may exist as a co-former, cocrystal, salt, or amorphous state. Each separate crystalline form has its physical, chemical, and physicochemical properties, such as dissolution rate, vapor pressure, melting point, solubility, hygroscopicity, density, stability, and particle shape. Solid forms may differ in significant ways, leading to the generation of different pharmaceutical products. As a point of formulation development and regulatory concern, it is vital to have a dosage form that meets the safety and efficacy with enhanced pharmacokinetic and dynamic properties. We have attempted to enumerate all the possible forms of compounds in our present review.
5.1. Polymorphism, microcrystal, and Co-crystal
The importance of polymorphs or cocrystals is that any new forms may offer an opportunity to improve the pharmaceutical performance of the drug. Concerning the new forms, formulation scientists can work on multiple dosage forms to achieve their objectives. Roxadustat shows different polymorphic and cocrystal forms. Table 2 shows different salt, amorphous, and crystal forms of [(4-hydroxyl-methyl-7-phenoxy -isoquinoline-3-carbonyl)-amino]-acetic acid with an analytical outcome like X-ray powder diffractogram and DSC have been mentioned [13]. Kallem et al. hold several patents on crystalline form- γ and crystalline form-δ of the Roxadustat [14]. Xinshan et al. has also described several crystalline forms, including preparation methods, i.e., forms I, II, III, IV, V, VI, and VII, in their patent [15]. Hanyue et al. have filed a patent for the microcrystalline form of Roxadustat as an anorthic system; the details include crystal X-ray diffraction results on cell parameters with a = 8.5830, b = 9.2790, c = 11.359: with angle α = 99.16(3°), β = 108.36(3°), γ = 102.16(3°), unit cell volume of 814.2(3)3 with molecular number Z = 2 in structure cell [16]. The different preparation methods employed for microcrystal Roxadustat are presented in Table 3. Due to the poor solubility (1.71 mg/L) of Roxadustat in water, several groups have reported the cocrystal form of Roxadustat [17]. The concept of cocrystals involves solid crystals of ionic compounds in a stoichiometric ratio. The structure comprises two or more compounds to form a unique crystal. In cocrystals, one component is an active pharmaceutical ingredient, and the other is a conformer. The selection of conformers is a critical task to achieve compatibility and effective formulation. Many of the reported suitable co-formers to form the water-soluble form of Roxadustat are from the group consisting of meglumine, N, N′-dibenzylethylenediamine, ter-butylamine, diethylamine, dicyclohexylamine, ammonia salt, magnesium, calcium, potassium, lithium, iron (III) salt, iron (II),2-naphthalene sulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, 3-ethyl-l-methyl-IH-imidazole-3-ium acetate, and caffeine [18]. A cocrystal form is a good option for effective formulation due to intrinsic barriers to drug delivery, insufficient solubility, dissolution, permeability, first-pass metabolism, and drug effect on bioavailability. At the molecular level of interaction of cocrystal drugs with different compounds, hydrogen bonding is involved without the formation of salts. This is a characteristic feature of a cocrystal since Brønsted acid-base chemistry is not required [19]. There exist multiple ways for cocrystal formation via hydrates, clathrate, and solvates based on the principle of host-guest chemistry. Also, there exist several methods for the preparation of cocrystals like solid-state methods that work on the principle of mixing two or more components, a controlled atmospheric environment that involves spontaneous mixing of API and conformer; grinding; extrusion methods; hot-melt extrusion (HME); high shear wet granulation; evaporation; cooling crystallization methods; isothermal slurry preparation; cocrystal with supercritical solvents; laser irradiation; freeze-drying; and electrospray technology [20]. Jetti et al. reported the formation of Roxadustat cocrystal with d-proline by mixing Roxadustat with d-proline in the presence of a solvent, followed by isolation of the cocrystal [21]. Jinchao synthesized and characterized four cocrystals of Roxadustat, cinnamate, benzamide, proline, and niacinamide by solution crystallization method [17]. Dr. Reddy’s Laboratories, India, has filed patents on the cocrystal form of RLP (Roxadustat with l-proline), RNM (Roxadustat with nicotinamide), and RU (Roxadustat with Urea) by using solution crystallization methods [13,[20], [21], [22], [23], [24], [25], [26], [27], [28]].
Table 2.
Solid-state properties.
| Compound | Form | X-ray powder diffractogram | DSC |
|---|---|---|---|
| Compound A Form A | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid | 8.5, 16.2, and 27.4 °2Θ ± 0.2 °2Θ | endotherm at 223 °C |
| Compound A Form B | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid hemihydrate | 4.2, 8.3, and 16.6 °2Θ ± 0.2 °2Θ | 222 °C |
| Compound A Form C | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid hexafluoropropylene-2-ol solvate | 4.5, 13.7, and 16.4 °2Θ ± 0.2 °2Θ | 222 °C |
| Compound A Form D | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid DMSO: water solvate | 8.4, 8.5, and 16.8 °2Θ ± 0.2 °2Θ | 222 °C |
| Compound A sodium salt | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid sodium salt | 5.3, 16.0, and 21.6 °2Θ ± 0.2 °2Θ | 314 °C |
| Compound A l-arginine salt) | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid l-arginine salt | 20.8, 21.8, and 25.4Ο2θ ± 0.2 °2Θ | 210 °C |
| Compound A l-lysine salt | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid l-lysine salt | 19.8, 20.7, and 21.2Ο2θ ± 0.2 °2Θ | 237 °C. |
| Compound A ethanolamine salt | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid ethanolamine salt | 21.8, 22.7, and 27.1Ο2θ ± 0.2 °2Θ | 171 °C |
| Compound A diethanolamine salt | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid diethanolamine salt | 16.9, 23.7, and 25.0 °2Θ ± 0.2 °2Θ | 150 °C |
| Compound A tromethamine salt | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid tromethamine salt | 10.1, 14.2, and 21.1 °2Θ ± 0.2 °2Θ | 176 °C. |
| amorphous Compound A | [(4-hydroxy-l-methyl-7-phenoxy- isoquinoline-3-carbonyl)-amino] -acetic acid | 291 °C | |
| Compound A potassium salt | [(4-hydroxy-l-methyl-7- phenoxy-isoquinoline-3-carbonyl)-amino] -acetic acid potassium salt | 291 °C |
Table 3.
Summary of microcrystal preparation.
| Sr.No | Preparation method | w/v of solvent | Method of dissolution | Condition | Dissolving agent | |
|---|---|---|---|---|---|---|
| Embodiment 1 | Drug dissolved in separate organic solvent acetonitrile, dehydrated alcohol, acetone | 1 g:1 mL | Ultrasonic dissolution | cool to room temperature | Hydrochloric acid or acetic acid. |  |
| Embodiment 2 | combination of any two solvents acetonitrile, dehydrated alcohol, acetone | 1 g:10 mL | Ultrasonic dissolution | |||
| Embodiment 3 | combination of three kinds in acetonitrile, dehydrated alcohol, acetone | 1 g:40 mL | Heating for dissolving at 30 °C | |||
| Embodiment 4 | combination of acetonitrile, dehydrated alcohol, acetone | 1 g:60 mL | Heating for dissolving at 80 °C | |||
| Embodiment 5 | acetonitrile, dehydrated alcohol, acetone | 1 g:50 mL | heating for dissolving at 50 °C |
6. Pharmaceutical formulation aspects
Roxadustat is available in the market under the brand Evrenzo in the form of a film-coated tablets with strengths of 20 mg, 50 mg, and 100 mg. The formulation contains excipients like microcrystalline (E460) cellulose, povidone (E1201), croscarmellose (E468), lactose hydrate, and sodium magnesium stearate (E470b), as shown in Table 4. The film coating excipient was used, such as titanium oxide (E171), partially hydrolyzed polyvinyl alcohol (E1203), macrogol (E1521), Allura red AC aluminum lake (E129), Lecithin (E322), talc, ferric oxide, ferric oxide [29]. Due to its photodegradation [30] nature, Roxadustat is used along with photo stabilizing agents in the formulation, such as titanium dioxide, along with additional dye agents like Allura red AC, aluminium lake, iron oxide, iron oxide yellow, sunset yellow, sunset yellow FCF, indigotin, and indigotin, aluminium lake are used to stabilize the molecule [30]..
Table 4.
Tablet formulations with some excipients.
| Roxadustat dose in tablet (mg) | Lactose (mg) | Allura Red AC aluminium lake (mg) | Soya lecithin (mg) |
|---|---|---|---|
| 20 | 40.5 | 0.9 | 0.21 |
| 50 | 101.2 | 1.7 | 0.39 |
| 70 | 141.6 | 2.1 | 0.47 |
| 100 | 202.4 | 2.8 | 0.63 |
| 150 | 303.5 | 3.7 | 0.84 |
7. Methods of analysis of Roxadustat and its metabolites
Various analytical and bioanalytical methods for analysis of Roxadustat are reported in the literature. All the available methods involving chromatographic conditions are summarized in Table 5. Notably, most of the analytical methodology for studying Roxadustat and the derivative is limited to biological samples. To our knowledge, there were only a few reported methods for analyzing API, pharmaceutical formulations, but no methods available for routing stability and degradation products. This review covered all the available bioanalytical methods for analyzing Roxadustat using various hyphenated techniques. In all the methods, the drug has been analyzed under different objectives like doping control, metabolite study, drug correlation study, and pharmacokinetics approach. In 2016, Buisson et al. [31], for the first time, published LC-MS/MS method for the analysis of Roxadustat, which was reported to be used by some athletes to increase their performances as an alternative doping agent. HIF stabilizers were listed as prohibited substances by the World Anti-Doping Agency (WADA) in 2011 [32]. As discussed earlier, HIF propyl-hydroxylase inhibitor increases erythropoiesis and RBC production, and this application is correlated with the possibility that a new erythropoiesis stimulator can be used to enhance athletic performance. Analysis was performed by collecting plasma and urine samples of an athlete before and after consumption of the Roxadustat drug. A direct identification approach considers monitoring the drug content. In this study, the authors used a sample preparation technique involving solid-phase extraction with the C18 cartridge. They achieved a low limit of detection (LLOD) of 400 pg/mL for the initial doping testing procedure [31].
Table 5.
Chromatographic methods for the analysis of the drug substance in different biological matrices.
| Matrix | Objective of analysis | Stationary phase | Mobile phase | Gradient | Detection | Flow rate | Column temperature | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Urine (Human) | Drug content | Zorbax SB-C8 Column 2.1 × 100mm,1.8 μm |
Mobile phase A-10 mM ammonium formate and acetic acid (pH 4) Mobile B- Acetonitrile |
Time | %B | Triple quadrupole mass spectrometer (Waters) Positive mode |
400 μL/min | 20 °C | [31] |
| 10 | |||||||||
| 8 | 55 | ||||||||
| 8.1 | 100 | ||||||||
| 8.6 | 10 | ||||||||
| 10.6 | 10 | ||||||||
| Urine (human) | Drug content | Zorbax XDB-C8 Column 2.1 × 150mm,5.0 μm |
Mobile phase A-10 mM ammonium formate and acetic acid (pH 4) Mobile B- Acetonitrile |
Time | %B | TSQ Quantum Ultra triple quadrupole mass spectrometer With positive mode |
250 μL/min | ||
| 0 | 40 | ||||||||
| 5 | 90 | ||||||||
| 9.5 | 90 | ||||||||
| 9.6 | 40 | ||||||||
| 15 | 40 | ||||||||
| Plasma and urine (human) | Drug and metabolite analysis | Biphenyl (100 × 2.1 mm, 2.7 μm | Mobile phase A-25 mM ammonium formate with 0.1% formic acid Mobile B- Acetonitrile | Time | %B | Triple quadrupole mass spectrometer | 400 μL/min | 40 °C | [7] |
| 0 | 14 | ||||||||
| 0.5 | 70 | ||||||||
| 6.5 | 70 | ||||||||
| 6.6 | 95 | ||||||||
| 7.5 | 95 | ||||||||
| 7.6 | 14 | ||||||||
| 9.0 | 14 | ||||||||
| Human hepatocyte | Metabolites study | Waters, BEH C18 Column 2.1 × 50mm,1.7 μm | Mobile phase A-5 mM ammonium acetate pH 3.5 Mobile B- Acetonitrile | Time | %B | Water Xevo TQ-S triple quadrupole mass spectrometer | 300 μL/min | 50 °C | [33] |
| 0 | 0.5 | ||||||||
| 0.5 | 5 | ||||||||
| 6.0 | 50 | ||||||||
| 8.0 | 50 | ||||||||
| 10.0 | 70 | ||||||||
| 12.0 | 95 | ||||||||
| 14 | 95 | ||||||||
| 16.5 | 5 | ||||||||
| Liver microsome and fungal sample | Acquity UPLC BEH C18 Column 2.1 × 100 mm,1.7 μm | Mobile phase A-0.1% Formic acid B- methanol | Time | %B | 500 μL/min | 65 °C | |||
| 0 | 5 | ||||||||
| 1.0 | 5 | ||||||||
| 6.0 | 95 | ||||||||
| 8.0 | 95 | ||||||||
| 8.1 | 5 | ||||||||
| 10.0 | 5 | ||||||||
| Plasma and urine(mice) | Metabolites study | Acquity HSS T3; C18, 150mmx2.1 mm,1.8 μm | Mobile phase A-0.01% Formic acid B- Acetonitrile with 0.01% Formic acid | Time | %B | Waters synapt G2-Si QTOF MS with ESI | 400 μL/min | 40 °C | [34] |
| 0 | 1 | ||||||||
| 1.0 | 1 | ||||||||
| 8.0 | 99 | ||||||||
| 13 | 85 | ||||||||
| 10.0 | 1 | ||||||||
| Plasma (human) | Pharmacokinetics and drug-drug interaction study | C18 Column | Mobile phase A-0.1% Formic acid B- acetonitrile 0.1% Formic acid | Isocratic – A: B (45:55) | [37] | ||||
| Urine | Antidoping analysis | Synchronis C18 (100 × 2.1 mm, 1.7 μm) | Mobile phase A-0.2% Formic acid B- acetonitrile 0.2% Formic acid | Time | %B | Thermo-scientific Q Exactive plus tandem mass spectrometer | 500 μL/min | [38] | |
| 0 | 2 | ||||||||
| 0.5 | 2 | ||||||||
| 8.5 | 95 | ||||||||
| 10.0 | 95 | ||||||||
| 12.0 | 2 | ||||||||
| Urine (human) | Simultaneous analysis of drug | Supelco AscentisR C18 (150 mm × 2.1mm × 2.7 μm) |
Mobile phase A-0.1% Formic acid B- acetonitrile 0.1% Formic acid | Time | %B | QExactive focus benchtop Orbitrap-based mass spectrometer | 250 μL/min | 30 °C | [39] |
| 0 | 5 | ||||||||
| 7 | 65 | ||||||||
| 8 | 100 | ||||||||
| 11.0 | 100 | ||||||||
| 13.0 | 5 | ||||||||
| Urine (Horse) | Metabolites study | Eclipse plus C18 (4.6 × 150mm, 3.5 μm) | Mobile phase A-5Mm ammonium acetate or 0.2% formic in water B- acetonitrile | Time | %B | QExactive high-resolution accurate mass spectrometer | 600 μL/min | [40] | |
| 0 | 2 | ||||||||
| 5 | 95 | ||||||||
| 8.0 | 95 | ||||||||
| 8.5 | 50 | ||||||||
| 13.5 | 50 | ||||||||
| 14.0 | 50 | ||||||||
| 15.5 | 2 | ||||||||
| Liposome formulation | HPLC assay | Supelco Discovery BIO wide pore C5, 250mmx4.6 mm,5 μm | Mobile phase A-5% acetonitrile and 0.1% trifluoroacetic acid in water Mobile phase B-95% acetonitrile and 0.1% trifluoroacetic acid in water |
Time | %B | UV 348 nm | 1 mL/min | [41] | |
| 0 | 30 | ||||||||
| 20 | 100 | ||||||||
| 21 | 30 | ||||||||
| Active pharmaceutical substance | Purity analysis | Kinetex C18 150mmX3.0 mm,2.6 μm |
Mobile phase A-10 mM ammonium formate pH 6.3 Mobile Phase B-acetonitrile |
Time | %B | LTQ XL Orbitrap Mass spectrometer (Thermo) | 0.6 mL/min | [42] | |
| 0 | 30 | ||||||||
| 4 | 30 | ||||||||
| 18 | 100 | ||||||||
| 23 | 100 | ||||||||
| 25 | 30 | ||||||||
| 30 | 30 | ||||||||
| Supelco- Ascentis Express C18 | Mobile phase A-0.01 M pH 3.0 Phase B-Acetonitrile |
Time | %B | UV 225 nm | |||||
| 0 | 10 | ||||||||
| 1 | 10 | ||||||||
| 10 | 90 | ||||||||
| 12 | 90 | ||||||||
| 13 | 10 | ||||||||
| 14 | 10 | ||||||||
Later, A doping control analysis was reported to study the drug's metabolites by performing urine analysis and analysis of plasma-derived metabolites from phase-I clinical trials using the UPLC-MS/MS technique [7]. The detection limit range was 0.05–1 ng/mL for urine and 1–5 ng/mL for plasma. Along with the metabolite study, the authors also mentioned a light-induced rearrangement product, a photo isomer of the drug Roxadustat. In order to prepare the sample, the following steps were considered: Methanolic solution of a stable isotope of the drug was added as an internal standard for urine sample. The solution was mixed using a vortex mixer and passed through HLB SPE cartridge using methanol as eluent. The plasma sample was also spiked with internal isotope standard and isopropanol was added. The vortexed sample was centrifuged, and the supernatant was collected and concentrated. The concentrate was further diluted with pH 5.0 sodium acetate buffer. Solid-phase extraction was carried out on bond Elut nexus SPE cation exchange sorbent with washing solution as water and elution solution as methanol. The sample was evaporated and reconstituted with a diluent before injection in the LC-MS system. Mass spectrometric conditions include electrospray ionization in positive mode with probe maintained at 1500 V, the source temperature was 150 °C, desolvation was carried out at a temperature of 450 °C and cone gas flow of 150 L/h. The mass values reported were M1- (m/z 369), M4 (m/z 296), M8 (m/z 529), and M11 (m/z 449) (see Table-6) corresponds to the metabolites with mention of one photo isomer (see Fig. 2). It was also reported that glucuronic acid conjugated metabolites show the longest response, up to 167 h, which is a good indication for doping analysis in the athlete. The other three metabolites can be traced for up to 24 h. All possible metabolites with the possible mechanism pathways for metabolism are mentioned in Fig. 4. Roxadustat undergoes phase-I Biotransformation to form M1-M6 metabolites, most of which occur via cytochrome P-450. M8-M10 metabolites form by glucuronidation conjugates via UDP-glucuronosyltransferases, while M11-M13 are formed by sulfation via sulfotransferases.
Table 6.
Metabolites of roxadustat.
| Name | RT | m/z [M+H]+ | Chemical Structure |
|---|---|---|---|
| M (Roxadustat) | 11.04 | 353.1125 | 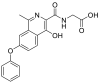 |
| M1 | 8.82 | 369.1075 |  |
| M2 | 7.53 | 369.1074 | |
| M3 | 9.82 | 369.1075 | |
| M4 | 8.03 | 296.0911 |  |
| M5 | 11.05 | 295.1074 | 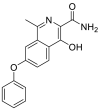 |
| M6 | 11.82 | 252.1016 |  |
| M7 | 10.99 | 312.0860 |  |
| M8 | 7.56 | 529.1446 |  |
| M9 | 7.15 | 545.1397 |  |
| M10 | 8.38 | 472.1231 | 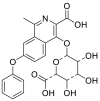 |
| M11 | 7.74 | 449.0636 | 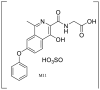 |
| M12 | 7.33 | 390.0293 |  |
| M13 | 8.34 | 389.0451 |  |
Fig. 2.
Schematic representation of a pathway related to Photo isomeric impurity.
Fig. 4.
Possible Mechanistic pathway of Metabolism.
In another method [33], performed several in-vitro metabolite studies involving liver microsomes, S9 fraction, human hepatocytes, equine liver microsomes, fungus model Cunninghamella elegance, and in-vivo urine samples. They identified a total of 12 metabolites in their study using LC-MS. Further, they reported that monohydroxylated metabolite (m/z 369.1079), one of the metabolites, was common in 3 in-vitro studies, which is present in equine liver microsomes, Cunninghamella elegans, and human liver microsomes. On the contrary, eleven other metabolites formed due to the sulfonation, dihydroxylation, monohydroxylated, mono sulfonated, and glycosylated of Roxadustat and its photo isomer are observed in Cunninghamella elegans. In the urine sample, the observed metabolite was glucuronide metabolite (m/z 529.144). No metabolites were observed in other in-vitro studies with S9 fraction and human hepatocyte.
Saigusa et al. [34], developed different chromatographic methods with mass spectrometric analysis of Roxadustat and its novel metabolites (Rox-methyl and Rox-Gluc) in mice. The authors used principal component analysis and partial least square discrimination analysis tools for the study design. For the first time in literature, using a mouse model, the G-met protocol was applied to detect unknown metabolites in doping. The authors also included a pharmacokinetic analysis that has not been published in earlier studies.
In another approach, a bioanalytical method was developed for drug-drug interaction studies involving lanthanum carbonate and Roxadustat. Lanthanum carbonate acts as a phosphate binder and is recommended for hyperphosphatemia in patients with kidney disease. It has been reported that lanthanum carbonate reduces the bioavailability of some drugs, like ciprofloxacin [35] and levothyroxine [36]. To check a similar effect on Roxadustat, the pharmacokinetic study was carried out in healthy, non-elderly adult males [37]. Pharmacokinetic parameters like Cmax, AUC, and Tmax were studied using UPLC/MS/MS techniques. No drug-drug interaction was reported when the drug was given separately and along with lanthanum carbonate.
In the sixth method by Kim et al. [38], UPLC/MS/MS was developed as a simple and cost-effective method for anti-doping analysis. The researchers used the novel extraction technique, QuEChERS, to screen for anti-doping for a human urine sample. After several trials, it was observed that the double extraction methodology with 1% formic acid in acetonitrile provided the highest recovery for accurate analysis. The researchers employed Mass spectrometric analysis with positive and negative modes with the capillary temperature set at 300 °C and spray voltage as 4000 V for positive and 3500 V for negative. The retention time was found as 6.3 min.
In another method [39], developed a single analytical LCMS technique for the determination of 9 HIF propyl-hydroxylase inhibitors, which are good references for anti-doping analysis of Roxadustat, vadadustat, molidustat, desidustat, daprodustat, FG2216, IOX2, IOX4, and JNJ-42041935. In this method, the retention time for Roxadustat was observed as 10.79 min.
In 2021 [40], identified drug metabolites in horse urine using LC with HR-MS and found 13 metabolites. Phase I biotransformation converts the parent molecule into a more polar form by oxidation, reduction, or hydrolysis mechanism. The authors found seven metabolites in phase I. In phase II, the authors detected one metabolite and five conjugated metabolites in phase I. All the metabolites were detected in positive mode mass spectrometry. Similar to earlier reports, the major metabolite was hydroxylated in phase I and glucuronic acid conjugated in phase II. The metabolites were hydroxylated, dealkylated, hydrolyzed, glucuronic acid conjugated, and sulfonic acid conjugated. Metabolites with m/z are mentioned in Table 6. This analytical technique can be an essential method for doping analysis in sports.
For all the above methods, the mass spectrometric parameters used were capillary voltage range from 1.5 to 4.0 kV for positive mode and −2.5-4.5 kV for negative mode, cone voltage between 10 and 40 V, desolvation temperature between 320 and 550 °C, nebulization gas flow 1000 L/h, and collision energy between 15 and 30eV.
8. Analytical methodology for API, process impurities, and formulation
In our literature search, we found only a few research articles supporting chromatographic analysis for liposomes as novel formulations [41] and details on impurities generated during five-step synthesis [42]. As it is known that liposome formulation demonstrates potential effects with fewer side effects, efficient dosing, and targeted delivery, Cheng-bang Jian et al. developed Roxadustat-loaded liposomes and studied drug loading efficiency along with liposome assay using HPLC.
Considering scalable synthesis, Pisa et al. synthesized Roxadustat and all the possible in-process impurities in the laboratory and monitored purity by HPLC. All the potential in-process impurities are mentioned in Fig. 1. In Fig. 2, we have suggested the possible route for generating isomeric photo impurities.
Fig. 1.
In-process impurities during the synthesis of Roxadustat.
In the present review, we have extended our topic of study by including the information related to substances and in-process impurities from different suppliers like Aozeal certified standards [43], Clearsynth [44], TLC Pharmaceutical standards [45], and Synzeal research [46], which have been mentioned in their official websites. It is hypothesized that the present review will serve as a reference for researchers to conduct future analytical studies like impurity profiling and stability studies for the characterization of unknown impurities (Table 7).
Table 7.
Related substance and possible In-process Impurity of Roxadustat.
| Compound name | Exact mass (Da) | Chemical Structure |
|---|---|---|
| 5-phenoxyisobenzofuran-1(3H)-one Chemical Formula: |
226.0630 |  |
| 1-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)cyclobutane-1-carboxylic acid Chemical Formula: C13H15FN2O3 |
266.1067 | 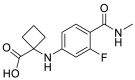 |
| methyl 2-(chloromethyl)-4-phenoxybenzoate Chemical Formula: C15H13ClO3 |
276.0553 | 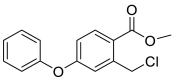 |
| 1-(4-hydroxy-1-methyl-7-phenoxyisoquinolin-3-yl)ethan-1-one Chemical Formula: C18H15NO3 |
293.1052 |  |
| methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate Chemical Formula: C17H13NO4 |
295.0845 | 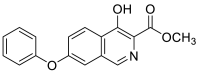 |
| 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylic acid | 295.0845 | 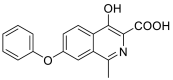 |
| 2,4-diphenoxybenzoic acid | 306.0892 |  |
| methyl 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylate | 309.1001 | 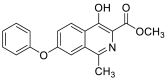 |
| ethyl 4-hydroxy-6-phenoxyisoquinoline-3-carboxylate | 309.1001 |  |
| methyl 4-hydroxy-1-oxo-7-phenoxy-1,2-dihydroisoquinoline-3-carboxylate | 311.0794 | 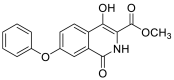 |
| 4-hydroxy-3-(methoxycarbonyl)-7-phenoxyisoquinoline 2-oxide | 311.0794 | 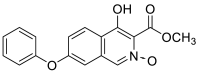 |
| methyl 1,4-dihydroxy-7-phenoxyisoquinoline-3-carboxylate | 311.0794 |  |
| methyl 4-hydroxy-1-methyl-7-phenoxy-5,6,7,8-tetrahydroisoquinoline-3-carboxylate | 313.1314 | 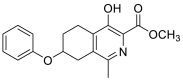 |
| methyl 4-hydroxy-1-methyl-7-phenoxy-4a,5,6,7,8,8a-hexahydroisoquinoline-3-carboxylate | 315.1471 | 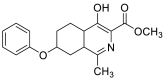 |
| ethyl 4-hydroxy-1-oxo-7-phenoxy-1,2-dihydroisoquinoline-3-carboxylate | 325.0950 |  |
| methyl 7-(4-chlorophenoxy)-4-hydroxyisoquinoline-3-carboxylate | 329.0455 | 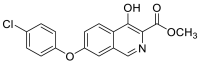 |
| (4-hydroxy-7-phenoxyisoquinoline-3-carbonyl)glycine | 338.0903 |  |
| methyl 7-(4-chlorophenoxy)-4-hydroxy-1-oxo-1,2-dihydroisoquinoline-3-carboxylate | 345.0404 | 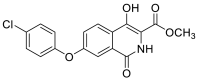 |
| (E)-2-((1-(4-hydroxy-1-methyl-7-phenoxyisoquinolin-3-yl)ethylidene)amino)acetic acid | 350.1267 | 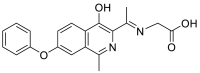 |
| (1a-methyl-6-oxo-3-phenoxy-1,1a,6,6a-tetrahydroindeno [1,2-b]azirine-6a-carbonyl)glycine | 352.1059 | 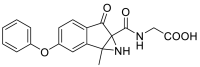 |
| (4-hydroxy-1-methyl-7-phenoxy-5,6,7,8-tetrahydroisoquinoline-3-carbonyl)glycine | 356.1372 | 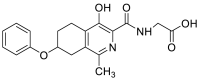 |
| (4-hydroxy-1-methyl-7-phenoxy-4a,5,6,7,8,8a-hexahydroisoquinoline-3-carbonyl)glycine | 358.1529 | 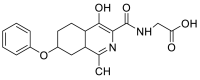 |
| (1a-methyl-6-oxo-3-phenoxydecahydroindeno [1,2-b]azirine-6a-carbonyl)glycine | 358.1529 |  |
| methyl (4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carbonyl)glycinate | 366.1216 |  |
| methyl 1-(acetoxymethyl)-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate | 367.1056 | 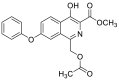 |
| (4-hydroxy-1-(hydroxymethyl)-7-phenoxyisoquinoline-3-carbonyl)glycine | 368.1008 |  |
| 3-((carboxymethyl)carbamoyl)-4-hydroxy-1-methyl-7-phenoxyisoquinoline 2-oxide | 368.1008 | 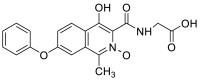 |
| methyl 1-(acetoxymethyl)-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate | 367.1056 |  |
| (1-chloro-4-hydroxy-7-phenoxyisoquinoline-3-carbonyl)glycine | 372.0513 | 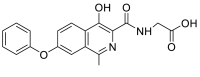 |
| (4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carbonyl)glycylglycine | 409.1274 | 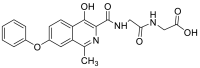 |
| methyl 1-(acetoxymethyl)-7-(4-chlorophenoxy)-4-hydroxyisoquinoline-3-carboxylate | 401.0666 |  |
| benzyl (4-(benzyloxy)-1-methyl-7-phenoxyisoquinoline-3-carbonyl)glycinate | 532.1998 | 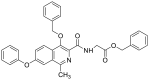 |
| dimethyl 4,4′-dihydroxy-7,7′-diphenoxy-[1,8′-biisoquinoline]-3,3′-dicarboxylate | 588.1533 | 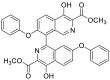 |
8.1. Other methods
Roxadustat pKa parameter has been analyzed by two analytical techniques potentiometric pH-metric titration and Spectrophotometric-UV method. Meloun et al. described the spectrophotometric analysis method as more sensitive among the methods, with a detection range between 10−5 to 10−6 M concentration [47]. A GC-MS method is also described in the literature to study the effect of Roxadustat on retinopathy treatment under hypoxia conditions. The authors studied the role of the serine metabolic pathway in the disease and the behaviour of Roxadustat [48]. Roxadustat was analyzed using NMR, XRPD, IR, and DSC in one research paper detailing synthetic process development [49]. A literature search was conducted to investigate the analytical methodologies and formulation aspects. A great deal of scope was identified for developing analytical methods for assays, dissolution tests, and related substance tests. No single analytical method exists for impurity profiling, process-related impurity, and stability tests. Even hyphenated techniques like GC are not mentioned anywhere in the literature for solvent analysis.
9. Pharmacology
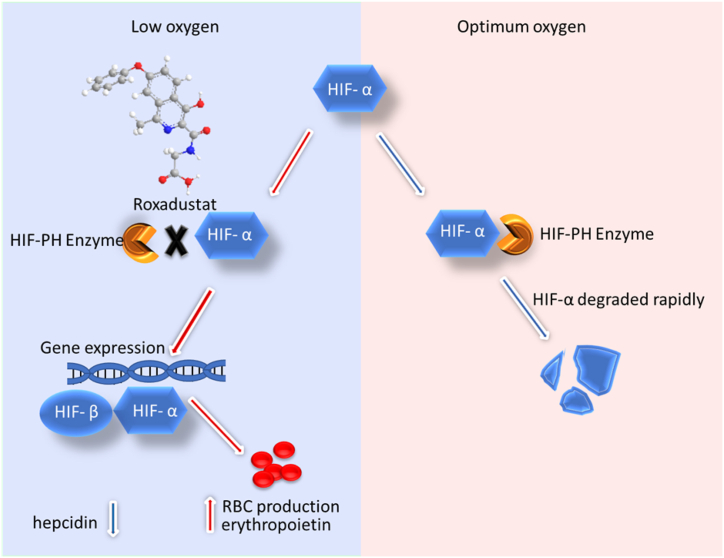
Mechanism of action: Roxadustat is hypoxia-inducible factor propyl hydroxylase (HIF-PH) enzyme inhibitor, which regulates the degradation of transcription factors in HIF and enhances endogenous erythropoietin production through erythropoietin gene expression, which causes an increase in RBC production, and is helpful to maintain the haemoglobin level in patients with chronic kidney diseases and anaemia (Fig. 3) [50]. In the reported study, the drug also showed an increase in iron bioavailability by suppressing hepcidin levels in the body [51].
Fig. 3.
Mechanism of action of Roxadustat.
9.1. Pharmacokinetics
Roxadustat showed rapid absorption when studied in dialysis patients. Pharmacokinetic parameters like the apparent volume of distribution after oral dose administration revealed 22–57 L, apparent renal clearance of 0.030–0.026 L/h, and apparent clearance of 1.2–2.65 L/h in healthy volunteers. The elimination half-life was 9.6–16 h. Plasma binding was 99%, and the fraction eliminated by hemodialysis was 2.34% [52]. The maximum plasma concentration (Cmax) was achieved between 2.0 and 3.5 h after drug administration. In a clinical trial, patients were studied for the treatment of efficacy of Roxadustat. Evaluation parameters considered the amount of endogenous erythropoietin, iron content, iron-binding capacity, transferrin saturation, and hepcidin level [51].
10. Clinical trial details
The drug Roxadustat was investigated in clinical trials in a variety of pathophysiological conditions, including anemic patients, anemic patients with chronic kidney disease (CKD), CKD patients on dialysis, CKD patients without dialysis, chemotherapy-induced anemics, healthy patients, and end-stage renal disease patients.). Different study goals involved investigating dosing efficacy, safety, and tolerability aspects, as well as pharmacokinetics, and multiple-dose studies. A few studies investigated Roxadustat’s effect compared to other treatments, such as erythropoietin recombinant and darbepoetin alfa. In two clinical studies, Roxadustat was studied as a drug-drug interaction with warfarin and Rosiglitazone. In one of the clinical studies, a pediatric formulation was studied in which the bioavailability of Roxadustat after a single dose of an azo dye-free formulation was assessed. The interventions were considered drugs and were compared with placebo, erythropoietin, recombinant human erythropoietin, epoetin alfa, darbepoetin alfa, and iron in all studies. In one of the articles from J. Barratt et al. he investigated Roxadustat versus erythropoiesis-stimulating agents (ESAs) in dialysis-dependent patients in a phase 3 clinical study, and it was found Roxadustat shows non-inferiors effects as compared to ESA in dialysis-dependent chronic kidney diseases patients [53]. He also highlighted cardiovascular safety aspects in his study. In another study, the same efficacy and cardiovascular safety were asses for Roxadustat in a patient with non-dialysis-dependent CKD [54]. All 38 completed clinical trials are listed in Table 8 with all essential details, including NCT Number, Interventions, Conditions, and Sponsor [55].
Table 8.
Summary of all the 38 completed clinical trials related Roxadustat.
| Sr No. | NCT Number | Interventions | Study Title | Conditions | Sponsor: |
|---|---|---|---|---|---|
| 1 | NCT04076943 | Drug: Roxadustat | Evaluation of Efficacy and Safety of Roxadustat for the Treatment of Chemotherapy Induced Anemia Has Results |
|
|
| 2 | NCT04454879 | Drug: Roxadustat | Different Doses of Roxadustat Treatment for Anemia in Peritoneal Dialysis Patients |
|
|
| 3 | NCT04484857 | Drug: Roxadustat | Study of Roxadustat Conversion in Participants Receiving Stable ESA or as Initial Anemia Treatment in Hemodialysis Participants |
|
|
| 4 | NCT04410198 | Drug: Roxadustat | Study of Roxadustat Conversion in Participants Receiving Stabe Erythropoiesis-Stimulating Agent (ESA) or as Initial Anemia Treatment in Chronic Dialysis Participants |
|
|
| 5 | NCT04655027 | Drug: Roxadustat Drug: rHuEPO |
A Study to Investigate the Effect of Roxadustat Versus Recombinant Human Erythropoietin (rHuEPO) on Oral Iron Absorption in Chinese Patients with Anemia of Chronic Kidney Disease (CKD) |
|
|
| 6 | NCT02273726 | Drug: Epoetin Alfa Drug: Roxadustat |
Study to Evaluate the Efficacy and Safety of Roxadustat in the Treatment of Anemia in Participants with ESRD on Stable Dialysis | •CKD Anemia in Stable Dialysis
|
|
| 7 | NCT02965040 | Drug: Roxadustat | A Phase 1 Study of Roxadustat in Subjects with Different Degrees of Renal Function |
|
|
| 8 | NCT04059913 | Drug: Roxadustat | Evaluate the Efficacy and Safety of Multiple Roxadustat Dosing Regimens for the Treatment of Anemia in Dialysis Participants with Chronic Kidney Disease | CKD Anemia in Dialysis
|
|
| 9 | NCT03960489 | Drug: Roxadustat | A Study to Assess the Relative Bioavailability of Roxadustat Following a Single Dose of Pediatric Azo Dye-free Tablet Formulation and Pediatric Azo Dye-free Mini-tablet Formulation Compared to a Single Dose of Azo Dye-containing Tablet Formulation in Healthy Adult Subjects |
|
|
| 10 | NCT01887600 | Drug: Roxadustat Drug: Placebo |
Roxadustat in the Treatment of Anemia in Chronic Kidney Disease Patients Not Requiring Dialysis |
|
|
| 11 | NCT02052310 | Drug: Roxadustat Drug: Epoetin Alfa |
Safety and Efficacy Study of Roxadustat (FG-4592) for the Treatment of Anemia in End-Stage Renal Disease (ESRD) Newly Initiated Dialysis Participants |
|
|
| 12 | NCT01630889 | Drug: Roxadustat | Open-Label Extension Study for the Long-Term Efficacy and Safety of Roxadustat in Participants with Dialysis and Non-Dialysis Chronic Kidney Disease |
|
|
| 13 | NCT02161224 | Drug: FG-4592 | A Study to Investigate the Exposure and Safety and Tolerability of a Single Dose of FG-4592 in Subjects With Moderately Diminished Liver Function Compared to Those with Normal Liver Function |
|
|
| 14 | NCT02021318 | Drug: Roxadustat Drug: Darbepoetin alfa |
Roxadustat in the Treatment of Anemia in Chronic Kidney Disease (CKD) Patients, Not on Dialysis, in Comparison to Darbepoetin Alfa |
|
|
| 15 | NCT02161796 | Drug: FG-4592 Drug: Placebo |
A Study to Evaluate the Dose proportionality and Effects of FG-4592 in Healthy Young and Elderly Male and Female Subjects |
|
|
| 16 | NCT02278341 | Drug: Roxadustat Drug: Epoetin alfa Drug: Darbepoetin alfa Drug: Iron |
Roxadustat in the Treatment of Anemia in End Stage Renal Disease (ESRD) Patients on Stable Dialysis |
|
|
| 17 | NCT01750190 | Drug: Roxadustat Drug: Placebo |
A Study of Roxadustat for the Treatment of Anemia in Participants With Chronic Kidney Disease and Not Receiving Dialysis |
|
|
| 18 | NCT01244763 | Drug: Roxadustat | Study of Roxadustat in Non- Dialysis Chronic Kidney Disease Participants with Anemia |
|
|
| 19 | NCT02252731 | Drug: Warfarin Drug: FG-4592 |
A Study to Evaluate the Effects of Multiple Doses of FG-4592 on the Exposure, Safety and Tolerability and Effect of Warfarin in Healthy Subjects | Pharmacokinetics of FG-4592
|
|
| 20 | NCT02174731 | Drug: Roxadustat Drug: Epoetin alfa |
Safety and Efficacy Study of Roxadustat to Treat Anemia in Patients with Chronic Kidney Disease, on Dialysis. |
|
|
| 21 | NCT01147666 | Drug: Roxadustat Drug: Epoetin Alfa Other: Placebo |
Study of Roxadustat (FG-4592) in Participants with End-Stage Renal Disease Receiving Maintenance Hemodialysis |
|
|
| 22 | NCT02174627 | Drug: Roxadustat Drug: Placebo |
Safety and Efficacy Study of Roxadustat to Treat Anemia in Patients with Chronic Kidney Disease (CKD), Not on Dialysis |
|
|
| 23 | NCT00761657 | Drug: Roxadustat Drug: Placebo |
Phase 2 Study of Roxadustat in Participants with Anemia and Chronic Kidney Disease Not Requiring Dialysis |
|
|
| 24 | NCT01414075 | Drug: Roxadustat Drug: Oral Iron Drug: IV Iron |
Study of Roxadustat (FG-4592) to Correct Anemia in Newly Initiated Dialysis Participants Not on Erythropoiesis-Stimulating Agent Treatment |
|
|
| 25 | NCT01376063 | Drug: FG-4592 | Study to Investigate the Interaction Between FG-4592 and Rosiglitazone in Healthy Adult Subjects |
|
|
| 26 | NCT01596855 | Drug: FG-4592 Drug: Epoetin Alfa |
Study of FG-4592 in Subjects with End-Stage Renal Disease Receiving Maintenance Hemodialysis in China |
|
|
| 27 | NCT01599507 | Drug: FG-4592 Drug: Placebo |
Study of FG-4592 in Subjects with Chronic Kidney Disease in China |
|
|
| 28 | NCT02652806 | Drug: FG-4592 Drug: Epoetin Alfa |
FG-4592 for Treatment of Anemia in Subjects with Chronic Kidney Disease |
|
|
| 29 | NCT02652819 | Drug: FG-4592 Drug: Placebo |
FG-4592 for Treatment of Anemia in Subjects with Chronic Kidney Disease Not on Dialysis |
|
|
| 30 | NCT02988973 | Drug: Roxadustat Drug: DA |
A Study of Intermittent Oral Dosing of ASP1517 in Non-Dialysis Chronic Kidney Disease Patients with Anemia |
|
|
| 31 | NCT02780726 | Drug: Roxadustat | A Study of Intermittent Oral Dosing of ASP1517 in Peritoneal Dialysis Chronic Kidney Disease Patients With Anemia |
|
|
| 32 | NCT02780141 | Drug: Roxadustat | A Study of Intermittent Oral Dosing of ASP1517 in Erythropoiesis Stimulating Agent (ESA)-Naive Hemodialysis Chronic Kidney Disease Patients with Anemia |
|
|
| 33 | NCT02964936 | Drug: Roxadustat | A Study of Intermittent Oral Dosing of ASP1517 in ESA untreated Chronic Kidney Disease Patients with Anemia |
|
|
| 34 | NCT02779764 | Drug: Roxadustat | A Long-Term Study of Intermittent Oral Dosing of ASP1517 in Hemodialysis Chronic Kidney Disease Patients with Anemia Converted From Erythropoiesis Stimulating Agent (ESA) Treatment |
|
|
| 35 | NCT01083888 | Drug: Roxadustat | ASP1517 Pharmacokinetics Study in Anemia Patients on Hemodialysis |
|
|
| 36 | NCT02952092 | Drug: Roxadustat Drug: Darbepoetin alfa |
A Study of Intermittent Oral Dosing of ASP1517 in Hemodialysis Chronic Kidney Disease Patients with Anemia |
|
|
| 37 | NCT00978198 | Drug: ASP1517(Roxadustat) Drug: Placebo |
Safety, Tolerability, and Pharmacokinetic Study of ASP1517 in Healthy Non-elderly Male Volunteers |
|
|
| 38 | NCT01888445 | Drug: Roxadustat Drug: darbepoetin alfa |
A Study to Investigate the Effect of ASP1517 After Intermittent Oral Dosing in Dialysis Chronic Kidney Disease Patients with Anemia Compared With Darbepoetin as a Reference Drug |
|
|
11. Summary & future perspective
As Roxadustat is a relatively new therapeutic molecule, the information related to Roxadustat’s formulations manufacturers and API providers, and the country where the molecule is approved for clinical usage is the first time covered in this review. In addition, this review provides the reader with a complete picture of physicochemical properties, formulation details, etc. The information presented in this review shows a strong need for developing precise analytical methods to analyze available cocrystals and polymorphs of the drug apart from their forced degradation products. Given the increasing misuse of Roxadustat among sports personnel, more bioanalytical methods to analyze the drug and its metabolites are required from various biofluids such as blood, plasma, and urine matrix using hyphenated LC-MS/MS techniques. Much more can be done to develop formulations and study the impurity profiles of drugs and formulations with the additional scope for drug-excipient interaction studies.
12. Conclusion
This review provides comprehensive information regarding the drug Roxadustat in terms of drug developments. It provides guidelines for developing new formulations in the future. As a drug with a promising function in numerous anaemic conditions, it offers fresh hope for the creation of novel formulations. From the very beginning, such as the supplier, to the very end, such as the product patentee information, the review offers us all the information. While multiple conformers and co-crystal characteristics exist, it was discovered that solid state investigation also has a scope of work. The author discussed every bioanalysis technique that can potentially be utilised for QC departments for monitoring. Ultimately, this review includes all facts on a single platform.
Credit author statement
Rupali Mahajan: Writing original draft, writing review. Amit Asthana: Supervision, writing and editing Supervision, Writing - review & editing. Samanthula Gananadhamu and Saurabh Srivastava: Review, suggestion and editing.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements-
The authors are thankful to the National institute of pharmaceutical Education and Research Hyderabad, for research funding support and RM acknowledge funding for doctoral fellowship by the department of chemical and fertilizer India. NIPER Hyderabad communication number NIPER/2023/34.
Contributor Information
Rupali Mahajan, Email: mahajanrupalims@gmail.com.
Gananadhamu Samanthula, Email: gana@niperhyd.ac.in.
Saurabh Srivastava, Email: saurabh@niperhyd.ac.in.
Amit Asthana, Email: amit.asthana@niperhyd.ac.in, amitasthana4@gmail.com.
References
- 1.Vieth J.T., Lane D.R. Anemia. Emerg. Med. Clin. North Am. 2014;32:613–628. doi: 10.1016/j.emc.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Li Z.-L., Tu Y., Liu B.-C. Treatment of renal anemia with Roxadustat: advantages and achievement. Kidney Dis. 2020;6:65–73. doi: 10.1159/000504850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Vecchio L., Locatelli F. Roxadustat in the treatment of anaemia in chronic kidney disease. Expet Opin. Invest. Drugs. 2018;27:125–133. doi: 10.1080/13543784.2018.1417386. [DOI] [PubMed] [Google Scholar]
- 4.Evrenzo® (roxadustat) Tablets Approved in Japan for the Treatment of Anemia Associated with Chronic Kidney Disease in Dialysis Patients | Astellas Pharma US, Inc. https://www.astellas.com/us/news/4536 (accessed April 5, 2022)
- 5.Chen N., Hao C., Peng X., Lin H., Yin A., Hao L., Tao Y., Liang X., Liu Z., Xing C., Chen J., Luo L., Zuo L., Liao Y., Liu B.-C., Leong R., Wang C., Liu C., Neff T., Szczech L., Yu K.-H.P. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N. Engl. J. Med. 2019;381:1001–1010. doi: 10.1056/NEJMOA1813599. [DOI] [PubMed] [Google Scholar]
- 6.Update on US regulatory review of roxadustat in anaemia of chronic kidney disease. https://www.astrazeneca.com/media-centre/press-releases/2021/update-on-us-review-of-roxadustat.html (accessed April 3, 2023)
- 7.Eichner D., Van Wagoner R.M., Brenner M., Chou J., Leigh S., Wright L.R., Flippin L.A., Martinelli M., Krug O., Schänzer W., Thevis M. Lmplementation of the prolyl hydroxylase inhibitor Roxadustat (FG-4592) and its main metabolites into routine doping controls. Drug Test. Anal. 2017;9:1768–1778. doi: 10.1002/DTA.2202. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon S. Roxadustat: first global approval. Drugs. 2019;79:563–572. doi: 10.1007/S40265-019-01077-1. [DOI] [PubMed] [Google Scholar]
- 9.Roxadustat Approved in Japan for the Treatment of Anemia. https://www.globenewswire.com/news-release/2019/09/20/1918409/33525/en/Roxadustat-Approved-in-Japan-for-the-Treatment-of-Anemia-Associated-with-Chronic-Kidney-Disease-in-Dialysis-Patients.html (accessed April 5, 2022)
- 10.https://www.globenewswire.com/news-release/2021/08/19/2283933/33525/en/Astellas-Receives-European-Commission-Approval-for-First-in-Class-EVRENZO-roxadustat-for-Adult-Patients-with-Symptomatic-Anemia-of-Chronic-Kidney-Disease.html Astellas Receives European Commission Approval for. (accessed April 5, 2022)
- 11.Roxadustat API Manufacturers | Suppliers | Drug Master Files (DMF) | CEP | Pharmacompass.com https://www.pharmacompass.com/manufacturers-suppliers-exporters/roxadustat (n.d.) (accessed April 5, 2022)
- 12.Hirota K. HIF-α prolyl Hydroxylase inhibitors and their implications for biomedicine: a comprehensive review. Biomedicines. 2021;9 doi: 10.3390/BIOMEDICINES9050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AU2013290438B9 - Crystalline forms of the prolyl hydroxylase inhibitor [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid - Google Patents. https://patents.google.com/patent/AU2013290438B9/en (accessed April 5, 2022)
- 14.WO2019030711A1 - Polymorphs and co-crystals of roxadustat - Google Patents. https://patents.google.com/patent/WO2019030711A1/en (accessed April 5, 2022)
- 15.US9206134B2 - Polymorphic forms of compounds as prolyl hydroxylase inhibitor, and uses thereof - Google Patents https://patents.google.com/patent/US9206134B2/en (n.d.) (accessed April 5, 2022)
- 16.CN106187888A - FG 4592 monocrystalline and preparation method thereof - Google Patents https://patents.google.com/patent/CN106187888A/en (n.d.) (accessed April 5, 2022)
- 17.Jinchao Xu Z.L., Chen Yong, Huiqing Y.E., Zhang Jie, Zhang Ji. Synthesis, characterization and physicochemical properties study of roxadustat co-crystals. CIE J. 2020;71:1851–1858. doi: 10.11949/0438-1157.20191198. [DOI] [Google Scholar]
- 18.WO2019042485A1 - Solid forms of roxadustat - Google Patents. https://patents.google.com/patent/WO2019042485A1/en (accessed April 5, 2022)
- 19.Yadav A.V., Shete A.S., Dabke A.P., Kulkarni P.V., Sakhare S.S. Co-crystals: a novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J. Pharmaceut. Sci. 2009;71:359. doi: 10.4103/0250-474X.57283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimi-Jafari M., Padrela L., Walker G.M., Croker D.M. Creating cocrystals: a review of pharmaceutical cocrystal preparation routes and applications, Cryst. Growth Des. 2018;18:6370–6387. doi: 10.1021/ACS.CGD.8B00933/ASSET/IMAGES/ACS.CGD.8B00933.SOCIAL.JPEG_V03. [DOI] [Google Scholar]
- 21.WO2020178847A1 - Cocrystal of roxadustat and d-proline - Google Patents. https://patents.google.com/patent/WO2020178847A1/en (accessed April 5, 2022)
- 22.US20200247753A1 - Polymorphs and co-crystals of roxadustat - Google Patents. https://patents.google.com/patent/US20200247753A1/en (accessed April 5, 2022)
- 23.CA3072601A1 - Polymorphs and co-crystals of roxadustat - Google Patents. https://patents.google.com/patent/CA3072601A1/en (accessed April 5, 2022)
- 24.CN111511371A - Polymorphs and co-crystals of rosuvastatin - Google Patents. https://patents.google.com/patent/CN111511371A/en (accessed April 5, 2022)
- 25.JP2020530473A - Polymorphs and co-crystals of Lokidustat - Google Patents. https://patents.google.com/patent/JP2020530473A/en (accessed April 5, 2022)
- 26.US11168057B2 - Polymorphs and co-crystals of roxadustat - Google Patents. https://patents.google.com/patent/US11168057B2/en (accessed April 5, 2022)
- 27.BR112020002939A2 - roxadustat polymorphs and co-crystals - Google Patents. https://patents.google.com/patent/BR112020002939A2/en (accessed April 5, 2022)
- 28.EP3664805A4 - Polymorphs and co-crystals of roxadustat - Google Patents, https://patents.google.com/patent/EP3664805A4/en (accessed April 5, 2022)
- 29.https://www.pmda.go.jp/files/000234811.pdfhttps://www.pmda.go.jp/files/000234811.pdf (accessed August 25, 2022)
- 30.KR20160018514A - Pharmaceutical formulations of a hif hydroxylase inhibitor - Google Patents https://patents.google.com/patent/KR20160018514A/en (n.d.) (accessed April 5, 2022)
- 31.Buisson C., Marchand A., Bailloux I., Lahaussois A., Martin L., Molina A. Detection by LC–MS/MS of HIF stabilizer FG-4592 used as a new doping agent: investigation on a positive case. J. Pharm. Biomed. Anal. 2016;121:181–187. doi: 10.1016/J.JPBA.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Beuck S., Bornatsch W., Lagojda A., Schänzer W., Thevis M. Development of liquid chromatography-tandem mass spectrometry-based analytical assays for the determination of HIF stabilizers in preventive doping research. Drug Test. Anal. 2011;3:756–770. doi: 10.1002/DTA.365. [DOI] [PubMed] [Google Scholar]
- 33.Hansson A., Thevis M., Cox H., Miller G., Eichner D., Bondesson U., Hedeland M. Investigation of the metabolites of the HIF stabilizer FG-4592 (roxadustat) in five different in vitro models and in a human doping control sample using high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2017;134:228–236. doi: 10.1016/J.JPBA.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 34.Saigusa D., Suzuki N., Matsumoto Y., Umeda K., Tomioka Y., Koshiba S., Yamamoto M. Detection of novel metabolite for Roxadustat doping by global metabolomics. J. Biochem. 2018 doi: 10.1093/JB/MVY028. [DOI] [PubMed] [Google Scholar]
- 35.How P.P., Fischer J.H., Arruda J.A., Lau A.H. Effects of lanthanum carbonate on the absorption and oral bioavailability of ciprofloxacin. Clin. J. Am. Soc. Nephrol. 2007;2:1235–1240. doi: 10.2215/CJN.01580407. [DOI] [PubMed] [Google Scholar]
- 36.Weitzman S.P., Ginsburg K.C., Carlson H.E. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19:77–79. doi: 10.1089/THY.2008.0312. https://www.Home.Liebertpub.Com/Thy [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata T., Nomura Y., Takada A., Aoki S., Katashima M., Murakami H. Evaluation of the effect of lanthanum carbonate hydrate on the pharmacokinetics of roxadustat in non-elderly healthy adult male subjects. J. Clin. Pharm. Therapeut. 2018;43:633–639. doi: 10.1111/JCPT.12729. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.H., Lim N.R., Min H., Sung C., Bin Oh H., Kim K.H. Analysis of Hypoxia-inducible factor stabilizers by a modified QuEChERS extraction for antidoping analysis. Msletters.Accesson.Kr. 2020;11 doi: 10.5478/MSL.2020.11.4.118. [DOI] [Google Scholar]
- 39.Mazzarino M., Perretti I., Stacchini C., Comunità F., de la Torre X., Botrè F. UPLC–MS-Based procedures to detect prolyl-Hydroxylase inhibitors of HIF in urine. J. Anal. Toxicol. 2021;45:184–194. doi: 10.1093/JAT/BKAA055. [DOI] [PubMed] [Google Scholar]
- 40.Mathew B., Philip M., Perwad Z., Karatt T.K., Caveney M.R., Subhahar M.B., Karakka Kal A.K. Identification of Hypoxia-inducible factor (HIF) stabilizer roxadustat and its possible metabolites in thoroughbred horses for doping control. Drug Test. Anal. 2021;13:1203–1215. doi: 10.1002/DTA.3014. [DOI] [PubMed] [Google Scholar]
- 41.Liposomal H., Park J., Sik Choi S., Jian C.-B., Yu X.-E., Gao H.-D., Chen H.-A., Jheng R.-H., Chen C.-Y., Lee H.-M. Mdpi.Com; 2022. Liposomal PHD2 Inhibitors and the Enhanced Efficacy in Stabilizing HIF-1α. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Píša O., Rádl S., Čerňa I., Šembera F. A scalable synthesis of roxadustat (FG-4592) Org. Process Res. Dev. 2021 doi: 10.1021/ACS.OPRD.1C00281/SUPPL_FILE/OP1C00281_SI_001.PDF. [DOI] [Google Scholar]
- 43.Search Results for “Roxadustat” – Welcome to AOZEAL.COM. https://www.aozeal.com/?s=Roxadustat&post_type=product&type_aws=true&id=1&filter=1 (accessed April 20, 2022)
- 44.Roxadustat Impurities | Clearsynth https://www.clearsynth.com/en/api_categories.asp?category=&api=Roxadustat-Impurities&url=rewrite (accessed April 4, 2023)
- 45.TLC Pharmaceutical Standards - World leader for isotope labeled materials, metabolites and reference standards. https://www.tlcstandards.com/ProductsSearchList.aspx?key=Roxadustat (accessed April 4, 2023)
- 46.SearchSynZeal https://www.synzeal.com/en/search?q=roxadustat (n.d.) (accessed April 4, 2023)
- 47.Meloun M., Pilařová L., Javůrek M., Pekárek T. Multiwavelength UV-metric and pH-metric determination of the dissociation constants of the hypoxia-inducible factor prolyl hydroxylase inhibitor Roxadustat. J. Mol. Liq. 2018;268:386–402. doi: 10.1016/J.MOLLIQ.2018.07.076. [DOI] [Google Scholar]
- 48.Singh C., Sharma A., undefined . Iovs.Arvojournals.Org; 2018. Serine Metabolism Is a Key Pathway Involved in the Prevention of Oxygen-Induced Retinopathy by Roxadustat.https://iovs.arvojournals.org/article.aspx?articleid=2692824 (accessed April 20, 2022) [Google Scholar]
- 49.Píša O., Rádl S., Čerňa I., Šembera F. A scalable synthesis of roxadustat (FG-4592) Org. Process Res. Dev. 2022;26:915–924. doi: 10.1021/ACS.OPRD.1C00281/SUPPL_FILE/OP1C00281_SI_001.PDF. [DOI] [Google Scholar]
- 50.Gupta N., J.W.-A.J. of K. Diseases, undefined . Elsevier; 2017. Hypoxia-inducible Factor Prolyl Hydroxylase Inhibitors: a Potential New Treatment for Anemia in Patients with CKD.https://www.sciencedirect.com/science/article/pii/S0272638617301105 (accessed April 20, 2022) [DOI] [PubMed] [Google Scholar]
- 51.Becker K., Saad M. A new approach to the management of anemia in CKD patients: a review on roxadustat. Adv. Ther. 2017;34:848–853. doi: 10.1007/S12325-017-0508-9. [DOI] [PubMed] [Google Scholar]
- 52.Czock D., Keller F. Clinical pharmacokinetics and pharmacodynamics of roxadustat. Clin. Pharmacokinet. 2022;61:347–362. doi: 10.1007/S40262-021-01095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barratt J., Sulowicz W., Schömig M., Esposito C., Reusch M., Young J., Csiky B. Efficacy and cardiovascular safety of roxadustat in dialysis-dependent chronic kidney disease: Pooled analysis of four phase 3 studies. Adv. Ther. 2021;38:5345–5360. doi: 10.1007/S12325-021-01903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provenzano R., Szczech L., Leong R., Saikali K.G., Zhong M., Lee T.T., Little D.J., Houser M.T., Frison L., Houghton J., Neff T.B. Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non-dialysis-dependent CKD: Pooled results of three randomized clinical trials. Clin. J. Am. Soc. Nephrol. 2021;16:1190–1200. doi: 10.2215/CJN.16191020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Search of: roxadustat - list results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=&term=Roxadustat&cntry=&state=&city=&dist= (accessed April 1, 2023)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.