Abstract
Objective:
Studies have suggested a potential link between traumatic experiences, psychological stress, and autoimmunity, but the impact of stress on disease activity and symptom severity in systemic lupus erythematosus (SLE) remains unclear. We examined whether increases in perceived stress independently associate with worse SLE disease outcomes over three years of follow-up.
Methods:
Participants were drawn from the California Lupus Epidemiology Study (CLUES). Stress was measured annually using the 4-item Perceived Stress Scale (PSS). Participants with PSS increases ≥0.5 standard deviation were defined as having an increase in stress. Four outcomes were measured at the year 3 follow-up visit: physician-assessed disease activity (Systemic Lupus Disease Activity Index), patient-reported disease activity (Systemic Lupus Activity Questionnaire), pain (PROMIS Pain Interference), and fatigue (PROMIS Fatigue). Multivariable linear regression evaluated longitudinal associations of increase in stress with all four outcomes while controlling for potential confounders.
Results:
The sample (n=260) was 91% female, 36% Asian, 30% White, 22% Hispanic, and 11% African American; mean age 46 (±14) years. In adjusted longitudinal analyses, increase in stress independently associated with greater physician-assessed disease activity (p=0.015), greater self-reported disease activity (p<0.001), more pain (p=0.019), and more fatigue (p<0.001).
Conclusion:
In a racially diverse sample of persons with SLE, those who experienced an increase in stress had significantly worse disease activity and greater symptom burden at follow-up compared to those with stress levels that remained stable or declined. Findings underscore the need for interventions to bolster stress resilience and support effective coping strategies among individuals living with lupus.
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune condition characterized by autoantibody formation, multisystem organ involvement, and increased mortality. It is also characterized by periods of disease exacerbation and relative clinical quiescence, but the factors responsible for relapses in disease activity and disease-related symptoms remain poorly understood (1). For example, there are certain known triggers for SLE flares—such as infections, hormonal changes, tapering immunosuppressive treatments, and ultraviolet light exposure—but it is common for people with SLE to experience flares of their disease without a clear preceding trigger (2). Furthermore, even among SLE patients thought to be in low disease activity, there is a high prevalence of persistent and debilitating disease-related symptoms (3). It is critical to elucidate the causes for fluctuations in SLE disease activity and patient-reported symptoms to inform interventions that mitigate the risk of flares and improve quality of life for people living with this disease.
A prior epidemiologic study found that women with a history of trauma had a nearly three-fold increased risk of subsequent incident SLE compared to women without trauma exposure, suggesting a potential link between traumatic experiences, psychological stress, and lupus pathogenesis (4). Though other studies have attempted to investigate the relationship between stress and disease activity in people with an established diagnosis of SLE, those studies were limited by small sample sizes or omission of a validated physician-assessed disease activity measure (5–7). Therefore, more work is needed to determine whether high psychological stress confers an increased risk for active disease and severe symptoms, and whether stress-reduction is an appropriate target for improving outcomes in SLE.
In this study, we aimed to investigate the relationship between perceived stress, disease activity, and disease-related symptoms among people with lupus. We conducted a longitudinal observational study of a racially and ethnically diverse cohort of individuals with SLE to determine the independent association of changes in stress with four disease outcomes: physician-assessed disease activity, self-reported disease activity, pain, and fatigue.
Patients and Methods
Study Design and Participants:
Subjects were participants in the California Lupus Epidemiology Study (CLUES), a prospective longitudinal cohort of individuals with SLE. Briefly, starting in 2015, participants for CLUES were recruited through the California Lupus Surveillance Project, which used outpatient, hospital, and laboratory records to identify all SLE patients residing in San Francisco County from 2007 to 2009 (8). Additional participants in the geographic region were identified through academic and community rheumatology clinics, and from earlier studies of genetic risk factors for SLE outcomes (9, 10). SLE diagnoses were confirmed by study physicians based on (a) ≥4 of the 11 American College of Rheumatology (ACR) revised criteria for the classification of SLE (11, 12), (b) meeting 3 of the 11 ACR criteria with a documented rheumatologist’s diagnosis of SLE, or (c) a confirmed diagnosis of lupus nephritis.
Participants were assessed annually. The baseline (year 1) assessment was conducted in-person, the year 2 follow-up visit was conducted by telephone, and the year three follow-up visit was conducted in-person (Figure 1). The in-person visits included collection and review of medical records prior to the visit; a history and physical examination conducted by a physician specializing in lupus; collection of biospecimens for clinical and research purposes; and completion of a structured interview administered by an experienced research assistant. CLUES specifically aimed to include a diverse sample with representation from multiple racial and ethnic groups and people who speak different languages. Therefore, research clinic visits and interviews were conducted in one of four languages: English, Spanish, Mandarin, or Cantonese. The study was approved by the UCSF IRB, and all participants provided informed consent.
Figure 1. Timeline of Study Design and Analyses.
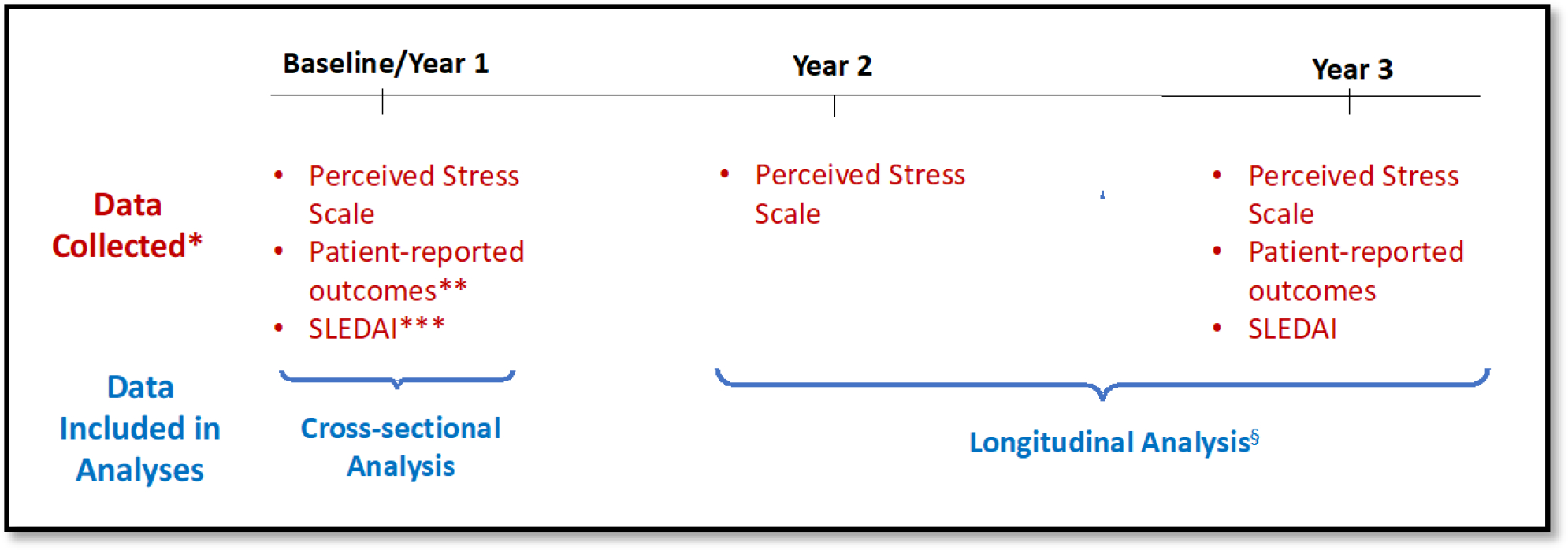
*Data listed in red represent key data collected to define predictor and outcome variables for each analysis; figure does not include an exhaustive list of all data collected at each study visit.
**Patient-reported outcomes (PROs) included patient-reported disease activity via the Systemic Lupus Activity Questionnaire (SLAQ), pain via the Patient-Reported Outcomes Measurement Information System (PROMIS) pain interference scale, and fatigue via the PROMIS fatigue scale.
***SLEDAI = Systemic Lupus Disease Activity Index.
§For the sensitivity longitudinal analysis, we included the score for the outcome of interest (PRO or SLEDAI) at baseline as a covariate in the multivariable regression model.
Given our objective to assess the independent association of changes in perceived stress with lupus disease activity and symptoms, participants were included in these analyses if they completed at least two stress measurements and underwent a physician-assessed lupus disease activity measurement during the in-person follow-up visit. There were 330 participants in the larger cohort, of whom 260 had the requisite stress and disease activity data and were included in this analysis.
Outcome Measures:
The primary outcome was disease activity, which was measured by both physician assessment and patient report. Physician-assessed disease activity was measured with the Safety of Estrogen in Lupus National Assessment (SELENA) version of the Systemic Lupus Disease Activity Index (SLEDAI), also known as the SELENA-SLEDAI tool, which is a validated physician-completed instrument consisting of data from 24 weighted clinical and laboratory variables from nine organ systems, resulting in a score ranging from 0 to 105 (13–16). Patient-reported disease activity was measured with the Systemic Lupus Activity Questionnaire (SLAQ), which includes questions on the presence and severity of 24 specific symptoms of disease activity representing disease involvement across nine organ systems, and which produces a numerical score ranging from 0 to 44 (17, 18).
The secondary outcome variables focused on symptoms commonly reported by patients with SLE, specifically pain and fatigue. Both pain and fatigue were measured using the Patient-Reported Outcome Measurement Information System (PROMIS), an NIH-funded program to develop validated patient reported outcomes (PROs) that can be used to study both the general population and individuals living with chronic conditions (19). Pain was assessed via the PROMIS 4-item Pain Interference scale, and fatigue was assessed via the PROMIS 4-item Fatigue scale. PROMIS scales are scored by adding the scores from individual questions and transforming the sum into a T-score with a population mean and standard deviation of 50 and 10, respectively. Therefore, a difference in PROMIS scores between two groups of 10 points represents one standard deviation. The range for PROMIS T-scores differs for each scale; the range for the 4-item Pain Interference scale is 41.6–75.6 and the range for the 4-item Fatigue scale is 33.7–75.89.
Independent Variable:
The primary predictor of interest was perceived stress, assessed using Cohen’s abbreviated 4-item Perceived Stress Scale (PSS), which yields scores ranging from 0 (low stress) to 16 (high stress). The PSS is a validated and widely used psychological instrument that measures the degree to which an individual perceives their life as uncontrollable, unpredictable, and overwhelming (20). Although the PSS was developed in the 1980s, it continues to be the gold standard instrument for assessing perceived stress and has been correlated with biological markers of stress and disease (21, 22). The principal investigators of the “Stress Measurement Network”, a National Institutes of Health (NIH)-sponsored project designed to improve the measurement of stress in research studies, recommend the PSS for measuring global stress perceptions (https://www.stressmeasurement.org/).
Because there is no standardized cut-off for PSS, in the cross-sectional analysis, participants with scores in the top quartile were identified as those with higher stress, while participants with PSS scores in the lower three quartiles were categorized as having low/moderate stress. For the main longitudinal analysis, the primary predictor variable was increase in stress, defined as an increase of ≥0.5 standard deviation in PSS from the second (study visit 2) to third (study visit 3) years of the study. There is no validated minimal clinically important difference (MCID) for the PSS, and therefore we utilized 0.5 standard deviation change in score, which has been shown to approximate an MCID (23).
Other measures:
Participants were asked about sociodemographic characteristics, including sex, age, race, educational attainment (categorized as at least a bachelor’s degree versus those with less education), and income (categorized for analysis as household income ≤ or > 125% of the federal poverty level). Height and weight were measured during the baseline in-person visit, and body mass index (BMI) was calculated as weight (kg) divided by height (m2). The presence of depression at baseline was defined by a score ≥10 on the Patient Health Questionnaire (PHQ)-8 (24). Participants were also queried regarding smoking status, age of lupus diagnosis, and major comorbidities such as cardiovascular disease, diabetes mellitus, and cancer. Disease damage was measured with the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index, a physician-assessed measure that provides a composite score of cumulative organ damage in SLE (25). Participants also provided information on SLE medication use, including glucocorticoids and other immunomodulatory medications.
Statistical analysis:
We first evaluated differences in the sample used for the current analysis compared to the larger CLUES cohort by comparing the characteristics of participants who met criteria for this analysis to the larger sample of 330 participants in the full study. Next, differences in characteristics of participants in the high stress versus low/moderate stress groups at baseline were tested with t-tests and chi-square analyses. For the baseline cross-sectional analysis, bivariate linear regression was used to quantify the cross-sectional association between high stress and each of the four disease outcomes at the time of the first study visit (Figure 1). Multiple linear regression was then used to model each of the outcomes as a function of high stress adjusting for age, sex, race and ethnicity, educational attainment, smoking, and disease duration. We did not include poverty-level income in the multivariable model because it was collinear with educational attainment. Similarly, we did not include depression as a covariate in the multivariable model because it was colinear with stress.
For the primary longitudinal analysis, we determined the independent association of experiencing an increase in perceived stress with disease outcome scores at the final in-person study visit. Disease outcome scores at follow-up were modeled as a function of increase in stress (minimum increase of 0.5 standard deviation) versus stable/decreased stress after adjusting for the following potential confounders: age, sex, race, educational attainment, smoking, and disease duration. We also conducted a sensitivity analysis that was identical to the primary longitudinal analysis except that it included the score for the outcome variable at baseline as an additional covariate. The primary analysis addressed the question of whether the stress trajectory of participants independently associates with the absolute value of disease outcome scores at follow-up while the sensitivity analysis aimed to determine whether stress trajectory associates with a change in the disease outcomes over time.
We did not include BMI as a covariate in the primary multivariable regression model because it did not associate with disease activity in the univariate analyses, and because we did not think it was a potential confounder in the primary relationship of interest. However, given that BMI was higher among the high-stress patients, we conducted a seccone sensitivity analysis that included obesity as an additional covariate in the multivariable regression model.
Several procedures were used to ensure the integrity of each of the models: the normality assumption was evaluated visually with boxplots and normal probability plots; collinearity was assessed by calculating a variance inflation factor (VIF) for each covariate and removing collinear variables based on VIF ≥ 10 from the final model; and homoscedasticity was confirmed by plots of fitted values versus residuals. For both the baseline and longitudinal analyses, in order to present the adjusted results in clinically meaningful terms, we calculated adjusted means for each outcome based on the multivariable regression models. All analyses were performed using Stata 14 (College Station, TX).
Results
Sample Characteristics:
The study sample (n=260) was 91% female and included representation from diverse racial and ethnic groups with 36% Asian, 30% White, 22% Hispanic, and 11% African American (Table 1). Participants had a mean age of 46 (± 14) years, a mean SLE disease duration of 17 years, and 18% met criteria for poverty-level income. At the time of the baseline assessment, 44% of participants had a history of lupus nephritis, 69% reported current treatment with hydroxychloroquine, and most participants had received treatment with a systemic steroid during the prior year. Few differences existed between CLUES participants who were and were not included in the analysis (Supplemental Table A). The only statistically significant differences between patients in the current analysis and those who were lost to follow-up is that the latter group had more men and a higher prevalence of prior lupus nephritis.
Table 1.
| Characteristics | Overall (N=260) | High Stress (N=64) | Low/Moderate Stress (N=193) | P |
|---|---|---|---|---|
|
| ||||
| Sociodemographic Factors: | ||||
| Age, mean ± SD | 45.6 ± 14.2 | 46.4 ± 13.1 | 45.4 ± 14.5 | 0.600 |
| Female | 91% | 94% | 90% | 0.377 |
| Race and ethnicity§ | 0.221 | |||
| Asian | 36% | 25% | 40% | |
| White | 30% | 34% | 28% | |
| Hispanic | 22% | 25% | 21% | |
| African American | 11% | 15% | 9% | |
| Unspecified or other | 2% | 2% | 2% | |
| Below poverty | 18% | 29% | 14% | 0.008 |
| Education: at least bachelor’s degree | 47% | 32% | 52% | 0.005 |
| Marital Status | 0.072 | |||
| Never married | 36% | 38% | 36% | |
| Married or living w/ partner | 53% | 45% | 56% | |
| Divorced | 10% | 17% | 7% | |
| Widowed | 1% | 0% | 2% | |
| Lupus Specific Characteristics: | ||||
| SLE disease duration, years, mean ± SD | 16.6 ± 10.5 | 17.5 ± 10.7 | 16.3 ± 10.5 | 0.437 |
| Disease damage by SDI, mean ± SD | 1.8 ± 2.0 | 2.4 ± 2.2 | 1.7 ± 1.9 | 0.012 |
| History of lupus nephritis (%) | 44% | 42% | 45% | 0.614 |
| Systemic steroid use over prior year | 59% | 66% | 56% | 0.159 |
| Current hydroxychloroquine use | 69% | 69% | 69% | 1.000 |
| Comorbidites and Health Status: | ||||
| Cardiovascular disease | 13% | 22% | 10% | 0.030 |
| Diabetes mellitus | 6% | 8% | 6% | 0.556 |
| History of malignancy | 8% | 12% | 7% | 0.205 |
| Depression by PHQ-8 score ≥ 10 | 26% | 62% | 14% | <0.001 |
| Obesity (BMI ≥ 30 kg/m2) | 24% | 28% | 23% | 0.401 |
| BMI (kg/m2), mean ± SD | 26.6 ± 6.4 | 28.0 ± 8.1 | 26.1 ± 5.7 | 0.038 |
| Current nicotine use | 4% | 8% | 3% | 0.107 |
Values are percent unless otherwise indicated. P-values calculated using chi-squared tests for categorical measures and t-tests for continuous measures.
High stress defined by scores in the top quartile of the 4-item Perceived Stress Scale (PSS) (range 8–16); low/moderate stress defined by scores in the three lower quartiles of the PSS (range 0–7).
This is a combined race/ethnicity variable. White, African American, and Asian are non-Hispanic.
BMI: body mass index
SDI: Systemic Lupus International Collaborating Clinics Damage Index
Cardiovascular Disease: history of stroke, coronary artery disease, and/or myocardial infarction
PHQ-8: 8-item Patient Health Questionnaire depression scale
Compared to the rest of the cohort, the patients in the high stress group were similar in age, sex, race, and ethnicity, but they had a greater prevalence of poverty-level income (29% versus 14%, p=0.008) and a lower level of educational attainment (32% versus 52% with at least a bachelor’s degree, p=0.005). The high-stress group also had a higher mean BMI, a marginally worse mean SLE disease damage index, and a greater prevalence of comorbid cardiovascular disease and depression (Table 1). The mean perceived stress score in the high stress group was 9.4 (± 1.6), compared with 3.6 (± 2.2) among the rest of the cohort.
Baseline adjusted associations of high perceived stress with lupus outcomes:
In the cross-sectional multivariable regression analysis, high stress was associated with significantly worse scores for patient-reported disease activity, pain interference, and fatigue, but not physician-assessed disease activity (SLEDAI), after adjustment for age, sex, race and ethnicity, educational attainment, smoking, and disease duration (Table 2). Among patients in the high stress group, the mean adjusted patient-reported disease activity score (via SLAQ) was 14.0 (95% CI 12.3, 15.6), compared to 7.4 (6.5, 8.3) for the rest of the cohort. Using the PROMIS scales to compare severity of pain and fatigue, the high stress group had a mean adjusted pain interference score of 58.4 (56.1, 60.6) compared to 50.2 (48.9, 51.5) among the rest of the cohort, and a mean adjusted fatigue score of 60.2 (57.6, 62.9) compared to 49.3 (47.8, 50.7) in the comparator group.
Table 2.
Adjusted Means for Lupus Outcomes at Baseline by Perceived Stress
| Adjusted Mean* (95% CI) | ||||
|---|---|---|---|---|
| Disease Outcomes | High Stress** (N=64) | Low/Moderate Stress (N=193) | P | Beta |
|
| ||||
| SLE Disease Activity: | ||||
| Physician-assessed disease activity1 | 2.5 (1.8, 3.3) | 3.0 (2.6, 3.5) | 0.251 | −0.51 |
| Self-reported disease activity2 | 14.0 (12.3, 15.6) | 7.4 (6.5, 8.3) | <0.001 | 6.56 |
| Symptoms: | ||||
| Pain Interference3 | 58.4 (56.1, 60.6) | 50.2 (48.9, 51.5) | <0.001 | 8.13 |
| Fatigue4 | 60.2 (57.6, 62.9) | 49.3 (47.8, 50.7) | <0.001 | 10.96 |
Adjusted means calculated from multivariable regression analysis adjusted for age, sex, race and ethnicity, educational attainment, smoking, and disease duration. The N for the multivariable regression was 257 (vs. 260 eligible participants) as 3 participants had missing data for smoking status.
Participants with scores in the top quartile for the 4-item Perceived Stress Scale.
Assessed by Systemic Lupus Disease Activity Index (SLEDAI), score range 0 – 105.
Assessed by Systemic Lupus Activity Questionnaire (SLAQ), score range 0 – 44.
Assessed by Patient-Reported Outcome Measurement Information System (PROMIS) 4-item Pain Interference scale, score range 41.6 – 75.6.
Assessed by PROMIS 4-item Fatigue scale, score range 33.7 – 75.89.
Longitudinal associations of increase in stress with lupus outcomes at three-year follow-up:
In the primary longitudinal multivariable regression analysis, participants who experienced an increase in stress had worse scores for all four disease outcomes at the time of follow-up, even after adjusting for age, sex, race and ethnicity, educational attainment, smoking, and disease duration (Table 3). Patients who experienced an increase in stress had worse disease activity—by both physician assessment and patient report—at the subsequent in-person follow-up visit. The mean adjusted SLEDAI score was 3.7 (CI 2.8, 4.5) among participants with an increase in stress compared to 2.5 (CI 2.1, 2.9) among the rest of the cohort (p=0.015), and the mean adjusted SLAQ score was 12.2 (CI 10.3, 14.0) versus 8.2 (CI 7.3, 9.1) (p=0.001). The participants who experienced an increase in stress also had greater symptom burden at follow-up compared to the rest of the cohort; the mean adjusted pain and fatigue scores were 54.7 (CI 51.8, 57.6) versus 50.8 (CI 49.4, 52.2) and 56.9 (CI 53.8, 60.0) versus 50.7 (CI 49.2, 52.2), respectively.
Table 3.
Association of Increase in Perceived Stress with Lupus Outcomes at Year 3 Follow-up
| Primary Analysis | Sensitivity Analysis | |||||
|---|---|---|---|---|---|---|
| Adjusted Mean* (95% CI) | Adjusted Mean** (95% CI) | P | ||||
| Disease Outcomes at Follow-up | Increase in Perceived Stress§ (N=50) | Stable/Decreased Perceived Stress (N=207) | P | Increase in Perceived Stress§ (N=50) | Stable/Decreased Perceived Stress (N=207) | |
|
| ||||||
| SLE Disease Activity: | ||||||
| Physician-assessed1 | 3.7 (2.8, 4.5) | 2.5 (2.1, 2.9) | 0.015 | 3.4 (2.6, 4.2) | 2.6 (2.2, 3.0) | 0.078 |
| Self-reported2 | 12.2 (10.3, 14.0) | 8.2 (7.3, 9.1) | <0.001 | 10.4 (9.1, 11.6) | 8.7 (8.1, 9.3) | 0.020 |
| Symptoms: | ||||||
| Pain Interference3 | 54.7 (51.8, 57.6) | 50.8 (49.4, 52.2) | 0.019 | 53.4 (49.8, 52.2) | 51.0 (49.8, 52.2) | 0.106 |
| Fatigue4 | 56.9 (53.8, 60.0) | 50.7 (49.2, 52.2) | <0.001 | 56.0 (53.6, 58.5) | 50.9 (49.7, 52.1) | <0.001 |
Adjusted means calculated from multivariable regression adjusted for age, sex, race and ethnicity, educational attainment, smoking, and disease duration. The N for the multivariable regression was 257 (vs. 260 eligible participants) as 3 participants had missing data for smoking status.
Adjusted means calculated based on covariates in primary analysis, as well as the outcome score at baseline.
Participants with an increase in Perceived Stress Scale score by ≥0.5 standard deviation (2 points) from year 2 to year 3.
Assessed by Systemic Lupus Disease Activity Index (SLEDAI), score range 0–105.
Assessed by Systemic Lupus Activity Questionnaire (SLAQ), score range 0–44.
Assessed by Patient-Reported Outcome Measurement Information System (PROMIS) 4-item Pain Interference scale, score range 41.6 – 75.6.
Assessed by PROMIS 4-item Fatigue scale, score range 33.7 – 75.89.
In the sensitivity analysis that adjusted for the score of the outcome variable at baseline in the regression model, the strength of some of the associations observed in the main analysis were attenuated (Table 3). The adjusted relationship between increase in stress and greater patient-reported disease activity at follow-up remained statistically significant (p=0.020). Similarly, the findings for the adjusted association of increase in stress with greater fatigue at follow-up were stable (p <0.001). The sensitivity analysis for increasing stress and the other two outcomes—physician-assessed disease activity and pain interference—showed that patients with more stress had numerically higher point estimates for disease activity and pain, but the differences were not statistically significant (p-values: 0.078 and 0.106, Table 3). The second sensitivity analysis that adjusted for obesity yielded results that were consistent with the results of the primary multivariable analysis (Supplemental Table B.)
Discussion
This is the largest longitudinal study to investigate the link between perceived stress and clinical outcomes in people with SLE. Though we did not see an independent association between high psychological stress and worse physician-assessed disease activity in the cross-sectional analysis, in the primary longitudinal analysis the patients who experienced an increase in stress had significantly worse physician-assessed disease activity at their final in-person visit. We also found that stress independently associated with worse patient-reported outcomes (PROs)—including greater patient-reported disease activity, pain, and fatigue—both in the cross-sectional and longitudinal analyses.
Our findings build on those of prior studies on stress and physician-assessed disease activity in SLE. Dobkin et al. conducted a cross-sectional study of 44 SLE patients and found a significant adjusted association between global psychological distress and physician-assessed SLE activity (measured with the Systemic Lupus Activity Measure-Revised instrument)(5). Similarly, in a study of 41 patients with SLE followed over six months, Pawlak et al. found that daily stress significantly associated with SLE activity the following month (7). The results presented here are most similar to those of Jung and colleagues who followed 100 SLE patients for 4–5 months and found that higher perceived stress scores at baseline did not associate cross-sectionally with SLEDAI, but that higher stress independently associated with worse SLEDAI scores at the subsequent visit (6). The difference that Jung et al. and our group observed in the relationship of stress to SLEDAI cross-sectionally versus longitudinally suggests there is a lag between experiencing high stress and developing worse lupus activity, and supports the possibility of a causal relationship. This difference also points to the superior predictive value of measuring trends in stress over time as opposed to a single measure for predicting risk of worse disease outcomes in the future.
Though the primary analysis revealed a significant relationship between increase in stress and worse absolute scores for all four disease outcomes at follow-up, the sensitivity analysis did not find a statistically significant association between worsening stress and change in the scores for SLEDAI or pain. By including the baseline score for the outcome variable as a covariate, the sensitivity analysis computed the adjusted association of stress with the change in outcomes from year 1 to year 3 (versus the absolute value of the outcome variables at year 3). Therefore, when considering the relationship of stress to SLEDAI in this study, we found that increase in stress associated with worse absolute scores at follow-up, but that it did not associate with an increase in SLEDAI from the first visit to the final in-person follow-up visit. This finding may be explained by the characteristics of the participants in the cohort—they had mostly low SLEDAI scores that were stable over time—or one could argue that baseline disease activity impacted both subsequent stress scores and disease activity at the follow-up visit. To address this uncertainty, we plan to pursue a similar future study after recruiting an SLE patient sample among whom there is greater variation in disease activity across the cohort and within individuals over time.
This study adds to a growing body of evidence that perceived stress is a strong independent predictor of worse PROs in SLE. For example, in a previous longitudinal study of 41 patients with SLE, report of high daily stress (via the Daily Stress Inventory) independently associated with worse symptom severity—including joint pain, abdominal distress, and rash—on the following day (26). In a study of 777 adults with lupus, perceived stress was identified as an important predictor of worse self-reported cognitive challenges, including forgetfulness and difficulty concentrating (27). Of particular interest given the worse health outcomes among African Americans compared to other racial groups with SLE, the investigators of the Black Women’s Experiences Living with Lupus (BeWELL) study found that racism-related psychological stress conferred an increased risk of worse patient-reported disease activity (28, 29). Taken together, our findings and the existing literature point to the critical role of psychological stress in the experience of living with SLE and help to explain the wide variability in symptom severity among patients with similar disease severity.
There are several potential mechanisms—biological, behavioral, and psychological—by which perceived stress contributes to worse outcomes in lupus. It is well established that stress exposure can lead to activation of the two physiologic stress response systems—the sympathetic autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis, both of which play an important role in regulating immune function (30). For example, the HPA axis regulates secretion of glucocorticoids (GCs), endogenous hormones with potent anti-inflammatory properties that inhibit expression of inflammatory genes both in periods of homeostasis and in disease states. One biologic mechanism by which stress may cause worse outcomes in SLE is via the “GC resistance” hypothesis: chronic stress causes reduced GC sensitivity in immune cells, thereby impairing the ability of the HPA axis to regulate the immune system (31). An alternative mechanism is that high stress can lead to sleep disturbance and unhealthy coping strategies such as smoking and substance abuse, which indirectly increase lupus activity (32–34). Furthermore, greater psychological distress has been associated with lower medication compliance (35, 36), and rapid withdrawal of immunosuppressive medications is an established cause of greater disease activity and flares in SLE (2). Finally, chronically elevated stress in the absence of sufficient social support may lead to depression and anxiety, which in turn cause impaired pain tolerance, more fatigue, and worse perceptions of overall health (37). Further studies are required to elucidate mechanisms of stress-illness effects in SLE to inform targeted interventions that improve both stress resilience and disease outcomes.
The primary limitation of this study is the observational design, which comes with the risk of unmeasured confounders and precludes the ability to make strong statements about causation between variables. We hypothesize that stress adversely impacts disease activity and PROs via both physiologic and psychosocial mechanisms, but we acknowledge that the relationship between stress and lupus activity is likely to be bidirectional given that living with more active lupus is a source of stress. However, we believe we were able to estimate the proximal effect of stress on downstream variables by evaluating the time ordering of predictor and outcome variables and using a time-lagged analytic approach to examine the relationship of changes in stress with outcomes at subsequent time points. Another limitation of this study is that most participants had long-standing disease (mean disease duration 16.6 years) and low SLEDAI scores that were stable during follow-up, thereby limiting our ability to detect predictors of changes in SLEDAI scores over time. It is uncertain how our results would differ if our cohort included a greater number of SLE patients with active disease. Finally, though the item content for the PSS and PROMIS Fatigue scales are quite distinct, it is possible that there may be some overlap in the content domains measured by these two instruments.
The limitations of this study are outweighed by several strengths. First, the sample included participants with physician-confirmed lupus who were recruited from a variety of practice settings, represented a diverse range of racial, ethnic, linguistic, and socioeconomic groups, and were followed longitudinally for at least three years. Second, we had a large sample size compared to other longitudinal studies in SLE. Third, all participants completed detailed clinical and laboratory assessments, providing an opportunity to compare the relationship of stress to objectively assessed and patient-reported disease activity. Finally, we leveraged the granular clinical and demographic data collected on each participant to adjust for potential confounders in every regression model in order to isolate the independent relationship of stress to SLE activity and PROs.
In conclusion, among a racially and ethnically diverse lupus cohort, we found that patients who experienced an increase in stress had significantly worse disease activity and greater symptom burden at follow-up compared to patients with stress levels that remained stable or declined. This finding has important clinical implications as the symptoms assessed in our study are known to have profound effects on quality of life and remain an important area of unmet need among many people with this disease (38). The relationship observed here between stress and lupus outcomes underscores the need to develop effective interventions that bolster stress resilience and support healthy coping strategies for people living with lupus. In addition to reducing psychological distress, such interventions may reduce disease activity and the severity of debilitating physical symptoms that are common in SLE.
Supplementary Material
Significance and Innovations:
This is the largest study to examine the longitudinal association between psychological stress and disease outcomes in SLE.
After adjusting for potential confounding factors, SLE patients who experienced an increase in perceived stress had greater disease activity and more severe symptoms at their follow-up visit compared to those with stress levels that remained stable or declined.
Interventions to increase stress resilience among people with SLE may improve both psychological distress and disease outcomes in this high-risk group.
Acknowledgements:
This work was funded by a grant from the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (5U01DP006486). Dr. Patterson’s effort on this study was funded by the National Center for Complementary and Integrative Health of the National Institutes of Health (K23AT011768-01).
Footnotes
Competing interests: The authors have no financial disclosures.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References:
- 1.Thanou A, Jupe E, Purushothaman M, Niewold TB, Munroe ME. Clinical disease activity and flare in SLE: Current concepts and novel biomarkers. J Autoimmun. 2021;119:102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez D, Kirou KA. What Causes Lupus Flares? Curr Rheumatol Rep. 2016;18(3):14. [DOI] [PubMed] [Google Scholar]
- 3.Pisetsky DS, Clowse MEB, Criscione-Schreiber LG, Rogers JL. A Novel System to Categorize the Symptoms of Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2019;71(6):735–41. [DOI] [PubMed] [Google Scholar]
- 4.Roberts AL, Malspeis S, Kubzansky LD, Feldman CH, Chang SC, Koenen KC, et al. Association of Trauma and Posttraumatic Stress Disorder With Incident Systemic Lupus Erythematosus in a Longitudinal Cohort of Women. Arthritis Rheumatol. 2017;69(11):2162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobkin PL, Fortin PR, Joseph L, Esdaile JM, Danoff DS, Clarke AE. Psychosocial contributors to mental and physical health in patients with systemic lupus erythematosus. Arthritis Care Res. 1998;11(1):23–31. [DOI] [PubMed] [Google Scholar]
- 6.Jung JY, Nam JY, Kim HA, Suh CH. Elevated Salivary Alpha-Amylase Level, Association Between Depression and Disease Activity, and Stress as a Predictor of Disease Flare in Systemic Lupus Erythematosus: A Prospective Case-Control Study. Medicine (Baltimore). 2015;94(30):e1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlak CR, Witte T, Heiken H, Hundt M, Schubert J, Wiese B, et al. Flares in patients with systemic lupus erythematosus are associated with daily psychological stress. Psychother Psychosom. 2003;72(3):159–65. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG. The Incidence and Prevalence of Systemic Lupus Erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis Rheumatol. 2017;69(10):1996–2005. [DOI] [PubMed] [Google Scholar]
- 9.Freemer MM, King TE Jr., Criswell LA. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. 2006;65(5):581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa A, Lovett DH, Peden EA, Zhu L, Seldin MF, Criswell LA. Renin-angiotensin system gene polymorphisms predict the progression to renal insufficiency among Asians with lupus nephritis. Genes Immun. 2005;6(3):217–24. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. [DOI] [PubMed] [Google Scholar]
- 13.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(12 Pt 1):953–62. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castrejon I, Tani C, Jolly M, Huang A, Mosca M. Indices to assess patients with systemic lupus erythematosus in clinical trials, long-term observational studies, and clinical care. Clin Exp Rheumatol. 2014;32(5 Suppl 85):S-85–95. [PubMed] [Google Scholar]
- 16.Petri M. Disease activity assessment in SLE: do we have the right instruments? Ann Rheum Dis. 2007;66 Suppl 3:iii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12(4):280–6. [DOI] [PubMed] [Google Scholar]
- 18.Yazdany J, Yelin EH, Panopalis P, Trupin L, Julian L, Katz PP. Validation of the systemic lupus erythematosus activity questionnaire in a large observational cohort. Arthritis Rheum. 2008;59(1):136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna D, Krishnan E, Dewitt EM, Khanna PP, Spiegel B, Hays RD. The future of measuring patient-reported outcomes in rheumatology: Patient-Reported Outcomes Measurement Information System (PROMIS). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 21.Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. J Appl Soc Psychol. 2012;42(6):1320–34. [Google Scholar]
- 22.Patterson SL, Sagui-Henson S, Prather AA. Measures of Psychosocial Stress and Stressful Exposures. Arthritis Care Res (Hoboken). 2020;72 Suppl 10:676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581–5. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–73. [DOI] [PubMed] [Google Scholar]
- 25.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. [DOI] [PubMed] [Google Scholar]
- 26.Adams SG Jr., Dammers PM, Saia TL, Brantley PJ, Gaydos GR. Stress, depression, and anxiety predict average symptom severity and daily symptom fluctuation in systemic lupus erythematosus. J Behav Med. 1994;17(5):459–77. [DOI] [PubMed] [Google Scholar]
- 27.Plantinga L, Lim SS, Bowling CB, Drenkard C. Perceived stress and reported cognitive symptoms among Georgia patients with systemic lupus erythematosus. Lupus. 2017;26(10):1064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter EA, Spears EC, Martz CD, Chung K, Fuller-Rowell TE, Lim SS, et al. Racism-related stress and psychological distress: Black Women’s Experiences Living with Lupus study. J Health Psychol. 2021;26(13):2374–89. [DOI] [PubMed] [Google Scholar]
- 29.Martz CD, Allen AM, Fuller-Rowell TE, Spears EC, Lim SS, Drenkard C, et al. Vicarious Racism Stress and Disease Activity: the Black Women’s Experiences Living with Lupus (BeWELL) Study. J Racial Ethn Health Disparities. 2019;6(5):1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–51. [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. [DOI] [PubMed] [Google Scholar]
- 32.Montes RA, Mocarzel LO, Lanzieri PG, Lopes LM, Carvalho A, Almeida JR. Smoking and Its Association With Morbidity in Systemic Lupus Erythematosus Evaluated by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index: Preliminary Data and Systematic Review. Arthritis Rheumatol. 2016;68(2):441–8. [DOI] [PubMed] [Google Scholar]
- 33.Palma BD, Gabriel A Jr., Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1527–32. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez Huerta MD, Trujillo-Martin MM, Rua-Figueroa I, Cuellar-Pompa L, Quiros-Lopez R, Serrano-Aguilar P, et al. Healthy lifestyle habits for patients with systemic lupus erythematosus: A systemic review. Semin Arthritis Rheum. 2016;45(4):463–70. [DOI] [PubMed] [Google Scholar]
- 35.Flower C, Hambleton I, Campbell M. The Effect of Psychosocial and Neuropsychiatric Factors on Medication Adherence in a Cohort of Women With Systemic Lupus Erythematosus. J Clin Rheumatol. 2016;22(8):411–7. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza-Pinto C, Garcia-Carrasco M, Campos-Rivera S, Munguia-Realpozo P, Etchegaray-Morales I, Ayon-Aguilar J, et al. Medication adherence is influenced by resilience in patients with systemic lupus erythematosus. Lupus. 2021;30(7):1051–7. [DOI] [PubMed] [Google Scholar]
- 37.IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, et al. Pain and Depression: A Systematic Review. Harv Rev Psychiatry. 2018;26(6):352–63. [DOI] [PubMed] [Google Scholar]
- 38.Danoff-Burg S, Friedberg F. Unmet needs of patients with systemic lupus erythematosus. Behav Med. 2009;35(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


