Abstract
Background
Pulmonary fibrosis (PF) is a lung disease characterized by scaring of lung tissue that impairs lung functions. The estimated survival time of patients with pulmonary fibrosis is 3–5 years. Bleomycin (BLM) is used clinically in the treatment of Hodgkin lymphoma and testicular germ-cell tumors. Bleomycin’s mechanism of action is the inhibition of DNA and protein synthesis. This happens when leukocytes induce the release of cytokines and chemokines which increase the pro-fibrotic and pro-inflammatory cytokines such as IL-6, TNF-alpha, IL-13, IL-1β and transforming growth factor-beta 1 (TGF-β). Crinum asiaticum L. bulbs (CAE) are widely found in parts of Africa, Asia and Indian Ocean Island. It is also prevalent in southern part of Ghana and traditionally used by the indigenes to treat upper respiratory tract infections, and for wound healing. Betulin (BET) is found in the bulbs of Crinum asiaticum L. but widely isolated from the external bark of birches and sycamore trees. Betulin as a lupine type triterpenes has been researched for their pharmacological and biological activities including anticancer, anti-inflammatory, antimicrobial activities and anti-liver fibrosis effects.
Aim of the study: The aim was to study the anti-pulmonary fibrosis effect of Crinum asiaticum L. bulbs extract and betulin in bleomycin-induced pulmonary fibrosis in mice.
Materials and method
There was a single oropharyngeal administration of bleomycin (80 mg/kg) in mice followed by the treatment of CAE and BET after 48 h of exposure to BLM.
Results
There was increased survival rate in CAE and BET treatment groups compared to the BLM induced group. There was a marked decreased in the levels of hydroxyproline, collagen I and III in the CAE and BET treatment groups compared to BLM-treated group. The treatment groups of CAE and BET significantly down regulated the levels of pro-fibrotic and pro-inflammatory cytokines concentrations such as TGF-β1, MMP9, IL-6, IL-1β and TNF-alpha compared to an increased in the BLM treated groups. The histological findings of the lungs suggested the curative effects of CAE and BET following BLM induced pulmonary fibrosis in mice, the study showed improved lung functions with wide focal area of viable alveolar spaces and few collagen fibers deposition on lungs of treatment groups.
Conclusion
CAE and BET attenuated pulmonary fibrosis by down regulating pro-fibrotic and pro-inflammatory cytokines as well as improving lung function. This could be a lead in drug discovery where compounds with anti-fibrotic effects could be developed for the treatment of lung injury.
1. Introduction
Pulmonary fibrosis (PF) is a lung disease characterized by scaring of lung tissues that impaired lung functions [1,2]. The estimated survival time of patients with pulmonary fibrosis is 3–5 years [3,4]. Bleomycin (BLM) is used clinically in the treatment of Hodgkin lymphoma and testicular germ-cell tumors, which are regarded as the most highly curable cancers [5]. The mechanism of action BLM exerts is via the inhibition of DNA and protein synthesis [6]. There is a limitation on the use of BLM in clinical setting for the treatment of cancers as there is a report on dose-dependent pneumonitis that progresses to interstitial pulmonary fibrosis [7]. It is reported that pathological changes associated with PF are oxidative stress, alveolar inflammation, fibroblast proliferation, collagen deposition, alveolar wall collapse, necrotizing alveolar and granulomatous lesion [8].
Leukocytes induce the release of cytokines and chemokines which upregulate the pro-fibrotic and pro-inflammatory cytokines such as IL-6, TNF-alpha, IL-13, IL-1β and transforming growth factor-beta 1 (TGF-β1) [9,10]. Moreover, there is proliferation and differentiation of fibrocytes derived in bone marrow or resident fibroblasts into myofibroblasts, which lead to high synthesis and accumulation of extracellular matrix (ECM), tissue reshaping and fibrotic lesions [11].
There are several causes of pulmonary fibrosis that vary etiologically and include chemicals, organic or inorganic dust, trauma and radiation [12]. The distinct pathological features are the inflammatory cell infiltration amidst honeycomb lung fibrosis within the pulmonary interstitium and alveolar cavities, which are mainly reflected in fibroblast proliferation and extracellular matrix (ECM) deposition, resulting in fibrotic foci formation [[13], [14], [15]]. When there is persistent stimulation of the accumulation of fibroblast or failure to regulate wound repair, there is the development of fibrosis that develops at any stage. Macrophages play vital role in pulmonary fibrosis in wound healing and tissue remodeling releasing several growth factors and cytokines including FGF, PDGF, TGF-β1, TGF-α, IGF-1, TNF-α, IL-6 and IL -1 [[16], [17], [18]]. Most of these soluble mediators recruit and activate fibroblasts, which will then synthesize, deposit, and organize the new tissue matrix [19,20].
Clinically, pulmonary fibrosis is a representative chronic fibrotic lung disease with the etiology unknown, characterized by a high disability rate and mortality, and a median survival time of only 3–5 years [13,21].
Pathological characteristics in PF indicate the mechanism that contribute to excessive extracellular matrix (ECM) deposition and tissue remodeling is induced by the pro-fibrotic and pro-inflammatory cytokines such as TGF-β1, IL-6, TNF-alpha, IL-1β in dysregulated processes.
The mechanism associated with pathogenesis of pulmonary fibrosis is not well elucidated [22] but the widely reported mechanism is the production of fibroblast and myofibroblast induced by the upregulation of TGF-β1 and MMP-9 that further enhance excessive accumulation of ECM deposition and remodeling [13,[23], [24], [25]]. The influx of pro-fibrotic cytokines are linked with some pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) produced [9]. The strategy to develop therapies to treat pulmonary fibrosis is to down regulate the levels of pro-fibrotic cytokines that are linked to excessive ECM deposition [26].
Irritants such as bleomycin, asbestos or silica may cause lung epithelial cell injury and are detected by macrophages. This stimulates the production of inflammatory cytokines such as IL-1β, TNF-α IL-18, IL-22, which mediate the activation of pro-fibrotic cytokines like TGF-β1 that triggers the proliferation and maturation of fibroblast. The TGF-β1 induces the activation of myofibroblast and excess ECM deposition on lungs. On the other hand, MMP9 contributes to ECM tissue remodeling and excessive deposition of ECM on lungs. Cytokines are key aggravators of BLM-induced lung injury and are secreted by alveolar macrophages. Fibroblasts are activated early in BLM-induced lung injury through stimulation of fibronectin which is produced by damaged endothelial cells or stimulation by cytokines such as TNF, platelet derived growth factor (PDGF) and TGF β1.
There is no cure for PF, the ultimate goal in treatment is to preserve patient’s lung function, decrease progression of the disease and the improvement of overall health-related quality of life for patient with PF [27,28]. Early and accurate diagnosis of PF is significant as well. Currently there are two anti-fibrotic drugs approved to slow the progression of the disease, these are nintedanib and pirfenidone which are noteworthy breakthrough in the treatment of PF [[29], [30], [31]].
It has been reported that nintedanib and pirfenidone have shown prospect in the improvement of lung function and the preservation of lung architecture but they have not shown an improvement in the survival rate of patients amidst numerous side effects [27], therefore the need to discover and develop newer therapeutic option is very important.
Natural products and compounds from plant sources have shown some prospects in the management of pulmonary fibrosis as reported recently [[32], [33], [34], [35], [36]].
Crinum asiaticum L. bulbs are widely found in some parts in Africa, Asia and in Indian Ocean Islands [37]. It is prevalent in southern part of Ghana and traditionally used by the indigenes to treat upper respiratory tract infections, and as wound healing agent [38]. The chloroform extract of Crinum asiaticum L. bulbs had been reported to be non-toxic with LD50 greater than 5000 mg/kg [38]. Other pharmacological effects of the Crinum asiaticum L. bulbs extracts reported include the anti-tubercular effects in an aerosol induced mice model [39] and the anti-efflux and anti-biofilm effects [40].
Triterpenes are important class of natural compounds with several biological and pharmacological effects [41]. Betulin is found in the bulbs of Crinum asiaticum L. but widely isolated from the external bark of birches and sycamore trees [42]. Betulin is a lupine type triterpene with various pharmacological and biological activities, including anticancer [[43], [44], [45], [46]], anti-inflammatory [47,48], antimicrobial [49,50] and anti-liver fibrosis effects [51]. The toxicity profile of betulin has been reported and the doses selected for this research work were below the lethal dose in a study conducted by Makarova in 2011 [52]. The aim of this work was to investigate the anti-pulmonary fibrosis effect of Crinum asiaticum and betulin in bleomycin induced pulmonary fibrosis in mice model therefore assessing some key pathological biomarkers.
2. Materials and methods
2.1. Materials
Crinum asiaticum L. bulbs, rotary evaporator (Buchi Labotechnik Rotavap R-210), autoclave (Sano clav), oral gavage, falcon tubes.
2.1.1. Drugs and reagents
Bleomycin sulfate (Celon Labs, India, CAT BMI2015AC), Betulin (purity >98% Chem Cruz, USA, CAT: D0522) Pirfenidone (PFD) (Cipla Pharma, India, CAT: P2011), Interleukin-6 ELISA KIT (R and D Systems, UK, CAT: M600B), TNF-alpha ELISA KIT (R and D Systems, UK, CAT: 401-MT), Interleukin-1β (Cusabio, China, CAT: CSB-E08054 m), Hydroxyproline kit (Cusabio, China, CAT: CSB-E08839 m), collagen I and III (Cusabio, China, CAT: CSB-EL005727MO and CSB-E07925 m respectively), TGF-β, MMP-9 (Cusabio, China CAT: CSB-E04726 m), chloroform (BDH Prolabo, CAT: 09H200510).
2.1.2. Processing of plant material
The bulbs of Crinum asiaticum were harvested from the Department of Horticulture garden, Kwame Nkrumah University of Science and Technology (KNUST) with GPS code 6.6790397, −1.5660286. The plant material was authenticated by Dr. Henry Sam, Department of herbal medicine, KNUST with a herbarium identification number KNUST/HM2020/B004 and was kept in the Herbarium, KNUST. The bulbs of Crinum asiaticum were washed with clean water, chopped, blended fresh with chloroform and cold macerated for 72 h with constant stirring followed by filtration. The filtrate containing the extracted bioactive compounds was concentrated using a rotary evaporator (Buchi Labotechnik Rotavap R-210). The concentrated extract was well dried, stored in containers, sealed and refrigerated.
2.1.3. Bioassay guided fractionation and the choice for betulin
Several fractions (1–56) were eluted from the bulbs extract of Crinum asiaticum L. in column chromatography. Fractions were concentrated and anti-pulmonary fibrosis activity was investigated. It was found out fraction (39) showed effects, and then TLC, GC-MS and NMR analytical techniques were conducted on the fraction and found out to contain betulin together with two other compounds. Further bioassays on the predicted compounds showed betulin as a sole contributor of the anti-fibrotic effect. Large quantity of betulin was needed therefore authors purchased pure betulin for this work.
2.1.4. Laboratory animals
Both male and female Swiss albino mice with age range 8–10 weeks and weight ranging from 15 to 20 g were purchased from Noguchi Memorial Institute for Medical Research, University of Ghana, Legon and were housed in the Animal House of the Department of Pharmacology (KNUST), Kumasi with conditions prescribed in the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Groupings and modeling
Mice were randomly divided into 6 groups with 8 mice (n = 8) per group: control (naïve), negative control (BLM-induced model), 100, 500 and 1000 mg/kg CAE treatment groups, 80 mg/kg PFD group, 25 and 50 mg/kg BET treatment groups. Mice received oropharyngeal injection of 80 mg/kg of BLM and 48 h later, mice were administered orally every day till the 20th day with 0.9% sodium chloride (naïve control), low dose of CAE (100 mg/kg), medium dose of CAE (500 mg/kg), high dose of (1000 mg/kg), low dose BET (25 mg/kg), high dose BET (50 mg/kg) and 80 mg/kg of PFD. Normal and model control mice were injected orally every day with 0.9% sodium chloride. On day 21, mice were sacrificed, lungs removed for biochemical and histological analysis.
2.3. Assessment of biomarkers for pulmonary fibrosis
Lung tissue homogenate was used to assess the level of biomarkers for pulmonary fibrosis. Such markers were hydroxyproline, collagen type I and collagen type III. Enzyme-linked immunoassay (ELISA) kits techniques were employed in their assessment. Kits purchased from Cusabio, China and assay conducted according to the commercial instruction of the kits.
2.4. Assessment of pro-fibrotic and pro-inflammatory cytokines
Pro-fibrotic and pro-inflammatory cytokines such as TNF-alpha, IL-6, IL-1β, TGF-β and MMP-9 were determined on the lung tissue exudates using ELISA kits purchased from Cusabio, China and R and D systems, UK. All experimental procedures were as prescribed by producers.
2.5. Histological analysis
The left lobe of the lung was preserved in 10% formalin, dehydrated and processed for Hematoxylin and eosin staining. Hematoxylin and eosin staining of lung morphology was performed and inflammation score, alveolitis grading and scoring criteria were calculated as reported [53,54]. Level 0: no alveolitis; level 1: mild alveolitis and the infiltrated alveolar septum were thick, but the lesions do not exceed 20% of the whole lung; level 2: moderate alveolitis and the area of alveolar inflammatory lesions accounts for 20–50% of the total lung; level 3: severe alveolitis and the lesion area exceeds 50% of the total lung area, and the alveolar cavity shows inflammatory cells and red blood cells. Masson's trichrome staining was stained according to the instructions and collagen was scored.
2.6. Statistical analysis
Results expressed as mean ± standard error of mean (SEM). Statistical analysis and graphing were carried out using Graph pad software Prism 8.01 (Graph pad Software Inc., San Diego, CA, USA). Significant differences between groups were determined using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons.
2.7. Ethical approval
Ethical clearance from the animal ethical committee Faculty of Pharmacy and Pharmaceutical Sciences, KNUST was issued with ethical code FFPS-AEC/CA05/18.
3. Results
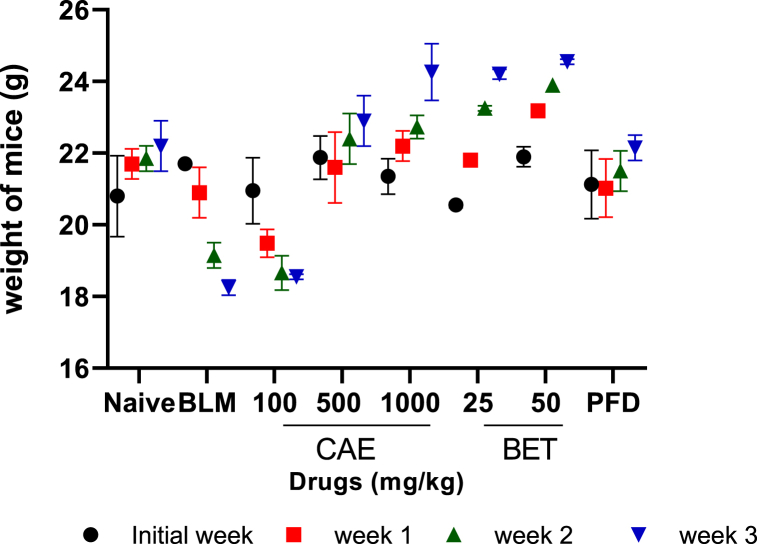
Effects of BET and CAE on body weight of mice.
The body weight of mice increased significantly (***ρ < 0.005) in the treatment groups of Crinum asiaticum chloroform extract and betulin when compared to the BLM control group which saw a steady decline in weight of the experimental animals. All animals in the naïve control group had their body weight increased.
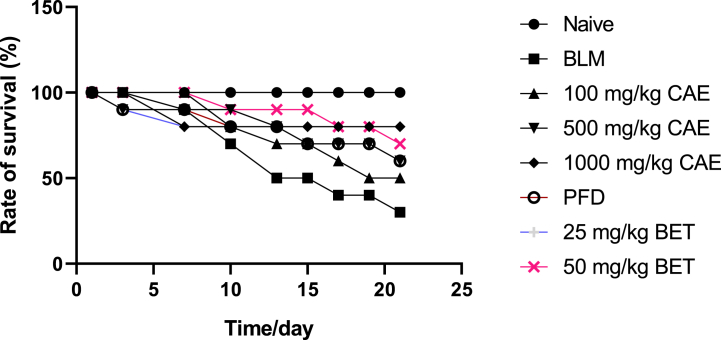
3.1. Survival rate analysis
Throughout the experimental period, the survival rate which is inversely proportional to the rate of mortality was determined and analyzed appropriately. High doses of the CAE and BET treatment groups produced higher rate of survival vis a vis a reduced mortality rate. The BLM treatment group produced significant reduced rate of survival (***ρ < 0.005) with a survival rate of 35%. The naïve group on the other hand produced 100% rate of survival.
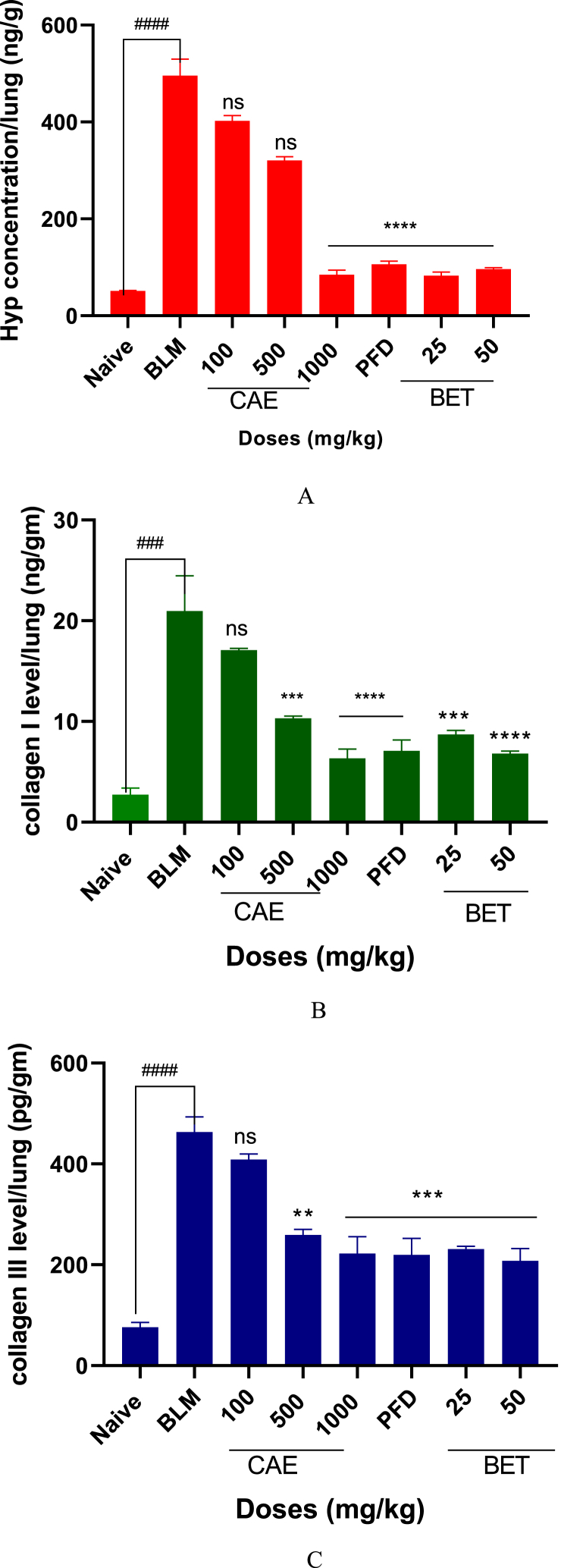
3.2. Effects of CAE and BET on hydroxyproline, collagen I and III levels
The levels of hydroxyproline, collagen I and III were significantly reduced by doses of BET and CAE (****ρ < 0.0001). The 1000 mg/kg CAE and 50 mg/kg BET produced greater effects as compared with PFD treatment group on all the biomarkers of pulmonary fibrosis.
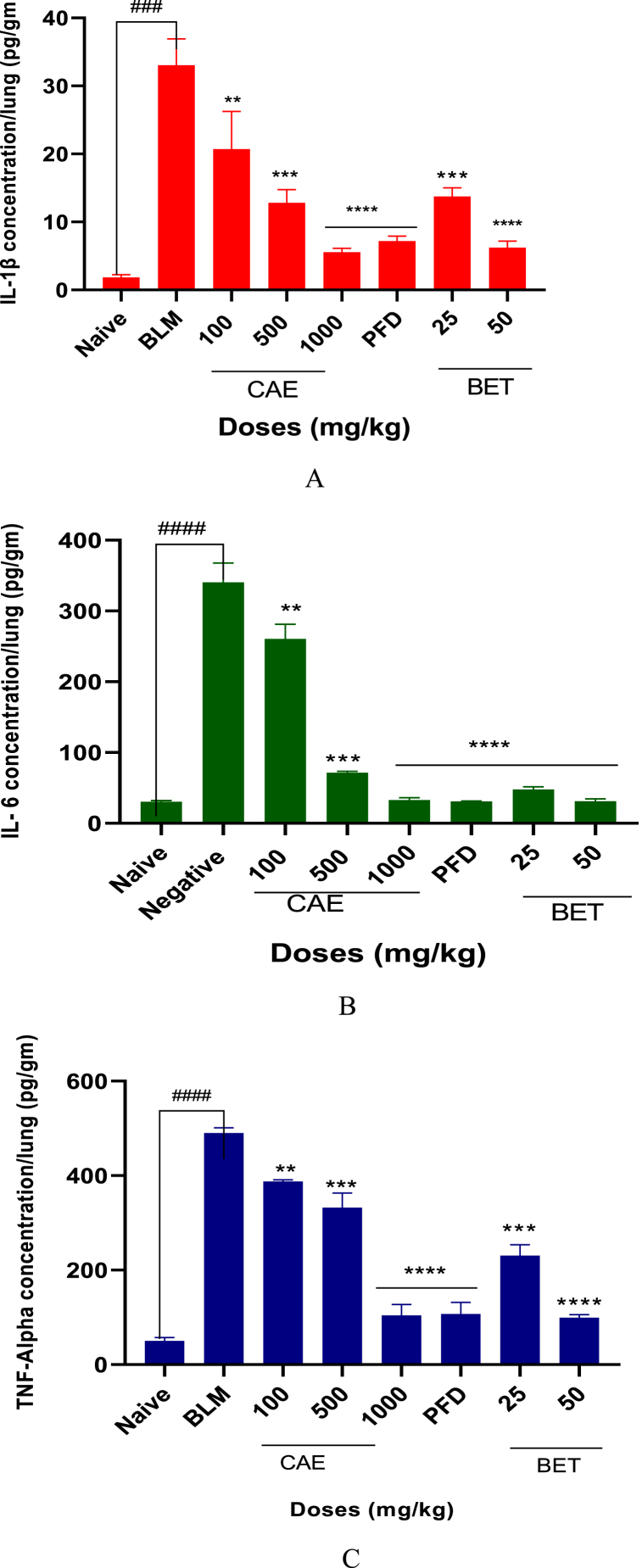
3.3. CAE and BET reduced pro-inflammatory cytokines level
Pro-inflammatory cytokines such as IL-6, IL-1β and TNF-alpha levels were reduced by the various doses of CAE and BET significantly as compared with the BLM-treated group (CAE and BET vrs BLM ***ρ < 0.005). The above listed cytokines are linked to the upregulation of TGF-β and MMP-9 and a reduced level of these cytokines are pharmacologically significant in the search for therapeutic remedies for pulmonary fibrosis.
3.4. CAE and BET reduced the level of TGF-β1 and MMP-9 in BLM induced pulmonary fibrosis in mice
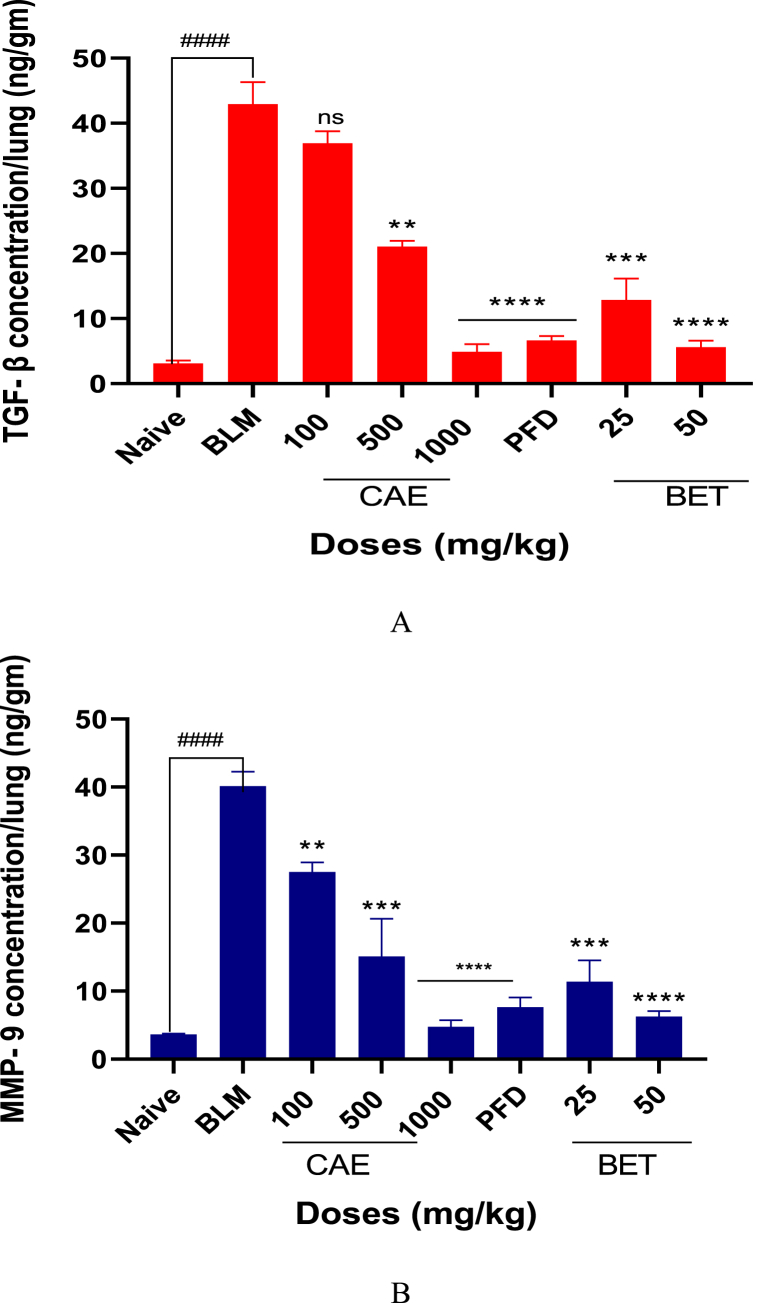
The levels of TGF-β1 and MMP-9 in the BLM induced pulmonary fibrosis in mice were significantly reduced by CAE and BET treatment groups (CAE and BET vrs BLM ****ρ < 0.0001) when compared with BLM treatment group. TGF-β1 and MMP-9 are reported to enhance fibroblast maturation, deposition and extracellular cell matrix remodeling which aggravate lung fibrosis.
3.5. Histological analysis during hematoxylin and eosin staining
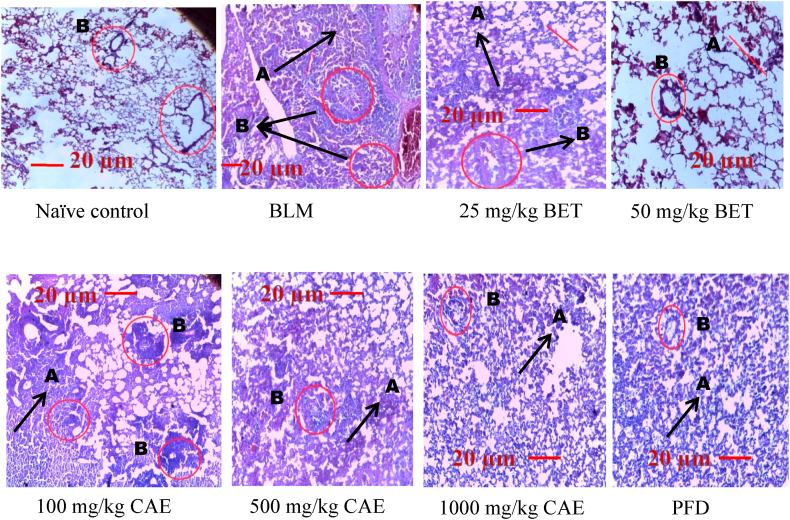
Careful observation was done carefully on the histology of the lungs from the naïve control group at x40 magnification revealed no damage to lung architecture. Alveolar morphology was intact without any defect or any signs of septal oedema, and inflammation. The BLM treated group presented with massive inflammatory cell infiltration within the bronchi and lung parenchyma, interstitial and alveolar oedema, and several blood vessels congestion. The alveoli were markedly thickened, accompanied by vascular congestion and fibrotic changes. Additionally, there were several alveolar spaces thickened with inflammatory cells infiltration, and remarkable destruction of alveolar structure was observed. The CAE and BET treatment groups showed a significant reduction in cellular infiltration and alveolar collapsed. There was improved lung function with large focal area of viable alveolar. Reduced bronchi infiltration and little vascular congestion and alveolar architecture was maintained.
3.5.1. Inflammatory score
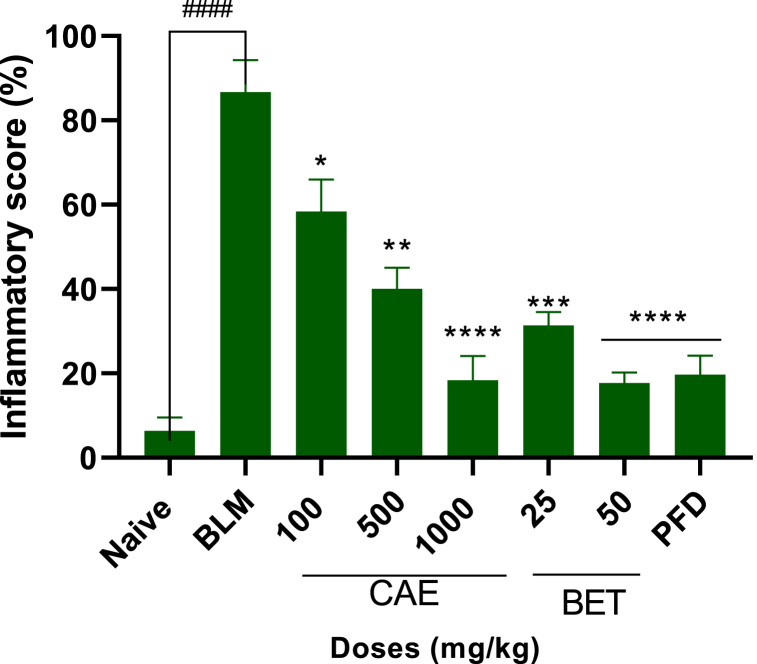
Inflammatory score was determined based on some indicators such as the severity of alveolitis and fibrosis observed on the lungs of CAE and BET treated groups. The inflammatory score for CAE and BET treatment groups was reduced significantly compared with the BLM treated group (****ρ < 0.0001).
3.6. Mason’s trichrome staining
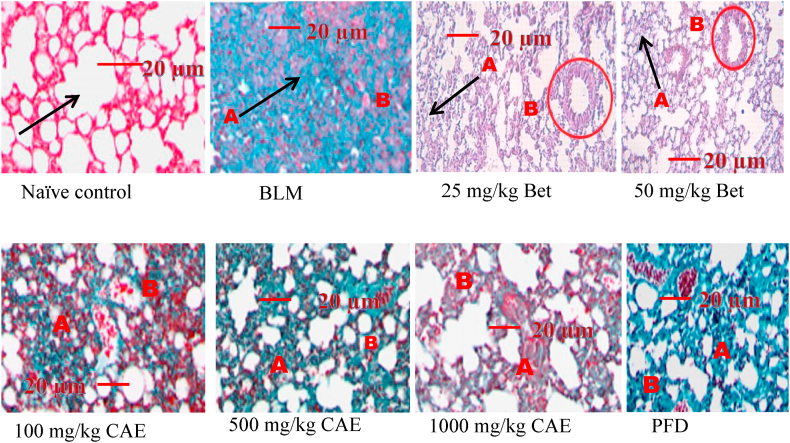
This staining technique helps in identification of collagen deposition on the lungs. This collagen forms scars during the healing process in pulmonary fibrosis and eventually impairs lung function. The BLM treated group showed high collagen deposition with several focal areas been affected. Lung architecture was seriously altered and function was eminently affected. The CAE and BET treatment groups showed reduced collagen deposition and several viable alveolar spaces. Positive index of collagen deposition was analyzed and plotted as percentage of positive index.
3.6.1. Percentage of positive index
The CAE and BET treated groups markedly showed reduced collagen deposition on the lungs improving lung function. Higher doses of CAE and BET significantly (****ρ < 0.0001) reduced fibrosis and therefore with improved lung architecture.
4. Discussion
Crinum asiaticum bulbs extract (CAE) and its active constituent betulin (BET) have shown several pharmacological effects which have been reported. The CAE extract has been reported to possess anti-tuberculosis effects in-vivo [39] and resistant modulatory effects in-vitro [39]. The compound on the other hand possess anti-tumor effects [57] and anti-liver fibrosis effect by binding Lck and suppressing Lck in HSC activation and proliferation [58]. The use of bleomycin clinically as an anticancer drug for the management of a myriad of human malignancies, has been hampered due to its detrimental effects [56]. One major side effect of bleomycin use is the induction of lung fibrosis in patients undergoing bleomycin chemotherapy. Pulmonary fibrosis is progressive and commonly an untreatable disease with an increasingly fatal outcome [59,60]. Animals models and experimentations present ways to understand the mechanisms by which pulmonary fibrosis develops as well as screening the efficacy of various compounds as potential therapeutic agents against this pathological manifestation [61,62]. The bleomycin-rodent animal model of lung fibrosis is an established and widely used surrogate model of human lung fibrosis (63–65). The use of animal model has again contributed partly in establishing the pathways of lung damage leading to fibrosis [66,67]. Inflammatory response to BLM administration which subsequently proceed to lung fibrosis has been reported [68,69]. Inflammation affected cell recruitment, pro-inflammatory cytokine, pro-fibrotic cytokines production and abnormal tissue repair contribute greatly to the fibrogenesis and extracellular matrix remodeling (Fig. 1) on the lungs [70,71].
Fig. 1.
Effect of CAE and BET on weight of mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
In this study, the survival rate and weight analysis were determined (Fig. 1, Fig. 2) and was found out that 500, 1000 mg/kg doses of CAE and 25, 50 mg/kg BET markedly increased the survival rate of the experimental animals compared to the BLM-treatment group. Again there was gradual increased in the body weight of mice in the CAE and BET treatment groups compared to the BLM-treated group which was significantly analyzed (CAE, BET vrs BLM ***ρ < 0.005). This confirms what was reported recently that agents that could be used to manage or treat PF were able to increase the survival rate of patients [71]. In the case of muscle wasting that resulted in the loss of body weight, this was attributed to the production of pro-inflammatory cytokines that reduce intracellular plasma leptin. Leptin reduction is linked with loss of appetite which vehemently affects the body weight [72,73].
Fig. 2.
Effect of CAE and BET on survival rate analysis of mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. (N = 8), ####ρ < 0.0001 for BLM vrs naïve, ***ρ < 0.005 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, **ρ < 0.005 for 25 mg/kg BET and 500 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
Hydroxyproline, collagen I and collagen III are notable biomarkers in pulmonary fibrosis [[74], [75], [76]]. Their levels are increased in PF. In a bleomycin (BLM)-induced lung fibrosis model, assessment of these biomarkers in the lung exudate showed a significant increase (Figs. 3A, B and C). The doses of CAE and BET significantly reduced the levels of these biomarkers as compared with the BLM-treated group (CAE, BET vrs BLM ****ρ < 0.0001). Type I and III collagen are produced by the fibroblasts and hydroxyproline plays essential role in the stability of collagen deposition on the lungs making them major players in pulmonary fibrosis [77,78]. It is reported that hydroxyproline, collagen I and III are accepted biomarkers in predicting drug efficacy in pulmonary fibrosis [[79], [80], [81], [82]]. Therefore their reduction is therapeutically essential in the screening of potential anti-pulmonary fibrosis agents.
Fig. 3.
A. Hydroxyproline level in CAE, BET, PFD and BLM treated mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 vrs naïve, ****ρ < 0.0001 25, 50 mg/kg BET and 1000 mg/kg CAE vrs BLM. Hydroxyproline – Hyp, Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
B. Collagen I level in CAE, BET, PFD and BLM treated mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, ***ρ < 0.005 for 25 mg/kg BET and 500 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
C. Collagen III level in CAE, BET, PFD and BLM treated mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ***ρ < 0.005 for 25, 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, **ρ < 0.05 for 500 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
Pro-inflammatory cytokines are crucially involved in the fibrogenesis process as they up-regulate TGF-β1 and MMP-9 which in turn enhance fibroblast synthesis, maturation and accumulation on the lungs as the lung epithelial is damaged [69,83]. The lung resident fibroblasts are activated by the pro-inflammatory cytokines secreted from macrophages and T cells (e.g., IL-6, TNF-α, IL-1β) and they achieve a spindle-shaped phenotype and start to produce collagen and interestingly, macrophages are able to exacerbate fibrosis by IL-1β activation [84,85]. Despite the complexity of the interplay of cytokines in fibrotic tissues, the breadth of the currently ongoing research targeting cytokines suggests that these may hold the key to mitigating tissue fibrosis and reducing organ damage in the future. In this research work, doses of BET and CAE (Figs. 4A, B and C) markedly reduced the level of IL-6, IL-1β and TNF-α compared to the BLM-treated group. The highest doses of CAE and BET, that is 1000 and 50 mg/kg respectively, significantly reduced these pro-inflammatory cytokines (CAE, BET vrs BLM ****ρ < 0.0001). This suggests that the upregulation of TGF-β and MMP-9 as the results of IL-6, IL-1β and TNF-α activation would be reduced and thereby reducing extracellular matrix accumulation.
Fig. 4.
A. IL-1β level reduced in CAE, BET, PFD and BLM treated mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ***ρ < 0.005 for 25, 50 mg/kg BET and 500 mg/kg vrs BLM, ****ρ < 0.0001 for 1000 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
B. IL-6 level reduced in CAE, BET, PFD and BLM treated mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 25, 50 mg/kg BET and 1000 mg/kg vrs BLM, ***ρ < 0.005 for 500 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
C. TNF-alpha level reduced in CAE, BET, PFD and BLM treated mice. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, ***ρ < 0.005 for 500 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
There are several findings examining the role of macrophages in pulmonary fibrosis regulation, being a major source of TGF-β1 during fibrogenesis [85,86]. They also promote fibroblast trans-differentiation and proliferation through the secretion of growth factors. Matrix metalloproteinase-9 on the other hand promotes and regulates myofibroblast activation in pulmonary fibrosis. Therefore, these pro-fibrotic cytokines are therapeutic strategies in treating pulmonary fibrosis [87].
The levels of TGF-β and MMP-9 were significantly reduced (****ρ < 0.0001) when doses of BET and CAE were administered to BLM-induced fibrosis mice model (Figs. 5A and B). This significantly showed that CAE and BET were able to treat pulmonary fibrosis by down regulating TGF-β1 and MMP-9. All statistical parameters supporting the various data sets are in the supplementary data (appendix A).
Fig. 5.
A. TGF-β1 level reduced in CAE, BET, PFD and BLM treated mice. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, ***ρ < 0.005 for 25 mg/kg BET vrs BLM. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
B. MMP-9 level reduced in CAE, BET, PFD and BLM treated mice. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, ***ρ < 0.005 for 25 mg/kg BET vrs BLM. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
Lung injury and its treatment were further confirmed through histological observation conducted on the lung tissue. Hematoxylin and eosin staining revealed focal area presented with inflammation. Indicators recorded were alveolitis, blood vessels congestion, infiltration of inflammatory mediators within the bronchi and granulomatous necrotizing within the lung parenchyma. Inflammatory score was quantified and graded in percentages as per what was reported earlier [56]. The histological results of this current study demonstrated clearly that BET and CAE exerted significant attenuation of the extent and severity of the histological signs of tissue damage as seen in the BLM-treatment group (Fig. 6, Fig. 7). There was improved lung function, reduced alveolitis, little or no blood vessels congestion and a reduced inflammatory cells infiltration in the bronchi. Inflammatory score based on inflammatory score guidelines (Table 1) suggested that BET and CAE produced significant reduction in inflammation as compared with the BLM-treatment group (CAE, BET vrs BLM ****ρ < 0.0001).
Fig. 6.
Histological analysis during H & E staining in BLM-treated group, CAE, BET and PFD treatment groups. Magnification x40. A: collapsed alveolar, B: bronchi infiltration with inflammatory cells. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
Fig. 7.
Inflammatory score for CAE, BET, PFD and BLM treated mice in BLM – induced pulmonary fibrosis in mice model. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, ***ρ < 0.005 for 25 mg/kg BET vrs BLM. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
Table 1.
Guide for inflammation score.
| Severity scores | Alveolitis | Fibrosis |
|---|---|---|
| None – 0 | No | No |
| Mild – 1 | <20% of the lung | <20% of the lung |
| Moderate – 2 | 20–50% of the lung | 20–50% of the lung |
| Severe – 3 | >50% of the lung | >50% of the lung |
In identification and quantification of collagen fibers deposition, mason’s trichrome staining technique was conducted on lung tissues [88]. It is a fact that fibroblast synthesizes collagen fibers and ground substances of extracellular matrix and moreover contributes to extracellular cell matrix remodeling [11]. Increase amount of fibroblast directly increases the amount of collagen fibers and vice versa. CAE and BET were able to reduce collagen deposition on the lung (Fig. 8) with percentage of positive index significantly reduced (Fig. 9) compared to the negative control group (****ρ < 0.0001).
Fig. 8.
Mason’s trichrome staining in BLM-treated group, CAE, BET and PFD treatment groups. Magnification x40. A: collapsed alveolar due to collagen deposition. B: collagen depositing around bronchi. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
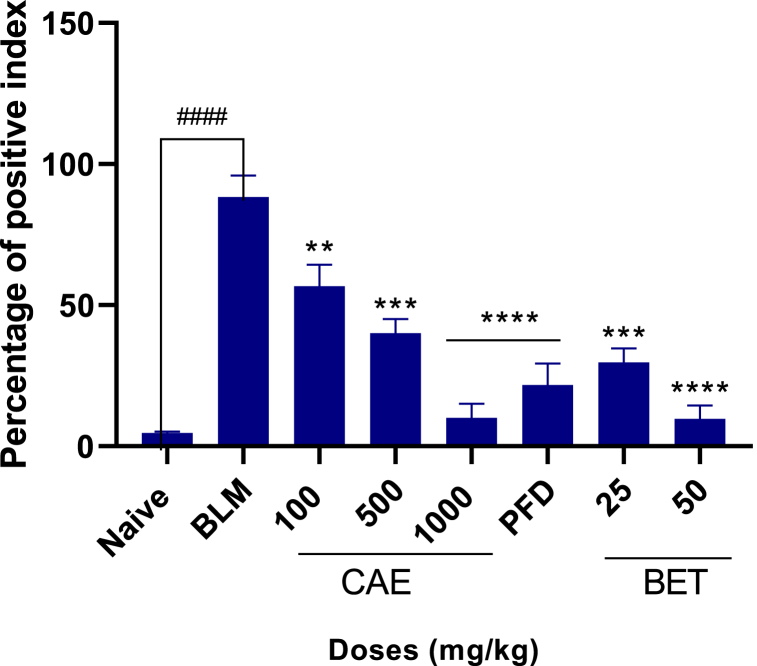
Fig. 9.
Positive index depicting collagen deposition on lungs of mice for CAE, BET, PFD after BLM – induced pulmonary fibrosis in mice model. Data were expressed as mean ± SEM with Dunnett’s multiple comparison test. N = 8, ####ρ < 0.0001 for BLM vrs naïve, ****ρ < 0.0001 for 50 mg/kg BET and 1000 mg/kg CAE vrs BLM, ***ρ < 0.005 for 25 mg/kg BET and 500 mg/kg CAE vrs BLM. Bleomycin induced group – BLM, Crinum asiaticum bulbs extract – CAE, Betulin – BET and Pirfenidone – PFD.
In summary, CAE and BET markedly reduced lung fibrosis biomarkers such as collagen and hydroxyproline deposition, pro-fibrotic cytokines such as TGF-β1 and MMP9 significantly, pro-inflammatory cytokines such as IL–1β, IL–6 and TNF–α and improved lung histology by reducing fibrotic foci and lung inflammation.
5. Conclusion
Betulin and Crinum asiaticum bulbs extract mitigated pulmonary fibrosis by improving lung function histologically, reduction of biomarkers of PF such as hydroxyproline and collagen fibers and down regulated pro-inflammatory and pro-fibrotic cytokines. This suggests that the therapeutic target of BET and CAE in PF could be attributed to down regulation of TGF-β and MMP9 which are key components in extracellular matrix remodeling and deposition on lungs.
These could serve as lead in drug discovery in developing compounds that could tackle pulmonary fibrosis.
Author contribution statement
Michael Ofori: Conceived and designed the experiment; Perform the experiment; Wrote the paper; Contributed reagents, materials, analysis tools or data.
Cynthia A. Danquah: Conceived and designed the experiment; Contributed reagents, materials, analysis tools or data.
Joshua Asante, Isaac Newton Nugbemado: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Selase Ativui, Adwoa Nkrumah Mensah: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Peace Doe: Performed the experiments; Analyzed and interpreted the data.
Alhassan Abdul-Nasir Taribabu: Conceived and designed the experiments; Performed the experiments.
Funding
This research work was not funded by any organization either been internal or external.
Data availability
Data sets are part of a PhD thesis and is made available in Kwame Nkrumah University of Science and Technology repository unit. https://ir.knust.edu.gh and also published at figshare. https://doi.org/10.6084/m9.figshare.22970378 Data would also be made available upon request from the corresponding author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- PF
Pulmonary fibrosis
- BLM
Bleomycin
- IL-6
Interleukin 6
- TNF-alpha
Tumor necrosis factor - alpha
- TGF
Transforming growth factor
- CAE
Crinum asiaticum bulbs extract
- BET
Betulin
- MMP9
Matrix metalloproteinase 9
- PDGF
Platelet derived growth factor
- ECM
Extracellular matrix
- LD50
Lethal dose 50
- PFD
Pirfenidone
- ELISA
Enzyme linked immunosorbent assay
Appendix A.
Supplementary data.
References
- 1.Li X., Liu S., Zhai Y., Cao X., Gao S., Huang M., et al. In vitro screening for compounds from Hypericum longistylum with anti-pulmonary fibrosis activity. Bioorg. Med. Chem. Lett. 2019;29(22) doi: 10.1016/j.bmcl.2019.126695. [DOI] [PubMed] [Google Scholar]
- 2.Gan W., Huang K., Lv Z., Gao S., Su C., Li X., et al. Comparison of in-vitro anti-fibrotic effects of pirfenidone and nintedanib. Eur. Respir. J. 2019;54(63):PA1282. [Google Scholar]
- 3.Zisman D.A., Keane M.P., Belperio J.A., Strieter R.M., Lynch J.P., 3rd Pulmonary fibrosis. Methods Mol. Med. 2005;117:3–44. doi: 10.1385/1-59259-940-0:003. https://pubmed.ncbi.nlm.nih.gov/16130230 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luppi F., Kalluri M., Faverio P., Kreuter M., Ferrara G. Idiopathic pulmonary fibrosis beyond the lung: understanding disease mechanisms to improve diagnosis and management. Respir. Res. 2021;22(1):1–16. doi: 10.1186/s12931-021-01711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini S., Imenshahidi M., Hosseinzadeh H., Karimi G. Biomedicine & Pharmacotherapy E ff ects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fi brosis. Biomed. Pharmacother. 2018;107:1454–1465. doi: 10.1016/j.biopha.2018.08.111. [DOI] [PubMed] [Google Scholar]
- 6.Twentyman P.R. Bleomycin--mode of action with particular reference to the cell cycle. Pharmacol. Ther. 1983;23(3):417–441. doi: 10.1016/0163-7258(83)90022-0. [DOI] [PubMed] [Google Scholar]
- 7.Chandler D.B. Possible mechanisms of bleomycin-induced fibrosis. Clin. Chest Med. 1990;11(1):21–30. [PubMed] [Google Scholar]
- 8.Kaminski N., Belperio J.A., Bitterman P.B., Chen L., Chensue S.W., Choi A.M.K., et al. vol. 29. 2003. Idiopathic Pulmonary Fibrosis. [Google Scholar]
- 9.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208(7):1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L., Li Q., Wei L., Wang Z., Ma W., Liu F., et al. Dexamethasone combined with berberine is an effective therapy for bleomycin-induced pulmonary fibrosis in rats. Exp. Ther. Med. 2019:2385–2392. doi: 10.3892/etm.2019.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson M.S., Wynn T.A. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009 Mar;2(2):103–121. doi: 10.1038/mi.2008.85. https://pubmed.ncbi.nlm.nih.gov/19129758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Liu S., Zhai Y., Cao X., Gao S. Bioorganic & Medicinal Chemistry Letters in vitro screening for compounds from Hypericum longistylum with anti- pulmonary fibrosis activity. Bioorg. Med. Chem. Lett. 2019;29(22) doi: 10.1016/j.bmcl.2019.126695. [DOI] [PubMed] [Google Scholar]
- 14.Wolters P.J., Collard H.R., Jones K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borensztajn K., Crestani B., Kolb M. Idiopathic pulmonary fibrosis : from epithelial injury to biomarkers – insights from the bench side. Respiration. 2013;18:441–452. doi: 10.1159/000357598. [DOI] [PubMed] [Google Scholar]
- 16.Sziksz E., Pap D., Lippai R., Béres N.J., Fekete A., Szabó A.J., et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. https://pubmed.ncbi.nlm.nih.gov/29765329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. https://pubmed.ncbi.nlm.nih.gov/26982353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracy L.E., Minasian R.A., Caterson E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care. 2016;5(3):119–136. doi: 10.1089/wound.2014.0561. https://pubmed.ncbi.nlm.nih.gov/26989578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olczyk P., Mencner Ł., Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res. Int. 2014;2014:12–14. doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet (London, England) 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 22.Todd N.W., Luzina I.G., Atamas S.P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):1. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biernacka A., Dobaczewski M., Frangogiannis N.G. TGF-β signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T., Yoshimi M., Maeyama T., Hagimoto N., Kuwano K., Hara N. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur. Respir. J. 2002;20(2):359–368. doi: 10.1183/09031936.02.00252602. [DOI] [PubMed] [Google Scholar]
- 25.Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta A., Scotton C.J., Chambers R.C. 2011. Themed Issue : Respiratory Pharmacology Novel Therapeutic Approaches for Pulmonary Fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalnins P., Brucker M., Spears D. 2019. Prolonged Survival in a Patient with Idiopathic Pulmonary Fibrosis Receiving Acupuncture and DHEA-Promoting Herbs with Conventional Management : A Case Report; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vega-Olivo M., Criner G.J. Idiopathic pulmonary fibrosis: a guide for nurse practitioners. Nurs. Pract. 2018;43(5):48–54. doi: 10.1097/01.NPR.0000531121.07294.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto H., Kobayashi T., Azuma A. Idiopathic pulmonary fibrosis: treatment and prognosis. Clin. Med. Insights Circulatory, Respir. Pulm. Med. 2015;9(1):179–185. doi: 10.4137/CCRPM.S23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes G., Toellner H., Morris H., Leonard C., Chaudhuri N. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J. Clin. Med. 2016;5(9) doi: 10.3390/jcm5090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cottin V., Richeldi L. Neglected evidence in idiopathic pulmonary fibrosis and the importance of early diagnosis and treatment. Eur. Respir. Rev. an Off. J. Eur. Respir. Soc. 2014;23(131):106–110. doi: 10.1183/09059180.00008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rong Y., Cao B., Liu B., Li W., Chen Y., Chen H., et al. 2018. International Immunopharmacology A Novel Gallic Acid Derivative Attenuates BLM-Induced Pulmonary Fibrosis in Mice; pp. 183–191. 64(June) [DOI] [PubMed] [Google Scholar]
- 33.Beigh S., Rashid H., Sharma S., Parvez S., Raisuddin S. ScienceDirect Bleomycin-induced pulmonary toxicopathological changes in rats and its prevention by walnut extract. Biomed. Pharmacother. 2017;94:418–429. doi: 10.1016/j.biopha.2017.07.124. [DOI] [PubMed] [Google Scholar]
- 34.Ghorashi M., Rezaee M.A., Rezaie M.J., Jalili A., Rahmani M.R. 2017. The Attenuating Effect of Aqueous Extract of Licorice on Bleomycin-Induced Pulmonary Fibrosis in Mice. [DOI] [Google Scholar]
- 35.Yao Y., Yuan Y., Lu Z., Ma Y., Xie Y., Wang M. 2021. Effects of Nervilia Fordii Extract on Pulmonary Fibrosis through TGF- β/Smad Signaling Pathway; pp. 1–16. 12(April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Y hui, Cheng M han, yu Liu X., Zhu D wei, Gao J. 2021. Sodium Houttuyfonate Inhibits Bleomycin Induced Pulmonary Fibrosis in Mice; pp. 1–12. 12(February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haque M., Jahan S., Rahmatullah M. Ethnomedicinal uses of Crinum asiaticum : a review. World J. Pharm. Pharmaceut. Sci. 2014;3(9):119–128. [Google Scholar]
- 38.Ofori M., Danquah C.A., Ossei P.P.S., Rahamani G., Asamoah W.A., Ativui S., et al. Acute and sub-acute toxicity studies of the chloroform extract of Crinum asiaticum bulbs in mice. South Afr. J. Bot. 2021;143:133–140. https://www.sciencedirect.com/science/article/pii/S0254629921003136 [Google Scholar]
- 39.Ofori M., Danquah C.A., Ossei P.P.S., Rahamani G., Nugbemado I.N., Doe P., et al. Antitubercular activities of Crinum asiaticum bulb extract using aerosolinduced Mycobacterium smegmatis in mice model. J. Appl. Pharmaceut. Sci. 2022;12(5):156–164. [Google Scholar]
- 40.Ofori M., Danquah C.A., Ativui S., Doe P., Asamoah W.A. In-vitro anti-tuberculosis, anti-efflux pumps and anti-biofilm effects of Crinum asiaticum bulbs. Biomed. Pharmacol. J. 2021;14(4):1905–1915. [Google Scholar]
- 41.Bachořík J., Urban M. Biocatalysis in the chemistry of lupane triterpenoids. Molecules. 2021;26(8) doi: 10.3390/molecules26082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csuk R., Barthel A., Sczepek R., Siewert B., Schwarz S. Synthesis, encapsulation and antitumor activity of new betulin derivatives. Arch. Pharm. (Weinheim) 2011;344(1):37–49. doi: 10.1002/ardp.201000232. [DOI] [PubMed] [Google Scholar]
- 43.Hordyjewska A., Ostapiuk A., Horecka A. Betulin and betulinic acid in cancer research. J. Pre-Clinical Clin. Res. 2018;12(2):72–75. [Google Scholar]
- 44.Gupta N., Rath S.K., Singh J., Qayum A., Singh S., Sangwan P.L. Synthesis of novel benzylidene analogues of betulinic acid as potent cytotoxic agents. Eur. J. Med. Chem. 2017;135:517–530. doi: 10.1016/j.ejmech.2017.04.062. https://www.sciencedirect.com/science/article/pii/S0223523417303367 [DOI] [PubMed] [Google Scholar]
- 45.Park C., Jeong J.W., Han M.H., Lee H., Kim G.Y., Jin S., et al. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Anim. Cell Syst. 2021;25(2):119–127. doi: 10.1080/19768354.2021.1915380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan X.K., Li J.L., Zhang S., Xing P.Y., Xia M.F. Betulinic acid exerts potent antitumor effects on paclitaxel-resistant human lung carcinoma cells (H460) via G2/M phase cell cycle arrest and induction of mitochondrial apoptosis. Oncol. Lett. 2018;16(3):3628–3634. doi: 10.3892/ol.2018.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su C.H., Lin C.Y., Tsai C.H., Lee H.P., Lo L.C., Huang W.C., et al. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. J. Funct.Foods. 2021;86 https://www.sciencedirect.com/science/article/pii/S1756464621003789 [Internet] [Google Scholar]
- 48.Chunhua M., Long H., Zhu W., Liu Z., Jie R., Zhang Y., et al. Betulin inhibited cigarette smoke-induced COPD in mice. Biomed. Pharmacother. 2017;85:679–686. doi: 10.1016/j.biopha.2016.11.079. https://www.sciencedirect.com/science/article/pii/S0753332216322636 [DOI] [PubMed] [Google Scholar]
- 49.Viszwapriya D., Subramenium G.A., Radhika S., Pandian S.K. Betulin inhibits cariogenic properties of Streptococcus mutans by targeting vicRK and gtf genes. Antonie Leeuwenhoek. 2017;110(1):153–165. doi: 10.1007/s10482-016-0785-3. [DOI] [PubMed] [Google Scholar]
- 50.Amiri S., Dastghaib S., Ahmadi M., Mehrbod P., Khadem F., Behrouj H., et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020;38 doi: 10.1016/j.biotechadv.2019.06.008. December 2018. [DOI] [PubMed] [Google Scholar]
- 51.Wan Y., Jiang S., Lian L.H., Bai T., Cui P.H., Sun X.T., et al. Betulinic acid and betulin ameliorate acute ethanol-induced fatty liver via TLR4 and STAT3 in vivo and in vitro. Int. Immunopharmacol. 2013;17(2):184–190. doi: 10.1016/j.intimp.2013.06.012. http://europepmc.org/abstract/MED/23816536 [DOI] [PubMed] [Google Scholar]
- 52.Makarova M., Shikov A., Avdeeva O. Evaluation of acute toxicity of betulin. Planta Med. 2011;77 [Google Scholar]
- 53.Guide for the care and use of laboratory animals . 2011. Guide for the Care and Use of Laboratory Animals. 8th Editio. National Academies Press. [Google Scholar]
- 54.Yang F., Hou Z feng, Zhu H yue, Chen X xuan, yang Li W. 2021. Catalpol Protects against Pulmonary Fibrosis through Inhibiting TGF- β 1/Smad3 and Wnt/β -Catenin Signaling Pathways; pp. 1–14. 11(January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma R., Kushwah L., Gohel D., Patel M. 2017. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin- Induced Pulmonary Fibrosis in Wistar Rats Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. October 2013. [Google Scholar]
- 57.Zhang X., Hu J., Chen Y. Betulinic acid and the pharmacological effects of tumor suppression (Review) Mol. Med. Rep. 2016;14(5):4489–4495. doi: 10.3892/mmr.2016.5792. [DOI] [PubMed] [Google Scholar]
- 58.Zhao H., Wu L., Zhang Y., Feng S., Ding Y., Deng X., et al. Betulinic acid prevents liver fibrosis by binding Lck and suppressing Lck in HSC activation and proliferation. J. Ethnopharmacol. 2022:296. doi: 10.1016/j.jep.2022.115459. https://www.sciencedirect.com/science/article/pii/S0378874122004986 115459. [DOI] [PubMed] [Google Scholar]
- 59.Fraser E., Hoyles R.K. Therapeutic advances in idiopathic pulmonary fibrosis. Clin. Med. 2016;16(1):42–51. doi: 10.7861/clinmedicine.16-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans C.M., Fingerlin T.E., Schwarz M.I., Lynch D., Kurche J., Warg L., et al. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol. Rev. 2016;96(4):1567–1591. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore B B., Lawson W.E., Oury T.D., Sisson T.H., Raghavendran K., Hogaboam C.M. Animal models of fibrotic lung disease. Am. J. Respir. Cell Mol. Biol. 2013;49(2):167–179. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walters D.M., Kleeberger S.R. Mouse models of bleomycin-induced pulmonary fibrosis. Curr. Protoc. Pharmacol. 2008;(40):1–17. doi: 10.1002/0471141755.ph0546s40. [DOI] [PubMed] [Google Scholar]
- 66.Tashiro J., Rubio G.A., Limper A.H., Williams K., Elliot S.J., Ninou I., et al. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front. Med. 2017;4:1–11. doi: 10.3389/fmed.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miles T., Hoyne G.F., Knight D.A., Fear M.W., Mutsaers S.E., Prêle C.M. The contribution of animal models to understanding the role of the immune system in human idiopathic pulmonary fibrosis. Clin. Transl. Immunol. 2020;9(7):e1153. doi: 10.1002/cti2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotani T., Ikemoto M., Matsuda S., Masutani R., Takeuchi T. Human MIKO-1, a hybrid protein that regulates macrophage function, suppresses lung fibrosis in a mouse model of bleomycin-induced interstitial lung disease. Int. J. Mol. Sci. 2022;23(17):1–9. doi: 10.3390/ijms23179669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oatis D., Simon-Repolski E., Balta C., Mihu A., Pieretti G., Alfano R., et al. Cellular and molecular mechanism of pulmonary fibrosis post-COVID-19: focus on galectin-1, -3, -8, -9. Int. J. Mol. Sci. 2022;23(15) doi: 10.3390/ijms23158210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian F., Jiang T., Qi X., Zhao Z., Li B., Aibibula M., et al. Role of cytokines on the progression of liver fibrosis in mice infected with echinococcus multilocularis. Infect. Drug Resist. 2021;14:5651–5660. doi: 10.2147/IDR.S344508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steen E.H., Wang X., Balaji S., Butte M.J., Bollyky P.L., Keswani S.G. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv. Wound Care. 2020;9(4):184–198. doi: 10.1089/wound.2019.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webster J.M., Kempen L.J.A.P., Hardy R.S., Langen R.C.J. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.597675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Londhe P., Guttridge D.C. Inflammation induced loss of skeletal muscle. Bone. 2015;80:131–142. doi: 10.1016/j.bone.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stainer A., Faverio P., Busnelli S., Catalano M., Zoppa M Della, Marruchella A., et al. Molecular biomarkers in idiopathic pulmonary fibrosis: state of the art and future directions. Int. J. Mol. Sci. 2021;22(12) doi: 10.3390/ijms22126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terashima H., Aonuma M., Tsuchida H., Sugimoto K., Yokoyama M., Kato M. Attenuation of pulmonary fibrosis in type I collagen-targeted reporter mice with ALK-5 inhibitors. Pulm. Pharmacol. Ther. 2019;54:31–38. doi: 10.1016/j.pupt.2018.11.005. https://www.sciencedirect.com/science/article/pii/S1094553918300932 [DOI] [PubMed] [Google Scholar]
- 76.Wybranowski T., Pyskir J., Bosek M., Napiórkowska M., Cyrankiewicz M., Ziomkowska B., et al. The mortality risk and pulmonary fibrosis investigated by time-resolved fluorescence spectroscopy from plasma in COVID-19 patients. J. Clin. Med. 2022;11(17) doi: 10.3390/jcm11175081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Onursal C., Dick E., Angelidis I., Schiller H.B., Staab-Weijnitz C.A. Collagen biosynthesis, processing, and maturation in lung ageing. Front. Med. 2021;8 doi: 10.3389/fmed.2021.593874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karna E., Szoka L., Huynh T.Y.L., Palka J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020;77(10):1911–1918. doi: 10.1007/s00018-019-03363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Organ L.A., Duggan A.M.R., Oballa E., Taggart S.C., Simpson J.K., Kang’ombe A.R., et al. Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir. Res. 2019;20(1):148. doi: 10.1186/s12931-019-1118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu X., Luo S., Li B., Dai H., Zhang J. Feature Article: IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Exp. Biol. Med. 2019;244(9):770–780. doi: 10.1177/1535370219843827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Kashef D.H. Nicorandil ameliorates pulmonary inflammation and fibrosis in a rat model of silicosis. Int. Immunopharm. 2018;64:289–297. doi: 10.1016/j.intimp.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 82.Weiskirchen R., Weiskirchen S., Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol. Aspect. Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Chuliá-Peris L., Carreres-Rey C., Gabasa M., Alcaraz J., Carretero J., Pereda J. Matrix metalloproteinases and their inhibitors in pulmonary fibrosis: EMMPRIN/CD147 comes into play. Int. J. Mol. Sci. 2022;23(13) doi: 10.3390/ijms23136894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bignold R., Johnson J.R. Effects of cytokine signaling inhibition on inflammation-driven tissue remodeling. Curr Res Pharmacol drug Discov. 2021;2 doi: 10.1016/j.crphar.2021.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yue X., Shan B., Lasky J.A. TGF-Β: titan of lung fibrogenesis. Curr. Enzym. Inhib. 2010;6(2) doi: 10.2174/10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogawa T., Shichino S., Ueha S., Matsushima K. Macrophages in lung fibrosis. Int. Immunol. 2021;33(12):665–671. doi: 10.1093/intimm/dxab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Craig V.J., Zhang L., Hagood J.S., Owen C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015;53(5):585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zorina A., Zorin V., Kudlay D., Kopnin P. Molecular mechanisms of changes in homeostasis of the dermal extracellular matrix: both involutional and mediated by ultraviolet radiation. Int. J. Mol. Sci. 2022;23(12) doi: 10.3390/ijms23126655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets are part of a PhD thesis and is made available in Kwame Nkrumah University of Science and Technology repository unit. https://ir.knust.edu.gh and also published at figshare. https://doi.org/10.6084/m9.figshare.22970378 Data would also be made available upon request from the corresponding author.