Abstract
Key Clinical Message
Although intravascular lymphoma rarely presents with peripheral neuropathy, learning about this presentation can lead to timely diagnosis and improved prognosis in patients with intravascular lymphoma.
Abstract
A 64‐year‐old man presented with asymmetric paresthesia and subsequent weakness of his feet and a 10 kg weight loss over 40 days. Electrodiagnostic studies revealed distal axonal sensory‐motor polyneuropathy with ongoing axonal loss. A peroneal nerve biopsy showed intravascular proliferation of CD‐20 positive lymphocytes, which suggested intravascular large B‐cell lymphoma.
Keywords: case report, diagnosis, intravascular large B‐cell lymphoma, mononeuritis multiplex, neuropathy
1. INTRODUCTION
Mononeuropathy multiplex (MM) is a painful neuropathy involving the sensory and motor peripheral nerves in two separate nerve areas simultaneously. 1 , 2
The differential diagnoses associated with MM include a wide range of systemic disorders such as diabetes mellitus, vasculitis, amyloidosis, systemic lupus erythematosus (SLE), viral infections such as AIDS (acquired immunodeficiency syndrome), hepatitis, parvovirus B19, multiple compression neuropathies, and paraneoplastic syndromes. 3 , 4 , 5 , 6 One possible, albeit rare, cause is lymphoma. 7
Intravascular lymphoma (IVL), a rare B‐cell lymphoma, involves an aggressive intravascular overgrowth of neoplastic B‐lymphocytes in small to medium‐sized vessels. 8 The resulting deficits in vascular supply to organs produce a range of systemic and neurologic symptoms that often overlap with those of other diseases, especially vasculopathies. 9 The most common symptoms include skin lesions and fever/chills. The majority of patients with intravascular lymphoma who develop neurologic symptoms show central nervous symptoms such as cognitive or motor deficits. 9 A systematic review of reported cases estimated that the peripheral nervous system was involved in a minority of 9.5% of patients who experienced some degree of involvement as a late finding discovered only after diagnosis. 9
Diagnosing intravascular lymphoma is challenging because it is rare and it presents with a wide variety of symptoms. 11 Moreover, its diagnosis relies on clinical suspicion and tissue biopsy. 10 On the other hand, this type of non‐Hodgkin lymphoma is lethal within a year unless diagnosed and treated early. 11 These issues highlight the importance of early accurate diagnosis and treatment and the role of a high index of suspicion for this life‐threatening disease in patients presenting with symptoms suggesting vascular involvement. 11 , 12 , 13
As intravascular lymphoma and its neurologic manifestations are rare, and the disease course is short with a fatal outcome, the study of their clinical course is limited to case reports and case series. 11 Previous case reports have reported patients with intravascular lymphoma presenting with peripheral neuropathy; however, MM as the primary presentation is extremely rare. 7 , 14 , 15 , 16 , 17 , 18 Previously reported patients were diagnosed in postmortem autopsies after an initial misdiagnosis of vasculitis. 18 , 19
We report a case in which MM was the core manifestation of intravascular large B‐cell lymphoma (IVLBCL) and was diagnosed based on a nerve biopsy.
2. CASE PRESENTATION
A 64‐year‐old Iranian man presented to our outpatient neurology clinic with paresthesia of distal lower extremities that had started in the left lower extremity and progressed to the right side. Within 1 week, he developed asymmetrical weakness in distal lower extremities that sequentially involved both proximal lower extremities over 1 month.
He also reported a loss of appetite and a 10 kg weight loss within one and a half months.
He did not take any medications, did not smoke or use illicit drugs, and had no history of exposure to chemicals or toxins. He had no history of autoimmune or neoplastic diseases and his family history was unremarkable.
Upon physical examination, he was a middle‐aged man with average body habitus. His general physical examination, including examination of the skin and lymph nodes, was unremarkable. The neurologic exam was significant for decreased muscle force in lower extremities that was more severe on the left side and absent deep tendon reflexes in the lower limbs. He had asymmetric distal hypoesthesia in both upper and lower limbs. His first dorsal interosseous muscle was atrophic on both sides.
The patient was admitted to the neurology ward for further workup and emergency treatment with a clinical diagnosis of multiple mononeuropathy. Electrophysiologic studies revealed distal axonal sensory‐motor polyneuropathy with ongoing axonal loss and multiple mononeuropathy (details of EMG–NCS can be found in Appendix S1). Initial lab tests revealed microcytic anemia. He underwent chest and abdominopelvic CT with contrast and left superficial peroneal nerve biopsy. Moreover, laboratory investigations were done in search of an underlying systemic disease that could cause anemia and multiple mononeuropathy (Table 1).
TABLE 1.
Para‐clinical work‐up for our patient who presented with mononeuropathy multiplex.
| Para‐clinical assessment | Normal range | |
|---|---|---|
| Hematologic | ||
| CBC | ||
| Hb | 9.9 | M: 14–18 |
| F: 12–16 | ||
| WBC | 4.6 | 4–10 |
| Plt | 236,000 | 140–440 |
| MCV | 69.4 | 77–97 |
| RDW | 21 | 11.8–14.5% |
| Retic count | 2.8% | 0.5–2.5% |
| Serum iron | 10 | 65–175 |
| Ferritin | 600 | 30–300 |
| Transferrin | 170 | 200–360 |
| ESR | 45 | 0–22 |
| Serum electrophoresis | Monoclonal IgA negative | |
| Urine electrophoresis | Monoclonal IgA negative | |
| Rheumatologic and vasculitis | ||
| ANA | Normal | |
| Anti‐Ro | Normal | |
| Anti‐La | Normal | |
| RF | Normal | |
| C3 | Normal | |
| C4 | Normal | |
| p‐ANCA | Normal | |
| c‐ANCA | Normal | |
| Paraneoplastic | ||
| CA19‐9 | Negative | |
| CA15‐3 | Negative | |
| CEA | Negative | |
| PSA | Negative | |
| AFP | Negative | |
| βHCG | Negative | |
| Infectious | ||
| HCV Ab | Negative | |
| HIV | Negative | |
| Viral markers | Negative | |
| VDRL | Negative | |
| PPD | Negative | |
| Toxins | ||
| Serum Pb | Normal | |
| Metabolic | ||
| HbA1C | Normal | |
| Neoplastic | ||
| Abdominopelvic CT | Splenomegaly | |
| Endoscopy and colonoscopy | No significant abnormalities | |
| Bronchoscopy | No malignant cell in biopsy | |
| Bone marrow aspiration and biopsy | Normocellular marrow with mild megaloid changes in erythroid series | |
Note: Cell counts were performed using the automated cell counter Sysmex® KP300. Biochemistry analyses were done using the Roche Hitachi 917 Rack Chemistry Analyzer, Japan; Serologic markers were checked using the ELISA kits from Autobio Diagnostics Co. and Liason Autobio A 2000 automated ELISA reader.
Abbreviations: AFP, alpha‐fetoprotein; ANA, antinuclear antibody; CBC, complete blood count; CEA, carcinoembryonic antigen; CT, computed tomography; ESR, erythrocyte sedimentation rate; HbA1C, glycated hemoglobin; HCV, hepatitis C virus; HIV, human immunodeficiency virus; p‐ANCA, perinuclear antineutrophil cytoplasmic antibodies; Pb, lead; PPD, purified protein derivative; PSA, prostate‐specific antigen; RF, rheumatoid factor; VDRL, venereal disease research laboratory (VDRL); βHCG, beta‐human chorionic gonadotropin.
He was treated empirically with 5 g of iv methylprednisolone followed by 50 mg daily oral prednisolone for 2 weeks.
The histopathological assessment of peroneal nerve biopsy revealed intravascular proliferation of large atypical lymphocytes. The cells were positive for CD‐20 on IHC staining (Figure 1). Further IHC specification was not feasible due to the few number of available cells.
FIGURE 1.
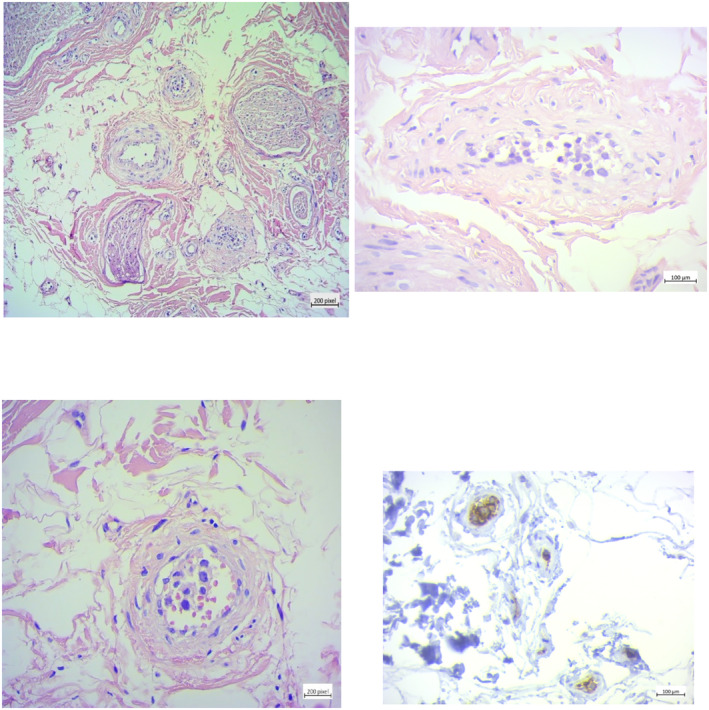
Histopathologic assessment of peroneal nerve biopsy; sections show unremarkable nerve bundles. Adjacent vessels are stuffed with large pleomorphic lymphoid cells. Cells have a high N/C ratio and hyperchromatic nuclei, and scant cytoplasm. Immunohistochemical (IHC) staining shows positive reactivity for CD20, and the diagnosis was reported as intravascular large B‐cell lymphoma(CD20+).
The final diagnosis was intravascular large B‐cell lymphoma, presenting as mononeuritis multiplex. The patient was referred to an oncology clinic where he received chemotherapy with a combination of doxorubicin, rituximab, cyclophosphamide, and vincristine for about 6 months. Along with chemotherapy, the patient continued receiving oral prednisolone at a dose of 100 mg per day.
2.1. Outcome and follow‐up
We visited the patient for follow‐up 4 months after he completed his first cycle of chemotherapy. His weakness had subjectively improved and his Overall Neuropathy Limitations Scale (ONLS) had improved from 1 to 0 in arms and from 4 to 2 in legs.
However follow‐up electrodiagnostic studies showed progression in axonal loss and worsening of polyneuropathy. We postulate that the reason was disease progression and chemotherapy‐induced axonal damage.
He came back to the clinic after 2 years of his first symptoms complaining of worsening weakness; his ONLS had improved to 2 in arms and 3 in legs. We performed a head‐to‐toe examination and found new skin lesions in his abdomen (Figure 2). The skin lesion was biopsied and the histopathologic study confirmed the recurrence of intravascular large‐B‐cell lymphoma. The timeline in Appendix S2 summarizes the disease course in our patient.
FIGURE 2.
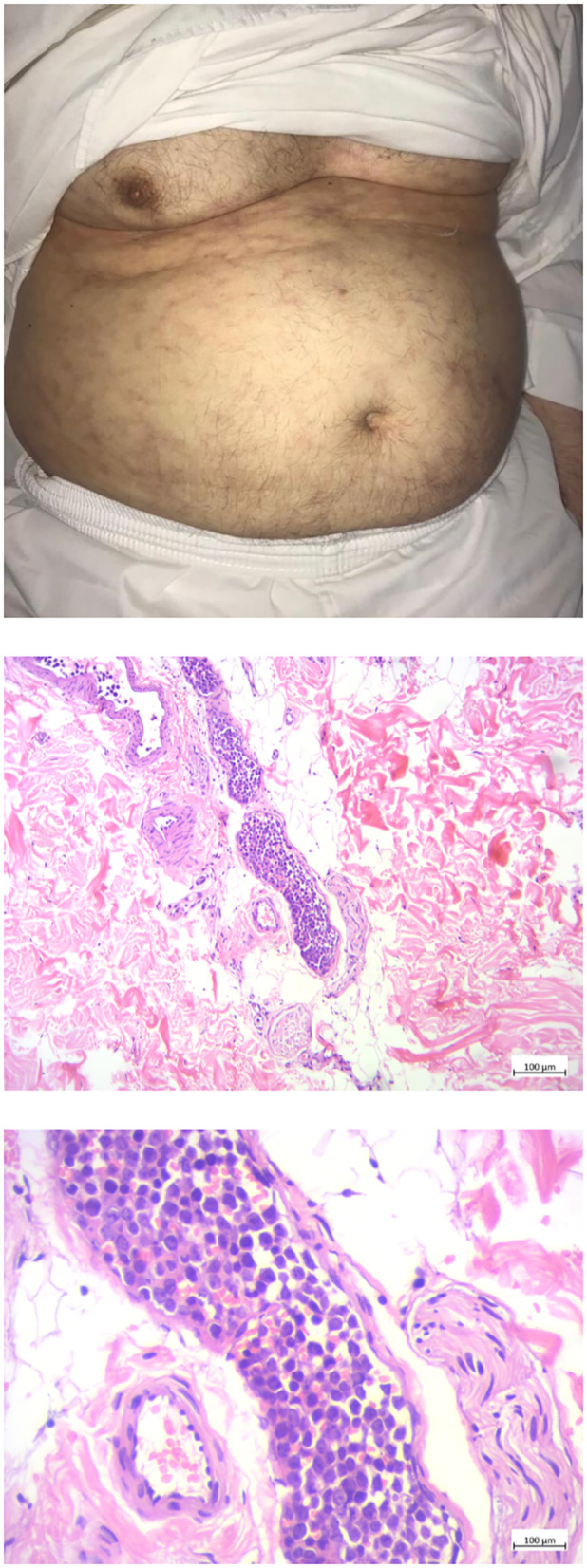
The abdominal skin lesion and the biopsy specimens of abdominal skin lesions; Left: Patient's abdomen upon examination on follow‐up 2 years post‐presentation. The skin shows scattered violaceous telangiectasiae and retiform purpurae. Middle and right: sections show intravascular proliferation of atypical lymphocytic cells inside the dermis. Left: low magnification, right: high magnification.
3. DISCUSSION AND CONCLUSION
We report a 64‐year‐old Iranian man with intravascular large B‐cell lymphoma who was referred to our clinic with paresthesia of distal lower extremities and motor symptoms that developed subsequently. These findings and later electromyography and nerve conduction studies were clinically compatible with a multiple mononeuritis pattern of involvement. The patient underwent a thorough work‐up, the results of which were inconclusive. A nerve biopsy was done, and the findings led to a diagnosis of intravascular lymphoma.
Notably, our patient did not have any specific symptoms that specifically pointed to a diagnosis of lymphoma. A few cases of intravascular B‐cell lymphoma have been reported that were associated with multiple mononeuropathy during the course of the illness. However, in most of these cases, MM was a late finding in the course of the disease, following weeks to months after the diagnosis that is usually based on other symptoms such as skin lesions, fever, or rigors. 7 , 15 , 16 Another case received an inaccurate diagnosis of vasculitis, which was revealed only after autopsy. 7
Patients with intravascular lymphoma most commonly present with symptoms related to the involvement of the central nervous system (39%) and skin (39%). Fever and skin lesions are common. 5 , 8 , 20 Bone marrow (32%), spleen (26%), and liver (26%) are less frequently involved. 17 Our patient did not have any evidence of CNS involvement at presentation, nor did he have fever or skin lesions. Also, bone marrow biopsy and aspiration did not show any significant pathological changes. However, splenomegaly was seen in his abdominal CT scan. Previous reports of similar cases are compatible with associated infiltration of the spleen and liver. 7
More importantly, our patient presented with mononeuropathy multiplex and weight loss without other symptoms. This is a rare presentation in IVLBCL but has been previously reported. 13 , 16 In a case series of 26 patients with lymphoma‐associated neuropathy, 6 patients had an MM pattern. 7 , 21 , 22 Most of these patients had a favorable hematological prognosis except for one patient who did not respond to chemotherapy and died as a result of infectious complications of bone marrow transplantation. Half of the patients experienced neurological improvement after chemotherapy. 16 In general, the prognosis is very poor for patients with intravascular lymphoma, with most of them dying within 1 year of their diagnosis. 7 , 9 , 15 , 16 Although our patient's neurological disability did not completely respond to chemotherapy and he experienced a relapse of IVLBCL in the skin, he had a favorable survival of more than 2 years after the initial presentation, partly due to timely diagnosis and early treatment.
Our case report, in line with previous reports, highlights the importance of considering neurolymphomatosis and intravascular lymphoma as possible causes of MM. Specifically, a nerve biopsy with an assessment of clonal perivascular infiltrates may aid clinicians in differentiating between intravascular neoplastic infiltration from vasculitis.
AUTHOR CONTRIBUTIONS
Bahram Haghi‐Ashtiani: Conceptualization; data curation; validation; writing – review and editing. Parichehr Moghaddam: Data curation; writing – review and editing. Farzaneh Barzkar: Data curation; validation; writing – original draft; writing – review and editing. Ali Z. Mehrjerdi: Data curation; formal analysis; resources; writing – review and editing. Mostafa Almasi‐Dooghaee: Conceptualization; data curation; supervision; visualization; writing – review and editing.
CONSENT TO PUBLISH
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Supporting information
Appendix S1.
Appendix S2.
ACKNOWLEDGMENTS
The authors did not receive any funding for this article. The authors declare that they have no competing interests.
Haqi‐Ashtiani B, Moghaddam P, Barzkar F, Zare Mehrjerdi A, Almasi‐Dooghaee M. Mononeuropathy multiplex as an uncommon presentation of intravascular lymphoma: A case report. Clin Case Rep. 2023;11:e7575. doi: 10.1002/ccr3.7575
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Böck K, Pschaid C, Topakian R, et al. Mononeuritis multiplex: association with infectious condition and familial background in a tropical environment: a case report. Wien Klin Wochenschr. 2014;126:42‐45. [DOI] [PubMed] [Google Scholar]
- 2. Donofrio PD. Textbook of Pperipheral neuropathy. Demos Medical Publishing; 2012. [Google Scholar]
- 3. Ekiz E, Ozkok A, Ertugrul NK. Paraneoplastic mononeuritis multiplex as a presenting feature of adenocarcinoma of the lung. Case Rep Oncol Med 2013;2013, 2013, 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huda S, Krishnan A. An unusual cause of mononeuritis multiplex. Pract Neurol. 2013;13:39‐41. [DOI] [PubMed] [Google Scholar]
- 5. Ferrari S, Vento S, Monaco S, et al. Human immunodeficiency virus‐associated peripheral neuropathies. Mayo Clin Proceed. 2006;81:213‐219. [DOI] [PubMed] [Google Scholar]
- 6. Bougea A, Anagnostou E, Spandideas N, Triantafyllou N, Kararizou E. An update of neurological manifestations of vasculitides and connective tissue diseases: a literature review. Einstein (Sao Paulo). 2015;13:627‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roux S, Grossin M, De Bandt M, Palazzo E, Vachon F, Kahn MF. Angiotropic large cell lymphoma with mononeuritis multiplex mimicking systemic vasculitis. J Neurol Neurosurg Psychiatry. 1995;58:363‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chapin JE, Davis LE, Kornfeld M, Mandler RN. Neurologic manifestations of intravascular lymphomatosis. Acta Neurol Scand. 1995;91:494‐499. [DOI] [PubMed] [Google Scholar]
- 9. Fonkem E, Dayawansa S, Stroberg E, et al. Neurological presentations of intravascular lymphoma (IVL): meta‐analysis of 654 patients. BMC Neurol. 2016;16:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang QL, Pytel P, Rowin J. Disseminated intravascular large‐cell lymphoma with initial presentation mimicking Guillain–Barré syndrome. Muscle Nerve. 2010;42:133‐136. [DOI] [PubMed] [Google Scholar]
- 11. Kamiya‐Matsuoka C, Shroff S, Gildersleeve K, Hormozdi B, Manning JT, Woodman KH. Neurolymphomatosis: a case series of clinical manifestations, treatments, and outcomes. J Neurol Sci. 2014;343:144‐148. [DOI] [PubMed] [Google Scholar]
- 12. Petluri G, Goyal MK, Singla V, et al. Neurolymphomatosis: a rare cause of multiple mononeuropathy. World. J Neurosci. 2014;04:190‐193. [Google Scholar]
- 13. Chamberlain MC, Fink J. Neurolymphomatosis: a rare metastatic complication of diffuse large B‐cell lymphoma. J Neurooncol. 2009;95:285‐288. [DOI] [PubMed] [Google Scholar]
- 14. Ho S, Tang B, Chai J, Tan S. Cutaneous large B‐cell lymphoma of the leg: presenting initially as mononeuritis multiplex. Singapore Med J. 2009;50:e158‐e160. [PubMed] [Google Scholar]
- 15. Pokharna R, Sen S, Schaefer C. Intravascular large b‐cell lymphoma as mono‐neuritis multiplex. Open Access J Neurol Neurosurg. 2017;4:59‐62. [Google Scholar]
- 16. Lynch KM, Katz JD, Weinberg DH, Lin DI, Folkerth RD. Isolated mononeuropathy multiplex—a rare manifestation of intravascular large B‐cell lymphoma. J Clin Neuromuscul Dis. 2012;14:17‐20. [DOI] [PubMed] [Google Scholar]
- 17. Le Clech L, Rizcallah MJ, Alavi Z, Hutin P. Mononeuritis multiplex in a patient with B‐cell prolymphocytic leukaemia: a diagnostic challenge. BMJ Case Rep. 2013;2013:bcr2013009425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sheikh AAE, Sheikh AB, Tariq U, et al. Paraneoplastic mononeuritis multiplex: a unique presentation of non‐Hodgkin lymphoma. Cureus. 2018;10:e2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aznar AO, Montero MA, Rovira R, Vidal FR. Intravascular large B‐cell lymphoma presenting with neurological syndromes: clinicopathologic study. Clin Neuropathol. 2007;26:180‐186. [DOI] [PubMed] [Google Scholar]
- 20. Detsky AS. Sources of bias for authors of clinical practice guidelines. CMAJ. 2006;175(1033):1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B‐cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109:478‐485. [DOI] [PubMed] [Google Scholar]
- 22. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’1. Br J Haematol. 2004;127:173‐183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Appendix S2.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.


