Abstract
Objectives:
To examine associations between sustained ownership of a pet and cognitive outcomes among a national sample of U.S. adults.
Methods:
Weighted linear mixed models were estimated using the Health and Retirement Study (2010–2016, n=1,369) to compare repeated measures of cognitive function between respondents who endorsed owning a pet in a sustained manner (>5 years), versus those who owned a pet ≤5 years, and non-pet owners.
Results:
Respondents age 65+ who owned a pet >5 years demonstrated higher composite cognitive scores, compared to non-pet owners (β=0.76, p=0.03). Sustained pet ownership was associated with higher immediate (β=0.3, p=0.02) and delayed (β=0.4, p=0.007) word recall scores. There were no significant differences in cognitive scores between pet owners and non-owners aged ≤65.
Discussion:
Sustained ownership of a pet could mitigate cognitive disparities in older adults. Further studies are needed to examine potential causal pathways, including physical activity and stress buffering, versus selection effects.
Keywords: Pets, cognitive health, cognitive function, pet ownership, companion animals
Introduction
Approximately 5.8 million Americans are currently living with dementia (Plassman et al., 2007; Zhang et al., 2021) – a term that describes a group of progressive, incurable neurological syndromes associated with irreversible cognitive decline and behavioral changes. The number of Americans living with Alzheimer’s dementia and other cognitive disorders is expected to more than double in the next 30 years, as the proportion of U.S. older adults increases (Zhang et al., 2021). By 2050, costs associated with Alzheimer’s and related dementias could top $1.1 trillion (Alzheimer’s Association, 2021), and caregiver burden carries high psychological and physical morbidity (Zhu et al., 2015).
Approximately one-third of dementia cases are attributable to modifiable causes, including physical inactivity, isolation, cardiovascular disease and hypertension, depression/anxiety, and chronic stress (Livingston et al., 2020). Efforts to support lifestyle habits that reduce the likelihood or severity of these conditions offer an important strategy to optimizing cognitive health for older adults. However, lifestyle interventions must be paired with robust evidence and subsequent public policy to support equitable implementation of any recommended strategies (Phelan et al., 2010), given known disparities in cognitive health by social position (e.g., race, ethnicity, gender, socioeconomic status [SES] (Farina et al., 2020; Garcia et al., 2019; Mayeda et al., 2016; Turrell et al., 2002; Weden et al., 2018))
Pet ownership represents an important yet understudied aspect of older adults’ lifestyle and social environment that could influence cognitive health. Approximately 50% of older U.S. adults are pet owners (Applebaum, Peek, et al., 2020; Bibbo et al., 2019). Pet ownership is thought to influence many health and disease outcomes (e.g., cardiovascular health, loneliness, and depression) via emotional support and stress buffering, but little is known about the impact of pet ownership on cognitive health or dementia risk (Barroso et al., 2021; Gee & Mueller, 2019; Tomlinson et al., 2021). This is a critical gap in knowledge, as a growing body of evidence links pet ownership to several dementia risk factors and modifiers (e.g., Allen et al., 2001, 2002; Barroso et al., 2021; Christian et al., 2013; Gee & Mueller, 2019; Handlin et al., 2011; MacLean et al., 2017; Miller et al., 2009; O’Haire et al., 2019; Pendry & Vandagriff, 2019; Rehn et al., 2014; Tomlinson et al., 2021). Prospective research that focuses on causal pathways between pet ownership and cognitive health could enhance the development of programs to support older adults who are interested in maintaining or initiating pet ownership, but such work must first be informed by longitudinal population-based data that are generalizable to the U.S. population.
Human-animal interaction (HAI) refers to the dynamic and mutual exchanges between human and non-human animals (Griffin et al., 2019). Much of the HAI literature to date has focused on how HAI (i.e., dogs) benefits humans’ affect and stress (Allen et al., 2001, 2002; Beetz et al., 2012; Crossman et al., 2020; McConnell et al., 2011). Significantly less attention has been given to how interactions with companion animals impact human cognition or other neurological outcomes, particularly in adult samples (Gee, Fine, et al., 2015; Gee, Friedmann, et al., 2015; Thayer & Stevens, 2021; Trammell, 2019). However, some investigators hypothesize that HAI (e.g., gazing at pets, directed attention to pets) could promote short-term cognitive health through enhanced attentional control, working memory, and reduced autonomic responses to stress (Thayer & Stevens, 2021).
Despite hypotheses that interactions with companion animals may benefit cognition, short-term studies have found no impact of HAI on select cognitive domains such as working memory (Gee, Fine, et al., 2015; Gee, Friedmann, et al., 2015) and long-term memory (e.g., Trammell, 2019). Potential explanations for the lack of observed associations between HAI and cognition have been proposed (Thayer & Stevens, 2021). First, the threshold for the impact of HAI on cognition may be hard to reach using study designs that focus on short-term interactions with unfamiliar (and therefore unbonded) animals (Thayer & Stevens, 2021). Second, there may be a time lag for the effects of HAI on cognition (e.g., Handlin et al., 2011; Thayer & Stevens, 2021) and the duration of exposure and number of interactions may be particularly important to account for in the context of examining the impact of HAI on cognitive functioning. Third, most studies have focused on short-term interactions with unfamiliar dogs vs. the impact of interactions with individuals’ own companion animals, with whom individuals’ likely have more enjoyable and/or stressful experiences. Thus, it may be particularly beneficial to examine whether and how pet ownership, rather than short-term HAI, is related to human cognition over time. To this end, the dearth of studies on the effect of owned pets on long-term cognitive decline in older adults stands out as a substantial knowledge gap.
In the current study, we evaluated associations between ownership of a pet and repeated measures of cognitive health among a national sample of U.S. adults aged 50+. We hypothesized that ownership of a pet would be associated with disparities in cognitive performance, and that this association would be strongest among older adults who endorsed sustained ownership of a pet (i.e., >5 years). Building on prior findings regarding cognitive health disparities, we also examined differential effects of long-term pet ownership among sociodemographic subpopulations.
Methods
The Health and Retirement Study
The Health and Retirement Study (HRS) is a large, nationally representative and diverse prospective cohort of U.S. adults aged 50+, designed to investigate the health, social, and economic implications of aging of the American population. This biennial survey collects a wide range of data on health, cognition, family, employment, and wealth, with oversampling of households with Black, Hispanic, and other racial and/or ethnic minority participants. Half of the sample was randomly assigned to face-to-face interviews while the other half was assigned to telephone interviews in each wave. The HRS follows respondents longitudinally until death and replenishes the sample every six years with younger cohorts. If a respondent is unable or unwilling to participate in the survey, the HRS attempts to identify a proxy respondent (usually a spouse or adult child) to complete the survey for them (Sonnega et al., 2014).
The present study utilized health information from the core HRS dataset, starting with the 2010 survey wave (baseline) and followed HRS participants up to 2016. Detailed companion animal questions became available in the 2012 wave and were administered to a subsample of participants.
Approvals
This study was deemed “non-regulated” by the Medical School Institutional Review Board at the University of Michigan, as only publicly available, de-identified data were used.
Variables
Pet ownership
The 2012 HRS wave included questions about companion animals, which were included in a module that was randomly assigned to a subset of study participants. Pet ownership was assessed based on participants’ responses to the question “Do you currently have any pets?” Participants were further asked “How long have you had your (pet/pets)?” If participants had more than one pet, HRS interviewers asked the participants to answer based on the pet they had owned the longest. Participants chose one of the answers (less than 1 year [y], 1–2 y, 3–5 y, 6–9 y, 10+ y, and “always” was volunteered by 33 participants). Based on the cutoffs and distribution of the answers, as well as studies of potential behavioral or environmental modifiers of dementia risk that demonstrate the impact of cumulative exposure on dementia incidence, this variable item was categorized into non-pet owners, pet owners ≤5 years, and pet owners >5 years. Although the duration of which stress or protective factors affect dementia risk has yet to be determined, chronic activation of physiological systems such as the HPA axis are postulated to have the largest impact on risk of dementia and other comorbidities including metabolic dysfunction and cardiovascular disorders (Mayeux & Stern, 2012; Yuede et al., 2018). Additionally, a pivotal study on the exposure of physical activity and diet as protective factors for Alzheimer’s dementia demonstrated a cumulative effect over a 12-year timespan (individuals were followed for a mean (SD) of 5.4 (3.3) years), with benefits of diet and exercise increasingly evident beyond the five-to-six-year time interval (Scarmeas et al., 2009). Consequently, we chose to study similar time intervals for our exposure of interest (pet ownership) on cognition.
Cognitive outcomes: composite cognitive score and individual cognitive test score
Only respondents who had normal cognition in 2010 (per composite score) were included in the analyses. The HRS core interview objectively assessed cognitive function with a range of individual tests including immediate and delayed 10-noun recall, serial seven subtraction test, backwards number counting, vocabulary, and mental status. Using an algorithm described in Iwashyna et al. (2010) and developed by Langa and Weir (2010), scores from the immediate and delayed 10-noun recall test, serial seven subtraction test, and a backwards count from 20 test were summed to yield a composite cognitive score (see Table 1 for assessment details and range). The composite score was then converted to a 27-point scale which allowed for the classification of respondents into 3 categories: dementia (composite score 0–6), cognitive impairment, not dementia (CIND, composite score 7–11), and normal cognition (composite score 12–27). Participants with normal cognition at baseline (2010) were included in the study.
Table 1:
Cognitive tests included in the study
| Name | Description | Score range |
|---|---|---|
| Immediate word recall | The interviewer read one of four possible lists of 10 nouns to the respondent. The lists do not overlap in word content and the initial list was randomly assigned to the respondent, in a longitudinal manner such that each respondent was assigned a different set of words in each of four successive waves of data collection. The respondent was asked to recall the nouns previously presented | 0–10 |
| Delayed word recall | After approximately 5 minutes of asking other survey questions (e.g., depression, and cognition items including backwards count, and serial 7’s) the respondent was asked to recall the nouns previously presented as part of the immediate recall task | 0–10 |
| Serial 7’s task | The interviewer asked the respondent to subtract 7 from 100, and continue subtracting 7 from each subsequent number for a total of five trials | 0–5 |
| Backwards count | Respondents were asked to count backwards for 10 continuous numbers beginning with the number 20 | 0–2 |
To account for missing data, we utilized imputed datasets of cognitive scores provided by the HRS. The imputed cognitive data were provided by the HRS. Imputation procedures of cognitive measures included non-changing baseline demographics, wave-specific demographics, and other wave-specific predictor variables in addition to the cognitive measures. First, the HRS assembled the baseline demographic variables, and imputed missing values. As the next step, they assembled the wave-specific variables and imputed missing values where necessary. Finally, cognitive measures were assembled, and missing values were imputed with non-changing baseline demographics, wave-specific demographics, and other wave-specific predictor variables.
Covariates
Baseline (2010) demographic variables included age, sex, and race/ethnicity. Age was coded as a binary variable: <65 years and ≥ 65 years based on the age distribution of the participants. Race/ethnicity was categorized into four groups: Hispanic; non-Hispanic White; non-Hispanic Black/African American; and non-Hispanic other race/ethnicity (American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, and other race/ethnicity). Common comorbidities (hypertension, diabetes, and depression) were defined by either of two criteria: self-report of physician diagnosis, or self-reported use of condition-specific medications. Relationship status was categorized as married/cohabiting or other. Smoking status was categorized as current smoker, ex-smoker, and non-smoker. Alcohol consumption was divided into three categories: current, past, and none. Physical activity was presented as a Metabolic Equivalent (MET) score, accounting for frequency of mild activity, moderate activity, and vigorous activity (He & Baker, 2005; Wen et al., 2014). The final MET score ranged from 0 to 16.33. Finally, family income was retrieved from RAND HRS files (RAND Center for the Study of Aging, 2021) and categorized into two groups (<15,900, 15,000+). As obesity (BMI), hypertension, and diabetes (variables hypothesized to be affected by pet ownership) are established dementia risk factors (Livingston et al., 2020; Ma et al., 2020), each was considered a potential mediator between the relationship between pet ownership and cognitive function and thus not included in the regression models to avoid over-adjustment.
Statistical analysis
Descriptive statistics were calculated as proportions for categorical variables and as means and standard errors for continuous variables. The weighted prevalence and mean, accounting for strata, 2010 HRS household-level and respondent-level weights, were calculated for the entire sample and by pet ownership duration. We normalized the individual weights within a household, so that the sum of the individual weights within a given household is equal to the number of people in that household. Sub-sample analyses were performed by adding a DOMAIN statement in proc surveymeans and DOMAIN=ROW within the TABLE statement in proc surveyfreq. This allowed us to retain all clusters of the entire sample. These statements appropriately include those clusters to properly estimate standard errors while properly filtering down to the subgroup of interest.
Associations between ownership of a pet and repeated cognitive measures were then examined. Participant-specific composite cognitive scores, and each individual cognitive test score from 2010 to 2016, were treated as repeated outcomes in the models. We evaluated pet ownership in 2012 in relation to cognitive performance that were repeatedly measured over a 6-year time span, every other year from 2010 to 2016. These temporal associations were analyzed with mixed linear models. We incorporated survey weights to account for differential selection probabilities and potential non-response bias in all regression models. Likelihood ratio tests were performed among models with different structure of covariance, with/without random intercept and slope to determine which model should be the final model. Sandwich variance estimators were obtained for the fixed effect and the variance. Weighted mixed models with a random intercept for each individual and a random slope for pet ownership category and variance component covariance were used to estimate the crude and adjusted coefficient (b) and p-value. In our regression models, we have included covariates based on their relevance to pet ownership and cognition. Rather than a stepwise approach that is data driven, we have included all relevant covariates in our models simultaneously. Models were adjusted for age, race/ethnicity, education, marital status, family income, and survey year. Furthermore, we examined these associations in a sample stratified by sociodemographic variables (age, race/ethnicity, marital status, education, gender, and family income) by using the WHERE statement in proc glimmix. Models were adjusted for all covariates above except for the stratification variable. Cardiovascular risk factors (diabetes, hypertension, and BMI) were further included in additional models to examine their potential mediating role in the association (Table S1). In 2010, in addition to non-pet owners (n=38), 38 respondents reported having pets for less than a year in 2012. To address this potential misclassification, we also conducted a sensitivity analysis classifying these 38 as non-pet owners (Table S2). Though imputed cognitive data were not available for 2018 and 2020, we conducted a sensitivity analysis including those with nonmissing cognitive data in 2018 and 2020 (n=969) (Table S3).
All analyses were performed in SAS version 9.4 and accounted for the strata (STRATUM), 2010 HRS household-level (MWGTHH) and respondent-level survey weights (MWGTR), and household ID in mixed linear models.
Results
Almost half of the participants (weighted prevalence: 47%) reported current pet ownership in 2012. Weighted summary statistics of demographic, health, and lifestyle characteristics are presented in Table 2. Among pet owners, 19% had pets for one to five years, and 28% had pets for more than five years. The mean age of the participants was 65.5 years (±0.3), 37% were men, 63% were women (other gender identities and/or expressions were not examined in the HRS). About 78% identified as non-Hispanic White. Long-term pet owners were more likely to be younger, women, married or cohabiting, identify their race and ethnicity as non-Hispanic White, report higher family income, have lower BMI, report higher physical activity, and have lower blood pressure, compared to non-owners. Long-term pet owners were more likely to have depression than non-owners.
Table 2:
Summary statistics—weighteda proportion (%) or weighted mean (and standard error)—of selected variables, by ownership of a pet; 2010 study population
| Total (n=1,369) |
Percentage of missing (%) |
Pet owner over 5 years (n=387) |
Pet owner 1–5 years (n=272) |
Non pet owner (n=707) |
|
|---|---|---|---|---|---|
| Men (%) | 37.0 | 0 | 36.1 | 36.3 | 38.0 |
| Age (years) | 65.5 (0.3) | 0 | 63.3 (0.5) | 64.2 (0.7) | 67.3 (0.5) |
| Race/ethnicity (%) | 0 | ||||
| White | 78.2 | 89.1 | 80.4 | 72.5 | |
| Black | 15.6 | 5.4 | 12.7 | 21.3 | |
| Hispanic | 3.6 | 4.0 | 4.3 | 3.1 | |
| Otherb | 2.6 | 1.5 | 2.6 | 3.1 | |
| Education (%) | 0.4 | ||||
| Below college | 67.3 | 66.9 | 69.8 | 66.4 | |
| College | 22.3 | 19.9 | 19.0 | 24.9 | |
| Above college | 10.4 | 13.2 | 11.2 | 8.7 | |
| Married (%) | 63.8 | 0.2 | 71.1 | 66.9 | 58.6 |
| BMI (kg/m2) | 29.0 (0.2) | 35.0 | 28.6 (0.4) | 28.8 (0.5) | 29.3 (0.3) |
| Diabetes (%) | 19.1 | 0.07 | 14.6 | 24.7 | 19.5 |
| Hypertension (%) | 54.6 | 0.07 | 46.3 | 55.0 | 59.0 |
| Depression (%) | 19.3 | 0.2 | 21.8 | 21.7 | 17.1 |
| Smoking (%) | 43.8 | ||||
| Non smoker | 56.3 | 54.5 | 55.4 | 58.2 | |
| Ex-smoker | 16.2 | 17.9 | 16.1 | 14.4 | |
| Current smoker | 27.5 | 27.6 | 28.5 | 27.4 | |
| Alcohol (%) | 0.4 | ||||
| Non drinker | 39.9 | 33.0 | 43.0 | 42.2 | |
| Past drinker | 19.7 | 22.9 | 18.8 | 18.3 | |
| Current drinker | 40.4 | 44.1 | 38.2 | 39.5 | |
| MET scorec | 4.4 (0.1) | 0.2 | 4.9 (0.2) | 4.4 (0.2) | 4.3 (0.1) |
| Family income | 15,937 (1,964) | 0 | 17,388 (3,128) | 14,750 (3,124) | 15,719 (3,153) |
BMI=body mass index
Accounted for strata and 2010 survey weights (household-level and respondent-level weights)
Includes American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, and other race/ethnicity.
Metabolic equivalent of task. Ranged from 0 to 16.33.
The weighted mean composite cognitive score from 2010–2016 by pet ownership duration is shown in Figure 1. As indicated in this figure of unadjusted data among individuals aged 65+, those who owned pets more than five years, in comparison to those did not own pets, had a higher mean composite cognitive score at baseline and each subsequent wave across the 6-year follow-up interval. With the exception of the 2016 wave, those with pet owners more than 5 years, in contrast to pet owners who owned pets less than 5 years, also had a higher mean composite cognitive score at baseline and each subsequent wave.
Figure 1: Weighted mean composite cognitive score from 2010 to 2016 by duration of ownership of a pet.
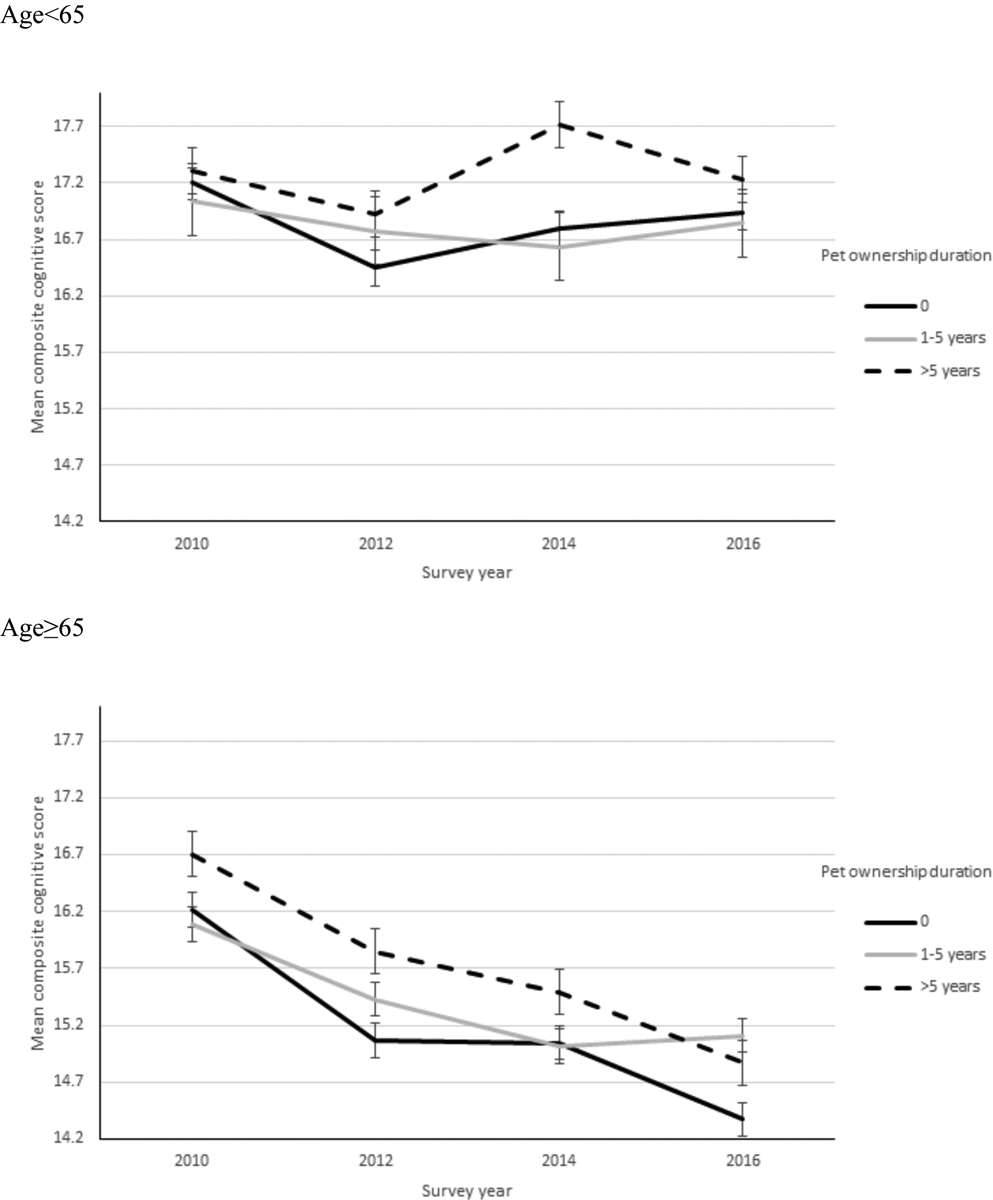
*Accounted for strata and 2010 survey weights (household-level and respondent-level weights)
Table 3 displays associations between ownership of a pet and repeated cognitive measures among all respondents from adjusted linear mixed models with a random intercept for each individual and a random slope for pet ownership, stratified by age group. Covariates included age, gender, education, marital status, race/ethnicity, family income, survey year, and depression (see Table S4 for the full model estimates). No significant differences were seen in cognitive test scores across the entire sample.
Table 3:
Estimated association (adjusted coefficient and stand error) between ownership of a pet over five years/1–5 years vs. no pets (exposure) and cognitive test score (outcome) for the total study population from the linear mixed model with a random intercept for each individual and a random slope for each pet ownership group
| Cognitive test | Total (n=1,369) | Age<65 (n=725) | Age≥65 (n=644) | |||
|---|---|---|---|---|---|---|
| Adjusted coefficienta (standard error) | P-value | Adjusted coefficientb (stand error) | P-value | Adjusted coefficientb (stand error) | P-value | |
| Composite cognitive score | ||||||
| 0 year | Reference | Reference | Reference | |||
| 1–5 years | −0.11 (0.2) | 0.64 | −0.29 (0.4) | 0.50 | 0.14 (0.3) | 0.68 |
| >5 years | 0.02 (0.2) | 0.94 | −0.41 (0.3) | 0.16 | 0.76 (0.3) | 0.03 |
| Immediate word recall | ||||||
| 0 year | Reference | Reference | Reference | |||
| 1–5 years | 0.08 (0.1) | 0.39 | −0.03 (0.2) | 0.84 | 0.20 (0.1) | 0.045 |
| >5 years | 0.01 (0.08) | 0.89 | −0.17 (0.1) | 0.13 | 0.30 (0.1) | 0.02 |
| Delayed word recall | ||||||
| 0 year | Reference | Reference | Reference | |||
| 1–5 years | 0.04 (0.1) | 0.74 | −0.06 (0.2) | 0.73 | 0.20 (0.2) | 0.27 |
| >5 years | 0.06 (0.1) | 0.54 | −0.14 (0.1) | 0.34 | 0.40 (0.2) | 0.007 |
| Serial 7’s task | ||||||
| 0 year | Reference | Reference | Reference | |||
| 1–5 years | −0.2 (0.1) | 0.02 | −0.17 (0.1) | 0.18 | −0.30 (0.2) | 0.05 |
| >5 years | −0.05 (0.08) | 0.58 | −0.07 (0.1) | 0.54 | 0.02 (0.1) | 0.86 |
| Backward count | ||||||
| 0 year | Reference | Reference | Reference | |||
| 1–5 years | −0.006 (0.02) | 0.80 | −0.02 (0.03) | 0.55 | 0.008 (0.03) | 0.81 |
| >5 years | −0.01 (0.02) | 0.48 | −0.03 (0.02) | 0.16 | 0.02 (0.03) | 0.51 |
Adjusted for age, gender, education level, marital status, race/ethnicity, family income, survey year, depression.
Adjusted for gender, education level, marital status, race/ethnicity, family income, survey year, depression.
Accounted for strata and 2010 survey weights (household-level and respondent-level weights)
Upon stratification of the sample by age (≥65 years vs <65), among those who were ≥ 65 years, respondents who owned a pet >5 years demonstrated a 0.76 higher mean difference in composite cognitive score in comparison to non-pet owners, in any given survey year (adjusted p=0.03). Similarly, in any given survey year, respondents aged ≥ 65 years who owned a pet >5 years also demonstrated a 0.30 higher mean difference in immediate word recall score (adjusted p=0.02), and 0.40 higher mean difference delayed word recall score (adjusted p=0.007) in comparison to non-pet owners. No significant differences were seen with the serial 7’s task or backward count scores. The associations between ownership of a pet and repeated cognitive measures among all respondents from linear mixed models with a random intercept and a random slope from the sensitivity analyses remained similar to the results from the primary analyses (Table S1, S2, S3).
We further stratified the sample by race/ethnicity, marital status, education, gender, and family income to examine their potential role as moderators in associations between ownership of a pet and total composite cognitive scores (Table 4). Aside from age (discussed above), after accounting for strata, 2010 HRS household-level and respondent-level weights, other sociodemographic variables did not modify the associations between ownership of a pet and cognitive scores.
Table 4:
Estimated association (adjusted coefficient and stand error) between ownership of a pet over five years vs. no pets (exposure) and composite cognitive score (outcome) for the total study population, stratified by demographic variables, from the linear mixed model with a random intercept for each individual and a random slope for each pet ownership group
| Stratification variable | Adjusted coefficient (stand error) | P-value |
|---|---|---|
| By age | ||
| Age<65 | −0.41 (0.3) | 0.16 |
| Age≥65 | 0.76 (0.3) | 0.03 |
| By race | ||
| White | 0.001 (0.2) | 0.99 |
| Black | 0.79 (0.7) | 0.29 |
| Hispanic | 0.43 (1.3) | 0.73 |
| Other | 0.50 (0.8) | 0.54 |
| Marital status | ||
| Marry/Cohabitate | −0.11 (0.3) | 0.67 |
| Unmarried | 0.24 (0.4) | 0.58 |
| By education | ||
| Below college | 0.12 (0.3) | 0.68 |
| College | −0.22 (0.5) | 0.63 |
| Above college | 0.18 (0.6) | 0.76 |
| Gender | ||
| Men | 0.20 (0.3) | 0.65 |
| Women | −0.12 (0.3) | 0.69 |
| Family income | ||
| <15,900 | 0.002 (0.2) | 0.92 |
| ≥15,900 | −0.08 (0.6) | 0.92 |
Adjusted for age, gender, education level, marry, race/ethnicity, family income, survey year, depression, except for the stratification variable
Accounted for strata and 2010 survey weights (household-level and respondent-level weights)
Discussion
In this study, we examined associations between sustained ownership of a pet and cognitive function among a national sample of U.S. adults. We found that, among a national, longitudinal cohort of U.S. adults with normal cognition, almost half owned pets. Among participants who were ≥ 65 years old, those who owned their pet for longer durations (>5 years) had higher mean composite cognitive scores over time in comparison to those who owned their pet for shorter periods (≤5 years), and those who did not own pets. This association was strongest for tests of verbal memory. These findings provide early evidence to suggest that long-term pet ownership could be protective against cognitive disparities, providing a novel and fundamental step to examine how sustained relationships with companion animals may contribute to brain health among older adults. We expand on potential mechanisms for these findings below.
Notably, our findings are somewhat inconsistent with those from a recent study also using the HRS: Branson and Cron found no evidence of associations between pet caretaking and cognitive function among the HRS participants (2021). Importantly, their study measured time spent taking care of a pet regardless of ownership status (i.e., those who reported >0 hours spent taking care of a pet on a given week, compared to those who reported 0 hours), rather than self-reported pet ownership, and they did not examine age-specific effects (Branson & Cron, 2021). A key finding of our study was the effect of pet ownership on cognitive function by age. Indeed, only participants aged 65 and above experienced relationships between pet ownership and cognition. Given that late-onset Alzheimer’s and other forms of dementia/cognitive impairment are most likely to manifest after age 65 (Plassman et al., 2007), we postulate that if a causal pathway between pet ownership and cognitive decline exists, benefits of pet ownership would be most apparent in participants in their seventh decade and above. In addition to this age-specific finding, we also found that many sociodemographic and health characteristics differed between long-term pet owners, short-term pet owners, and non-owners; however, there were no significant findings related to long-term pet ownership in models stratified by race, marital status, education, gender, and family income.
Although causal biological pathways cannot be determined from these population-based data, our findings allow speculation about mechanisms by which sustained ownership of a pet could protect cognitive function. A growing number of studies have identified associations between pet ownership and factors linked to cognitive function in humans. One area of increasing interest is the effect of oxytocin on brain function. Oxytocin is a neuropeptide produced in the hypothalamus that influences lactation, reproductive function, and mother-infant bonding. Oxytocin has also been shown to affect social cognition and memory encoding in humans (Guastella et al., 2008). Several studies have identified associations between oxytocin levels and pet interaction and bonding among dog owners (Handlin et al., 2011; MacLean et al., 2017; Miller et al., 2009; Rehn et al., 2014), raising questions about oxytocin as a mechanism by which pet ownership could benefit cognitive health.
We also found that respondents who endorsed sustained ownership of a pet tended to show indicators of greater physical activity (i.e., MET score), lower BMI, and lower incidence of diabetes and hypertension than short-term and non-owners, providing other potential mechanisms for our results. These findings are consistent with previous literature often showing that pet owners tend to be healthier on a variety of physical health measures than non-owners (e.g., Allen et al., 2001; Friedmann et al., 2013; Gee & Mueller, 2019). Although findings regarding the association between pet ownership and physical activity are mixed, pet ownership, and particularly lifestyle habits associated with dog ownership, could provide cognitive as well as physical benefits (Christian et al., 2013). Higher levels of exercise in mid-life are associated with reduced cognitive decline and brain atrophy in older adults (Bugg & Head, 2011; Vemuri et al., 2012). A physically active lifestyle is also associated with reductions in biomarkers β-amyloid burden, hippocampal atrophy and total brain atrophy associated with Alzheimer’s dementia, among at-risk late-middle-aged individuals (Okonkwo et al., 2014; Rovio et al., 2010).
The impact of stress on cognition, and potential stress-buffering effects of pet ownership, also deserve consideration in the context of our findings. The detrimental effect of chronic stress on cognitive decline has been extensively studied, with a growing body of literature linking cumulative stress to Alzheimer’s and other forms of dementia. Further, brain regions involved in verbal memory, including the dorsolateral cortex and hippocampus, may be particularly vulnerable to effects of long-term stress (Arnsten, 2009; Kim et al., 2015). Although reasons for this association are likely multifactorial, disturbances in the hypothalamic-pituitaryadrenocortical (HPA) axis, which lead to elevations in the stress hormone cortisol, are believed to detrimentally affect hippocampal function and other brain regions associated with cognition (Kim et al., 2015). Previous experimental research has indicated that pets may buffer physiological responses to mental stress, such as blood pressure (Allen et al., 2001) and cardiovascular reactivity (Allen et al., 2002). Other studies have identified associations between pet ownership and other stress indicators (heart rate, blood pressure) in the absence of significant changes in cortisol level, suggesting that stress reduction through interactions with companion animals may arise through multiple pathways (Handlin et al., 2011; Pendry & Vandagriff, 2019).
It should be acknowledged that findings supporting the potential benefits of pet ownership, particularly in relation to stress reduction, are historically based on physiological responses to short-term interactions with animals, and thus may fail to capture the multifaceted nature of individuals’ relationships with their pets (Rodriguez et al., 2021). Indeed, other research has found pet ownership to cause or exacerbate stress under certain conditions (e.g., Applebaum, Tomlinson, et al., 2020). For example, some studies have shown that pet-friendly housing can be difficult to find and maintain due to extra costs and less availability, particularly in low-income communities and communities of color, which could cause economic stress related to pet ownership (Applebaum et al., 2021; Rose et al., 2020). Furthermore, a study by Buller and Balantyne showed that managing a pet with behavioral issues can negatively impact owner wellbeing (Buller & Ballantyne, 2020). Notably, our findings were most compelling among those who had a long-term relationship with their pet, which suggests that older pets may be less likely to cause stress due to behavioral challenges, which could in turn promote the bond between pet and owner and thus potentially provide stress-buffering effects.
Beyond the physiological responses discussed above, pets could provide social support and thus promote cognitive health via psychological wellbeing. Perceived social support between humans is thought to provide benefits to mental health both directly and indirectly via stress buffering (Thoits, 2011). Although pets are not capable of providing tangible or instrumental support (e.g., a car ride to a doctor’s appointment), some studies suggest that they may provide owners with emotional support, particularly when owners are otherwise socially isolated (McDonald, Matijczak, et al., 2021). That said, it is important to note that pet owners in this study tended to have a higher incidence of depression than non-owners. Previous findings regarding the impact of pets on mental health, and particularly depression, have been mixed (Gee & Mueller, 2019; Herzog, 2011; Scoresby et al., 2021). While pets may provide social support that could have a positive influence on mental health, it is possible that those with depression are more likely to seek companionship from a pet in substitution of other important sources of social support (i.e., from humans). Prior research has shown that it is important to consider how human and pet social support function together in relationships between stress and mental health (McDonald, O’Connor, et al., 2021).
Both reverse causality and selection effects should be acknowledged as possible explanations for our findings. First, those with better cognitive function are more likely to maintain tasks associated with pet ownership. Therefore, it is possible that individuals with more cognitive reserve are more likely to own pets for longer periods of time and experience slower rates of cognitive decline. Second, many social factors contribute to an ability to maintain long-term pet ownership, including access to veterinary care, stable pet-friendly housing, and a supportive community and built environment. Our findings showed that nearly 90% of the long-term pet owners were White, while only 5.4% were Black. Furthermore, long-term pet owners represented the highest proportion of highly educated individuals, as well as the highest family incomes. Populations who are less likely to have access to pet-supportive resources and environments are likely the same as those who may be subject to other risks to cognitive health, and broader health disparities. Recent research has suggested that there may be differences between pet owners and non-owners in terms of demographics, social and economic resources, and lifestyle (Applebaum, Peek, et al., 2020; Halbreich & Mueller, 2022; Mueller et al., 2018, 2021; Saunders et al., 2017), all of which are also known to impact health. For example, pet ownership is more than twice as common among White Americans, compared to Black Americans (Applebaum, Peek, et al., 2020); similarly, White Americans are more than twice as likely to have better cognitive health in late life, compared to Black Americans (Garcia et al., 2019). Additionally, dog ownership is more common among high-SES groups, compared to low-SES. Income and education, two elements of SES, are well-known to influence cognitive health in later life (Turrell et al., 2002). Thus, the implementation of social programs that promote health equity would also likely increase access to and support for pet ownership among marginalized groups.
Future Directions
The findings from this study could inform several avenues for future research. More granular and repeated collection of historical and longitudinal measures of pet ownership in large health studies such as the HRS would be a significant step in determining if a causal link exists between pet ownership and cognition. Clearer and more extensive measures of duration of pet ownership, such as cumulative historical pet ownership, duration of ownership of one’s favorite pet, and duration of sustained exposure to others’ pets, could each potentially have differential effects on cognition. Furthermore, future observational studies and studies of lifestyle interventions on cognition could benefit from more in-depth assessments of the quality and intensity of pet-owner relationships, to provide insight into the efficacy of pets to provide stress relief and thus promote cognitive health. Finally, although we did not find any evidence of differential effects by sociodemographic subgroups (aside from age), future research about pet ownership and health should consider disparities in disease and mortality as a result of social conditions (e.g., racism, access to resources) and how these factors may interact with the impact of pets on health and well-being.
Strengths and Limitations
This study harnessed the strengths of the HRS - a large, racially and ethnically diverse national sample, which allowed analysis of the associations between pet ownership and objective cognitive function, in a manner that also included stratified samples by important demographic variables. Some limitations also deserve mention. First, cognitive outcomes had missing values that ranged between 0.3% and 10.8%. To address missingness, we utilized imputed cognitive data provided by HRS. This approach increased the statistical power of our study and generated unbiased results. Second, to avoid over-adjustment, our models did not adjust for cardiovascular risk factors such as diabetes, hypertension, and BMI. However, we performed a sensitivity analysis and these potential mediators did not significantly change the results. Third, the smaller sample of racial and ethnic minority participants could lead to limited power to detect the differences in cognitive decline by duration of pet ownership. Given the potential mechanisms by which long-term pet ownership could be beneficial to cognitive health are likely cumulative (i.e., not immediate), we utilized information about length of pet ownership leading up to 2012 with the assumption that effects would be observable through the subsequent waves of data collection of cognitive function. Finally, the question administered to participants to determine length of pet ownership queried the length of time that they had their current pet, which did not allow for the systematic assessment of cumulative and historical pet ownership. While some participants volunteered that they had always had pets, it is presumed that most responded based on the length of time they’d had the individual pet they’d had the longest at that time. It is possible that some of the newer owners who were coded into the <5 years category based on their responses did previously have other pets.
Policy Implications
We do not recommend pet ownership as a therapeutic intervention; however, if a causal link exists between sustained pet ownership and cognitive health, older adults who are interested in or committed to pet ownership could benefit from social policies and community partnerships to provide support for owners. A recent example of an innovative, community-based pet support program for older adults in Calgary, Canada was described by McLennan and colleagues (McLennan et al., 2022). The program is volunteer-driven and provides broad assistance to low-income older adults in caring for their pets in order to support and maintain bonds between older adults and their pets (2022). Expanded social safety net programs aimed at reducing economic and social barriers to health-supportive services among older adults (e.g., expanding affordable housing programs) would also likely make strides in supporting pet ownership among older adults.
Supplementary Material
Funding
Research reported in this publication was supported by The National Center for Advancing Translational Sciences of the National Institutes of Health under the University of Florida and Florida State University Clinical and Translational Science Awards [TL1TR001428 and UL1TR001427], The National Center for Complementary and Integrative Health, the National Institute on Aging, and the Patient Centered Outcomes Research Institute [R01AT011341, R01AG074342, and MS-1610-36980], The National Heart, Lung, and Blood Institute [T32 HL110952], from the National Institute on Aging [R01AG074342] and The National Heart, Lung, and Blood Institute [K01 HL144914].
References
- Allen K, Blascovich J, & Mendes WB (2002). Cardiovascular Reactivity and the Presence of Pets, Friends, and Spouses: The Truth About Cats and Dogs. Psychosomatic Medicine, 64(5). https://journals.lww.com/psychosomaticmedicine/Fulltext/2002/09000/Cardiovascular_Reactivity_and_the_Presence_of.5.aspx [DOI] [PubMed] [Google Scholar]
- Allen K, Shykoff BE, & Izzo JL (2001). Pet ownership, but not ACE inhibitor therapy, blunts home blood pressure responses to mental stress. Hypertension, 38(4), 815–820. 10.1161/hyp.38.4.815 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2021). https://www.alz.org/alzheimers-dementia/facts-figures
- Angevaren M, Aufdemkampe G, Verhaar H, Aleman A, & Vanhees L (2008). Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews, 3. 10.1002/14651858.CD005381.PUB3/INFORMATION/EN [DOI] [PubMed] [Google Scholar]
- Applebaum JW, Horecka K, Loney L, & Graham TM (2021). Pet-Friendly for Whom? An Analysis of Pet Fees in Texas Rental Housing. Frontiers in Veterinary Science, 8, 767149. 10.3389/FVETS.2021.767149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum JW, Peek CW, & Zsembik BA (2020). Examining U.S. pet ownership using the General Social Survey. The Social Science Journal, March, 1–10. 10.1080/03623319.2020.1728507 [DOI] [Google Scholar]
- Applebaum JW, Tomlinson CA, Matijczak A, Mcdonald SE, & Zsembik BA (2020). The Concerns, Difficulties, and Stressors of Caring for Pets during COVID-19: Results from a Large Survey of U.S. Pet Owners. Animals, 10(10), 1882. 10.3390/ani10101882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso CS, Brown KC, Laubach D, Souza M, Daugherty LM, Dixson M, Johnson A, Ng Z, & Winkle M (2021). Cat and/or Dog Ownership, Cardiovascular Disease, and Obesity: A Systematic Review. Veterinary Sciences 2021, Vol. 8, Page 333, 8(12), 333. 10.3390/VETSCI8120333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetz A, Uvnäs-Moberg K, Julius H, & Kotrschal K (2012). Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. In Frontiers in Psychology (Vol. 3, Issue JUL, p. 234). Frontiers. 10.3389/fpsyg.2012.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbo J, Curl AL, & Johnson RA (2019). Pets in the Lives of Older Adults: A Life Course Perspective. Anthrozoos, 32(4), 541–554. 10.1080/08927936.2019.1621541 [DOI] [Google Scholar]
- Branson S, & Cron S (2021). Pet Caretaking and Risk of Mild Cognitive Impairment and Dementia in Older US Adults. Anthrozoos. 10.1080/08927936.2021.1986259 [DOI] [Google Scholar]
- Bugg JM, & Head D (2011). Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging, 32(3), 506–514. 10.1016/J.NEUROBIOLAGING.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian HE, Westgarth C, Bauman A, Richards EA, Rhodes RE, Evenson KR, Mayer JA, & Thorpe RJ (2013). Dog Ownership and Physical Activity: A Review of the Evidence. Journal of Physical Activity and Health, 10(5), 750–759. 10.1123/JPAH.10.5.750 [DOI] [PubMed] [Google Scholar]
- Crossman MK, Kazdin AE, Matijczak A, Kitt ER, & Santos LR (2020). The Influence of Interactions with Dogs on Affect, Anxiety, and Arousal in Children. Journal of Clinical Child & Adolescent Psychology, 49(4), 535–548. 10.1080/15374416.2018.1520119 [DOI] [PubMed] [Google Scholar]
- Farina MP, Hayward MD, Kim JK, & Crimmins EM (2020). Racial and Educational Disparities in Dementia and Dementia-Free Life Expectancy. The Journals of Gerontology: Series B, 75(7), e105–e112. 10.1093/GERONB/GBZ046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann E, Thomas SA, Son H, Chapa D, & McCune S (2013). Pet’s Presence and Owner’s Blood Pressures during the Daily Lives of Pet Owners with Pre- to Mild Hypertension. Anthrozoos, 26(4), 535–550. 10.2752/175303713X13795775536138 [DOI] [Google Scholar]
- Garcia MA, Downer B, Chiu CT, Saenz JL, Rote S, & Wong R (2019). Racial/Ethnic and Nativity Differences in Cognitive Life Expectancies Among Older Adults in the United States. The Gerontologist, 59(2), 281–289. 10.1093/GERONT/GNX142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee NR, Fine AH, & Schuck S (2015). Animals in Educational Settings: Research and Practice. In Handbook on Animal-Assisted Therapy (pp. 195–210). Academic Press. 10.1016/B978-0-12-801292-5.00014-6 [DOI] [Google Scholar]
- Gee NR, Friedmann E, Stendahl M, Fisk A, & Coglitore V (2015). Heart Rate Variability During a Working Memory Task: Does Touching a Dog or Person Affect the Response? Anthrozoos, 27(4), 513–528. 10.2752/089279314X14072268687763 [DOI] [Google Scholar]
- Gee NR, & Mueller MK (2019). A Systematic Review of Research on Pet Ownership and Animal Interactions among Older Adults. Anthrozoos, 32(2), 183–207. 10.1080/08927936.2019.1569903 [DOI] [Google Scholar]
- Graham TM, & Glover TD (2014). On the Fence: Dog Parks in the (Un)Leashing of Community and Social Capital. Leisure Sciences, 36(3), 217–234. 10.1080/01490400.2014.888020 [DOI] [Google Scholar]
- Griffin JA, Hurley K, & McCune S (2019). Human-Animal Interaction Research: Progress and Possibilities. Frontiers in Psychology, 10. 10.3389/fpsyg.2019.02803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Mathews F (2008). Oxytocin Enhances the Encoding of Positive Social Memories in Humans. Biological Psychiatry, 64(3), 256–258. 10.1016/J.BIOPSYCH.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Halbreich ED, & Mueller MK (2022). Profiles of family pet ownership during the COVID-19 pandemic. Current Psychology 2021, 1, 1–5. 10.1007/S12144-021-02574-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlin L, Hydbring-Sandberg E, Nilsson A, Ejdebäck M, Jansson A, & Uvnäs-Moberg K (2011). Short-term interaction between dogs and their owners: Effects on oxytocin, cortisol, insulin and heart rate-an exploratory study. Anthrozoos, 24(3), 301–315. 10.2752/175303711X13045914865385 [DOI] [Google Scholar]
- He XZ, & Baker DW (2005). Differences in leisure-time, household, and work-related physical activity by race, ethnicity, and education. Journal of General Internal Medicine 2005 20:3, 20(3), 259–266. 10.1111/J.1525-1497.2005.40198.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog H (2011). The Impact of Pets on Human Health and Psychological Well-Being: Fact, Fiction, or Hypothesis? Current Directions in Psychological Science, 20(4), 236–239. 10.1177/0963721411415220 [DOI] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, & Langa KM (2010). Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA, 304(16), 1787–1794. 10.1001/JAMA.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Pellman B, & Kim JJ (2015). Stress effects on the hippocampus: a critical review. Learning & Memory, 22(9), 411. 10.1101/LM.037291.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Kabeto M, & Weir D (2010). Report on race and cognitive impairment using HRS. http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf
- LaVallee E, Mueller MK, & McCobb E (2017). A Systematic Review of the Literature Addressing Veterinary Care for Underserved Communities. Journal of Applied Animal Welfare Science, 20(4), 381–394. 10.1080/10888705.2017.1337515 [DOI] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, … Mukadam N (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. 10.1016/S0140-6736(20)30367-6/ATTACHMENT/CEE43A30-904B-4A45-A4E5-AFE48804398D/MMC1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ajnakina O, Steptoe A, & Cadar D (2020). Higher risk of dementia in English older individuals who are overweight or obese. International Journal of Epidemiology, 49(4), 1353–1365. 10.1093/ije/dyaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Gesquiere LR, Gee NR, Levy K, Martin WL, & Carter CS (2017). Effects of Affiliative Human–Animal Interaction on Dog Salivary and Plasma Oxytocin and Vasopressin. Frontiers in Psychology, 8, 1606. 10.3389/fpsyg.2017.01606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, & Whitmer RA (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12(3), 216–224. 10.1016/J.JALZ.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, & Stern Y (2012). Epidemiology of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine, 2(8), a006239. 10.1101/CSHPERSPECT.A006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell AR, Brown CM, Shoda TM, Stayton LE, & Martin CE (2011). Friends with benefits: On the positive consequences of pet ownership. Journal of Personality and Social Psychology, 101(6), 1239–1252. 10.1037/a0024506 [DOI] [PubMed] [Google Scholar]
- McDonald SE, Matijczak A, Nicotera N, Applebaum JW, Kremer L, Natoli G, O’Ryan R, Booth LJ, Murphy JL, Tomlinson CA, & Kattari SK (2021). “He was like, my ride or die”: Sexual and Gender Minority Emerging Adults’ Perspectives on Living With Pets During the Transition to Adulthood. Emerging Adulthood, 216769682110253. 10.1177/21676968211025340 [DOI] [Google Scholar]
- McDonald SE, O’Connor K, Matijczak A, Murphy J, Applebaum JW, Tomlinson CA, Wike TL, & Kattari SK (2021). Victimization and psychological wellbeing among sexual and gender minority emerging adults: Testing the moderating role of emotional comfort from companion animals. Journal of the Society for Social Work and Research. 10.1086/713889 [DOI] [Google Scholar]
- McLennan K, Rock MJ, Mattos E, & Toohey AM (2022). Leashes, Litterboxes, and Lifelines: Exploring Volunteer-Based Pet Care Assistance Programs for Older Adults. Frontiers in Psychology, 0, 1617. 10.3389/FPSYG.2022.873372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Kennedy C, DeVoe D, Hickey M, Nelson T, & Kogan L (2009). An examination of changes in oxytocin levels in men and women before and after interaction with a bonded dog. Anthrozoos, 22(1), 31–42. 10.2752/175303708X390455 [DOI] [Google Scholar]
- Mueller MK, Gee NR, & Bures RM (2018). Human-animal interaction as a social determinant of health: descriptive findings from the health and retirement study. BMC Public Health, 18(1), 305. 10.1186/s12889-018-5188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MK, King EK, Callina K, Dowling-Guyer S, & McCobb E (2021). Demographic and contextual factors as moderators of the relationship between pet ownership and health. Health Psychology and Behavioral Medicine, 9(1), 701–723. 10.1080/21642850.2021.1963254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Haire ME, Tedeschi P, Jenkins M, Braden S, & Rodriguez KE (2019). The impact of human-animal interaction in trauma recovery. In New Directions in the Human-Animal Bond (pp. 15–53). Purdue University Press. [Google Scholar]
- Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, & Sager MA (2014). Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology, 83(19), 1753–1760. 10.1212/WNL.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, & Vandagriff JL (2019). Animal Visitation Program (AVP) Reduces Cortisol Levels of University Students: A Randomized Controlled Trial: AERA Open, 5(2), 233285841985259. 10.1177/2332858419852592 [DOI] [Google Scholar]
- Phelan JC, Link BG, & Tehranifar P (2010). Social Conditions as Fundamental Causes of Health Inequalities: Theory, Evidence, and Policy Implications. Journal of Health and Social Behavior, 51(1_suppl), S28–S40. 10.1177/0022146510383498 [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, & Wallace RB (2007). Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology, 29(1–2), 125–132. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polick CS, Applebaum JW, Hanna C, Darnysus Jackson I, Tsaras-Schumacher S, Hawkins R, Conceicao A, O’Brien LM, Chervin RD, & Braley TJ (2021). The Impact of Pet Care Needs on Medical Decision-Making among Hospitalized Patients: A Cross-Sectional Analysis of Patient Experience. Journal of Patient Experience, 8, 1–7. 10.1177/23743735211046089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND Center for the Study of Aging. (2021). RAND HRS Longitudinal File 2018 (V1). National Institute on Aging and the Social Security Administration. [Google Scholar]
- Rehn T, Handlin L, Uvnäs-Moberg K, & Keeling LJ (2014). Dogs’ endocrine and behavioural responses at reunion are affected by how the human initiates contact. Physiology & Behavior, 124, 45–53. 10.1016/J.PHYSBEH.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Rodriguez KE, Herzog H, & Gee NR (2021). Variability in Human-Animal Interaction Research. Frontiers in Veterinary Science, 7, 619600. 10.3389/fvets.2020.619600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, McMillian C, & Carter O (2020). Pet-Friendly Rental Housing: Racial and Spatial Inequalities. Space and Culture, 120633122095653. 10.1177/1206331220956539 [DOI] [Google Scholar]
- Rovio S, Spulber G, Nieminen LJ, Niskanen E, Winblad B, Tuomilehto J, Nissinen A, Soininen H, & Kivipelto M (2010). The effect of midlife physical activity on structural brain changes in the elderly. Neurobiology of Aging, 31(11), 1927–1936. 10.1016/J.NEUROBIOLAGING.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Saunders J, Parast L, Babey SH, & Miles J. v. (2017). Exploring the differences between pet and non-pet owners: Implications for human-animal interaction research and policy. PLoS ONE, 12(6). 10.1371/journal.pone.0179494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, & Stern Y (2009). Physical Activity, Diet, and Risk of Alzheimer Disease. JAMA, 302(6), 627–637. 10.1001/JAMA.2009.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoresby KJ, Strand EB, Ng Z, Brown KC, Stilz CR, Strobel K, Barroso CS, & Souza M (2021). Pet Ownership and Quality of Life: A Systematic Review of the Literature. Veterinary Sciences, 8(12), 332. 10.3390/VETSCI8120332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, & Weir DR (2014). Cohort Profile: the Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. 10.1093/IJE/DYU067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer E, & Stevens J (2021). Effects of Human-Animal Interactions on Affect and Cognition. Human-Animal Interaction Bulletin, 10(2), 73–98. https://www.apahai.org/haib/download-info/effects-of-human-animal-interactions-on-affect-and-cognition/ [Google Scholar]
- Thoits PA (2011). Mechanisms Linking Social Ties and Support to Physical and Mental Health. Journal of Health and Social Behavior, 52(2), 145–161. 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Tomlinson CA, Matijczak A, McDonald SE, & Gee NR (2021). The Role of Human-Animal Interaction in Child and Adolescent Health and Development. Reference Module in Biomedical Sciences. 10.1016/B978-0-12-818872-9.00003-0 [DOI] [Google Scholar]
- Toohey AM, & Krahn TM (2018). ‘Simply to be let in’: opening the doors to lower-income older adults and their companion animals. Journal of Public Health, 40(3), 661–665. 10.1093/PUBMED/FDX111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell JP (2019). Therapy Dogs Improve Student Affect but Not Memory. Anthrozoös, 32(5), 691–699. 10.1080/08927936.2019.1645514 [DOI] [Google Scholar]
- Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, & Salonen JT (2002). Socioeconomic Position Across the Lifecourse and Cognitive Function in Late Middle Age. The Journals of Gerontology: Series B, 57(1), S43–S51. 10.1093/GERONB/57.1.S43 [DOI] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, Kantarci K, Senjem ML, Gunter JL, Boeve BF, Petersen RC, & Jack CR (2012). Effect of lifestyle activities on alzheimer disease biomarkers and cognition. Annals of Neurology, 72(5), 730–738. 10.1002/ANA.23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weden MM, Shih RA, Kabeto MU, & Langa KM (2018). Secular Trends in Dementia and Cognitive Impairment of U.S. Rural and Urban Older Adults. American Journal of Preventive Medicine, 54(2), 164–172. 10.1016/J.AMEPRE.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Li L, & Su D (2014). Physical Activity and Mortality Among Middle-Aged and Older Adults in the United States. Journal of Physical Activity and Health, 11(2), 303–312. 10.1123/JPAH.2011-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuede CM, Timson BF, Hettinger JC, Yuede KM, Edwards HM, Lawson JE, Zimmerman SD, & Cirrito JR (2018). Interactions between stress and physical activity on Alzheimer’s disease pathology. Neurobiology of Stress, 8, 158–171. 10.1016/J.YNSTR.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX, Tian Y, Wang ZT, Ma YH, Tan L, & Yu JT (2021). The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. The Journal of Prevention of Alzheimer’s Disease, 8(3), 313–321. 10.14283/JPAD.2021.15 [DOI] [PubMed] [Google Scholar]
- Zhu CW, Scarmeas N, Ornstein K, Albert M, Brandt J, Blacker D, Sano M, & Stern Y (2015). Health-care use and cost in dementia caregivers: Longitudinal results from the Predictors Caregiver Study. Alzheimer’s & Dementia, 11(4), 444–454. 10.1016/J.JALZ.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


