Abstract
Background:
Reduced antibiotic susceptibility (RS) in Neisseria gonorrhoeae (GC) may increase treatment failure. Conducting tests of cure (TOC) for patients with RS-GC may facilitate identification of treatment failures.
Methods:
We examined 2018 to 2019 data from 8 jurisdictions participating in the US Centers for Disease Control and Prevention’s Strengthening US Response to Resistant Gonorrhea project. Jurisdictions collected GC isolates and epidemiological data from patients and performed antimicrobial susceptibility testing. Minimum inhibitory concentrations of ceftriaxone, 0.125 μg/mL or greater; cefixime, 0.250 μg/mL or greater; or azithromycin, 2.0 μg/mL or greater were defined as RS. Patients with RS infections were asked to return for a TOC 8 to 10 days posttreatment. We calculated a weighted TOC return rate and described time to TOC and suspected reasons for any positive TOC results.
Results:
Overall, 1165 patients were diagnosed with RS infections. Over half returned for TOC (weighted TOC, 61%; 95% confidence interval, 50.1%–72.6%; range by jurisdiction, 32%–80%). Test of cure rates were higher among asymptomatic (68%) than symptomatic patients (53%, P = 0.001), and men who have sex with men (62%) compared with men who have sex with women (50%; P < 0.001). Median time between treatment and TOC was 12 days (interquartile range, 9–16). Of the 31 (4.5%) TOC patients with positive results, 13 (42%) were suspected because of reinfection and 11 (36%) because of false-positive results. There were no treatment failures suspected to be due to RS-GC.
Conclusions:
Most patients with a RS infection returned for a TOC, though return rates varied by jurisdiction and patient characteristics. Test of cure can identify and facilitate treatment of reinfections, but false-positive TOC results may complicate interpretation and clinical management.
Gonorrhea is one of the most commonly reported notifiable diseases in the United States, 616,392 cases were reported in 2019 and case rates have increased by more than 50% since 2015.1 Although symptoms are common in male urethral gonococcal infections, endocervical, rectal, and pharyngeal gonorrhea are often asymptomatic. If left untreated, gonorrhea may cause pelvic inflammatory disease and severe reproductive health complications.
Neisseria gonorrhoeae, the causative agent of gonorrhea, has developed resistance to each class of antibiotic used to treat it, and the US Centers for Disease Control and Prevention (CDC) classifies antibiotic-resistant gonorrhea as an urgent public health threat.2 In 2020, CDC removed azithromycin as part of treatment of gonorrhea due in part to concerns about declining azithromycin susceptibility. Currently, only 1 first-line treatment option remains, a single dose of ceftriaxone (500 mg to 1 g, depending on patient weight) by intramuscular injection, with oral cefixime (800 mg) as an alternate treatment.3 To date, no confirmed cases of gonorrhea treatment failures due to ceftriaxone-resistant infections have been confirmed in the United States. However, a small number of treatment failures due to ceftriaxone-resistant infections have been documented in other countries, and a case with similar resistant mutations but that responded to recommended therapy was recently documented in the United States.4–9 The threat of ceftriaxone-resistant infections and untreatable gonorrhea warrants robust surveillance for detection of resistance.
Test of cure (TOC), in which patients diagnosed with gonorrhea return within 1 to 2 weeks after treatment for retesting, is an approach to detect persistent infections despite treatment (ie, treatment failures), including resistant infections. In particular, conducting TOCs in patients whose infections demonstrate elevated antimicrobial minimum inhibitory concentrations (MICs)—a patient population that might be at elevated risk of treatment failure—may facilitate rapid detection of resistance. However, N. gonorrhoeae antimicrobial susceptibility testing (AST) is not typically performed outside of sexually transmitted disease (STD) clinics participating in antimicrobial resistance sentinel surveillance. Consequently, current CDC guidelines focus on criteria, other than AST results, that may correlate with treatment failures. At present, CDC only recommends a TOC for pharyngeal gonorrhea infections because of the asymptomatic presentation of most infections and the suspicion of increased resistance development at this anatomic site.3
Data on the proportion of patients who return for a recommended TOC (TOC visit return rates) in the United States have been reported between 8.5% and 44.2%, with 3.4% to 4.6% of nucleic acid amplification test (NAAT) specimens collected at TOC visits positive for N. gonorrhoeae.10–12 However, although reexposure is sometimes assessed (10–11), AST on specimens collected is not often performed, and reasons for TOC NAAT positivity, other than reinfection, are often not investigated. To date, no data have been published on TOC visit return rates among persons with infections with reduced antibiotic susceptibility (RS). The objectives of this evaluation are to describe the TOC visit return rate among patients identified as having gonorrhea with RS to azithromycin, ceftriaxone, or cefixime from 42 health centers in 8 city or county jurisdictions across the United States. We also sought to describe patient characteristics associated with higher TOC visit return rates, days from treatment to TOC, test results among persons who returned for a TOC visit, and suspected reasons for any positive TOC results based on review of patient records.
METHODS
Study Setting and Population
We used 2018 to 2019 data from Strengthening the US Response to Resistant Gonorrhea (SURRG) for this analysis. Strengthening the US Response to Resistant Gonorrhea is a CDC-supported multisite project designed to strengthen local laboratory, clinic, and epidemiological capacity to rapidly detect and respond to antimicrobial-resistant gonorrhea. The 8 funded SURRG grantees included the following: California (San Francisco County), Colorado (Denver County/Denver), Indiana (Marion County/Indianapolis), Hawaii (Honolulu County/Honolulu), New York City, North Carolina (Guilford County), Washington (King County/Seattle), and Wisconsin (Milwaukee City). Project methods are described in another article in this supplement.13
Based on locally developed SURRG specimen collection criteria, each participating jurisdiction collected urogenital, rectal, and pharyngeal specimens for NAAT, culture, and AST from patients attending partnering STD clinics (n = 16) and other selected health care settings, such as emergency rooms, human immunodeficiency virus (HIV) care providers, LGBTQ-focused, and Planned Parenthood health centers (hereafter referred to as non-STD clinics) (n = 26). All but 1 STD clinic and many non-STD clinics used the Hologic Aptima Combo 2 NAAT assay. Cultures were obtained from patients presumptively treated at the time of testing and from patients who returned to the clinic for treatment after a positive NAAT result. Patients diagnosed with gonorrhea were treated in accordance with clinic protocols and CDC treatment guidelines. Local public health laboratories performed gonorrhea NAATs and cultures and conducted AST using Etest gradient strips (bioMérieux, France) for azithromycin, ceftriaxone, and cefixime on any identified N. gonorrhoeae isolates. Patients with isolates exhibiting elevated azithromycin MICs of 2.0 μg/mL or greater, ceftriaxone MICs of 0.125 μg/mL or greater, or cefixime MICs of 0.25 μg/mL or greater were categorized as having an RS infection.
Health department disease investigation staff (or nursing staff in the case of Denver) attempted to contact (at least 3 times) any patient with RS gonorrhea and recommended the patient return for a TOC clinical visit with testing by NAAT and culture 8 to 10 days after treatment to ensure the infection was cured. The SURRG protocol to conduct a TOC 8 to 10 days after treatment was developed early in SURRG based on the best available evidence on time to clearance for RNA-based NAATs (eg, Aptima Combo 2) and expert opinion among clinicians from SURRG jurisdictions.14,15 Patients with positive gonorrhea NAATs at TOC visits were managed per CDC treatment guidelines and local protocols (ie, retreatment with the recommended regimen, and a second TOC by NAAT and culture).15 Per SURRG protocols, demographic, clinical, epidemiological, and laboratory data were collected from all patients with specimens collected for culture; these deidentified data were subsequently submitted to CDC.
Definitions and Measures
Tests of cure visits were classified by local project staff based on the documented reason for the visit and review of clinical records. No limit was imposed on the number of days after initial treatment a local program could classify a subsequent visit as a TOC visit. At TOC visits, specimens for NAAT and culture were collected from the anatomic site(s) of the recently diagnosed RS infection. We defined the TOC visit return rate as the percentage of patients with RS gonorrhea who returned for a TOC visit following treatment. We limited this analysis to patients’ first TOC visit (ie, data on any subsequent TOC visits for patients who tested gonorrhea-positive at their initial TOC visit are not included in this analysis).
We defined patients as having positive TOC results if they had a positive gonorrhea NAAT at 1 or more of the same anatomic sites as their initial RS infection, and defined days to TOC as the number of days from treatment to the TOC visit. Patients were considered symptomatic if, at the initial testing or treatment visit, they reported symptoms commonly associated with gonorrhea at 1 or more of the infected anatomic sites (eg, dysuria, genital discharge, or throat, rectal, or abdominal pain).
Based on review of patient records, we designated the most likely suspected reason for each NAAT-positive TOC case into 1 of the following 6 categories using definitions described in Table 3: (1) “pharyngeal gonorrhea treatment failure due to alternative treatment,” (2) “treatment failure due to suboptimal treatment,” (3) “reinfection,” (4) “false-positive due to residual genetic material/nonviable organism,” (5) “treatment failure due to pharmacokinetic/pharmacodynamic or host factor issues,” and (6) “treatment failure due to ceftriaxone resistance.”
TABLE 3.
Suspected Reason for GC NAAT-Positive TOC Result Among Patients With RS Gonorrhea Infections, SURRG, 2018–2019
| Suspected Reason* | Definition†‡ | n (%) TOC GC-NAAT Positive (N = 31) |
|---|---|---|
|
(1) Pharyngeal gonorrhea treatment failure due to alternative treatment§ |
Patient had a RS-pharyngeal gonococcal infection treated with an alternative regimen (eg, gentamycin plus azithromycin) | 4 (12.9)¶ |
|
(2) Treatment failure due to suboptimal treatment |
Patient treated with a nonrecommended treatment (eg, doxycycline) | 1 (3.2)¶ |
|
(3) Reinfection |
Patient received the recommended treatment and reported sexual exposure between treatment and TOC visit |
13 (41.9) |
| (4) False-positive due to residual genetic material/nonviable organism | Patient received recommended treatment and denied interval sex; TOC culture was negative, and the initial isolate tested had low ceftriaxone MICs | 11 (35.5) |
|
(5) Treatment failure due to PKPD modeling or host factor issues |
Patient received recommended treatment and denied interval sex; TOC culture was positive, and the initial isolate had low ceftriaxone MICs | 2 (6.5)|| |
|
(6) Treatment failure due to ceftriaxone resistance |
Patient received recommended treatment, initial isolate had elevated ceftriaxone MICs, and no other reason was deemed likely | 0 (0) |
Categories of suspected reason are not mutually exclusive; however, we applied a hierarchy of classifications based on the numbered reasons listed in the table to assign 1 determination. For example, if the case met the definition for suspected reason number 1, it was assigned to that reason. Only cases that were not assigned to suspected reason number 1 were then eligible to be assigned to suspected reason number 2, and so on.
Recommended, alternative, and nonrecommended treatment classifications were based on CDC’s 2015 STD treatment guidelines.16
Data on interval sexual exposure were not required for all TOC patients, but data were extracted from medical records for patients who tested GC NAAT-positive at their TOC visit.
During the study period, CDC TOC recommendations were for pharyngeal GC treated with an alternate regime only—due to documented lower treatment success for pharyngeal infections not treated with ceftriaxone-based therapies.16
None of these patients reported sexual exposure between their initial treatment and TOC visit.
Includes 1 pharyngeal RS infection and 1 rectal RS infection.
GC, Neisseria gonorrhoeae; PKPD, pharmacokinetic/pharmacodynamic.
Analysis
This analysis included patients diagnosed with RS gonorrhea at participating clinics during 2018 to 2019. Among patients diagnosed with RS gonorrhea, we calculated the percent of patients who returned for a TOC and reported the unadjusted and weighted rates (with 95% confidence intervals). We used inverse-variance weighting to estimate the overall return rate, accounting for sample size differences across jurisdictions. We also performed χ2 tests to compare differences in TOC visit return rates across key characteristics, including gender, gender of recent sex partners among men, symptomatology, and anatomic site of RS infection. To assess for changes in TOC visit return rates over time, we conducted a Cochran-Armitage trend test. All analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Human Subjects Protection
The CDC’s institutional review board reviewed the SURRG protocol and determined the project to be a public health activity and not human subject research.
RESULTS
Study Population
During 2018 to 2019, 1165 patients were diagnosed with gonococcal infections with RS to azithromycin, ceftriaxone, and/or cefixime (Table 1). The number of patients with RS infections varied by jurisdiction, ranging from 27 in Guilford County, NC to 460 in New York City. Patients from New York City constituted 39.5% of the study population. Most patients (94%) had an infection with RS to azithromycin (MIC ≥2.0 μg/mL) and less than 4% had an infection with RS to ceftriaxone (0.125–0.25 μg/mL) or cefixime (0.25–0.50 μg/mL), respectively. Most patients self-identified as male (94%), and less than 1% identified as transgender or “other gender” identity. Two thirds of male patients (cis and trans males) reported recent sex only with other men (MSM); 20% reported sex only with women (MSW). The majority of patients were non-Hispanic Black (35%) or non-Hispanic White (33%), and median age was 29 years (interquartile range [IQR], 25–36 years). Most patients had urethral RS infections (57%). Only 3% of patients with RS gonorrhea had endocervical infections. Sixty-one percent of patients with RS gonorrhea reported symptoms. Symptoms were more commonly reported by male patients with RS urethral infections (84.7%; 599 of 707) than by patients with RS endocervical (46.9%; 23 of 48), rectal (20.1%; 62 of 256), or pharyngeal infections (18.4%; 25 of 228) (results not shown). Ninety percent of patients with RS gonorrhea received their care at STD clinics. Additional data on collected specimens, isolates, and overall patient populations in SURRG are reported elsewhere.13
TABLE 1.
Characteristics of Patients With Reduced Susceptible Gonococcal* Infections by Test of Cure Visit Status, SURRG, 2018–2019
| Returned for Test of Cure Visit |
|||
|---|---|---|---|
| Total Patients (N = 1165) |
No (n = 475) |
Yes (n = 690) |
|
| Characteristics | n (Col %) | n (Row %) | n (Row %) |
| Jurisdiction | |||
| Denver County, CO | 126 (10.8) | 25 (19.8%) | 101 (80.2) |
| Guilford County, NC | 27 (2.3) | 13 (48.1) | 14 (51.9) |
| Honolulu County, HI | 28 (2.4) | 19 (67.9) | 9 (32.1) |
| Marion County, IN | 83 (7.2) | 28 (33.7) | 55 (66.3) |
| Milwaukee City, WI | 149 (12.8) | 57 (38.3) | 92 (61.7) |
| New York City, NY | 460 (39.5) | 250 (54.3) | 210 (45.7) |
| San Francisco County, CA | 180 (15.5) | 58 (32.2) | 122 (67.8) |
| Seattle-King County, WA | 112 (9.6) | 25 (22.3) | 87 (77.7) |
| Reduced susceptible antibiotic† | |||
| Azithromycin | 1099 (94.3) | 444 (40.4) | 655 (59.6) |
| Ceftriaxone | 43 (3.7%) | 21 (48.8) | 22 (51.2) |
| Cefixime | 40 (3.4) | 19 (47.5) | 21 (52.5) |
| Gender‡ | |||
| Male | 1096 (94.1) | 451 (41.1) | 645 (58.9) |
| Female | 62 (5.3) | 22 (35.5) | 40 (64.5) |
| Transgender male | 2 (0.2) | 0 (0) | 2 (100) |
| Transgender female | 1 (0.1) | 0 (0) | 1 (100) |
| Other gender identity | 4 (0.3) | 0 (50.0) | 2 (50.0) |
| Gender of males’ sex partners | |||
| MSM | 785 (67.4) | 296 (37.7) | 489 (62.3) |
| MSMW | 50 (4.3) | 18 (36.0) | 32 (64.0) |
| MSW | 230 (19.7) | 115 (50.0) | 115 (50.0) |
| Unknown | 37 (3.2) | 22 (64.9) | 37 (35.1) |
| Age, y | |||
| Median [IQR] | 29 [25–36] | 29 [24–36] | 29 [25–36] |
| Race/Hispanic ethnicity | |||
| AIAN | 4 (0.3) | 2 (50.0) | 2 (50.0) |
| Asian | 79 (6.8) | 29 (36.7) | 50 (63.3) |
| Black | 402 (34.5) | 186 (46.3) | 216 (53.7) |
| NHOPI | 3 (0.3) | 0 (0) | 3 (100) |
| White | 379 (32.5) | 144 (38.0) | 235 (62.0) |
| Hispanic/Latino | 230 (19.7) | 81 (35.2) | 149 (64.8) |
| Other | 23 (2.0) | 13 (56.5) | 10 (43.5) |
| Multirace | 30 (2.6) | 10 (33.3) | 20 (66.7) |
| Unknown | 15 (1.3) | 10 (66.7) | 5 (33.3) |
| Infection source (N = 1239§) | |||
| Urethral (male) | 707 (57.1) | 326 (46.1) | 381 (53.9) |
| Endocervical | 48 (3.4) | 16 (33.3) | 32 (66.7) |
| Rectal | 256 (20.6) | 82 (32.0) | 174 (68.0) |
| Pharyngeal | 228 (18.4) | 71 (31.1) | 157 (68.9) |
| Symptom status¶ | |||
| Symptomatic | 706 (60.6) | 329 (46.6) | 377 (53.4) |
| Asymptomatic | 459 (39.4) | 146 (31.8) | 313 (68.2) |
| Clinic type|| | |||
| STD clinic | 1051 (90.2) | 424 (40.3) | 627 (59.7) |
| Non-STD health center | 114 (9.8) | 51 (44.7) | 63 (55.3) |
| HIV status‡ | |||
| Positive | 116 (10.0) | 53 (45.7) | 63 (54.3) |
| Negative | 627 (53.8) | 260 (41.5) | 367 (58.5) |
| Not tested/unknown | 422 (36.2) | 162 (38.4) | 260 (61.6) |
| History of gonorrhea‡ | |||
| Yes | 300 (25.8) | 115 (38.3) | 185 (61.7) |
| No | 777 (66.7) | 314 (40.4) | 463 (59.6) |
| Unknown | 88 (7.6) | 46 (52.3) | 42 (47.7) |
All patients had 1 or more gonococcal isolate with azithromycin MIC ≥ 2.0 μg/mL, ceftriaxone MIC ≥ 0.125 μg/mL, and/or cefixime MIC ≥ 0.250 μg/mL.
Categories not exclusive; 15 patients diagnosed as having an infection with reduced susceptibility to more than 1 antibiotic.
Self-reported gender, last HIV result, and history of gonorrhea.
Instances of initial reduced susceptible gonococcal infections at more than 1 anatomic site (1239 infections among 1165 patients).
Symptomatic at the anatomic site(s) from which the specimen(s) was collected.
Non-STD health centers included: emergency rooms, infectious disease practices, LGBTQ-focused health centers, HIV testing sites, federally qualified health centers, Planned Parenthood health centers, and women’s health practices.
AIAN, American Indian/Alaskan Native; NHOPI, Native Hawaiian and Other Pacific Islander.
Comparison of Patients Who Did and Did Not Return for a TOC Visit
Among patients with RS gonorrhea, 59% (690 of 1165) returned for a TOC visit, with a wide variability in TOC visit return rates by jurisdiction, ranging from 32% (9 of 28) in Honolulu to 80% (101 of 126) in Denver (Table 1). The weighted overall TOC return rate was 61% (95% confidence interval, 50.1%–72.6%), and unweighted return rates were steady over time (Figure S1, http://links.lww.com/OLQ/A740). Asymptomatic patients were more likely to return for a TOC visit than symptomatic patients (68% vs 53%, respectively; P = 0.001). A higher proportion of women (cis and trans females) returned for a TOC visit than men (65% vs 59%, respectively, P = 0.32), but this difference was not statistically significant. Among men (cis and trans males), MSM and cis and trans males who reported recent sex with both men and women (MSMW) were more likely to return for a TOC than MSW (62% vs 50%, respectively, P < 0.001). Patients with RS urethral infections were less likely to return for a TOC than patients with nonurethral RS infections (54% vs 68%, statistical test not performed as observations are not independent with some people infected at multiple anatomic sites) (Table 1). When stratified by symptom status, asymptomatic male patients, regardless of gender of sex partners and anatomic site of infection, were more likely to return for a TOC visit than symptomatic male patients (68.7% vs 52.8%) (Table 2). Overall, asymptomatic patients with RS gonorrhea were more likely to return for a TOC visit than symptomatic patients with RS gonorrhea at the same anatomic site. We did not observe clinically meaningful differences in TOC return rates by clinic type, HIV status, or history of gonorrhea (Table 1).
TABLE 2.
TOC Clinic Visit Return Rates by Symptomatic Status and Patient Characteristics, SURRG, 2018–2019
| Asymptomatic* |
Symptomatic* |
|||||
|---|---|---|---|---|---|---|
| Characteristics | n | N | % | n | N | % |
| Sex | ||||||
| Female (cis and trans female) | 25 | 39 | 64.1 | 16 | 24 | 66.7 |
| Male (cis and trans male) | 287 | 418 | 68.7 | 360 | 680 | 52.9 |
| Sex of males’ sex partners | ||||||
| MSM/MSMW | 246 | 341 | 72.1 | 275 | 494 | 55.7 |
| MSW only | 32 | 53 | 60.4 | 83 | 117 | 46.9 |
| Infection source† | ||||||
| Urethral | 66 | 108 | 61.1 | 315 | 599 | 52.6 |
| Endocervical | 17 | 25 | 68.0 | 15 | 23 | 65.2 |
| Rectal | 140 | 194 | 72.2 | 34 | 62 | 54.8 |
| Pharyngeal | 142 | 203 | 70.0 | 15 | 25 | 60.0 |
N, number of patients who were asymptomatic or symptomatic with or without a TOC visit; n, number of patients who returned for a TOC visit within that category.
Includes reduced susceptible infections at more than 1 anatomic site.
MSM/MSMW, cis and transgender males who reported recent sex with men or both men and women.
Days From Treatment to TOC Visit
Dates of treatment were available for 87% (597 of 690) of patients with a TOC visit documented. For these patients, the median time between treatment and TOC visit was 12 days (range, 4–48 days; IQR, 9–16 days) (Fig. 1) and was shorter for patients who tested positive at their TOC visit (11 days) than those who tested negative (13 days) (results not shown). Thirty-three percent (202 of 597) returned for their TOC visit during the recommended interval of 8 to 10 days after treatment, and 97% (579 of 597) returned within 30 days of treatment (Fig. 1).
Figure 1.
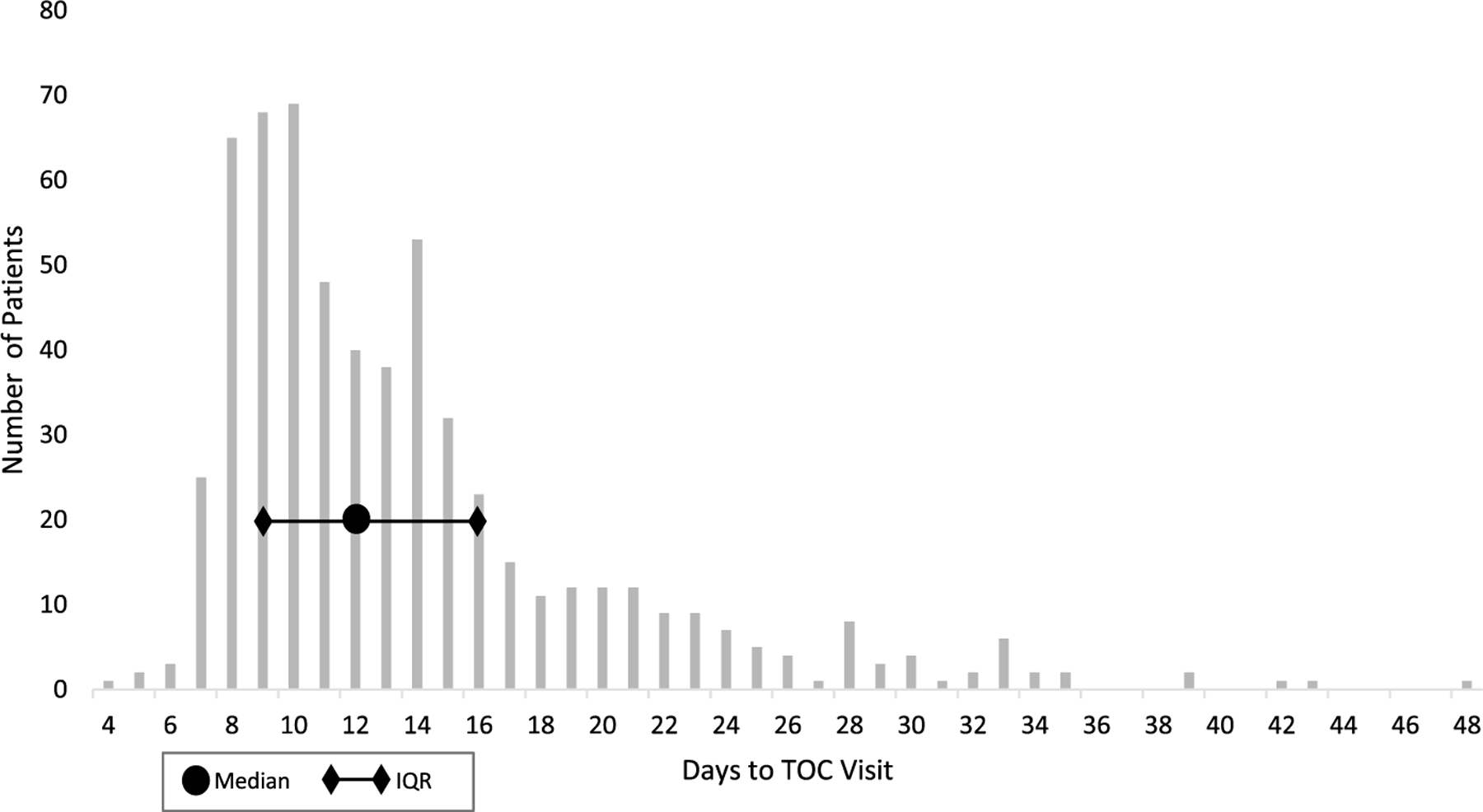
Days between treatment and TOC visit among patients returning for a TOC visit, SURRG, 2018–2019 (N = 597)*.
TOC Results and Suspected Reasons for Positive Results
Among the 690 patients who returned for a TOC visit (representing 744 RS infections due to some patients being infected at multiple anatomic sites), 31 (4.5%) tested gonorrhea NAAT-positive at one or more of the same anatomic site where they were previously diagnosed as having an RS infection. Test of cure NAAT positivity was highest for RS pharyngeal infections (12.1%; 19 of 157). Test of cure positivity for urethral, endocervical, and rectal RS infections was 2.4% (9 of 381), 0% (0 of 32), and 1.7% (3 of 174), respectively ( P < 0.001 nonpharyngeal vs pharyngeal).
We described the suspected reasons for each of the 31 patients with a positive gonorrhea NAAT at their TOC visit (Table 3). Ceftriaxone resistance was not a suspected reason for NAAT positivity for any of the patients. Most positive results at TOC visits were suspected to be due to reinfection (41.9%) or detection of genetic material from nonviable organisms (false-positives) (35.5%). In addition, 7 patients (22.6%) were suspected of having a positive TOC NAAT and persistent infection owing to inadequate treatment with a nonrecommended or alternative treatment or host factor issues. Among the 11 patients with suspected false-positive NAATs, the median time between treatment and TOC was 9 days (range, 5–13 days). Among the other 20 TOC NAAT-positive patients, the median days between treatment and TOC visit was 13 days (range, 8–33 days). Most TOC NAAT-positive patients (29 of 31) received care at clinics that used the Hologic Aptima Combo-2. Two TOC NAAT-positive patients received care at a clinic that used the Roche cobas 4800 CT/NG assay; both of these patients were suspected of testing positive due to reinfection.
DISCUSSION
We found 61% of patients diagnosed with RS gonorrhea returned for a TOC visit overall, with wide variability across the 8 jurisdictions participating in SURRG. Return rates were higher among asymptomatic patients, women, and MSM compared with symptomatic patients, men, and MSW, respectively. The median time from treatment to TOC visit was 12 days, and 5% of patients tested NAAT-positive at their TOC visit. We did not identify any treatment failures due to ceftriaxone-resistant infections and determined that most positive results at TOC visits were likely due to reinfection or false positive NAAT results. Our findings suggest that the majority of patients diagnosed with and notified of RS gonorrhea will return for a TOC visit to facilitate detection of treatment failures, and that while TOC can identify and facilitate treatment of reinfections, false-positive TOC results can complicate interpretation and clinical management.
The TOC visit return rates we observed in our analysis are similar to the rates reported in the United Kingdom (58%), where TOC is recommended for all cases of gonorrhea.16 However, our TOC visit return rates were considerably higher than rates observed in other United States–based TOC evaluations, which reported rates of 44% and 26% among MSM with urethral, rectal or pharyngeal gonococcal infections in evaluations in New York City and Los Angeles, respectively, and 9% among STD clinic patients (from 17 clinics participating in the STD Surveillance Network) diagnosed with pharyngeal gonorrhea and treated with alternate regimes.10–12 It is unclear why the TOC visit return rates we observed were higher than other United States–based TOC analyses, although ours was the first to assess the TOC visit return rate among patients identified with RS gonorrhea, and patients in our study may have been more likely to return for a TOC visit after being informed their infection demonstrated RS. Further, the reasons for the wide variability we observed in TOC rates across the 8 jurisdictions were not immediately clear, as TOC protocols across the jurisdictions were similar. Additional investigation would be useful to determine if these differences are due to variability in how the TOC model was implemented locally, demographics of patient populations, and/or other factors, such as clinic access.
Although our overall TOC visit return rate was higher than other United States–based studies, nearly 40% of patients with RS gonorrhea did not return for a TOC visit. Thus, detection of treatment failures, even when patients are informed of having RS gonorrhea can be difficult. Given that most gonococcal infections are asymptomatic, patients with RS gonorrhea who do not return for a TOC visit may unknowingly spread these RS infections and challenge containment efforts. Many confirmed ceftriaxone-resistant treatment failure cases from around the world (with documented AST results) reported ceftriaxone MICs of ≥0.50 μg/mL, which is higher than any of the ceftriaxone MICs we reported.8 It is unclear if patients would be more likely to return for a TOC if they were informed they had an infection with a level of resistance at or above levels observed in cases that led to treatment failures elsewhere.
Consistent with previous analyses, we reported a higher TOC visit return rate among asymptomatic patients than symptomatic patients and speculate that patients who were initially symptomatic and whose symptoms cleared are less motivated to return for a TOC visit than asymptomatic patients.10 We also found differences in the TOC visit return rate by gender, gender of sex partners among men, and anatomic site of infection. One hypothesis for the observed difference in TOC return rates between MSM and MSW only is that some MSM (perhaps through provider or media messaging) may be aware that antimicrobial-resistant gonorrhea rates are highest among MSM and, therefore, more motivated to return for a TOC visit when told they have a RS infection compared with MSW only. A second hypothesis is that MSM have higher TOC return rates than MSW only because of a greater likelihood of having asymptomatic infections (ie, rectal and pharyngeal infections) than MSW only. Further study of TOC visit return rate differences by patient characteristics, and the reasons for those differences may help guide patient TOC messaging. Our findings on TOC visit return rates, days between treatment and TOC visits, and TOC positivity have implications for clinic operations (eg, patient volume, clinic flow, testing resources, and protocols) particularly as providers implement or consider implementing the updated 2020 gonorrhea TOC guidelines, which recommend a TOC 7 to 14 days after treatment for all patients diagnosed with pharyngeal gonorrhea.3 Anecdotally, a small number of SURRG clinics reported that implementing a TOC for patients with RS gonorrhea led to strain on clinic staff and reduced clinic availability for walk-in patients. Future work could investigate TOC burden on staff, clinic operations, resources, and patients, including assessing if TOC leads to turning away other priority patients. Documenting the value and cost-effectiveness of conducting TOC in different scenarios and evaluating if different models to streamline TOC specimen collection (eg, self-collected specimens, home-based testing) can reduce staff involvement and patient time in the clinic, or increase TOC visit return rates, are important next steps to support widespread integration of any current or future gonorrhea TOC recommendations into clinic operations.
In this evaluation, TOC activities did not identify any treatment failures due to ceftriaxone-resistant infections. This finding was not unexpected because there have not been any confirmed cases of ceftriaxone-resistant gonorrhea in the United States (although we did not know this would be the case when we established the SURRG TOC protocol). In SURRG, we purposefully set AST thresholds for RS to include MICs lower than current CLSI breakpoints for cephalosporin nonsusceptibility. This strategy allowed us to investigate emerging resistant infections and to pilot and evaluate response efforts including TOC. Our TOC activities, however, identified patients with persistent infections because of inadequate treatment and reinfection. Among the 5% of patients who tested TOC-positive, 65% were suspected of having persistent infections due to nonrecommended or alternative treatment regimens, host factor issues, or reinfection, and so retreating these patients has the potential both to prevent infection sequalae and to prevent onward transmission.
At the same time, we found that about one-third of positive TOC results were likely false-positives. This finding was not surprising, given the well-documented issue of false-positive NAAT results from residual nonviable genetic material and ongoing debate about the most appropriate timing for gonorrhea TOC recommendation.14–20 Also, potentially germane to this analysis, RS infections might be associated with delayed clearance of genetic material.19 In our analysis, the 11 patients who likely had false-positive NAATs at their TOC visit returned for their TOC visit less than 14 days after treatment; and 8 of the 11 patients returned for their TOC visit less than 10 days after treatment. These results suggest that waiting to perform TOC using NAATs until at least 10 days after treatment would likely reduce false-positive results. However, even if TOC were consistently performed at least 10 days after treatment, it may still be difficult for clinicians to differentiate between false-positive results, persistent infections, and reinfections. Recommendations for management of patients with a positive TOC can be found in the 2021 CDC STI treatment guidelines and include a careful sexual history to elicit sexual activity since initial treatment, retreatment, culture and antibiotic susceptibility testing, a second TOC, and consultation if cephalosporin treatment failure is suspected.21 Further, although DNA-based NAAT assays have been associated with higher rates of false positivity than RNA-based assays,14,19 we do not think the use of different assays across participating clinics impacted our results, as the RNA-based Aptima combo 2 was used in all suspected false-positive TOC cases in our analysis.
Our evaluation has several strengths including being a multisite project conducted over a 2-year period across 42 heath centers in 8 jurisdictions, using standardized data collection, and a large sample size. It is also the first analysis to our knowledge of TOC visit return rates and outcomes among patients identified with RS gonorrhea.
This analysis has several limitations. First, TOC return rates reported may not be generalizable to all persons with gonorrhea in the general population or in local jurisdictions. Strengthening the US Response to Resistant Gonorrhea was conducted in 8 US jurisdictions and 90% of patients came from STD clinics, whereas nationally, in 2019, only 8.6% of nationally reported gonorrhea cases were diagnosed in STD clinics.1 Second, some patients may have returned for a TOC visit at a facility not participating in SURRG, which may have underestimated the proportion of patients with a TOC visit. Third, small numbers of patients with RS infections identified at non-STD clinics may have limited our ability to detect differences in the TOC return percent by clinic setting. Fourth, the time between treatment and TOC visit was missing or not usable for 93 patients who returned for a TOC visit. And third, designations of suspected reasons for gonorrhea NAAT-positive specimens were based on best-available evidence in the medical record and may not represent the true reasons for NAAT-positive TOC results. For example, TOC-positive patients may have underreported interval sexual exposure, and thus, we may have undercounted likely reinfections. Also, because gonorrhea culture is less sensitive than NAAT, interpretation of a negative TOC-culture with a positive TOC NAAT alone does not necessarily indicate a false positive NAAT.
With the threat of ceftriaxone-resistant gonorrhea and limited alternative treatment options, conducting TOC on patients with known RS gonorrhea may be an important strategy to rapidly detect resistant infections, ensure infections are cured, and thus reduce the onward spread of resistant infections. Results of this analysis may also be useful to guide implementation of the recent TOC recommendations for all patients treated for pharyngeal gonorrhea.3,21 This evaluation provides data to support that a high proportion of patients with RS gonorrhea will return for a TOC within a reasonable time frame and could facilitate surveillance for treatment failures due to ceftriaxone resistance. Tests of cure can also identify cases of treatment failure due to inadequate treatment or host factor issues, as well as cases of reinfection—all situations from which patients can benefit from retreatment. Notably, our results also suggest that TOC conducted at the middle or later end of the recommended interval of 7 to 14 days might reduce false-positive NAAT results in patients whose infections demonstrate reduced antibiotic susceptibility. However, decisions about timing of TOC may also balance implications for possible reinfection and loss to follow-up and pragmatic decisions about clinic scheduling and patient availability. Further efforts to pilot and evaluate the most effective and efficient TOC models and messaging may enhance our ability to monitor for treatment failures and contain the spread of ceftriaxone-resistant gonorrhea that is anticipated to appear in the United States in the near future.
Acknowledgments:
SURRG Working Group: Rebecca Abelman, Lizzete Alvarado, Janet Arno, Tamara Baldwin, Lindley A. Barbee, Kyle T. Bernstein, Ruthie Burich-Weatherly, Lance Chinna, Alberto Clemente, Stephanie Cohen, Caitlin Conrad, Michael M. Denny, Rose Finney, Kim M. Gernert, Karen Gieseker, Alesia Harvey, Christine Heumann, Chi Hua, Sopheay Hun, Kimberly Johnson, Roxanne P. Kerani, Ellen Kersh, Noah Leigh, Jennifer Ludovic, Kerry Mauk, Candice J McNeil, Christie Mettenbrink, Victoria Mobley, Evelyn Nash, Trang Nguyen, Melissa Pagaoa, Elizabeth Palavecino, Ruchi Pandey, Rushlenne Pascual, Preeti Pathela, Zachary Perry, Elisabeth Phillips, Brian Raphael, Jennifer Reimche, Brad Roland, Maddie Sankaran, Kevin Sellers, Brandy Sessoms, Samera Sharpe, Olusegun O. Soge, Katy Town, Erica Terrell, Cindy Toler, Chun Wang, Karen Wendel, Wendy Wittmann; and Christine Khosropour for her assistance with this manuscript.
Sources of Funding:
Funding for the Strengthening the US Response to Resistant Gonorrhea activities described in this article was supported with federal Antibiotic Resistance Initiative funding and administered through the US Centers for Disease Control and Prevention’s (CDC) Epidemiology and Laboratory Capacity for the Prevention and Control of Infectious Diseases (ELC) Cooperative Agreement [CK19-1904].
Footnotes
Conflict of Interest: None declared.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019 Atlanta, GA: US Department of Health and Human Services, CDC, 2021. Available at: https://www.cdc.gov/std/statistics/2019/default.htm. Accessed April 13, 2021. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic Resistant Threats in the United States, 2019 Available at: https://www.cdc.gov/drugresistance/biggest-threats.html. Accessed March 26, 2021.
- 3.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fifer H, Natarajan U, Jones L, et al. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016; 374:2504–2506. [DOI] [PubMed] [Google Scholar]
- 5.Eyre DW, Sanderson ND, Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23:1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unemo M, Seifert HS, Hook EW 3rd, et al. Gonorrhoea. Nat Rev Dis Primers 2019; 5:79. [DOI] [PubMed] [Google Scholar]
- 7.Unemo M, Golparian D, Nicholas R, et al. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unemo M, Lahra MM, Cole M, et al. World Health Organization global gonococcal antimicrobial surveillance program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; 16:412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picker MA, Knoblock RJ, Hansen H, et al. Notes from the field: first case in the United States of Neisseria gonorrhoeae harboring emerging mosaic penA60 allele, conferring reduced susceptibility to cefixime and ceftriaxone. MMWR Morb Mortal Wkly Rep 2020; 69:1876–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beymer MR, Llata E, Stirland AM, et al. Evaluation of gonorrhea test of cure at 1 week in a Los Angeles community-based clinic serving men who have sex with men. Sex Transm Dis 2014; 41:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okah E, Westheimer EF, Jamison K, et al. Frequency of nucleic acid amplification test positivity among men who have sex with men returning for a test-of-cure visit 7 to 30 days after treatment of laboratory-confirmed Neisseria gonorrhoeae infection at 2 public sexual health clinics, New York City, 2013 to 2016. Sex Transm Dis 2018; 45:177–182. [DOI] [PubMed] [Google Scholar]
- 12.Llata E, Braxton J, Asbel L, et al. Pharyngeal gonococcal infections: A cross-sectional study in a network of sexually transmitted disease clinics; sexually transmitted disease surveillance network—January 2016 to June 2018. Sex Transm Dis 2019; 46:777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlanger K, Learner ER, Pham CD, et al. Strengthening the US Response to Resistant Gonorrhea: An overview of a multisite program to enhance local response capacity for antibiotic-resistant Neisseria gonorrhoeae. Sex Transm Dis 2021; 48(12S):S97–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wind CM, Schim van der Loeff MF, Unemo M, et al. Test of cure for anogenital gonorrhoea using modern RNA-based and DNA-based nucleic acid amplification tests: A prospective cohort study. Clin Infect Dis 2016; 62:1348–1355. [DOI] [PubMed] [Google Scholar]
- 15.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 16.Public Health England. Antimicrobial resistance in Neisseria gonorrhoeae in England and Wales: Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP 2019). Available at: https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report. Accessed March 26, 2021.
- 17.Barbee LA, Soge OO, Khosropour CM, et al. The duration of pharyngeal gonorrhea: a natural history study. Clin Infect Dis 2021; 73: 575–582. Available at: 10.1093/cid/ciab071/6123922. Accessed March 26, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbee LA, Golden MR. Editorial commentary: when to perform a test of cure for gonorrhea: Controversies and evolving data. Clin Infect Dis 2016; 62:1356–1359. [DOI] [PubMed] [Google Scholar]
- 19.Bissessor M, Whiley DM, Fairley CK, et al. Persistence of Neisseria gonorrhoeae DNA following treatment for pharyngeal and rectal gonorrhea is influenced by antibiotic susceptibility and reinfection. Clin Infect Dis 2015; 60:557–563. [DOI] [PubMed] [Google Scholar]
- 20.Zenilman JM. Editorial commentary: Persistent gonococcal DNA: Artifact or real? Further insights into the biology of a remarkable pathogen. Clin Infect Dis 2015; 60:564–565. [DOI] [PubMed] [Google Scholar]
- 21.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70: 1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]


