Abstract
Objectives:
To investigate whether the micronutrients that were shown to reduce the risk of development of age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) can have the same effect on the development of diabetic retinopathy in rats, and to understand the possible mechanisms.
Methods:
Streptozotocin-induced diabetic rats received a powdered diet with or without supplemental micronutrients (ascorbic acid, vitamin E, beta-carotene, zinc, and copper). The retina was used after the rats had diabetes for 12 months to detect vascular histopathology and to measure the biochemical parameters and messenger RNA levels of the genes involved in oxidative and nitrative stress.
Results:
The AREDS-based micronutrients prevented a diabetes-induced increase in the number of retinal acellular capillaries. In the same rats, micronutrients inhibited increases in retinal oxidatively modified DNA and nitrotyrosine and decreases in manganese superoxide dismutase. Diabetes-induced alterations in the messenger RNA expression of mitochondrial electron transport complex III (coenzyme Q cytochrome-c reductase) and inducible nitric oxide synthase were also prevented.
Conclusion:
Age-Related Eye Disease Study–based micronutrients inhibit the development of diabetic retinopathy in rodents by inhibiting oxidative and nitrative stress.
Clinical Relevance:
Micronutrients that slow down the onset and progression of age-related macular degeneration have the potential to inhibit the development of diabetic retinopathy.
DIABETIC RETINOPATHY IS the major cause of blindness in working adults in developed countries, and high glucose is considered the main instigator in its development. Good glycemic control can prevent and/or retard diabetic retinopathy,1–3 but such control is difficult to achieve and maintain. Many biochemical and molecular sequelae of hyperglycemia have been implicated in the pathogenesis of diabetic retinopathy;4–8 however, the exact mechanism remains elusive.
Oxidative stress is elevated in the retina in diabetes, and increased oxidative stress contributes to the development of diabetic retinopathy.7,8 Increase in reactive oxygen species is considered a causal link between elevated glucose and other metabolic abnormalities important in the development of diabetic retinopathy.9 Various antioxidants and nutrients have provided encouraging results in experimental models of diabetic retinopathy,7,8,10–13 though the results from clinical trials have been less conclusive.14–16 In diabetic mice, overexpression of the enzyme responsible for scavenging mitochondrial superoxide, manganese superoxide dismutase (MnSOD), prevents early lesions of retinopathy.10 In diabetic rats, supplementation with multiantioxidants or lipoic acid inhibits the development of retinopathy, and green tea and benfotiamine (a vitamin B1 derivative) inhibit the formation of increased acellular capillaries.7,8,10–12
The Age-Related Eye Disease Study (AREDS) has demonstrated that micronutrients, including antioxidants and trace metals, can reduce the risk of developing a blinding disease, age-related macular degeneration (AMD).17–19 The purpose of our study is to investigate the effect of the same AREDS-based micronutrients on the development of retinopathy in rats with streptozotocin-induced diabetes and to understand the possible mechanism through which these nutrients elicit their beneficial effects.
METHODS
RATS
Diabetes was induced in Lewis rats (weight, 200-220 g; male) by streptozotocin (dosage by body weight, 55 mg/kg). To allow slow weight gain while maintaining hyperglycemia (blood glucose, 360-450 mg/dL [to convert to mmol/L multiply by 0.0555]), a small dose of insulin (1-2 IU) (Humulin N; Eli Lilly, Indianapolis, Indiana) was administered 3 to 5 times per week. A group of rats with diabetes received a powder diet (LabDiet 5001; TestDiet, Richmond, Indiana) supplemented with AREDS-based micronutrients (50 mg/kg of ascorbic acid; 0.5 g/kg of vitamin E; 1.5 mg/kg of beta carotene; 8 mg/kg of zinc oxide; and 0.2 mg/kg of copper oxide) (diabetes and AREDS group); the rats with diabetes (diabetes group) and the age-matched control rats (without diabetes; control group) received the LabDiet 5001 powder diet without any supplementation. These diets were initiated soon after establishment of diabetes (3-4 days after administration of streptozotocin). Each group had 12 or more rats, and the entire rat colony was housed in metabolic cages. The rats were weighed 2 times per week and their feeders were weighed once every week to calculate the amount of food consumed. The entire rat colony received a new diet every other week. Glycated hemoglobin was measured after 2 months with diabetes11 and every 3 months thereafter. Twelve months after initiation of the experiment, a duration when histopathology can be observed in rats with streptozotocin-induced diabetes,7,11,20 the rats were euthanized by an overdose of pentobarbital and the eyes were removed. One eye was suspended in 10% formalin to prepare trypsin-digested microvessels, and the retina was isolated from the other eye for biochemical measurements by gently separating it from the choroid under a dissecting microscope. Institutional guidelines and the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research were followed.
VASCULAR HISTOPATHOLOGY IN RETINA
The retina was removed from the formalin-fixed eyes (8-10 eyes/group) and digested with 3% crude trypsin in Tris-HCl (tromethamine hydrochloric acid) buffer (pH, 7.8) containing 0.2M sodium fluoride for 90 minutes to isolate the microvessels.7,10,11 The vessels were stained with periodic acid-Schiff and hematoxylin for histological evaluation. The number of acellular capillaries (representing basement membrane tubes lacking cell nuclei and maintaining at least one-fourth the normal capillary caliber over their lengths), which is considered one of the hallmarks of early signs of diabetic retinopathy,7,10,11,20 was counted in a masked manner in multiple midretinal fields with 1 field adjacent to each of the 5 to 7 retinal arterioles radiating out from the optic disc; counts are expressed per square millimeter of retinal area examined.7,10,11,20
OXIDATIVE STRESS
Oxidative stress was quantified in the retina by measuring oxidatively modified DNA (8-hydroxy-2′ deoxyguanosine [8-OHdG]), nitrotyrosine, and the enzyme activity of MnSOD.
The DNA was digested with deoxyribonuclease and used for enzyme-linked immunosorbent assay of 8-OHdG as routinely employed in our laboratory.11,21,22 The 8-OHdG standard (0.5-40 ng/mL) or 15 to 20 μg of DNA was incubated for 1 hour with a monoclonal antibody against 8-OHdG in a plate precoated with 8-OHdG. The final color was developed by the addition of 3, 3′, 5, 5′-tetramethylbenzidine, and absorbance was measured at 450 nm.
Nitrotyrosine, a measure of peroxynitrite formed by reaction between superoxide and nitric oxide (NO), was quantified in the retina by enzyme immunoassay.21,22 Nitrotyrosine standard or retinal homogenates were incubated with a nitrotyrosine antibody in the microplate for 1 hour, followed by incubation with streptavidin peroxidase for 1 hour. The samples were incubated with tetramethylbenzidine substrate for 30 minutes. The reaction was stopped by 2.0M citric acid, and absorbance at 450 nm was monitored.
The enzyme activity of superoxide dismutase was measured by a method used in our laboratory.22 Activity of MnSOD was calculated by performing the assay in the presence of potassium cyanide to inhibit copper-zinc superoxide dismutase, thus measuring the residual MnSOD activity.
TOTAL RNA ISOLATION AND MESSENGER RNA QUANTIFICATION
Total retinal RNA was isolated by TRIZol reagent (Invitrogen, Carlsbad, California). The concentration and integrity of RNA was determined spectrophotometrically. The RNA (1 μg) was converted to complementary DNA (cDNA) using the High Capacity cDNA Reverse Transcription Kit with RNase inhibitor (Applied Biosystems, Foster City, California). The cDNA was synthesized using the GeneAmp PCR 9700 thermal cycler (Applied Biosystems), quantified spectrophotometrically, and diluted to 10 ng/μL.22
Quantitative real-time polymerase chain reaction (PCR) for genes for MnSOD, catalase, complex III (coenzyme Q cytochrome-c reductase), and an inducible form of NO synthase (iNOS) was performed using the TaqMan Assays on Demand for rats (Applied Biosystems). The β2-macroglobulin was used as a housekeeping gene. Real-time PCR was performed in 96-well plates using the ABI-7500 sequence detection system (Applied Biosystems), and each sample was analyzed in triplicate. The cDNA templates were combined with the TaqMan assay and TaqMan Universal PCR Master Mix (Applied Biosystems) and amplified. The standard PCR conditions were 2 minutes at 50°C and 10 minutes at 95°C followed by 40 cycles of extension at 95°C for 15 seconds and 1 minute at 60°C. Threshold lines were automatically adjusted to intersect amplification lines in the linear portion of the amplification curves. Data were normalized with β2-macroglobulin, and the fold change in gene expression relative to normal was calculated using the ddCt (δ-δ cycles threshold) method.23
STATISTICAL ANALYSIS
Each measurement was made at least in duplicate, and the values are expressed as mean (standard deviation). Parameters were analyzed using the nonparametric Kruskal-Wallis test followed by Mann-Whitney U test. Histopathology data were also analyzed by analysis of variance and similar conclusions were yielded. A P value of less than .05 was considered statistically significant.
RESULTS
PREVENTION OF DIABETES-INDUCED HISTOPATHOLOGY IN THE RETINA
Duration of diabetes of 12 months in rats increased the number of degenerative (acellular) capillaries in the retinal vasculature 2.5-fold compared with control rats (Figure 1). This increase was prevented in rats with diabetes who received micronutrients (P = .03); the number of acellular capillaries was similar in the retinas of control rats and those in the diabetes and AREDS group (P =.92).
Figure 1.
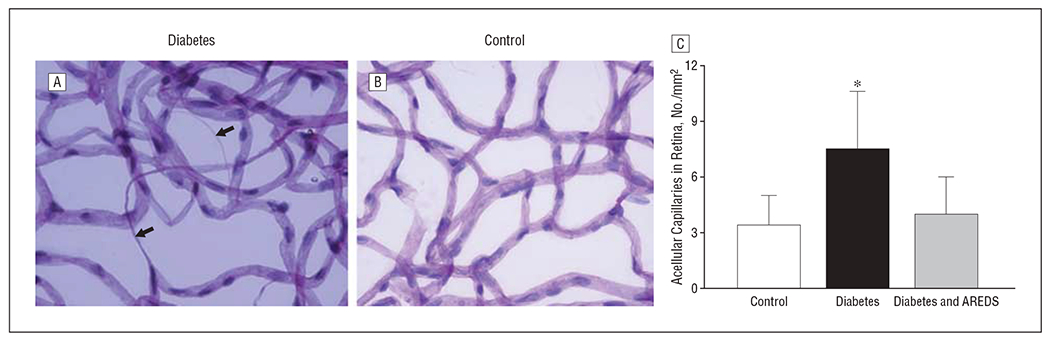
Inhibition of diabetes-induced capillary degeneration by Age-Related Eye Disease Study (AREDS)–based micronutrients in the retina of rats with diabetes (A) compared with a retina from a rat in the control group (B). Trypsin-digested retinal microvasculature was stained with periodic acid–Schiff and hematoxylin. Arrows indicate acellular capillaries. C, The number of acellular capillaries was counted in multiple midretinal fields and standardized to retinal area (per millimeter squared). Results are expressed as mean (standard deviation) of 8 rats in the control group and 9 rats each in the diabetes and diabetes and AREDS groups. * P<.05 compared with rats in control group and diabetes and AREDS group.
INHIBITION OF OXIDATIVE STRESS IN THE RETINA
Because oxidative stress is implicated in the development of diabetic retinopathy, the effect of the micronutrients on the parameters of oxidative stress in the retina was investigated.
The levels of 8-OHdG were elevated by 45% in the retinas obtained from rats with diabetes compared with those of the control rats. Elevation in retinal 8-OHdG levels was prevented when rats with diabetes were administered AREDS-based micronutrients; 8-OHdG levels in the control rats were comparable with those in the diabetes and AREDS group (P > .05; Figure 2A).
Figure 2.
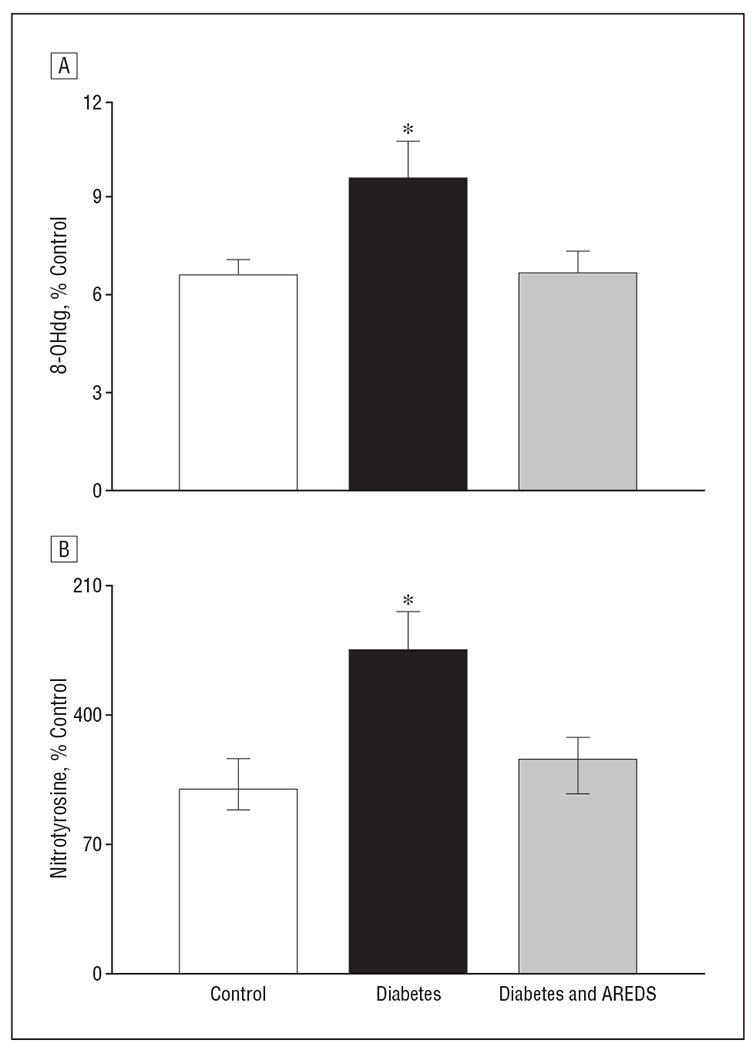
Inhibition of diabetes-induced increase in oxidatively modified DNA and nitrotyrosine accumulation in the retina by Age-Related Eye Disease Study (AREDS)–based micronutrients. A, Levels of 8-hydroxy-2′deoxyguanosine (8-OHdG) were quantified in 10 to 15 μg of retinal DNA using enzyme-linked immunosorbent assay (ELISA) for 8-OHdG. B, Nitrotyrosine quantified in the retinal homogenate by ELISA. Each sample was measured in duplicate. The values are mean (standard deviation) of 6 or more rats in each of the 3 groups. % Control indicates percentage compared with the control group; * P<.05 compared with rats in control group and diabetes and AREDS group.
Nitrotyrosine levels were elevated by 80% in the retinas of rats who had diabetes for 12 months, and supplementation with AREDS-based micronutrients had a significant beneficial effect on such increases. Retinal nitrotyrosine levels in rats in the diabetes and AREDS group were significantly lower than those in the rats with diabetes who were not given micronutrients (Figure 2B).
As expected, 12 months of having diabetes decreased the activity of MnSOD by about 35% in the retinas of rats with diabetes compared with control rats (Figure 3). Micronutrients prevented this diabetes-induced decrease in retinal MnSOD; the values of the control group and the group with diabetes and AREDS were not different from each other (P > .05).
Figure 3.
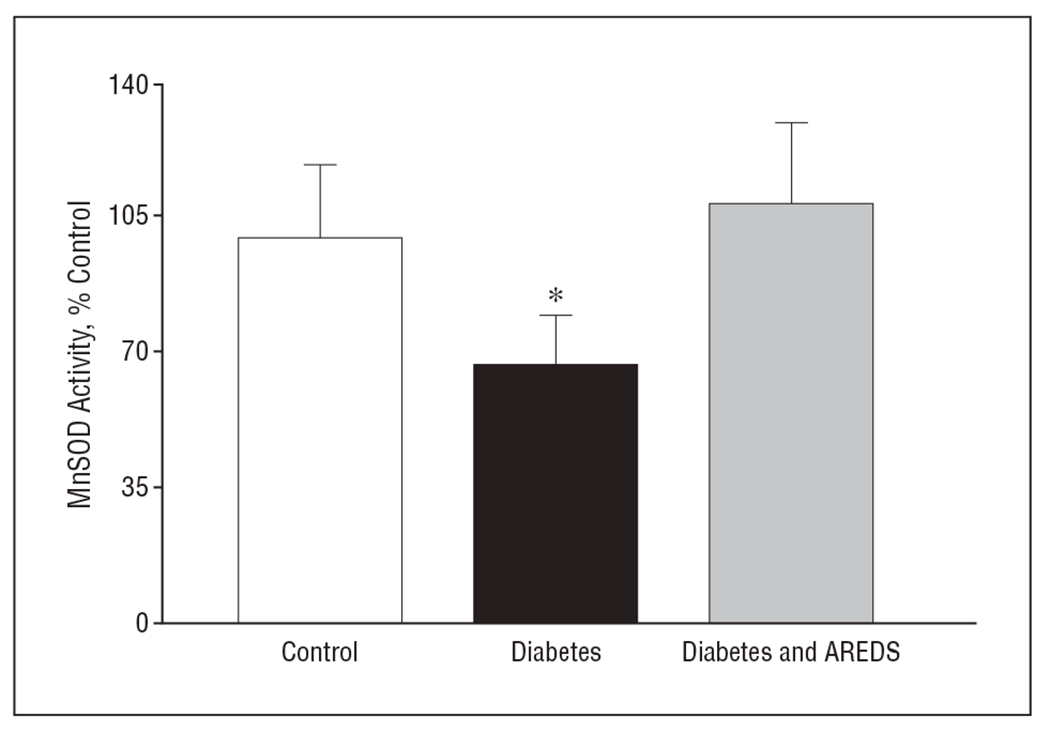
Inhibition by Age-Related Eye Disease Study (AREDS)–based micronutrients of diabetes-induced inhibition of retinal manganese superoxide dismutase (MnSOD) activity. Retinal protein (5-10 μg) was used to measure the activity of superoxide dismutase (SOD) using tetrazolium salt to quantify superoxide radicals generated by xanthine oxidase and hypoxanthine. The activity of MnSOD was calculated by subtracting the potassium cyanide–inhabitable activity from total SOD activity. The values are mean (standard deviation) of 5 to 6 rats in each group. % Control indicates percentage compared with the control group; * P<.05 compared with rats in control group and diabetes and AREDS group.
MODULATION OF ALTERATIONS IN THE EXPRESSION OF OXIDATIVE STRESS–RELATED GENES
To determine if the micronutrients had beneficial effects on the prevention of diabetes-induced alterations in mRNA levels of MnSOD, gene expression of MnSOD was quantified. Twelve months of having diabetes decreased mRNA levels of MnSOD in rats by about 30%, and the micronutrients prevented a diabetes-induced decrease in MnSOD messenger RNA (mRNA) levels (Figure 4A). The expression of MnSOD (determined by Western blot) also decreased by about 35% in the retinas of rats with diabetes compared with control rats; this reduction was restored to levels similar to normal values in the rats with diabetes who received the micronutrients (data not shown). Similarly, mRNA expression of another antioxidant defense enzyme, catalase, decreased by 30% in the retinas of rats with diabetes; however, AREDS-based micronutrients had no beneficial effect on this decrease (Figure 4B).
Figure 4.
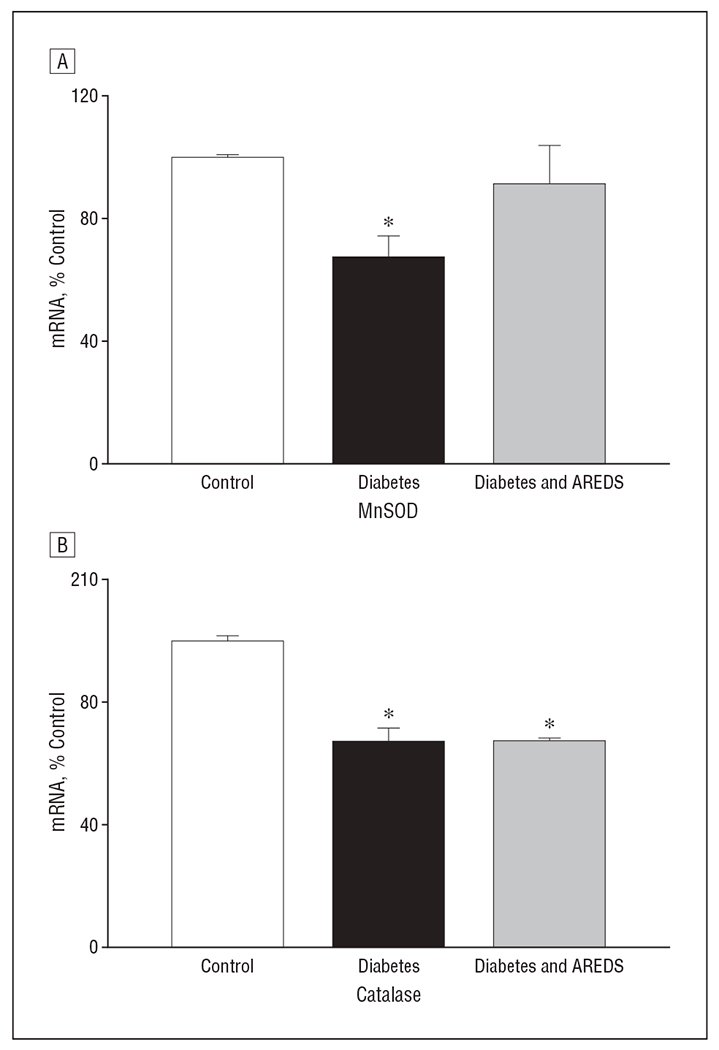
Divergent effects of Age-Related Eye Disease Study (AREDS)–based micronutrients on mRNA levels of retinal antioxidant defense enzymes in rats with diabetes. The content of mRNA for manganese superoxide dismutase (MnSOD) and catalase was determined in the retina by quantitative real-time polymerase chain reaction. The mRNA levels of MnSOD and catalase were normalized to those of β2-macroglobulin mRNA. A retina from each rat was analyzed at least in duplicate, and the results are the mean (standard deviation) of 6 rats each in the control, diabetes, and diabetes and AREDS groups. % Control indicates percentage compared with the control group; * P<.05 compared with control rats.
One of the sources of increased superoxide in the retinas of rats with diabetes is the impaired complex III activity,10 and increased retinal NO in diabetes is due to increased iNOS.8,24,25 Figure 5A shows that mRNA expression of complex III was decreased by 35% in rats with diabetes. The mRNA expression of iNOS was elevated approximately 7-fold (Figure 5B). Supplementation with micronutrients prevented a diabetes-induced decrease in complex III mRNA by more than 90% and partially, but significantly, prevented an increase in iNOS gene expression (approximately 50%; P < .002).
Figure 5.
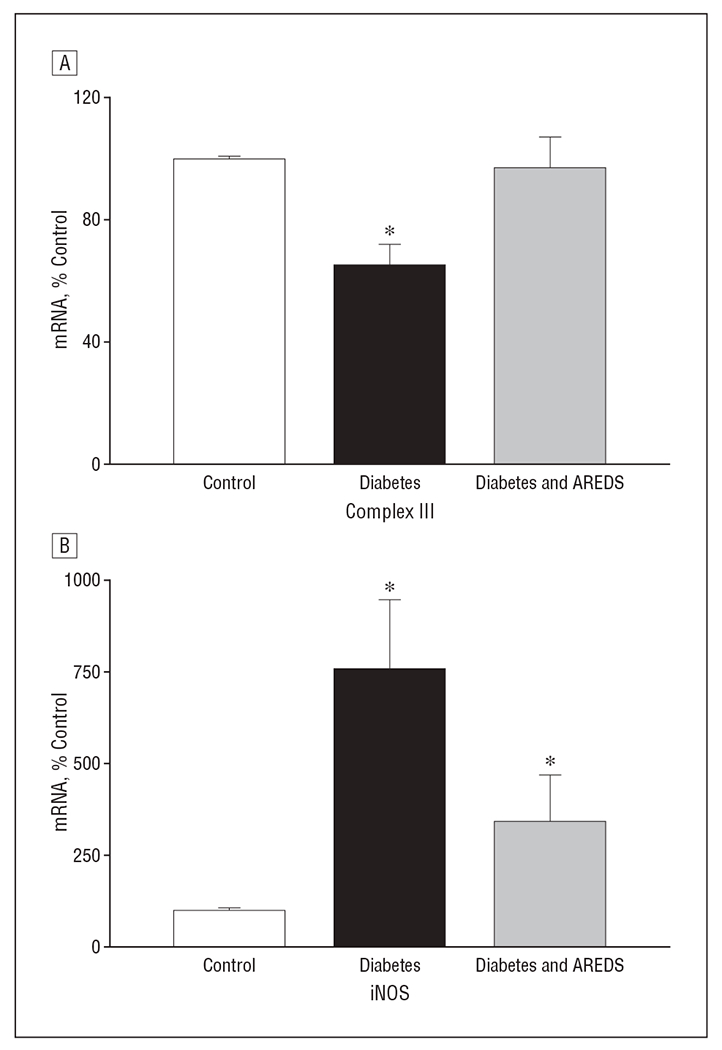
Inhibition of diabetes-induced decreases in complex III (coenzyme Q cytochrome-c reductase) and nitric oxide synthase (iNOS) mRNA levels by Age-Related Eye Disease Study (AREDS)–based micronutrients. Retinal mRNA for complex III and iNOS was determined by quantitative real-time polymerase chain reaction, and the values were normalized to β2-macroglobulin mRNA in each sample. % Control indicates percentage compared with the control group; * P<.05 compared with rats in control group and diabetes and AREDS group.
EFFECT OF AREDS-BASED MICRONUTRIENTS ON THE SEVERITY OF HYPERGLYCEMIA IN ANIMALS WITH DIABETES
To ensure that the severity of hyperglycemia was similar between the rats with diabetes that were and those that were not treated with AREDS-based micronutrients, insulin dosage was adjusted 3 to 4 times per week, depending on body weight and food consumption. Body weights for the entire duration of the experiment in the diabetes and AREDS group were not different from those of the diabetes group (Table). Glycated hemoglobin, an index of long-term glycemic control, was also similar in the 2 diabetic groups. Rats with diabetes had 10-fold higher urine output compared with control rats; this was not ameliorated by micronutrient supplementation. Similarly, blood glucose values in the diabetes and AREDS group were not different from those of the diabetes group; these values were significantly higher than those of the control rats (data not shown).
Table.
Effect of AREDS-Based Micronutrients on the Severity of Hyperglycemia in Rats
| Group | Mean (SD) |
|||
|---|---|---|---|---|
| Body Weight, g | Food Intake, g/d | GHb, %b | Urine Volume, mL/24 h | |
| Control (n = 8) | 412 (26) | 23 (3) | 4.2 (0.77) | 4 (2) |
| Diabetesa (n=7) | 260 (32) | 35 (4) | 11.5 (1.3) | 68 (18) |
| Diabetes and AREDSa (n = 9) | 259 (39) | 33 (5) | 10.7 (2.9) | 75 (25) |
Abbreviations: AREDS, Age-Related Eye Disease Study; GHb, glycated hemoglobin.
Glycated hemoglobin was quantified after 8 weeks of having diabetes and repeated every 3 months thereafter for 12 months.
To convert to proportion of total hemoglobin, multiply by 0.01.
COMMENT
This is the first study demonstrating that the nutritional supplements that slow down onset and/or progression of AMD, the leading cause of blindness in the elderly population, also inhibit the development of another sight-threatening disease, diabetic retinopathy (a major cause of blindness in young adults). The possible mechanism by which these nutrients inhibit diabetic retinopathy appears to involve inhibition of oxidative stress.
Formation of degenerative capillaries represents one of the early features of retinopathy seen in rodents with diabetes.10,20,25,26 Our exciting data show that the increased appearance of acellular capillaries in the retinal vasculature can be inhibited significantly by AREDS-based nutritional supplements. These results could have immense clinical implications because they suggest that the development and/or progression of a multifactorial complication that affects more than 80% of diabetic patients can be retarded by nutritional supplements that are already being tested for treatment of AMD.17–19,27
Oxidatively modified DNA is one of the most frequently used and reliable indicators of oxidative damage.28 Increased levels of 8-OHdG in the retina are implicated in the pathogenesis of diabetic retinopathy. Inhibition of the early lesions of diabetic retinopathy by lipoic acid administration in rats or by overexpression of MnSOD in mice is postulated to be caused by inhibition of retinal 8-OHdG levels.10,11 We show that AREDS-based micronutrients can also have beneficial effects on elevated retinal 8-OHdG levels, suggesting that these micronutrients could be inhibiting the development of diabetic retinopathy, in part by inhibiting the accumulation of oxidized DNA in the retina.
Peroxynitrite, produced from the diffusion-controlled reaction between NO and a superoxide anion, interacts with lipids, DNA, and proteins via direct oxidative reactions or indirect, radical-mediated mechanisms. These reactions, in turn, can modulate cell signaling and increase oxidative stress. Thus, the pathological implications of peroxynitrite have subtle and specific actions on cells.29,30 Nitrative stress is increased early in the course of development of retinopathy in diabetes, and the therapies that inhibit the activation of the apoptosis execution enzyme and the development of retinopathy in rats with diabetes decrease retinal nitrative stress.11,21,24 The AREDS-based micronutrients inhibit the development of diabetic retinopathy and nitrotyrosine level; this supports the role of peroxynitrite in the development of diabetic retinopathy.
Manganese superoxide dismutase is considered the first line of defense against increased mitochondrial superoxide. Its enzyme activity and mRNA are decreased in the retina in rats with hyperglycemia.31–33 Overexpression of MnSOD inhibits retinal oxidative stress and retinopathy in mice with diabetes, and supplementation with antioxidants prevents decreases in MnSOD in the retinas of rats.10,11 Beneficial effects of the micronutrients on MnSOD suggest that these micronutrients, by regulating MnSOD, help scavenge increased retinal superoxide. Furthermore, the micronutrients also prevented a decrease in the mRNA of complex III, an enzyme responsible for release of superoxide to both sides of the mitochondrial membrane. Our recent study10 has suggested that complex III is one of the sources of diabetes-induced increased retinal superoxide; its activity is decreased in mice with diabetes that can be prevented by overexpression of MnSOD. Thus inhibition of the decrease in the mRNA expression of complex III strongly suggests that these micronutrients could have a beneficial effect on diabetic retinopathy by preventing mitochondrial dysfunction and decreasing accumulation of superoxide. In contrast, the same micronutrients failed to inhibit reduction of catalase mRNA expression. The reason for this failure is not clear, but is consistent with our previous results showing that supplementation with ascorbic acid and vitamin E protects the retinal vasculature from histopathology in rats with diabetes, but does not prevent a decrease in catalase activity.7,31
Diabetes upregulates iNOS in the retina.24,25,34 In rodents, increased NO levels are observed early in the pathogenesis of diabetic retinopathy and remain elevated when histopathology is developing.11,24 Mice with diabetes who are iNOS-deficient are protected from retinal vascular pathology.25 Here we show that the mRNA of iNOS is elevated in the retina of rats with diabetes; this can be prevented by these micronutrients. This suggests that the micronutrients could be inhibiting increased retinal peroxynitrite by decreasing both superoxide and NO levels.
The whole retina was used to analyze biochemical parameters and mRNA levels; this approach did not allow us to identify the specific cell type because the retina has multiple layers and cell types.35 Prevention of vascular histopathology by micronutrients, however, suggests that these micronutrients had access to the retinal vasculature and thus could have exerted their beneficial effects on the development of retinopathy by inhibiting the biochemical abnormalities also in the vasculature.
The AREDS-based micronutrients consisted of multiple antioxidants and trace metals; thus the mechanism by which these micronutrients could inhibit diabetic retinopathy cannot be clearly elucidated. Vitamin E, ascorbic acid, and beta carotene inhibit diabetic retinopathy in rats, possibly by inhibiting increased oxidative stress.7 In addition to the antioxidants, the micronutrients had zinc oxide and copper oxide. Zinc has been shown to protect the retina from diabetes-induced increased lipid peroxidation and decreased glutathione levels in rats either by stabilizing the membrane structure or by inducing metallothionein synthesis.13 Zinc is essential for copper-zinc superoxide dismutase and inhibits diabetes-induced increases in plasma malondialdehyde and decreases in erythrocyte antioxidant defense enzymes.36 Copper oxide functions as the active center of many cuproenzymes, including copper-zinc superoxide dismutase,37 and copper deficiency results in oxidative damage to lipids, DNA, and proteins.38 The exact mechanism by which zinc and copper exerted their protective effect against retinal damage is not clear, but the possibility that these nutrients are helping to decrease oxidative damage remains very strong.
Our results demonstrate that AREDS-based micronutrients inhibit the lesions associated with diabetic retinopathy in rats, despite similar severity of hyperglycemia in the diabetes and diabetes and AREDS groups. These beneficial effects cannot be attributed to amelioration of the severity of hyperglycemia. Glycated hemoglobin values were similar in the 2 diabetic groups and were significantly higher than those obtained from age-matched control rats, suggesting that glycation of proteins is not influenced by these micronutrients.
Inhibition of the development of diabetic retinopathy, a long-term disease, requires a therapy that is safe and easily tolerable. We did not observe any significant effect of these micronutrients on the morbidity and mortality of rats with diabetes, suggesting that the nutrients were not toxic, but in clinical settings, patients should be made aware of any possible shortcomings from a long-term use of this therapy for this progressive disease. Animal models have provided promising results with antioxidants, though results from clinical studies have been ambiguous. Intake of antioxidants, based on diet recall by diabetic patients, is ineffective in treating diabetic retinopathy.15,16 The differences for such discrepancies are not clear, but the possibility that the initiation of antioxidants could be subsequent to the development of background retinopathy, in contrast to the animal studies in which antioxidants are administered soon after establishment of diabetes, or that the antioxidant concentrations achieved in the retina of patients were not sufficient to produce beneficial effects, cannot be ruled out. The results presented here are from a preventive study, but the effect of these micronutrients in diabetic animals with early alteration in the blood-retinal barrier could be very informative. Nutritional supplements have become the first line of defense for clinicians in treating dry AMD.17–19 We show that the same micronutrients have the potential to prevent the development of diabetic retinopathy. Our novel findings are the first step toward testing the same micronutrients in clinical settings; this could be welcome news for patients with diabetes. The use of nutritional supplements, if successful, will be a major step forward in fighting this sight-threatening complication that patients with diabetes fear.
Funding/Support:
This study was supported in part by grants 014370 and E017313 from the National Institutes of Health, grant 2004-287 from the Juvenile Diabetes Research Foundation, the Thomas Foundation, and Research to Prevent Blindness.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–812. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 3.Hammes H-P, Klinzing I, Wiegand S, Bretzel RG, Cohen AM, Federlin K. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol Vis Sci. 1993;34(6):2092–2096. [PubMed] [Google Scholar]
- 4.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of DAG-PKC pathway in diabetes and hypergalactosemia. Diabetes. 1994;43(9):1122–1129. [DOI] [PubMed] [Google Scholar]
- 5.Robison WG, Laver NM, Jacot JL, Glover JP. Sorbinil prevention of diabetic-like retinopathy in the galactose-fed rat model. Invest Ophthalmol Vis Sci. 1995;36(12):2368–2380. [PubMed] [Google Scholar]
- 6.Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75(1):95–108. [DOI] [PubMed] [Google Scholar]
- 7.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia VII: effect of long-term administration of anti-oxidants on the development of retinopathy. Diabetes. 2001;50(8):1938–1942. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Kennedy A. Therapeutic potential of anti-oxidants and diabetic retinopathy. Expert Opin Investig Drugs. 2001;10(9):1665–1676. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. [DOI] [PubMed] [Google Scholar]
- 10.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48(8):3805–3811. [DOI] [PubMed] [Google Scholar]
- 11.Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53(12):3233–3238. [DOI] [PubMed] [Google Scholar]
- 12.Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9(3):294–299. [DOI] [PubMed] [Google Scholar]
- 13.Moustafa SA. Zinc might protect oxidative changes in the retina and pancreas at the early stage of diabetic rats. Toxicol Appl Pharmacol. 2004;201(2):149–155. [DOI] [PubMed] [Google Scholar]
- 14.Bursell SE, Clermont AC, Aiello LP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22(8):1245–1251. [DOI] [PubMed] [Google Scholar]
- 15.Millen AE, Gruber M, Klein R, Klein BE, Palta M, Mares JA. Relations of serum ascorbic acid and alpha-tocopherol to diabetic retinopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(3):225–233. [DOI] [PubMed] [Google Scholar]
- 16.Millen AE, Klein R, Folsom AR, Stevens J, Palta M, Mares JA. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2004;79(5):865–873. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007;86(1):180–188. [DOI] [PubMed] [Google Scholar]
- 18.SanGiovanni JP, Chew EY, Clemons TE, et al. ; Age-Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report No. 20. Arch Ophthalmol. 2007;125(5):671–679. [DOI] [PubMed] [Google Scholar]
- 19.SanGiovanni JP, Chew EY, Clemons TE, et al. ; Age-Related Eye Disease Study Research Group. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report No. 22. Arch Ophthalmol. 2007;125(9):1225–1232. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56(2):337–345. [DOI] [PubMed] [Google Scholar]
- 21.Kowluru RA, Kanwar M. Effect of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond). 2007;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowluru RA, Menon B, Gierhart DL. Beneficial effect of Zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci. 2008;49(4):1645–1651. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 24.Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37(11):1169–1180. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L, Du Y, Miller C, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50(9):1987–1996. [DOI] [PubMed] [Google Scholar]
- 26.Kern TS, Engerman RL. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res. 1994;13(12):863–867. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol. 2006;124(7):1038–1045. [DOI] [PubMed] [Google Scholar]
- 28.Alper G, Irer S, Duman E, Caglayan O, Yilmaz C. Effect of I-deprenyl and gliclazide on oxidant stress/antioxidant status and DNA damage in a diabetic rat model. Endocr Res. 2005;31(3):199–212. [DOI] [PubMed] [Google Scholar]
- 29.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virág L, Szabó E, Gergely P, Szabó C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140-141:113–124. [DOI] [PubMed] [Google Scholar]
- 31.Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia IV: antioxidant defense system. Free Radic Biol Med. 1997;22(4):587–592. [DOI] [PubMed] [Google Scholar]
- 32.Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1594–1599. [DOI] [PubMed] [Google Scholar]
- 33.Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Qiushi Ren, Ho PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res. 2006;83(4):807–816. [DOI] [PubMed] [Google Scholar]
- 34.Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC. Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood-retinal barrier permeability. Nitric Oxide. 2000;4(6):590–596. [DOI] [PubMed] [Google Scholar]
- 35.Cheng H, Nair G, Walker TA, et al. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A. 2006;103(46):17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duzguner V, Kaya S. Effect of zinc on the lipid peroxidation and the antioxidant defense systems of the alloxan-induced diabetic rabbits. Free Radic Biol Med. 2007;42(10):1481–1486. [DOI] [PubMed] [Google Scholar]
- 37.Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol. 2002;6(2):171–180. [DOI] [PubMed] [Google Scholar]
- 38.Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26(4-5):268–298. [DOI] [PubMed] [Google Scholar]


