Abstract
In modern lifestyles, high-fat diets and prolonged inactivity lead to more people developing type 2 diabetes (T2D). Based on the modern pathogenesis of T2D, food, and its components have become one of the top concerns for patients. Recent studies have found that dysbiosis and gut-related inflammation are more common in T2D patients. Probiotics and prebiotics play complementary roles in the gut as dietary supplements. Together, they may help improve dysbiosis and intestinal inflammation in people with T2D, increase the production of blood glucose-lowering hormones such as incretin, and help reduce insulin resistance and lower blood glucose. Therefore, changing the dietary structure and increasing the intake of probiotics and prebiotics is expected to become a new strategy for the adjuvant treatment of T2D.
Keywords: type 2 diabetes, probiotics, prebiotics, microbiota
Introduction
Diabetes is one of the top ten causes of death globally, and 90% of patients have type 2 diabetes (T2D) (Holman et al. 2015). The International Diabetes Federation estimated that in 2015 10% of the global population had diabetes. Most patients have had hyperglycemia and/or insulin resistance (IR) for several years prior to diagnosis, and patients with undiagnosed and untreated diabetes are at higher risk of developing more severe complications than patients receiving treatment (Magliano et al. 2019). In modern society, unhealthy lifestyles such as long-term high-fat and high-sugar diets, lack of physical exercise, and excessive work pressure lead to obesity and T2D (Zheng et al. 2018; Hosomi et al. 2022). Among them, diet plays a vital role in the occurrence and development of T2D (Merino 2022). Changing unhealthy lifestyles, maintaining a healthy weight, eating healthy, maintaining physical activity, not smoking, and moderate alcohol consumption can prevent and improve T2D.
With the discovery that gut microbiota changes during the diet, exercise, traditional hypoglycemic drugs, and bariatric surgery, the relationship between gut microbiota and T2D has attracted widespread attention in the medical community. It has also been confirmed that the development of T2D is closely related to intestinal microbiota (Sonnenburg and Bäckhed 2016). The gut microbiota affects many biological functions, and its characterization has become a central area of biomedical research. Recent studies have shown that the gut microbiota plays an important role in diseases such as obesity, diabetes, and non-alcoholic fatty liver disease (Canfora et al. 2019). In many human and animal studies, T2D is associated with severe gut dysbiosis, and specific gut bacteria and their metabolites are significantly altered in T2D. Both the abundance of butyrate-producing microorganisms and butyrate production is reduced in diabetic patients; high-fat diet-induced T2D can activate the lipopolysaccharide (LPS) component of Gram-negative bacteria and cause endotoxemia (Sun et al. 2010). LPS plays a role in metabolic disorders by initiating innate immune mechanisms; certain antidiabetic drugs such as metformin can improve metabolic dysfunction by activating the bile acid glycoursodeoxycholic acid (GUDCA)-intestinal farnesoid X receptor (FXR) pathway through Bacteroides fragilis (Sun et al. 2018).
Studies of the gut microbiome have shown that the microbiota is an important regulator of host energy and substrate metabolism. Abnormal composition and/or function of gut microbes can lead to disturbances in energy metabolism, affecting the metabolism of fat, liver, muscle, and other tissues. Microbial composition and abundance depend on the integrity of the gut itself, intrinsic factors such as genetics, and extrinsic factors such as diet and drug intake (Schmidt et al. 2018). Disturbed gut microbiota is widespread in patients with T2D and contributes to the development of insulin resistance. The digestion of food is inextricably linked to the microbiome. Dietary control is selecting foods that are beneficial to the beneficial bacteria in the gut to achieve the purpose of controlling blood glucose. Altering the energy-dense diet of people with T2D to promote a diet rich in whole grains, fruits, vegetables, nuts, and legumes, and reducing intake of refined grains, red or processed meat, and sugar-sweetened beverages maybe a preventive measure and an effective method to improve T2D. A healthy diet includes a high amount of dietary fiber (in vegetables and fruits) and oligosaccharides, which belong to the category of prebiotics, and supplementation with prebiotics is beneficial in maintaining intestinal microbial balance (Kanazawa et al. 2021). In addition, clinical studies have shown that probiotics supplementation can improve the intestinal microbiota and have an adjuvant effect on T2D (Zhang et al. 2020). Specific microorganisms and their macro-, micro-, and non-nutritional components play an important role in regulating glucose homeostasis. Therefore, we review studies of dietary supplementation with probiotics and/or prebiotics in patients, which may be an adjunctive prevention and management strategy for treating T2D.
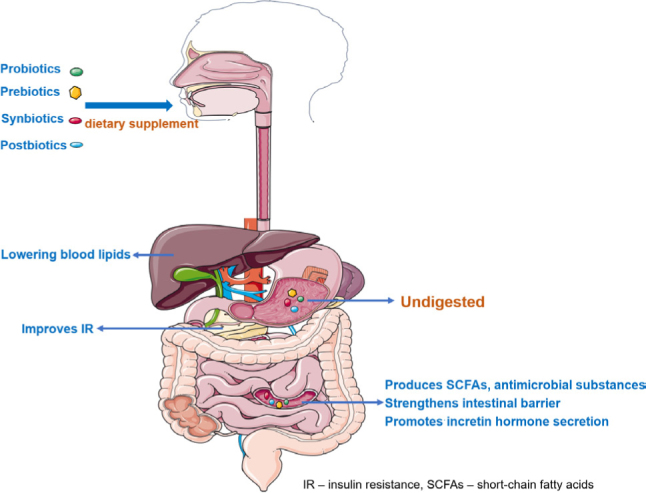
Probiotics
International Scientific Association for Probiotics and Prebiotics (ISAPP) defines probiotics as live micro-organisms that, when administered in adequate numbers, confer a health benefit on the host (Hill et al. 2014). It benefits health by modulating host mucosal immune function, improving dysbiosis, or promoting nutrient absorption. The colon is where most bacteria live in the digestive tract, so the colon is the primary place for probiotics to be active. Metabolism in the colon largely depends on nutrients, the number of complex foods, the composition of the gut microbiome, and colonic transit time. The proximal colon prefers to utilize fiber, resistant starch, and prebiotics for fermentation to obtain the energy needed for survival. In contrast, the distal colon utilizes residual peptides and proteins for proteolytic fermentation, which can be further metabolized by microbial cross-feeding (Canfora et al. 2019). T2D is characterized by microbial dysbiosis, intestinal barrier damage, and a low-grade inflammatory state. A healthy microbial community generally maintains healthy barrier function, including mucosal integrity, tight junctions, and a normal mucosal immune system. Dietary changes have the most significant impact on diabetes risk and blood glucose regulation in the long run. Regarding dietary composition, probiotics, master regulators of gut microbiota, were most strongly associated with reduced risk of T2D. There are two main methods for screening probiotics with glucose-lowering potential: inhibition of α-glucosidase (Balaich et al. 2021) and inhibition of dipeptidyl peptidase IV (DPPIV). The structure of α-glucosidase inhibitors is similar to that of oligosaccharides, mainly through the competitive binding to carbohydrate-binding sites on α-glucosidase, reducing the production of monosaccharides and reducing post-prandial blood glucose. Probiotics-fermented blueberry juices increased α-glucosidase inhibitory and α-amylase inhibitory activities, and enhanced antioxidant, anti-microbial, and antidiabetic activities (Zhong et al. 2021). DDPIV inhibitors induce glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) inactivation and increase their endogenous levels. The cell-free excretory supernatants and cell-free extracts of Lactobacillus acidophilus KLDS1.0901 has better DPP-IV inhibitory activity, antioxidative activities, and biological characteristics than Lactobacillus rhamnosus GG (Yan et al. 2020).
The health benefits of probiotics should also be evaluated clinically or experimentally to study their mechanisms. A total of 15 randomized controlled trials and 902 patients showed that probiotics treatment could reduce glycated hemoglobin A1c (HbA1c), fasting blood glucose (FBG), and insulin resistance levels in T2D patients quite robust and stable (Tao et al. 2020). In randomized controlled trials of probiotics intervention in 50 T2D patients, probiotics supplementation effectively reduced inflammatory factors and increased acetic acid production (Tonucci et al. 2017). Probiotics in camel milk have antidiabetic effects on T2D mice by inducing GLP-1 secretion and increasing claudin-1 and mucin-2 proteins to repair the intestinal mucosal barrier (Wang et al. 2020). Probiotics can also regulate patients’ metabolism, improve dyslipidemia and hypertension, and reduce the risk of cardiovascular disease in T2D (Kocsis et al. 2020). Lactobacilli gasseri and Lactobacilli johnsonii improve systemic glucose metabolism by restoring fatty acid β-oxidation and reducing high-fat diet-induced hepatic mitochondrial damage (Rodrigues et al. 2021). Akkermansia muciniphila, a bacterium present in the mucus layer, is inversely associated with T2D disease, and increasing the activity of A. muciniphila has a positive effect on improving metabolism and mucus layer thickness. Probiotics can lower triglyceride and total cholesterol levels in gestational diabetes mellitus (Okesene-Gafa et al. 2020). Microbial metabolites, short-chain fatty acids (SCFAs), and total bile acids can activate hypoglycemic signaling pathways, improve endotoxemia, and increase GLP-1 and peptide tyrosine (PYY) levels. Probiotics can act as an adjunct to metformin by increasing butyrate production. Delayed-release metformin relies on the dissolution of the tablet enteric coating in the distal intestine, where a high density of L cells maintains a pH of 6.5 for maximal efficacy (Buse et al. 2016). In addition, probiotics can protect β-cell function by reducing IR and oxidative stress (Kesika et al. 2019). Ruminococcus ilealis was significantly increased in 80% of obese patients, suggesting that this microbe may be a common pathogen in obese individuals (Wu et al. 2011). These different studies explored the relationship between gut microbes and T2D by modulating metabolism, increasing bacterial abundance, and adjusting the timing of interventions, suggesting that certain probiotics can modulate glucose metabolism and improve microbial dysbiosis.
Prebiotics
ISAPP defines a prebiotic as a selectively utilized substrate by host microorganisms conferring a health benefit (Gibson et al. 2017). Prebiotics are organic substances that are not digested and absorbed by the host but can selectively promote the metabolism and proliferation of probiotics. They can be used as probiotic nutrients or materials, regulating microbiota abundance, and improving host health. Prebiotics can enhance the effect of probiotics, and the two complement each other to maintain the homeostasis of gut microbes. In addition, probiotics themselves can regulate intestinal function by producing SCFAs through carbohydrate fermentation, improving the intestinal microenvironment and increasing the production of GLP-1 (Zhao et al. 2018). Prebiotics mainly include oligosaccharides (fructose oligosaccharides, galactooligosaccharides, xylooligosaccharides, isomaltose, soybean oligosaccharides, inulin, etc.), microalgae (such as Spirulina, Arthrospira); polysaccharides (such as Coriolus versicolor polysaccharide), protein hydrolyzate (such as casein hydrolyzate, α-lactalbumin, lactoferrin, etc.) and natural vegetables, fruits, herbal medicine, wild plants, and other dietary fibers (Gibson et al. 2017; Peng et al. 2020). Prebiotics are entirely (100%) translocated to the colon, which balances the microbiome and provides beneficial effects.
Prebiotics improve insulin sensitivity by altering the composition of the microbiota, reducing endotoxemia, and reducing energy harvesting. Prebiotic treatment is designed to provide a selective advantage to beneficial bacteria compared to the direct administration of probiotics. Food enters the small and large intestines through the digestion of the mouth and stomach, and the indigestible dietary fiber is fermented and decomposed by various intestinal microorganisms to generate SCFAs such as acetic acid, propionic acid, butyric acid, and reduce the metabolism of harmful compounds (such as indole and hydrogen sulfide). Fermentation of gut microbes has positive effects on reducing inflammation, controlling body weight, improving IR, and improving glucose and lipid metabolism (Zhao et al. 2018). Harmful microbiota enters the bloodstream by disrupting the intestinal mucosa, creating a low inflammatory state, and increasing IR. Supplementation of prebiotics in the diet of T2D patients, which produce SCFAs through the fermentation of intestinal microorganisms, is important for maintaining the intestinal barrier (Robertson 2020). Prebiotic supplementation normalized the abundance of A. muciniphila and improved abnormal glucose metabolism (Everard et al. 2013). Resistant starch from Kudzu helps restore the expression of insulin receptor substrate 1 (IRS-1), phosphorylated-phosphatidylinositol 3-kinase (p-PI3K), phospho-Akt and glucose transporter 4 (GLUT4), thereby increasing the efficiency of insulin (Song et al. 2021). Cyclocarya paliurus polysaccharides can reduce diabetes symptoms and increase the abundance of probiotics (Li et al. 2021). The addition of oligofructose-enriched inulin decreased the ratio of Firmicutes to Bacteroidetes while increasing the abundance of Bifidobacterium (de Cossío et al. 2017). Arabinoxylan promoted the growth of fiber-degrading bacteria and increased SCFAs decreased the abundance of opportunistic pathogens. Arabinoxylan also decreased 12α-hydroxylated bile acids and increased equal, indole propionic acid, and behenic acid (Nie et al. 2022).
Dietary fiber is a large group of prebiotics; it is not a single substance. Digestive enzymes do not efficiently hydrolyze them, and fibers are divided into soluble (pectin, gum, mucilage) and insoluble (cellulose, hemicellulose, lignin) according to their water solubility. The definition of dietary fiber also includes those isolated from food materials or edible carbohydrates. Fiber is not easily digested and absorbed in the human small intestine but can be partially or fully fermented in the colon. In the gastrointestinal tract, it has been shown to improve bowel function, lower blood lipids, and lower blood glucose (Waddell and Orfila 2022). Individual differences in gut microbial composition were associated with individualized responses to dietary fiber intervention and significantly contributed to changes in probiotics abundance.
The dietary composition impacts the gut microbiota, so a specific dietary fiber diet can control the gut microbiota to a healthy gut pattern (Healey et al. 2016). While solubility is a fundamental determinant of physiological responses, viscosity, and fermentability play a more prominent role in physiological benefits, so high-viscosity dietary fiber makes chyme more cohesive and can slow glucose release (Kumar et al. 2012). In addition, soluble viscous fiber reduces gastric emptying by absorbing water to form a viscous gel and plays an important role in controlling postprandial blood glucose and insulin (Holt et al. 1979). The most substantial and most consistent effect in reducing the risk of developing T2D was observed in a diet high in insoluble and only moderately fermented grain fiber. At the same time, fruits and vegetables were important sources of soluble/fermentable fiber. The U.S. recommends 1.5–2 cup-equivalents of fruits and 2–3 cup-equivalents of vegetables daily for modern health (Lee et al. 2022). A prospective study of fruit and vegetable supplements has been shown to help reduce HbA1c in T2D patients (Gordon et al. 2022). A prospective study of 120,343 volunteers in the United Kingdom showed that increased intake of fresh fruits and vegetables reduced the incidence of T2D during an 8.4-year follow-up period (Gao et al. 2022).
Synbiotics and postbiotics
ISAPP defines synbiotics as a mixture comprising live microorganisms and substrates selectively utilized by host microorganisms that confer a health benefit on the host (Swanson et al. 2020). It not only exerts bacterial activity but also selectively and rapidly increases prebiotics, making the effect of probiotics more pronounced and long-lasting. As a new generation of micro-ecological regulators, synbiotics play the physiological functions of both probiotics and prebiotics to fight diseases and maintain micro-ecological balance. A study of 11 randomized controlled trials and 18 subjects showed that synbiotics supplementation lowered blood glucose levels and improved IR in patients with prediabetes and T2D (Naseri et al. 2022). The addition of synbiotics in T2D patients helps to improve their gut environment (Kanazawa et al. 2021) and taking synbiotics may be a potential adjunctive therapy to improve metabolism and lower HbA1c (Bock et al. 2021).
ISAPP defines a postbiotic as a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host. Effective postbiotics must contain inactivated microbial cells or cell components, with or without metabolites, that contributes to observed health benefits (Salminen et al. 2021). Postbiotics have the potential to modulate intestinal barrier stability and improve metabolic diseases. SCFAs can improve glucose homeostasis, and 24 weeks of inulin-propionate supplementation can enhance insulin release by inhibiting apoptosis while maintaining functional β-cell viability (Pingitore et al. 2017). SCFAs maintain the intestinal barrier by promoting the growth of intestinal epithelial cells, strengthening tight junctions, and modulating the activity of microbiota and immune cells (Liu et al. 2021). Butyrate alleviates dysbiosis induced by a methionine-choline-deficient diet, and the improvement of the microbiota by butyrate is closely related to intestinal barrier function (Ye et al. 2018). In addition, butyrate suppressed high-fat diet-induced obesity and IR, and the tight junction protein claudin-1 was significantly increased in the jejunum, ileum, and colon epithelium (Matheus et al. 2017).
Application of probiotics and prebiotics
In terms of probiotics supplementation, people should pay attention to the proper consumption of probiotics-containing foods, rather than relying too much on probiotics products. Fermented dairy products are the carrier of choice for probiotics. On the one hand, dairy products do not require changes in technology and manufacturing processes, and on the other hand, the dairy matrix can protect probiotics through gastrointestinal transport (Fernandez and Marette 2017). Surprisingly, minerals such as calcium and magnesium in dairy products were associated with a lower incidence of T2D (Madjd et al. 2016). Probiotic yogurt is a nutrient-dense food that may benefit patients with lactose intolerance, constipation, diarrhea, high blood pressure, cardiovascular disease, diabetes, and certain cancers. Probiotic yogurt overcomes host defense factors such as intestinal colonization resistance, reduces FBG, and improves oxidation. For lactose intolerance (Shaukat et al. 2010), Streptococcus salivarius ssp. thermophilus and Lactobacillus delbrueckii ssp. bulgaricus produce β-galactosidase, in which lactose is hydrolyzed by β-galactosidase into monosaccharides that are easily absorbed by the intestinal epithelium.
Antibiotics are one of the key therapeutic tools in the fight against bacterial infections and therefore deserve our attention in treating dysbiosis. Antibiotics can sometimes be used synergically with probiotics (Al-Dulaimi et al. 2021). Antibiotics can be used to remove or inhibit harmful microorganisms, probiotics can be used to introduce missing microorganisms, and prebiotics can be applied to enhance the proliferation of beneficial microorganisms to maximize sustained changes in the microbiome’s composition. Furthermore, probiotics can act as adjuvants for antibiotics, adhere to the intestinal layer to enhance barrier function, secrete antimicrobials to fight pathogen invasion, and coordinate immune function (Wan et al. 2019). Nevertheless, repeated clinical use or abuse of antibiotics can lead to the development of bacterial resistance, which will result in more significant harm to people with common bacterial infections. The use of probiotics is expected to improve this symptom, and a clinical trial evaluation showed that various probiotics (including Bacillus spp., Bifidobacterium spp., Clostridium butyricum, Lactobacilli spp.) have a protective effect against antibiotic-associated diarrhea as well as shortening the duration of diarrhea (Guo et al. 2019). For antibiotic-sensitive probiotics bacteria, altering their genetic modules may be a solution. Encoding plasmids in Lactobacillus to achieve independent high expression of multiple toxin-antitoxin system genes will help expand the use of Lactobacillus in healthcare (Dey et al. 2023).
Numerous clinical studies have shown that supplementation with probiotics, prebiotics, synbiotics, or postbiotics can reduce HbA1c and lipid levels and improve IR (Bock et al. 2021), while their use is more effective than placebo in reducing FBG, both in the short and long term (Rittiphairoj et al. 2021). While probiotics products can be taken alone, the best time to consume them is after meals (Wang et al. 2022). Food enters the stomach after meals, and the neutralization of gastric acid is more conducive to the effect of live probiotics. In addition, the intestinal microbiota should be maintained in a balanced state, not the more probiotics, the better. Excessive intake of probiotics has no positive effects, but overloading can also have serious adverse effects, wreaking havoc on the gut environment.
Conclusion
Many human and animal experiments have confirmed that probiotics can significantly regulate microbial dysbiosis, repair the intestinal barrier system, and have particular clinical significance in improving IR and reducing blood glucose. However, current research still needs in-depth characterization studies of the leading probiotic strains to treat T2D. Therefore, there is a greater need to study and explain these microorganisms’ different mechanisms of action to evaluate their application in functional foods and different food matrices in the future. On the other hand, another medical and nutritional therapy challenge that should be considered is managing T2D through whole foods without any side effects. Therefore, the long-term effects of prebiotics, synbiotics, and postbiotics on glucose metabolism may be a potential hope for treating T2D.
Funding Statement
Funding This research was funded by the National Natural Science Foundation of China, grant number 82074061.
Footnotes
Author contributions: Yuying Wang designed the concept and drafted the article. Yuying Wang, Lina Wen, Huazhen Tang, and Jinxiu Qu participated in the compilation of the article materials. Benqiang Rao made critical revisions to important content. All authors finally approved the submitted version.
Conflict of interest: The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- AL-Dulaimi M, Algburi A, Abdelhameed A, Mazanko MS, Rudoy DV, Ermakov AM, Chikindas ML Antimicrobial and anti-biofilm activity of polymyxin E alone and in combination with probiotic strains of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against clinical isolates of selected Acinetobacter spp.: a preliminary study Pathogens. 2021 NaN10(12):1574. doi: 10.3390/pathogens10121574. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaich J, Estrella M, Wu G, Jeffrey PD, Biswas A, Zhao L, Korennykh A, Donia MS The human microbiome encodes resistance to the antidiabetic drug acarbose Nature. 2021 NaN600(7887):110. doi: 10.1038/s41586-021-04091-0. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock PM, Telo GH, Ramalho R, Sbaraini M, Leivas G, Martins AF, Schaan BD The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis Diabetologia. 2021 NaN64(1):26. doi: 10.1007/s00125-020-05295-1. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies Diabetes Care. 2016 NaN39(2):198. doi: 10.2337/dc15-0488. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Canfora EE, Meex RCR, Venema K, Blaak EE Gut microbial metabolites in obesity, NAFLD and T2DM Nat Rev Endocrinol. 2019 NaN15(5):261. doi: 10.1038/s41574-019-0156-z. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- de Cossío LF, Fourrier C, Sauvant J, Everard A, Capuron L, Cani PD, Layé S, Castanon N Impact of prebiotics on metabolic and behavioral alterations in a mouse model of metabolic syndrome Brain Behav Immun. 2017 NaN64:33. doi: 10.1016/j.bbi.2016.12.022. . . ; : –. [DOI] [PubMed] [Google Scholar]
- Dey S, Blanch-Asensio M, Balaji Kuttae S, Sankaran S Novel genetic modules encoding high-level antibiotic-free protein expression in probiotic lactobacilli Microb Biotechnol. 2023. NaN, pp. 1751–7915. . . ; –. .14228. [DOI] [PMC free article] [PubMed]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity Proc Natl Acad Sci USA. 2013 NaN110(22):9066. doi: 10.1073/pnas.1219451110. . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MA, Marette A Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties Adv Nutr. 2017 NaN8(1):155S. doi: 10.3945/an.115.011114. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Jebb SA, Aveyard P, Ambrosini GL, Perez-Cornago A, Papier K, Carter J, Piernas C Associations between dietary patterns and incident type 2 diabetes: prospective cohort study of 120,343 UK Biobank participants Diabetes Care. 2022 NaN45(6):1315. doi: 10.2337/dc21-2258. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics Nat Rev Gastroenterol Hepatol. 2017 NaN14(8):491. doi: 10.1038/nrgastro.2017.75. . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Gordon B, Ridinger S, Krick R, Grosvenor L, Charron R Fruit and vegetable prescription program for diabetes control among community health centers in Rural Idaho and Oregon Am J Public Health. 2022 NaN112(7):975. doi: 10.2105/AJPH.2022.306853. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC Probiotics for the prevention of pediatric antibiotic-associated diarrhea Cochrane Database Syst Rev. 2019 NaN4(4):CD004827. doi: 10.1002/14651858.CD004827.pub5. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey G, Brough L, Butts C, Murphy R, Whelan K, Coad J Influence of habitual dietary fibre intake on the responsiveness of the gut microbiota to a prebiotic: protocol for a randomised, double-blind, placebo-controlled, cross-over, single-centre study BMJ Open. 2016 NaN6(9):e012504. doi: 10.1136/bmjopen-2016-012504. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic Nat Rev Gastroenterol Hepatol. 2014 NaN11(8):506. doi: 10.1038/nrgastro.2014.66. . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Holman N, Young B, Gadsby R Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK Diabet Med. 2015 NaN32(9):1119. doi: 10.1111/dme.12791. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Holt S, Carter DC, Tothill P, Heading RC, Prescott LF Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol Lancet. 1979 NaN313(8117):636. doi: 10.1016/S0140-6736(79)91079-1. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Hosomi K, Saito M, Park J, Murakami H, Shibata N, Ando M, Nagatake T, Konishi K, Ohno H, Tanisawa K, et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota Nat Commun. 2022 NaN13(1):4477. doi: 10.1038/s41467-022-32015-7. . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa A, Aida M, Yoshida Y, Kaga H, Katahira T, Suzuki L, Tamaki S, Sato J, Goto H, Azuma K, et al. Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: A randomized controlled study Nutrients. 2021 NaN13(2):558. doi: 10.3390/nu13020558. . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesika P, Sivamaruthi BS, Chaiyasut C Do probiotics improve the health status of individuals with diabetes mellitus? A review on outcomes of clinical trials BioMed Res Int. 2019 NaN2019:1. doi: 10.1155/2019/1531567. . . ; : –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, Garami A, Soós A, Márta K, Solymár M Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials Sci Rep. 2020 NaN10(1):11787. doi: 10.1038/s41598-020-68440-1. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Sinha AK, Makkar HPS, de Boeck G, Becker K Dietary roles of non-starch polysaccharides in human nutrition: a review Crit Rev Food Sci Nutr. 2012 NaN52(10):899. doi: 10.1080/10408398.2010.512671. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Lee SH, Moore LV, Park S, Harris DM, Blanck HM Adults meeting fruit and vegetable intake recommendations – United States, 2019 MMWR Morb Mortal Wkly Rep. 2022 NaN71(1):1. doi: 10.15585/mmwr.mm7101a1. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Hu J, Nie Q, Chang X, Fang Q, Xie J, Li H, Nie S Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration Sci China Life Sci. 2021 NaN64(1):117. doi: 10.1007/s11427-019-1647-6. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Liu W, Luo X, Tang J, Mo Q, Zhong H, Zhang H, Feng F A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: by changing gut barrier Eur J Nutr. 2021 NaN60(5):2317. doi: 10.1007/s00394-020-02431-w. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Madjd A, Taylor MA, Mousavi N, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: a randomized controlled trial Am J Clin Nutr. 2016 NaN103(2):323. doi: 10.3945/ajcn.115.120170. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, Tabesh M, Koye DN, Shaw JE Trends in incidence of total or type 2 diabetes: systematic review BMJ. 2019 NaN366:l5003. doi: 10.1136/bmj.l5003. . . ; : [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheus VA, Monteiro LCS, Oliveira RB, Maschio DA, Collares-Buzato CB Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice Exp Biol Med. 2017 NaN242(12):1214. doi: 10.1177/1535370217708188. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino J Precision nutrition in diabetes: when population-based dietary advice gets personal Diabetologia. 2022 NaN65(11):1839. doi: 10.1007/s00125-022-05721-6. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Naseri K, Saadati S, Ashtary-Larky D, Asbaghi O, Ghaemi F, Pashayee-Khamene F, Yari Z, de Courten B Probiotics and synbiotics supplementation improve glycemic control parameters in subjects with prediabetes and type 2 diabetes mellitus: A GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials Pharmacol Res. 2022 NaN184:106399. doi: 10.1016/j.phrs.2022.106399. . . ; : [DOI] [PubMed] [Google Scholar]
- Nie Q, Hu J, Chen H, Geng F, Nie S Arabinoxylan ameliorates type 2 diabetes by regulating the gut microbiota and metabolites Food Chem. 2022 NaN371:131106. doi: 10.1016/j.foodchem.2021.131106. . . ; : [DOI] [PubMed] [Google Scholar]
- Okesene-Gafa KA, Moore AE, Jordan V, McCowan L, Crowther CA Probiotic treatment for women with gestational diabetes to improve maternal and infant health and well-being Cochrane Database Syst Rev. 2020 NaN6(6):CD012970. doi: 10.1002/14651858.CD012970.pub2. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Tabashsum Z, Anderson M, Truong A, Houser AK, Padilla J, Akmel A, Bhatti J, Rahaman SO, Biswas D Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods Compr Rev Food Sci Food Saf. 2020 NaN19(4):1908. doi: 10.1111/1541-4337.12565. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, Morrison DJ, Preston T, Wallis GA, Tedford C, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro Diabetes Obes Metab. 2017 NaN19(2):257. doi: 10.1111/dom.12811. . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis Adv Nutr. 2021 NaN12(3):722. doi: 10.1093/advances/nmaa133. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MD Prebiotics and type 2 diabetes: targeting the gut microbiota for improved glycaemic control? Pract Diab. 2020 NaN37(4):133. doi: 10.1002/pdi.2285. . ; ( ): –. [DOI] [Google Scholar]
- Rodrigues RR, Gurung M, Li Z, García-Jaramillo M, Greer R, Gaulke C, Bauchinger F, You H, Pederson JW, Vasquez-Perez S, et al. Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes Nat Commun. 2021 NaN12(1):101. doi: 10.1038/s41467-020-20313-x. . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics Nat Rev Gastroenterol Hepatol. 2021 NaN18(9):649. doi: 10.1038/s41575-021-00440-6. . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TSB, Raes J, Bork P The human gut microbiome: from association to modulation cell. 2018 NaN172(6):1198. doi: 10.1016/j.cell.2018.02.044. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Shaukat A, Levitt MD, Taylor BC, MacDonald R, Shamliyan TA, Kane RL, Wilt TJ Systematic review: effective management strategies for lactose intolerance Ann Intern Med. 2010 NaN152(12):797. doi: 10.7326/0003-4819-152-12-201006150-00241. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Song X, Dong H, Zang Z, Wu W, Zhu W, Zhang H, Guan Y Kudzu resistant starch: an effective regulator of type 2 diabetes mellitus Oxid Med Cell Longev. 2021 NaN2021:1. doi: 10.1155/2021/4448048. . . ; : –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Bäckhed F Diet – microbiota interactions as moderators of human metabolism Nature. 2016 NaN535(7610):56. doi: 10.1038/nature18846. . . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin Nat Med. 2018 NaN24(12):1919. doi: 10.1038/s41591-018-0222-4. . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clément K, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese Diabetes Care. 2010 NaN33(9):1925. doi: 10.2337/dc10-0340. . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics Nat Rev Gastroenterol Hepatol. 2020 NaN17(11):687. doi: 10.1038/s41575-020-0344-2. . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF Effects of probiotics on type II diabetes mellitus: a meta-analysis J Transl Med. 2020 NaN18(1):30. doi: 10.1186/s12967-020-02213-2. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonucci LB, Olbrich dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study Clin Nutr. 2017 NaN36(1):85. doi: 10.1016/j.clnu.2015.11.011. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Waddell IS, Orfila C Dietary fiber in the prevention of obesity and obesity-related chronic diseases: from epidemiological evidence to potential molecular mechanisms Crit Rev Food Sci Nutr. 2022. NaN, pp. 1–16. . . ; –. [DOI] [PubMed]
- Wan MLY, Forsythe SJ, El-Nezami H Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges Crit Rev Food Sci Nutr. 2019 NaN59(20):3320. doi: 10.1080/10408398.2018.1490885. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Wang C, Wei S, Liu B, Wang F, Lu Z, Jin M, Wang Y Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota Gut Microbes. 2022 NaN14(1):2057779. doi: 10.1080/19490976.2022.2057779. . . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi XH Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice Biomed Pharmacother. 2020 NaN125:109914. doi: 10.1016/j.biopha.2020.109914. . . ; : [DOI] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes Science. 2011 NaN334(6052):105. doi: 10.1126/science.1208344. . ; ( ): –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Li N, Yue Y, Wang C, Zhao L, Evivie SE, Li B, Huo G Screening for potential novel probiotics with dipeptidyl peptidase IV-inhibiting activity for type 2 diabetes attenuation in vitro and in vivo Front Microbiol. 2020 NaN10:2855. doi: 10.3389/fmicb.2019.02855. . . ; : [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Lv L, Wu W, Li Y, Shi D, Fang D, Guo F, Jiang H, Yan R, Ye W, et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels Front Microbiol. 2018 NaN9:1967. doi: 10.3389/fmicb.2018.01967. . ; : [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, Gu X, Xue Y, Huang S, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study) Nat Commun. 2020 NaN11(1):5015. doi: 10.1038/s41467-020-18414-8. . ; ( ): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes Science. 2018 NaN359(6380):1151. doi: 10.1126/science.aao5774. . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ley SH, Hu FB Global aetiology and epidemiology of type 2 diabetes mellitus and its complications Nat Rev Endocrinol. 2018 NaN14(2):88. doi: 10.1038/nrendo.2017.151. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]
- Zhong H, Abdullah, Zhao M, Tang J, Deng L, Feng F Probiotics-fermented blueberry juices as potential antidiabetic product: anti-oxidant, antimicrobial and antidiabetic potentials J Sci Food Agric. 2021 NaN101(10):4420. doi: 10.1002/jsfa.11083. . . ; ( ): –. [DOI] [PubMed] [Google Scholar]


