Abstract
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) is a dementia-related proteinopathy common in the elderly population. LATE-NC stages 2 or 3 are consistently associated with cognitive impairment. A condensed protocol (CP) for the assessment of Alzheimer disease neuropathologic change and other disorders associated with cognitive impairment, recommended sampling of small brain portions from specific neuroanatomic regions that were consolidated, resulting in significant cost reduction. Formal evaluation of the CP for LATE-NC staging was not previously performed. Here, we determined the ability of the CP to identify LATE-NC stages 2 or 3. Forty brains donated to the University of Washington BioRepository and Integrated Neuropathology laboratory with known LATE-NC status were resampled. Slides containing brain regions required for LATE-NC staging were immunostained for phospho-TDP-43 and reviewed by 6 neuropathologists blinded to original LATE-NC diagnosis. Overall group performance distinguishing between LATE-NC stages 0–1 and 2–3 was 85% (confidence interval [CI]: 75%–92%). We also used the CP to evaluate LATE-NC in a hospital autopsy cohort, in which LATE-NC was more common in individuals with a history of cognitive impairment, older age, and/or comorbid hippocampal sclerosis. This study shows that the CP can effectively discriminate higher stages of LATE-NC from low or no LATE-NC and that it can be successfully applied in clinical practice using a single tissue block and immunostain.
Keywords: Condensed protocol, LATE, LATE-NC, Limbic predominant age-related TDP-43 encephalopathy neuropathologic change
INTRODUCTION
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) is a prevalent and clinically impactful proteinopathy involving the transactive response DNA binding protein of 43 kDa (TDP-43); it is frequently diagnosed in the elderly (≥80 years of age) (1). In the course of LATE-NC, the proteinopathy is believed to progress anatomically in a stereotypical manner starting from the amygdala (stage 1), followed by the hippocampus (stage 2), and finally to many other brain regions including the middle frontal gyrus (stage 3) (1, 2). Clinically, LATE-NC correlates with amnestic cognitive impairment independent of other copathologies, but the presence of LATE-NC is often associated with other comorbid neurodegenerative and age-related pathologies such as Alzheimer disease neuropathologic change (ADNC), Lewy body disease, and hippocampal sclerosis (HS) (3–5). In individuals with ADNC, the presence of comorbid LATE-NC is associated with more severe and faster rates of cognitive decline than in the setting of ADNC without LATE-NC (1, 2, 6, 7). In particular, studies suggest that LATE-NC stages 2 and 3 are strongly predictive of cognitive impairment, while the clinical implications of LATE-NC stage 1 are not as apparent (8–10).
In a recent study combining data from 13 community or population-based autopsy cohorts, LATE-NC was observed in nearly 40% of brain donors (9). Despite the high prevalence of LATE-NC in the community, sampling recommendations and immunohistochemical guidelines for community-based pathologists, particularly those working in general hospital and forensic autopsy services, are lacking. In 2017, a condensed protocol (CP) to assess for ADNC and related pathologies was developed at the University of Washington (UW). Based on the 2012 NIA-AA guidelines for the assessment of ADNC and related dementia pathologies, this protocol maintains sampling fidelity across 20 recommended brain regions, condensed into 5 tissue cassettes, resulting in a ∼75% cost-reduction (11). Despite substantially reducing the size of each sample, this protocol was shown to perform favorably in both academic hospital autopsy and forensic neuropathology settings where resources may be limited (12, 13). These studies have demonstrated the utility of the CP for the diagnosis of common neurodegenerative diseases, including ADNC, Lewy body disease, and microvascular brain injury. Diagnostic criteria for LATE-NC were not included in the first iteration of the CP because guidelines for the assessment of LATE-NC were not published in the form of a consensus recommendation until 2019 (1).
Since the publication of the original UW condensed protocol in 2017 (11), additional studies investigating alternative sampling and immunohistochemical staining schema have been developed, and some have incorporated phosphorylated TDP-43 (pTDP-43) immunostaining. Clement et al (14) devised a 6-block condensed protocol which utilized larger tissue sample sizes and applied pTDP-43 immunohistochemical staining to a single block containing unilateral hippocampus and frontal cortex in a subset that were known to have some type of TDP-43 proteinopathy (frontotemporal lobar degeneration with TDP-43 pathology [FTLD-TDP] or LATE-NC). Likewise, Multz et al (15) utilized the original UW condensed protocol sampling scheme and added immunohistochemical staining for pTDP-43 to 3 blocks in order to determine if TDP-43 proteinopathy could be detected, using a combination of cases diagnosed as either FTLD-TDP or LATE-NC. Both studies demonstrated high sensitivity for the detection of pathologic TDP-43 using a CP approach, however neither were designed to specifically test its effectiveness in screening for clinically relevant LATE-NC. Additionally, both studies were limited to a research brain bank population; there is no prior published study that demonstrated best practices for applying the CP in a community setting to identify clinically relevant LATE-NC.
In the current study, the original UW condensed protocol was compared with the gold-standard approach provided by the 2019 LATE-NC consensus working group report, which recommends sampling and TDP-43 immunohistochemical staining of the amygdala, hippocampus, and middle frontal gyrus (1). This sampling recommendation is followed by the UW Biorepository and Integrated Neuropathology (BRaIN) lab which samples and performs pTDP-43 immunohistochemical staining on these recommended brain regions, with sampled tissue measuring approximately 3.0×2.5×0.4 cm and bilateral hippocampi and bilateral amygdalae represented in a single tissue block each. The CP includes regions recommended by the 2019 LATE-NC consensus working group, but there are key differences compared with the gold standard approach: (1) in the CP, because samples from multiple brain regions are placed in a single tissue block, each region is represented by a smaller sample size; (2) instead of bilateral hippocampi residing in a single block, the left and right hippocampus are each placed in a separate block; and (3) the amygdala is sampled unilaterally instead of bilaterally. The goal of the CP is to accurately screen cases for neurodegenerative disease in a cost-effective manner; therefore, the primary aim of this study was to determine whether the minimalist sampling and staining approach of the CP could effectively distinguish clinically relevant high stages of LATE-NC (2 and 3) from no to low stage LATE-NC (0 and 1) in a research cohort.
A secondary aim of this project was to evaluate the performance of the CP for the assessment of pTDP-43 immunohistochemistry in the hospital autopsy setting. The goal of this aim was to describe the performance of applying the CP to diagnose clinically relevant LATE-NC in a resource-limited or clinical setting, in alignment with the original intent of the CP, with a patient population that differs substantially from that of a neurodegenerative disease-focused research autopsy cohort. As part of standard practice, the UW Medicine clinical autopsy service has been applying the CP for the assessment of neurodegenerative disease in brains from persons ≥65 years of age, as well as younger autopsied individuals with a clinical history of cognitive impairment/dementia. While pTDP-43 immunohistochemistry was not applied in the original CP published in 2017, pTDP-43 immunohistochemical staining has been routinely performed on a single block containing samples of right hippocampus, right middle frontal gyrus, right temporal lobe, and left occipital lobe for all UW Medicine autopsy CP cases since 2018. Here, we supplement our findings using the CP to assess LATE-NC in a neurodegenerative cohort with 4 years of hospital autopsy neuropathology data derived from the application of the CP with inclusion of pTDP-43 assessments.
MATERIALS AND METHODS
Ethics statement
The use of human subject material was performed in accordance with the Declaration of Helsinki and the guidelines set by the UW Institutional Review Board, including a waiver of informed consent.
Research case selection and slide preparation
Forty donor brains from the UW BRaIN laboratory with known LATE-NC status (stages 0–3) were selected. These brains had been previously evaluated according to the 2019 LATE-NC consensus guidelines, including immunohistochemical evaluation for pTDP-43 in amygdala, hippocampus, and prefrontal cortex (middle frontal gyrus). Cases from each LATE-NC stage were included (Stage 0, n = 11, Stage 1, n = 9, Stage 2, n = 10, Stage 3, n = 10). Case selection was made by first identifying all LATE-NC stage 3 cases, which is the least common. Ten LATE-NC stage 3 cases were identified in the research repository and the remaining stages were selected to match based on age, sex, postmortem interval, and year of death.
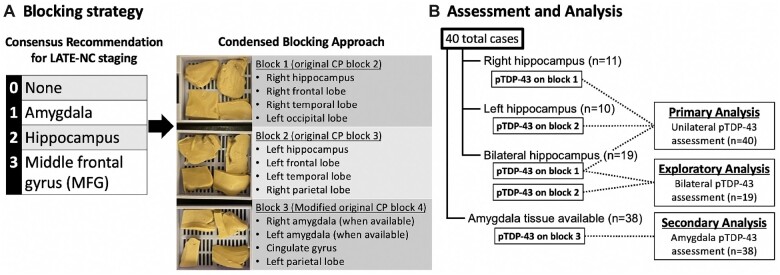
Sampling based on the CP was then performed for all cases using the remaining available tissues. Approximately half of the cases had only unilateral hippocampus available for CP sampling (n = 21) because the research brain service directed the entire hippocampus from one hemisphere to frozen sampling for most cases until late 2018. For those cases, CP sampling included one block that contained either right hippocampus, right middle frontal gyrus, right middle temporal gyrus, and left occipital cortex (n = 11) or left hippocampus, left middle frontal gyrus, left middle temporal gyrus, and right inferior parietal lobule (n = 10). For cases with bilateral hippocampus available (n = 19), both blocks were generated, and while the assessment of the right side was used for primary analyses, bilateral assessments were used in an exploratory analysis to determine whether bilateral assessments significantly improved sensitivity. Another CP block containing amygdala (bilateral when available) was generated for cases with available amygdala tissue (n = 38) for a secondary analysis to determine whether including this block would allow for the distinction between LATE-NC stages 0 and 1. These methods are illustrated in Figure 1.
Figure 1.
(A) For this study, blocks were prepared according to the original condensed protocol for the assessment of neurodegenerative disease in order to assess the use of this condensed blocking scheme for the identification of LATE-NC compared to standard research blocking protocols. Specifically, blocks 2, 3, and 4 from the original protocol contain the brain regions relevant to the assessment of LATE-NC and were the focus of this study. Blocks 1 and 2 in the current study exactly followed the original protocol for blocks 2 and 3, respectively. Block 3 in the current study is a modified version of block 4 from the original protocol that includes bilateral amygdala sampling when available. Immunohistochemistry for pTDP-43 was applied to all 3 blocks. (B) For primary analysis, only block 1 or 2, depending on tissue availability, was included for assessment of unilateral pTDP-43 pathology in the hippocampus and frontal cortex (n = 40). In an exploratory analysis, the 19 cases with bilateral hippocampal tissue available were analyzed to determine if adding a second block significantly improved sensitivity of the CP for detecting clinically relevant LATE-NC. In a secondary analysis, the 38 cases with available amygdala were assessed to determine if the CP amygdala samples were sufficient to accurately identify LATE-NC stage 1.
All tissue block processing and slide preparations were performed by the UW BRaIN lab following established protocols (16). Briefly, histologic sections were cut from formalin-fixed paraffin-embedded tissue blocks on a microtome at 5-µm thickness onto charged glass slides. A hematoxylin and eosin (H&E) stain and an immunohistochemical stain for pTDP-43 (clone 1D3; BioLegend, San Diego, CA) were performed on sections from each tissue block. H&E staining was performed manually and pTDP-43 immunohistochemical staining was performed on the BioCare intelliPATH semiautomated staining device at a dilution of 1:2000 following antigen retrieval with Diva decloaker at 110°C for 15 minutes.
Neuropathologic evaluation of research cases
For each case, the slides generated from the CP blocks containing regions relevant to the staging of LATE-NC were evaluated by neuropathologists (K.P.S., C.S.L., C.D.K, A.L.N.), a senior neuropathology fellow (H.M.) at UW, and a neuropathologist from the University of Kentucky (P.T.N.). Each neuropathologist evaluated the H&E and pTDP-43 immunohistochemical stains generated for each case and recorded the LATE-NC stage based on the 2019 LATE-NC consensus criteria (1). Although there has recently been an update to these guidelines (17), we followed the 2019 criteria in order to keep our approach consistent with the original assessments. This included not distinguishing pTDP-43 positive neurites only versus pTDP-43 intracellular inclusions.
Hospital autopsy case evaluation and data collection
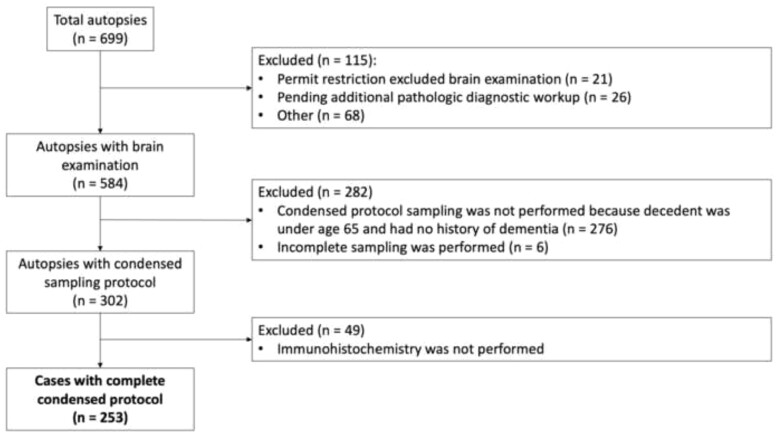
Not every hospital autopsy case routinely undergoes CP assessment. For this analysis, we used the same criteria as have been published previously for this cohort (13). Briefly, inclusion criteria included decedents aged 65 years or older, or those of any age with a clinical concern for dementia or neurodegenerative disease, with autopsies that included the complete CP performed at UW Medicine hospitals from 2018 to 2022 (n = 253) (Fig. 2). In accordance with our previously published protocol, 20 brain regions were consolidated into 5 blocks and special stains performed in compliance with the 2012 NIA-AA guidelines (11, 18). A pTDP-43 stain, added to block 2, which contains right hippocampus, right frontal lobe, right temporal lobe, and left occipital lobe, in 2018 (Table 1). This allows for a screen of any pTDP-43 present in hippocampus or cortex. Identification of pTDP-43 would either lead to a diagnosis of LATE or trigger a more extensive workup for FTLD-TDP or motor neuron disease. Tissue processing, H&E, and immunohistochemical staining were performed by the UW Department of Laboratory Medicine and Pathology. Retrospective hospital autopsy data were obtained utilizing PowerPath (Sunquest, Tucson, AZ) and SlicerDicer (Epic, Verona, WI) programs for cases that met inclusion criteria.
Figure 2.
Algorithm for NIA-AA condensed protocol work up in clinical autopsy cohort.
Table 1.
Original condensed protocol sampling guidelines with addition of pTDP-43 as applied to the hospital autopsy cohort
| Regions sampled | Immunohistochemical stain | ||
|---|---|---|---|
| Block 1 | Thal phase | Right occipital cortex | Beta-amyloid |
| Right basal ganglia | |||
| Midbrain | |||
| Cerebellum | |||
| Block 2 | Braak stage | Right hippocampus | Phosphorylated tau |
| LATE stage | Right temporal (scored 2×) | Phosphorylated TDP-43 | |
| Right frontal | |||
| Left occipital (scored 1×) | |||
| Block 3 | CERAD score | Left hippocampus | Bielschowsky |
| Left temporal (scored 2×) | |||
| Left frontal (scored 1×) | |||
| Right parietal | |||
| Block 4 | Lewy body stage | Pons | Alpha-synuclein |
| Amygdala | |||
| Cingulate | |||
| Left parietal | |||
| Block 5 | Vascular brain injury | Right thalamus | None |
| Left thalamus (scored 1×) | |||
| Left basal ganglia | |||
| Medulla |
Statistical analyses
Classification accuracy of the CP using the previously diagnosed LATE-NC staging based on the 2019 consensus guidelines as the “gold standard” was assessed with measurements of accuracy (% correct), sensitivity, and specificity for the classification into stage 2–3 versus stage 0–1. In order to obtain appropriate errors accounting for the clustered structure of our data (6 raters per case), mean percent accuracy and 95% confidence intervals (CIs) averaged across raters were estimated using generalized estimating equations. The unit of observation was each individual rating for each case and each rater (40 cases, 6 raters, 240 observations). The dependent variable was the rating correctness (1 = rater correctly classified stage; 0 = misclassification), the independent variable was stage (2–3 vs 0–1), and study case ID was the clustering variable, using binomial errors and an exchangeable correlation structure. A similar model was used to estimate average sensitivity and specificity with CIs. In this model, the dependent variable was the rater’s classification to stage 2–3 versus 0–1, and the independent variable was the “true” classification based on the gold standard. Sensitivity was obtained by estimating the marginal mean percent of classification to stage 2–3 for cases that had LATE-NC stage 2–3. Specificity was obtained by estimating the marginal mean percent of classification to stage 0–1 for cases that had LATE-NC stage 0–1. Details of these models are supplied in the supporting information. Inter-rater reliability in the classification of LATE-NC stage 2–3 versus 0–1 was assessed using Light kappa statistic.
In secondary analyses, classification accuracy for each of the 4 LATE-NC stages was estimated using generalized estimating equations as above. Inter-rater agreement for LATE-NC classification was measured with the intraclass correlation using the 2-way, agreement, single-measurement variant. We also carried out exploratory analysis comparing classification accuracy in bilateral versus unilateral assessments in the subsample of cases with bilateral assessments. Analyses were carried out using R 4.2.1 and packages tidyverse, geepack, emmeans, and irr (19).
RESULTS
In this study, we sought to evaluate the performance of the CP for the purposes of identifying clinically relevant high LATE-NC (at least stage 2). We identified 40 donor brains in the UW BRaIN Lab, with a mean age at death of 89 years (ranging from 74 to 99) autopsied during the years 2006–2019. These brains represented LATE-NC stages 0–3. Thirty-three (83%) of cases had postmortem fixation intervals under 24 hours, 5 cases had intervals between 32 and 48 hours, and 2 cases had intervals close to 80 hours. There were strong associations between LATE-NC and Braak NFT stages, HS, and a clinical history of dementia, as expected based on prior studies of LATE-NC (Table 2) (1, 6, 8, 9). There were no differences in year of death or postmortem interval between groups.
Table 2.
Characteristics of the BRaIN lab subjects
| 0 (n = 11) | 1 (n = 9) | 2 (n = 10) | 3 (n = 10) | Total (n = 40) | p value* | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 87.9 (7.9) | 91.4 (6.4) | 87.7 (5.5) | 90.1 (4.9) | 89.2 (6.3) | 0.51 |
| Sex, male | 5 (45%) | 5 (56%) | 5 (50%) | 4 (40%) | 19 (48%) | 0.97 |
| Year of death, mean (SD) | 2014 (5) | 2016 (4) | 2014 (4) | 2015 (5) | 2015 (4) | 0.87 |
| Postmortem time interval (hours), mean (SD) | 18.5 (22.8) | 16.8 (24.1) | 15.8 (17.0) | 12.9 (9.9) | 16.1 (18.6) | 0.93 |
| AD neuropathology | 0.26 | |||||
| N-Miss | 5 | 2 | 4 | 3 | 14 | |
| None/Low | 3 (50%) | 1 (14%) | 0 (0%) | 0 (0%) | 4 (15%) | |
| Med | 3 (50%) | 4 (57%) | 4 (67%) | 4 (57%) | 15 (58%) | |
| High | 0 (0%) | 2 (29%) | 2 (33%) | 3 (43%) | 7 (27%) | |
| Braak stage V–VI | 0.003 | |||||
| N-Miss | 0 | 0 | 1 | 0 | 1 | |
| 0–IV | 9 (82%) | 6 (67%) | 1 (11%) | 2 (20%) | 18 (46%) | |
| V–VI | 2 (18%) | 3 (33%) | 8 (89%) | 8 (80%) | 21 (54%) | |
| Hippocampal sclerosis | <0.001 | |||||
| N-Miss | 0 | 0 | 1 | 0 | 1 | |
| HS− | 10 (91%) | 9 (100%) | 4 (44%) | 2 (20%) | 25 (64%) | |
| HS+ | 1 (9%) | 0 (0%) | 5 (56%) | 8 (80%) | 14 (36%) | |
| Clinical dementia history | ||||||
| N-Miss | 1 | 1 | 0 | 1 | 3 | <0.001 |
| No | 9 (90%) | 5 (62%) | 1 (10%) | 0 (0%) | 15 (41%) | |
| Yes | 1 (10%) | 3 (38%) | 9 (90%) | 9 (100%) | 22 (59%) |
Based on ANOVA for age and the Fisher exact test for categorical variables.
BRaIN, University of Washington Biorepository and Integrated Neuropathology.
Condensed protocol detected LATE-NC stage 2–3 with high sensitivity and specificity
For our primary analysis, we retrospectively performed CP sampling for each of the donors in the cohort, followed by immunohistochemical staining for pTDP-43 on one CP block that contained regions relevant to the staging of LATE-NC unilaterally, comparing the performance of the CP against the original gold standard consensus assessment. The average overall performance of the CP was calculated based on the percent correct for the entire group and for each individual rater (Table 3). Overall performance was 85% (CI: 75%–92%) for the group, with 86% (CI: 70%–94%) of raters correctly classifying cases with stage 0–1 and 85% (CI: 68%–94%) correctly classifying cases with stage 2–3. Average sensitivity and specificity to classify LATE-NC stage 2–3 versus stage 0–1 was 85% (CI: 68%–94%) and 86% (CI: 70%–94%), respectively. Overall agreement among reviewers was moderate, with an average kappa of 0.76 (CI: 0.63–0.87) (20, 21). Raters in 28 out of 40 cases were in complete agreement. In the 12 cases where there was some disagreement, 9 of these consisted of only 1 out of 6 raters in disagreement with the other 5 raters, 1 case with 2 raters disagreeing with the other 4 raters, and the remaining 2 cases evenly split in their ratings. Of a possible 600 pairwise ratings agreements (15 pairs of ratings across 6 raters multiplied by 40 cases), there were 529 (88%) pairwise agreements (Supplementary DataFig. S1).
Table 3.
Percent correct classification and sensitivity and specificity for LATE-NC stage 2–3 versus 0–1 for each of 6 raters, and means (95% CI) across raters
| Rater 1 | Rater 2 | Rater 3 | Rater 4 | Rater 5 | Rater 6 | Mean (95% CI)* | |
|---|---|---|---|---|---|---|---|
| Stage 0–1 | 85 | 95 | 75 | 85 | 95 | 80 | 86 (70–94) |
| Stage 2–3 | 85 | 90 | 85 | 85 | 75 | 90 | 85 (68–94) |
| Mean across stage | 85 | 92 | 80 | 85 | 85 | 85 | 85 (75–92) |
| Sensitivity | 85 | 90 | 85 | 85 | 75 | 90 | 85 (68–94) |
| Specificity | 85 | 95 | 75 | 85 | 95 | 80 | 86 (70–94) |
Calculated from generalized estimating equations.
Bilateral assessments did not substantially increase sensitivity of CP
The 19 cases with bilateral hippocampus were also evaluated bilaterally to determine if this would substantially increase sensitivity and justify the routine addition of another immunohistochemical stain to the CP. Of the corresponding 114 assessments across the 6 raters using bilateral blocks, 6 were found to be LATE-NC stage 2–3 that were not detected using unilateral blocks. Sensitivity to detect LATE-NC using bilateral blocks was 94% (CI: 84%–98%) compared with 85% (CI: 62%–95%) using only unilateral blocks in the 19 cases.
Including pTDP-43 assessment of amygdala did not reliably identify LATE-NC stage 1 in CP
The 38 cases with sampled amygdala were also evaluated to determine if LATE-NC stage 1 could be reliably identified and justify the routine addition of another immunohistochemical stain to the CP. Our exploratory analysis revealed that overall performance for the identification of LATE-NC stage 1 (amygdala only) was low with reviewers correctly identifying LATE-NC stage 1 only 17% of the time (CI: 7%–34%) (Supplementary DataTable S1).
Application of condensed protocol identified LATE-NC in a hospital autopsy cohort
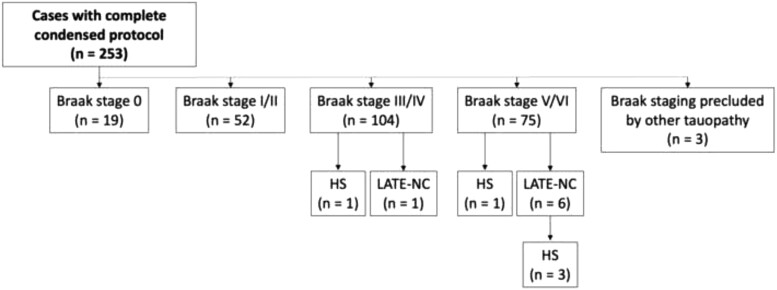
Postmortem brains from 253 patients from the UW Medicine diagnostic autopsy service were examined for LATE-NC using the Condensed Protocol by including immunohistochemical staining for pTDP-43 (Fig. 2; Table 1). The age range for this cohort was 44–94 years with a mean age of 73.7 years and a majority (n = 155; 61%) were men. Ninety-two percent of participants were 65 years of age or older, most of which did not have formal neurologic evaluations or cognitive data readily available. Conversely, the 23 subjects younger than 65 each had a clinical history of cognitive impairment/dementia. Neurodegenerative pathology was not uncommon in this cohort; only 19 cases lacked neurofibrillary neurodegeneration (Braak stage 0), and most had at least an intermediate Braak stage (Fig. 3). Seven cases with a LATE-NC stage of 2 or 3 were identified. HS was only rarely observed but was much more likely to occur when LATE-NC was present. The presence of LATE-NC was also associated with higher Braak stage. Table 4 summarizes the relevant known clinical and pathologic characteristics of the 7 subjects with LATE-NC. Notably, no cases were diagnosed with FTLD-TDP or motor neuron disease and all cases with TDP-43 pathology were determined to represent LATE-NC.
Figure 3.
Characteristics of the hospital autopsy subjects. Of the 253 hospital autopsy cohort subjects that underwent complete condensed protocol assessments, 7 with LATE-NC were identified, 6 of which had high Braak stage (Braak V/VI), and 1 of which had intermediate Braak stage (Braak IV). Three of seven subjects with LATE-NC also had HS (42.9%) compared to 2 of 246 subjects without LATE-NC that had HS (0.8%). HS, hippocampal sclerosis.
Table 4.
Demographic and pathologic data and in hospital cases with LATE-NC
| Case | Sex | Age at death | ADNC | Thal | Braak | CERAD | LATE stage | HS | Clinical history of dementia |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 89 | Intermediate | 5 | V | Sparse | 2 | No | Yes |
| 2 | M | 90+ | High | 5 | VI | Frequent | 2 | No | Yes |
| 3 | M | 77 | Intermediate | 3 | V | Sparse | 2 | Yes (R only) | No |
| 4 | M | 83 | High | 4 | VI | Frequent | 2 | Yes (BL) | Yes |
| 5 | M | 76 | Not ADNC | 0 | VI | None | 2 | Yes (BL) | Yes |
| 6 | M | 71 | Not ADNC | 0 | IV | None | 3 | No | No |
| 7 | M | 86 | High | 4 | VI | Moderate | 3 | No | Yes |
HS, hippocampal sclerosis; BL, bilateral; M, male; F, female.
DISCUSSION
We evaluated the performance of the CP for detection of higher stage LATE-NC (at least stage 2) and assessed 4 years of autopsy data at a large academic medical center to provide guidance for the use of pTDP-43 immunohistochemistry with the CP. Overall, our results in the research cohort show that the CP performs well for the detection of relatively severe LATE-NC stages with the use of only one immunostained section.
Although to capture the full possible spectrum of LATE-NC (including LATE-NC stage 1 and cases with unilateral pathology), up to 3 additional immunostains would need to be performed, our results demonstrate that the additional workup would not substantially improve the diagnostic sensitivity or accuracy. Specifically, our analyses show that the CP is not sensitive enough to identify stage 1, which is likely due to the limited and focal pTDP-43 pathology observed in LATE-NC stage 1 cases (22) combined with the small size of the amygdala sample in the CP. With respect to bilateral assessments of the hippocampus and frontal cortex, this is not specifically recommended in the consensus criteria (1) and our results do not show a substantial increase in sensitivity when bilateral assessments are taken into account in a subgroup analysis. While HS is reportedly unilateral in up to 40%–50% of cases when both sides are evaluated, the lateralization of pTDP-43 pathology in LATE-NC is not well characterized and requires further investigation (23–25). Therefore, we conclude that the CP is best suited for unilateral screening for LATE-NC stages 2 and 3, which is likely a clinically relevant distinction given that the neurocognitive consequences of LATE-NC stage 1 are not well defined (8–10).
Evaluation of the CP in a hospital autopsy cohort underscored that different autopsy settings may experience a distinct mix of autopsied subjects with corresponding differences in the brain pathologies. In a recent evaluation of 13 community-based or population-based research cohorts, approximately 25% of participants had a LATE-NC stage of 2 or 3, compared with the 2.8% in our hospital autopsy cohort (9). Notably, the age at death in the hospital autopsy cohort was markedly younger than the community and population-based research cohorts (73.7 years compared with 88.1 years). This is important because LATE-NC (as the acronym implies) has a strong tendency to affect individuals who are beyond age 85 years at death (1, 2, 6, 9, 26, 27). Among those in this cohort aged 85+ years (n = 27), 11.1% (n = 3) had LATE-NC stage 2 or 3. Additionally, although cognitive status was not definitively known for the vast majority of subjects in the hospital autopsy cohort, this is likely another major difference, as the research cohorts are substantially enriched for participants at risk for dementia unlike hospitalized patients where dementia is less likely but other factors may tend to cause them to come to autopsy. Thus, it can be argued that there is stronger (albeit less predictable) recruitment bias in a hospital-based autopsy sample than in a community-based autopsy cohort.
The demographic and comorbid neuropathological data from our 40 sampled research cases were consistent with prior studies, showing that LATE-NC was highly correlated with clinical history of dementia, higher ADNC severity, and HS (9). These findings were further supported by 4 years of hospital autopsy data where LATE-NC was seen most frequently in those with dementia and higher Braak NFT stages. These observations indicate that an assessment for LATE-NC may be particularly appropriate in decedents with a clinical history of cognitive decline.
Factoring in our results, a rational approach can be applied for the application of pTDP-43 immunohistochemistry using the CP. Performing pTDP-43 immunohistochemistry on only one block (containing one hippocampus and the ipsilateral frontal cortex) will maintain the cost-reducing benefits of the CP while still allowing for an effective assessment of this important neuropathology. Although all dementia cases are recommended to be assessed for LATE-NC, pTDP-43 immunohistochemistry may be performed only in cases with a high suspicion of LATE-NC if resources are severely limited (Table 5).
Table 5.
Recommendations for the application of phosphorylated TDP-43 immunohistochemistry to the condensed protocol
|
If hippocampal sclerosis (HS) is identified unilaterally, pTDP-43 should be performed on the side with HS.
This study had several strengths, including a large research cohort representing the complete spectrum of LATE-NC in which to assess the performance of the CP combined with 4 years of hospital autopsy data to examine the practical utility of assessing for pTDP-43 pathology using the CP. As a result, we were able to provide some guidance for the evaluation of LATE-NC in resource-limited, nonresearch settings. We also performed inter-rater reliability assessments by multiple raters, incorporating several institutions and career levels among raters, that helped assess the generalizability of our findings.
There also were some limitations relevant to this study. First, the study design was not oriented to carefully test the prevalence of high stage LATE-NC in the hospital autopsy cohort. Because the CP was applied as a screening tool, more extensive workup was not performed on these cases and there is a possibility that cases of unilateral LATE-NC were missed. However, as noted above, the analyses performed on the research cohort suggest it is unlikely that many cases were missed with this approach and performing unilateral assessments for pTDP-43 immunoreactivity in a block that contains small samples of hippocampus and frontal cortex is a sensitive and cost-effective screening approach for LATE-NC.
This study was not designed to study the ability of the CP to differentiate FTLD-TDP from severe LATE-NC. Indeed a recently published study highlighted this potential weakness in using the CP to diagnose LATE-NC (15). However, what we and others have demonstrated is that pTDP-43 pathology can be identified and characterized in a clinically relevant manner using the CP (14, 15). Given that LATE-NC is much more common than FTLD-TDP (1, 9), particularly in the hospital and forensic setting, this single immunostain will be sufficient in the great majority of cases. If clinical or pathologic suspicion for FTLD-TDP exists, an expanded workup should be performed to improve diagnostic accuracy. For example, in cases with a high burden of pTDP-43 in the frontal cortex, it may be prudent to sample additional brain regions, including additional cortical regions to further evaluate the pattern and relative burden of pTDP-43 pathology (28).
Finally, a technical limitation is that the cases in the research cohort were retrospectively sampled, which means that some the resampled blocks contained tissue sections that had been fixed in formalin for prolonged periods (>1 year). It was noted that older cases had weaker staining for pTDP-43 compared with newer cases (data not shown), suggesting that there is degradation of IHC signal for samples that had remained for long periods (up to 4 years) in formalin before undergoing paraffin processing and embedding. This may have affected inter-rater reliability due to faint immunostaining and is one possible source of discrepancy between the original diagnosis and that based on the CP evaluations.
Overall, our study demonstrates that the CP can reliably detect high-stage LATE-NC (stages 2 and 3) without adding additional tissue samples or multiple immunostains. While LATE-NC is relatively common in aging research cohorts enriched for neurodegenerative disease, the prevalence of LATE-NC in other populations, such as younger autopsy cohorts or forensic settings where the CP is most useful, is less clear. Our results from a large academic medical center suggest that clinically significant high-stage LATE-NC may be comparatively uncommon, which may be attributed to the younger average age at death and lower incidence of dementia in these cohorts. As such, we recommend that pTDP-43 immunohistochemistry be performed on block 2 or 3 of the original CP in select patients, including those with a clinical history of dementia, with hippocampal sclerosis, and/or those ≥80 years of age. By applying these criteria, LATE-NC can be effectively diagnosed using the CP in a cost-effective manner.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Ms Jessica Malmberg, and the University of Washington Autopsy and After Death Services team for outstanding clinical and technical support, Aimee Schantz and John Campos for outstanding administrative and data management support, and the UW BioRepository and Integrated Neuropathology lab tissue procurement and histology teams for technical and histology support.
Contributor Information
Heather Maioli, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Rhonda Mittenzwei, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Jane B Shofer, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, Washington, USA; Mental Illness Research, Education, and Clinical Center (MIRECC), VA Puget Sound Health Care System, Seattle, Washington, USA.
Kathryn P Scherpelz, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Desiree Marshall, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Amber L Nolan, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Peter T Nelson, Department of Pathology and Laboratory Medicine, University of Kentucky, Lexington, Kentucky, USA.
C Dirk Keene, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Caitlin S Latimer, Division of Neuropathology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
FUNDING
Sources of support include NIH K08 AG065426 (to C.S.L.), NIH K08 NS114170 (to A.L.N.), P30 AG066509 (UW ADRC), P30 AG072946 (UK ADRC), U19 AG066567 (Adult Changes in Thought Study), and RF1 NS118584 (to P.T.N.), the Nancy and Buster Alvord Endowment (to C.D.K.), and the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the Department of Veterans Affairs Puget Sound Mental Illness Research, Education, and Clinical Center (MIRECC fellowship to D.M., K.P.S., H.M., and R.M.).
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest.
SUPPLEMENTARY DATA
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus Working Group Report. Brain 2019;142:1503–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Besser LM, Teylan MA, Nelson PT.. Limbic predominant age-related TDP-43 encephalopathy (LATE): Clinical and neuropathological associations. J Neuropathol Exp Neurol 2020;79:305–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aoki N, Murray ME, Ogaki K, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP type A. Acta Neuropathol 2015;129:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arai T, Mackenzie IRA, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 2009;117:125–36 [DOI] [PubMed] [Google Scholar]
- 5. Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 2014;127:811–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kapasi A, Yu L, Boyle PA, et al. Limbic-predominant age-related TDP-43 encephalopathy, ADNC pathology, and cognitive decline in aging. Neurology 2020;95:e1951–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008;70:1850–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nag S, Yu L, Boyle PA, et al. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun 2018;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer’s disease neuropathology: Combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol 2022;144:27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nascimento C, Di Lorenzo Alho AT, Bazan Conceição Amaral C, et al. Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: Systematic review and meta-analysis. Neuropathol Appl Neurobiol 2018;44:286–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flanagan ME, Marshall DA, Shofer JB, et al. Performance of a condensed protocol that reduces effort and cost of NIA-AA guidelines for neuropathologic assessment of Alzheimer disease. J Neuropathol Exp Neurol 2017;76:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Priemer DS, Folkerth RD.. Dementia in the forensic setting: Diagnoses obtained using a condensed protocol at the Office of Chief Medical Examiner, New York City. J Neuropathol Exp Neurol 2021;80:724–30 [DOI] [PubMed] [Google Scholar]
- 13. Bharadwaj R, Cimino PJ, Flanagan ME, et al. Application of the condensed protocol for the NIA-AA guidelines for the neuropathological assessment of Alzheimer’s disease in an academic clinical practice. Histopathology 2018;72:433–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clement NF, DeWitt JC, Frosch MP, et al. A simplified brain blocking protocol optimized for the diagnosis of neurodegenerative disease saves time and money while preserving anatomic relationships. Arch Pathol Lab Med 2021;145:960–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Multz RA, Spencer C, Matos A, et al. What every neuropathologist needs to know: Condensed protocol work-up for clinical dementia syndromes. J Neuropathol Exp Neurol 2023;82:103–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Latimer CS, Melief EJ, Ariza-Torres J, et al. Protocol for the systematic fixation, circuit-based sampling, and qualitative and quantitative neuropathological analysis of human brain tissue. Methods Mol Biol 2023;2561:3–30 [DOI] [PubMed] [Google Scholar]
- 17. Nelson PT, Lee EB, Cykowski MD, et al. LATE-NC staging in routine neuropathologic diagnosis: An update. Acta Neuropathol 2023;145:159–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montine TJ, Phelps CH, Beach TG, et al. ; Alzheimer’s Association. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing [Internet]. Vienna, Austria: R Core Team; 2022. Available from: https://www.R-project.org/ [Google Scholar]
- 20. McHugh ML. Interrater reliability: The kappa statistic. Biochem Med 2012;22:276–82 [PMC free article] [PubMed] [Google Scholar]
- 21. Xie Q. Agree or Disagree? A Demonstration of an Alternative Statistic to Cohen’s Kappa for Measuring the Extent and Reliability of Agreement between Observers. Arlington, VA: MacroSys, LLC; 2013:1–12 [Google Scholar]
- 22. Cykowski MD, Arumanayagam AS, Powell SZ, et al. Patterns of amygdala region pathology in LATE-NC: Subtypes that differ with regard to TDP-43 histopathology, genetic risk factors, and comorbid pathologies. Acta Neuropathol 2022;143:531–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: Clinical and pathological features. Brain 2011;134:1506–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kero M, Raunio A, Polvikoski T, et al. Hippocampal sclerosis in the oldest old: A Finnish population-based study. J Alzheimers Dis 2018;63:263–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zarow C, Weiner MW, Ellis WG, et al. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav 2012;2:435–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cykowski MD, Powell SZ, Schulz PE, et al. Hippocampal sclerosis in older patients: Practical examples and guidance with a focus on cerebral age-related TDP-43 with sclerosis. Arch Pathol Lab Med 2017;141:1113–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelson PT, Trojanowski JQ, Abner EL, et al. “New old pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol 2016;75:482–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson JL, Porta S, Garrett FG, et al. Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain 2020;143:2844–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.