Abstract
Objective
To determine if ChatGPT can generate useful suggestions for improving clinical decision support (CDS) logic and to assess noninferiority compared to human-generated suggestions.
Methods
We supplied summaries of CDS logic to ChatGPT, an artificial intelligence (AI) tool for question answering that uses a large language model, and asked it to generate suggestions. We asked human clinician reviewers to review the AI-generated suggestions as well as human-generated suggestions for improving the same CDS alerts, and rate the suggestions for their usefulness, acceptance, relevance, understanding, workflow, bias, inversion, and redundancy.
Results
Five clinicians analyzed 36 AI-generated suggestions and 29 human-generated suggestions for 7 alerts. Of the 20 suggestions that scored highest in the survey, 9 were generated by ChatGPT. The suggestions generated by AI were found to offer unique perspectives and were evaluated as highly understandable and relevant, with moderate usefulness, low acceptance, bias, inversion, redundancy.
Conclusion
AI-generated suggestions could be an important complementary part of optimizing CDS alerts, can identify potential improvements to alert logic and support their implementation, and may even be able to assist experts in formulating their own suggestions for CDS improvement. ChatGPT shows great potential for using large language models and reinforcement learning from human feedback to improve CDS alert logic and potentially other medical areas involving complex, clinical logic, a key step in the development of an advanced learning health system.
Keywords: artificial intelligence, clinical decision support, large language model
INTRODUCTION
Clinical decision support (CDS) provides information and recommendations to healthcare professionals and patients at the point of care.1 As electronic health record (EHR) adoption has increased, in part due to more than $34 billion of government spending,2 the use of CDS has also increased. Rule-based CDS alerts that deliver patient- and task-specific recommendations are a required part of all certified EHRs.3 Such CDS alerts can improve clinical practice,1,4 standardize care to close quality gaps,5 and address racial and ethnic disparities.6 For example, a review of cardiovascular disease studies found that CDS alerts increased guideline-recommended testing and examinations by 9.6–45.6% in Black and Hispanic populations.7 A classic CDS design framework attempts to optimize CDS utility by ensuring that they are relevant to the right patient, to the right health professional, at the right time in the workflow, in the right intervention format, and through the right channel.8
Despite these potential benefits, approximately 90% of alerts are overridden or ignored by clinicians with justifiable reasons (eg, irrelevancy, poor timing, or incomplete characterization of clinical condition).9–11 Alert fatigue arises when clinicians encounter these poorly performing alerts, which threatens patient safety.12–14 Researchers have proposed several approaches for optimizing alerts. The first approach uses human review to optimize alert content, timing, and target audience,15–17 which can reduce 9–35% of alerts with no untoward consequences.18 For example, Vanderbilt University Medical Center (VUMC) conducted the Clickbusters program,19 where 24 physicians and informaticians reviewed alerts at VUMC using a structured process. They eliminated 70 000 unnecessary weekly alert firings, representing a 15% decrease. However, this approach is resource-intensive, subject to cognitive bias and premature closure, and requires periodic re-assessment. Marginal effects also diminish rapidly (ie, after all simple improvements have been identified, it takes a disproportionate effort for reviewers to identify further improvements).20,21 Experts involved in manual reviews are often clinicians who practice in a single area or use a particular workflow. Subsequently, they may not consider improvements that are relevant to other team members with different workflows. Automated tools using simple rules or machine learning techniques for identifying problematic alerts might allow for sustainable and scalable CDS maintenance.22,23
Valuable insights generated by ChatGPT or other large language models could greatly support experts in refining their suggestions and enhance the specificity of alerts, ultimately addressing the issue of alert fatigue. ChatGPT, an artificial intelligence (AI) chatbot created by OpenAI, has achieved attention for its ability to solve a wide range of natural language processing tasks and generate human-like responses. ChatGPT was built using the GPT-3.5 large language model and fine-tuned for general tasks using human feedback and supervised and reinforcement learning.24 ChatGPT was launched on November 30, 2022, and has gained widespread attention among the medical community due to its potential to semiautonomously perform tasks such as answering sample questions from United States Medical Licensing Exam (USMLE) and generating simplified radiology reports for patient consumption.25,26 As a novel AI algorithm, the goal of the large language model is to predict the next sequences of words based on the previous context. To achieve this goal, large language models are often pretrained with large text corpora. For example, the large language model used in ChatGPT, GPT3.5, was trained with 175 billion parameters, with a dataset including CommonCrawl and WebText (web page data until 2021), 2 Internet-based book corpora, and English-language Wikipedia.27 After generating the large language model, OpenAI sampled prompts and collected related demonstration data from humans. Then, they used this dataset for supervised learning to fine-tune the GPT3.5 language model. In a second step, they asked annotators to rank the model outputs based on quality, which was used as a reward function to further fine-tune the supervised learning model to maximize the reward.
To address current challenges in optimizing CDS alerts, we proposed that ChatGPT-based CDS alert tuning might enable fast and cost-effective analysis of a high volume of alerts. The objectives for this work were to determine if ChatGPT can generate useful suggestions for improving clinical decision support logic and to assess noninferiority compared to human-generated suggestions. Our goal was not to show that ChatGPT suggestions are superior to human suggestions but, rather, to show that the ChatGPT suggestions may enhance traditional techniques for CDS maintenance and optimization. This is consistent with the fundamental theorem of medical informatics,28 where the goal is not to create computer systems that are superior to human, but rather to create systems that augment human intelligence, such that the human and computer together perform better than the human alone.
MATERIALS AND METHODS
Setting
This project was conducted at VUMC, a large integrated delivery system in the Southeastern United States, which uses Epic (Epic Systems Co., Verona, WI) as its EHR. VUMC has more than 80 certified Physician Builders, who are trained and certified to develop and maintain CDS, have experience in using CDS tools, and are willing to participate in EHR-related projects within the organization. We previously conducted an alert optimization program—Clickbusters—involving a 10-step process of reviewing alert related data and clinical evidence, identifying possible improvements, discussing improvements with stakeholders, making changes in the test environment, testing, and evaluating.19 The entire process was documented and archived.
Human-generated suggestions
In this study, we analyzed 7 alerts (BestPractice Advisories) from the Epic EHR at VUMC. These alerts, described in Table 1, were selected from the previously described Clickbusters program, because they had previously been reviewed for suggested improvements by clinical informaticians.19 During the review process, the alert logic and human suggestions were documented.
Table 1.
Selected alerts and descriptions
| Alert title | Description |
|---|---|
| a1: Immunocompromised and Live Virus Immunization | To prevent ordering a live virus vaccine for patients who are immunosuppressed. |
| a2: Anesthesia Postoperative Nausea and Vomiting | To identify patients who have risk factors for postoperative nausea and vomiting (PONV). |
| a3: Pediatrics Bronchiolitis Patients with Inappropriate Order | To identify potentially inappropriate use of albuterol or chest X-rays in children with bronchiolitis. |
| a4: Artificial Tears Frequency >6/day | To identify patients who have been prescribed artificial tears with a frequency exceeding 6 administrations per day. |
| a5: IP Allergy Documentation | To identify inpatients over 8 weeks old who have documented allergies, but have not had their allergy list reviewed. |
| a6: RX NSAID/Pregnancy | To discourage ordering nonsteroidal anti-inflammatory drugs (NSAIDs) in pregnant patients. |
| a7: Warfarin No INR | To notify pharmacists upon order verification of warfarin if the patient does not have an international normalized ratio (INR) result in the past 7 days. |
AI-generated suggestions
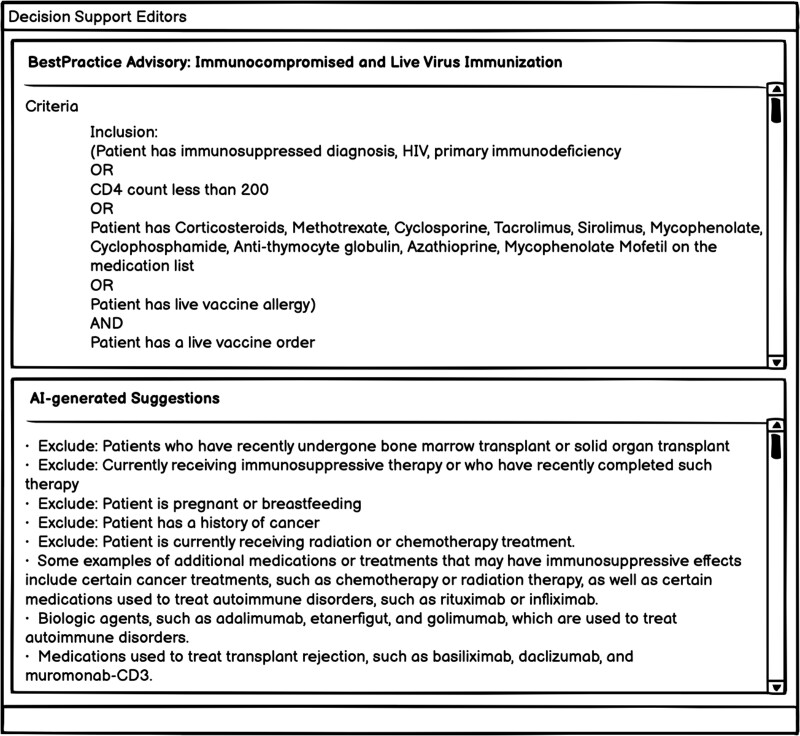
To generate potential improvements to the alerts automatically, we selected the ChatGPT chatbot. We transformed the documented alert logic into ChatGPT prompts in the following format: “I have a clinical decision support alert about [alert description]. [statement of guideline or standard, if available]. Current inclusion and exclusion criteria are listed below. Are there any additional exclusions that should be added? [Inclusion and exclusion criteria of the alert].” In addition, for groups of specific medications and diagnoses, we used additional prompts, such as “what other immunosuppressant medications should be added?” An example of using ChatGPT to generate suggestions to improve an alert is listed in Figure 1. All prompts used in this study are listed in Supplementary Appendix S1.
Figure 1.
An example of using ChatGPT to generate suggestions to improve an alert.
Expert review of suggestions
We mixed AI-generated suggestions with the suggestions previously generated by clinical informaticians (4 physicians and 1 pharmacist) during the Clickbusters program and grouped them by alert.19 Within each alert, we randomized the order of the AI- and human-generated suggestions. For the human-generated suggestions, we reformatted them if they included specific alert identifiers, since humans often included these identifiers but ChatGPT never did (because it did not have access to record IDs). For example, if a human suggested something like “Exclude patients with medications in grouper [1234]” and “grouper [1234]” corresponded to “immunosuppressant medications,” we transformed the human-generated suggestion to “Exclude patients with immunosuppressant medications.” The experts were blinded to whether the suggestions were generated by a human or ChatGPT. However, they were aware that the suggestions they were reviewing were a combination of both AI- and human-generated suggestions.
We created a semistructured questionnaire using REDCap.29 For each alert, we listed: (1) the alert description, (2) the logic of the alert, and (3) a link to more detailed information (eg, a screenshot of the alert). The questionnaire content was piloted within the research group (AW, AW, and AM) to improve its structure. Participants were a convenience sample of physicians and pharmacists with formal training in informatics and professional experience optimizing CDS tools. Participants were recruited from VUMC and UT Southwestern Medical Center. Each participant was assigned a unique number to ensure anonymity. Each suggestion was independently rated on a 5-point Likert scale (1—strongly disagree, 5—strongly agree) from 8 perspectives: (1) Understanding: I understand this suggestion. (2) Relevance: This suggestion includes relevant concepts. (3) Usefulness: This suggestion contains concepts that will be useful for improving the alert. (4) Acceptance: I can accept this suggestion without edits. (5) Workflow: Based on this suggestion, I will recommend a change to a clinical workflow/process outside of this alert. (6) Redundancy: This suggestion is redundant with the existing alert logic. (7) Inversion: This suggestion is inverted (eg, the suggested exclusion should be an inclusion). (8) Bias: This suggestion may contribute to bias. We also included a text box for each suggestion where participants could provide additional comments. For example, a common problem with AI-generated text is the tendency of language models to make up information, a phenomenon known as “hallucination.”30
Evaluation
We calculated the mean (standard deviation) for each item for each suggestion and generated box plots to show median and interquartile range, etc. In the overall score calculation, for Redundancy, Inversion, and Bias items, we used reversed scores, ie (1—strongly agree, 5—strongly disagree). The overall score was the average of the ratings in the 3 reversed items and the ratings from the remaining items. We used mean values to combine the summated effects of individual ratings to derive the scores in the interval measurement scale. This approach of using a composite score based on multiple items could provide more stable results.31 We performed a nonparametric Mann-Whitney Wilcoxon test to compare expert ratings of AI-generated suggestions and human-generated suggestions.32 In the alert-level analysis, we conducted Kruskal-Wallis H-tests to compare median values for each item. Statistical significance was set at P < .01. In addition, we calculated the intraclass correlation coefficient (ICC) to evaluate interrater reliability.33 ICC estimates and 95% CIs were reported based on a 2-way mixed-effects model (mean of k raters type and consistency definition). For ICC estimates, below 0.5 represents low reliability; 0.5–0.74 represents moderate reliability; 0.75–0.9 represents good reliability; and greater than 0.9 represents excellent reliability.34 Statistical analyses were conducted in Python3.6. Comments in free text were thematically analyzed through NVivo 12 using the inductive approach.35 An open coding scheme was used to guide the coding process. SL read and coded all comments. We reported summarized themes. We also reported descriptive statistics of participants’ characteristics (eg, clinical service, roles, and years of experience with CDS).
RESULTS
Five CDS experts trained in internal medicine, anesthesiology, pharmacy, emergency medicine, and pediatrics participated in the survey. The average values of clinical experience and EHR experience were 13.75 and 16.25 years, respectively. The characteristics of participants in the survey are listed in Table 2. The ICC value was 0.86, with a 95% CI ranging from 0.83 to 0.87, indicating good reliability.
Table 2.
Characteristic of 5 CDS experts participating in the survey
| Gender | |
| Male | 4 |
| Female | 1 |
| Clinical specialty | |
| Internal medicine | 1 |
| Anesthesiology | 1 |
| Pharmacy | 1 |
| Emergency medicine | 1 |
| Pediatrics | 1 |
| Clinical role | |
| Physician | 4 |
| Pharmacist | 1 |
| Years of clinical experience | 13.75 |
| Years of EHR experience | 16.25 |
Examples of AI-generated suggestions and human-generated suggestions
ChatGPT generated 36 suggestions across 7 alerts, while humans generated 29. All suggestions were included in the final questionnaire. The mean length of AI-generated suggestions was 134.0 ± 51.0 characters, while the mean length of human-generated suggestions was 57.8 ± 29.1 characters. Of the 20 suggestions that scored highest in the survey, 9 were generated by ChatGPT. These 20 suggestions and their scores for acceptance, relevance, understanding, and usefulness are presented in Table 3.
Table 3.
Top 20 suggestions and their ratings for acceptance (A), relevance (R), understanding (U1), and usefulness (U2)
| Alert | Suggestion | A | R | U1 | U2 | |
|---|---|---|---|---|---|---|
| H | Anesthesia postoperative nausea and vomiting | Scope of roles should include fellow. | 4.8 ± 0.5 | 4.8 ± 0.5 | 4.8 ± 0.5 | 4.8 ± 0.5 |
| H | Immunocompromised, live virus vaccine | Pregnant patients. | 3.0 ± 1.0 | 4.8 ± 0.5 | 4.4 ± 0.9 | 4.8 ± 0.5 |
| A | Immunocompromised, live virus vaccine | Some examples of additional medications or treatments that may have immunosuppressive effects include certain cancer treatments, such as chemotherapy or radiation therapy, as well as certain medications used to treat autoimmune disorders, such as rituximab or infliximab. | 2.4 ± 1.1 | 4.8 ± 0.5 | 4.8 ± 0.5 | 4.6 ± 0.6 |
| A | Immunocompromised, live virus vaccine | Exclude: Patient is currently receiving radiation or chemotherapy treatment. | 2.6 ± 1.5 | 4.4 ± 0.6 | 3.8 ± 1.6 | 4.5 ± 0.6 |
| H | Immunocompromised, live virus vaccine | Add an active chemotherapy criterion. | 2.4 ± 0.9 | 4.8 ± 0.5 | 4.8 ± 0.5 | 4.4 ± 0.6 |
| A | Immunocompromised, live virus vaccine | Add biologic agents, such as adalimumab, etanerfigut [sic]a, and golimumab, which are used to treat autoimmune disorders. | 2.2 ± 1.1 | 4.8 ± 0.5 | 4.6 ± 0.6 | 4.4 ± 0.6 |
| A | Immunocompromised, live virus vaccine | Exclude: Patients who have recently undergone bone marrow transplant or solid organ transplant. | 2.2 ± 1.3 | 4.6 ± 0.6 | 4.0 ± 1.7 | 4.3 ± 0.5 |
| H | Immunocompromised, live virus vaccine | Make the list of immunosuppression medications more complete. | 2.4 ± 1.1 | 4.6 ± 0.6 | 4.6 ± 0.6 | 4.2 ± 0.8 |
| H | IP allergy documentation | Exclude NICU and newborn departments. | 3.4 ± 1.3 | 4.2 ± 0.5 | 4.4 ± 0.6 | 4.2 ± 0.5 |
| A | Pediatrics bronchiolitis patients with inappropriate order | Exclude patients with other respiratory conditions, such as pneumonia or asthma, that may require chest radiography or albuterol treatment. | 2.6 ± 0.9 | 4.6 ± 0.6 | 4.8 ± 0.5 | 4.0 ± 1.2 |
| A | Immunocompromised, live virus vaccine | Add medications used to treat transplant rejection, such as basiliximab, daclizumab, and muromonab-CD3. | 2.4 ± 1.1 | 4.4 ± 0.6 | 4.6 ± 0.6 | 4.0 ± 1.2 |
| H | Anesthesia postoperative nausea and vomiting | Currently history of PONV looks to problem list, but it should also look to the last anesthesia preop evaluation. | 3.4 ± 0.9 | 4.8 ± 0.5 | 4.4 ± 0.9 | 4.0 ± 1.0 |
| A | Immunocompromised, live virus vaccine | Exclude: Currently receiving immunosuppressive therapy or who have recently completed such therapy. | 1.8 ± 1.1 | 4.2 ± 0.5 | 3.6 ± 1.5 | 4.0 ± 0.8 |
| H | Artificial tears frequency >6/day | Add additional eye drops. | 2.4 ± 0.9 | 4.2 ± 0.5 | 4.4 ± 0.6 | 4.0 ± 0.7 |
| H | Pediatrics bronchiolitis patients with inappropriate order | Add a 3rd OR statement if the ED bronchiolitis panel was used during this encounter. | 3.2 ± 1.6 | 3.8 ± 1.6 | 3.8 ± 1.6 | 3.8 ± 1.6 |
| H | Artificial tears frequency >6/day | Restrict inpatient options to only formulary. | 3.2 ± 0.8 | 3.8 ± 1.1 | 4.0 ± 1.2 | 3.8 ± 1.1 |
| H | Artificial tears frequency >6/day | Add inpatient orders. | 3.6 ± 0.9 | 3.8 ± 1.1 | 3.8 ± 1.1 | 3.8 ± 1.1 |
| A | Pediatrics bronchiolitis patients with inappropriate order | Exclude patients who are receiving palliative care or end-of-life care, as these patients may require chest radiography or albuterol treatment for symptom management. | 3.2 ± 1.6 | 3.6 ± 1.7 | 4.4 ± 0.9 | 3.6 ± 1.7 |
| H | Immunocompromised, live virus vaccine | Add patients with transplant on problem list. | 2.6 ± 1.3 | 4.6 ± 0.6 | 4.6 ± 0.6 | 3.6 ± 1.5 |
| A | Anesthesia postoperative nausea and vomiting | Patients who have had previous PONV episodes, as they may be at higher risk for developing PONV again. | 2.0 ± 1.0 | 4.6 ± 0.6 | 4.6 ± 0.6 | 3.6 ± 1.1 |
Etanerfigut is either a misspelling, which is hard to imagine from a computer, or a nonexistent medication hallucinated by ChatGPT.
NICU: neonatal intensive care unit; PONV: postoperative nausea and vomiting; ED: emergency department; CD3: cluster of differentiation 3; H: human-generated suggestions; A: AI-generated suggestions.
Results of expert review of AI-generated suggestions and human-generated suggestions
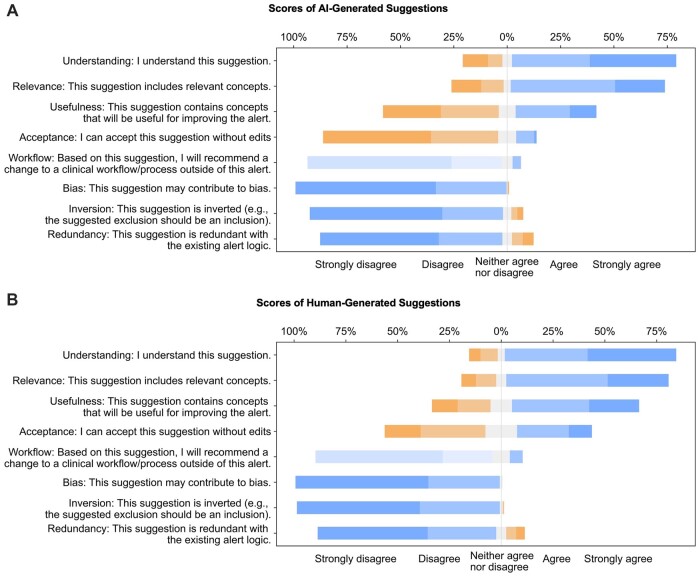
Out of the 36 AI-generated suggestions, 27 (75%) achieved an overall score of 3 or higher, with a maximum score of 4.0 ± 0.2 and a minimum score of 2.8 ± 0.4. The mean score was 3.3 ± 0.5. These AI-generated suggestions provided additional immunosuppressive medications and treatments and excluded additional patients (eg, patients with other respiratory conditions that may require chest radiography or albuterol treatment, and patients in palliative care). On average, the scores of AI-generated suggestions for relevance and understanding were rated as “agree,” usefulness as “neither agree nor disagree,” bias as “strongly disagree,” and workflow, inverted, and redundancy as “disagree.” Figure 2 shows 2 stacked bar charts of the scores for each item of the AI-generated suggestions (Figure 2A) and human suggestions (Figure 2B). In Figure 2A, AI-generated suggestions had high understanding and relevance, which were similar with human-generated suggestions. The acceptance rate for human suggestions was higher compared to AI-generated suggestions, while the ratings for workflow, bias, and inversion were similar for both sources of suggestions.
Figure 2.
Stacked bar charts of the scores of each item (understanding, relevance, usefulness, acceptance, workflow, bias, inversion, redundancy) for AI-generated suggestions (A) and human-generated suggestions (B).
AI-generated suggestions achieved high scores in understanding and relevance and did not differ significantly from human-generated suggestions. In addition, AI-generated suggestions did not show significant differences in terms of bias, inversion, redundancy, or workflow compared to human-generated suggestions. However, AI-generated suggestions received lower scores for usefulness (AI: 2.7 ± 1.4, human: 3.5 ± 1.3, P < .001) and acceptance (AI: 1.8 ± 1, human: 2.8 ± 1.3, P < .001). The overall scores were human: 3.6 ± 0.6 and AI: 3.3 ± 0.5 (P < .001). Values for mean and standard deviation for each item are presented in Table 4. Boxplots for each item are in Supplementary Appendix S2.
Table 4.
Means and SD for survey items using a 5-point Likert scale (1—strongly disagree, 5—strongly agree)
| AI-generated suggestions (mean ± SD) | Human-generated suggestions (mean ± SD) | P | |
|---|---|---|---|
| Understanding: I understand this suggestion. | 3.9 ± 1.3 | 4.1 ± 1.1 | .3 |
| Relevance: This suggestion includes relevant concepts. | 3.6 ± 1.3 | 3.8 ± 1.2 | .09 |
| Usefulness: This suggestion contains concepts that will be useful for improving the alert. | 2.7 ± 1.4 | 3.5 ± 1.3 | <.001 |
| Acceptance: I can accept this suggestion without edits. | 1.8 ± 1 | 2.8 ± 1.3 | <.001 |
| Workflow: Based on this suggestion, I will recommend a change to a clinical workflow/process outside of this alert. | 1.5 ± 0.8 | 1.6 ± 0.9 | .2 |
| Bias: This suggestion may contribute to bias. | 1.4 ± 0.6 | 1.4 ± 0.5 | .8 |
| Inversion: This suggestion is inverted (eg, the suggested exclusion should be an inclusion.) | 1.6 ± 0.9 | 1.4 ± 0.6 | .9 |
| Redundancy: This suggestion is redundant with the existing alert logic. | 1.7 ± 1.1 | 1.7 ± 1 | .8 |
| Overall | 3.3 ± 0.5 | 3.6 ± 0.6 | <.001 |
We further compared the scores of each item for AI-generated suggestions grouped by alert. The results showed significant variations in the scores for usefulness, acceptance, and relevance between alerts. On the other hand, the scores for understanding, workflow, bias, inversion, and redundancy did not vary significantly between the alerts.
According to the results, the AI-generated suggestions were found to have high levels of understanding (3.9 ± 1.3) and relevance (3.6 ± 1.3), both of which tended to “agree.” All AI-generated suggestions scored 3 or higher on the understanding item, with 29 (80.6%) of them scoring ≥3 on relevance. For example, the AI-generated suggestion for including patients with a history of PONV in the “postanesthesia nausea and vomiting” alert ranked third among all suggestions for both understanding and relevance. Similarly, excluding patients who have recently undergone bone marrow or solid organ transplantation from the “immunocompromised and live virus immunity” alert also ranked third for relevance.
The AI-generated suggestions had a moderate level of usefulness with a mean score of 2.7 ± 1.4. Nearly half of the AI-generated suggestions (15; 41.7%) received a score of 3 or higher on the usefulness item. Additionally, besides the suggestions previously mentioned, two other AI-generated suggestions for the “Immunocompromised and Live Virus Immunization” alert, regarding the exclusion of patients currently receiving radiation or chemotherapy treatment and the exclusion of patients currently receiving immunosuppressive therapy or who have recently completed such therapy, ranked second and fifth in terms of usefulness, respectively. The mean score for the acceptance item was 1.8 ± 1, and 2 AI-generated suggestions (5.6%) receiving a score of 3 or higher, indicating they could be accepted without changes. These suggestions were: (1) the exclusion of patients who are receiving palliative care or end-of-life care for the “Peds Bronchiolitis Patients with Inappropriate Order” alert and (2) the exclusion of patients with a pending INR test for the “Warfarin No INR” alert. It was unlikely to change workflow based on AI-generated or human-generated suggestions. Bias and redundancy were also low.
Qualitative analysis of comments on AI-generated suggestions
Lack of knowledge management and implementation
The lack of knowledge management and implementation understanding was a common barrier to the acceptance of AI-generated suggestions. For example, regarding the “Immunocompromised and Live Virus Immunization” alert, the AI-generated suggestion to “Exclude patients who are currently receiving immunosuppressive therapy or who have recently completed such therapy,” was commented on by experts that they liked the idea of excluding such patients for this alert, but they noted that it would require additional work to identify appropriate value sets and other specific implementation details in the EHR, before it could be included. Additional informatics work will be needed to operationalize these suggestions and implement them in the EHR.
Hallucination
We found the presence of hallucination in AI-generated suggestions, which involved generating made-up information. In the “Immunocompromised and Live Virus Immunization” alert, one of the AI-generated suggestions was to “add biologic agents, such as adalimumab, etanerfigut, and golimumab, which are used to treat autoimmune disorders.” Experts pointed out that “etanerfigut” was not a medication, and perhaps the intended term was “etanercept.”
Partially correct information
An expert commented that the AI-generated suggestion “Exclude: Patient is currently receiving radiation or chemotherapy treatment” for the “Immunocompromised and Live Virus Immunization” alert should only include chemotherapy.
Divergent opinions from experts
We also found that there was disagreement among experts when it came to AI-generated suggestions. One of the AI-generated suggestions for the “Anesthesia Postoperative Nausea and Vomiting” alert was “patients who are taking medications that may increase the risk of PONV, such as certain antidepressants or chemotherapy drugs.” While 2 participants found the suggestion useful, another expert pointed out that the suggestion was potentially misleading, as antidepressants are not typically associated with PONV, and some studies have found that they may actually decrease PONV.
DISCUSSION
In this study, we applied ChatGPT to generate suggestions to improve the logic of CDS alerts. To evaluate AI-generated suggestions, we mixed them with human-generated suggestions and asked CDS experts to rate all suggestions for their usefulness, acceptance, relevance, understanding, workflow, bias, inversion, and redundancy. While the AI-generated suggestions were not scored as highly as the human-generated suggestions, our findings demonstrated that AI-generated suggestions had high relevance and understanding scores, moderate usefulness scores, and low scores in ability to improve clinical workflow, bias, inversion, and redundancy. In addition, the lack of redundancy between the human- and AI-generated suggestions indicate that the AI could supplement traditional CDS optimization, as would be expected from the fundamental theorem of medical informatics.28
The results of this study indicate that ChatGPT could be used to automatically analyze alert logic and generate useful suggestions. Most of the AI-generated suggestions could not be accepted without modification, but they still offered valuable insights for experts to build upon. In addition, this approach enables the rapid analysis of many alerts, making it possible to scale CDS optimization efforts. Additionally, this approach could be well-integrated into the alert development process to provide AI-generated suggestions at the alert development stage.
A prototype is shown in Figure 3, where the top section displays the CDS alert being created by the user and its corresponding logic, and the bottom section displays the suggestions generated by AI. Based on the suggestions, the user could refine the alert logic to make it more specific and consider some easily overlooked aspects. It is worth noting that Epic provides “Build Inspectors” in its development interface offer suggestions to improve the alerts such as “Remove manual follow-up actions or configure this advisory to display to users.” However, these build inspectors provide feedback on data storage formats and specific functionality within the Epic system, rather than examining the alert logic itself. AI models like ChatGPT could provide similar recommendations, but for content.
Figure 3.
A prototype of potential implementation in EHR system—AI decision support editors.
In this project, we generated suggestions directly using the ChatGPT model, which was trained on a general dataset consisting of web pages, books, and Wikipedia for a variety of common use cases such as text generation, open-ended question-answers, brainstorming, chat, and rewriting in the form of conversations. Future research could therefore focus on improving language models and specifying training tasks. First, medical texts, such as clinical notes from the MIMIC-III dataset and PubMed articles, could be added to the language model. For example, using a publicly available clinical language model, GatorTron, based on 90 billion words of deidentified clinical notes from the University of Florida Health, PubMed articles, and Wikipedia, might be a good start.36 Another example is BioGPT, a GPT-like model trained on PubMed articles.37 In addition, UpToDate is an important source of CDS content for alert development and may have potential for adding to the language model. Nonetheless, due to its nonpublic availability, researchers may need to engage in additional steps to establish a collaborative partnership with UpToDate and obtain access. Second, based on the Reinforcement Learning from Human Feedback (RLHF) framework, researchers could train a model specifically for this task on the improved language model.38 Suggested steps include: (1) Human-generated suggestions: recruit CDS experts to generate suggestions for a number of CDS alerts (ie, the Clickbuster process).19 (2) Supervised fine-tuning (SFT): Use the selected alerts and human-generated suggestion dataset to fine-tune the pretrained language model to learn a supervised strategy (SFT model) to generate suggestions for selected alerts. (3) Mimic human preferences: recruit CDS experts to rank the output of the baseline model (ie, the generated suggestions). The generated suggestions and corresponding expert ranks are used as the reward model in reinforcement learning. (4) Proximal Policy Optimization (PPO): The reward model is used to further fine-tune and improve the SFT model to develop the final policy model. Third, researchers could use APIs from OpenAI to fine-tune GPT models on a specific task.
Limitations
This study has several limitations. First, the ChatGPT model is sensitive to the provided prompts and as a result, the AI-generated suggestions may vary based on changes in the input sentences. The prompt format used in our experiment was derived from an analysis of multiple input forms, but it is possible that there are other more effective ways to engage the ChatGPT model in this specific task. Second, we assessed the quality of AI-generated suggestions from the viewpoint of CDS experts, but the effect on clinical outcomes remains unknown. Third, ChatGPT was trained on text up to 2021 and did not include information on new drugs or clinical guidelines developed after that year. Consequently, ChatGPT is unable to provide suggestions regarding clinical guidelines and drugs that were developed after 2021. Fourth, the mean length of AI-generated suggestions was greater than the mean length of human-generated suggestions, and the tone of the AI-generated suggestions was somewhat different from human suggestions, which could potentially make it apparent to reviewers which suggestions were generated by AI, potentially impacting the rating process.
CONCLUSION
Alert fatigue is a pressing issue. In this study, we evaluated the feasibility of using ChatGPT to generate suggestions for improving the specificity of alert logic. The suggestions generated by AI were found to offer unique perspectives and were evaluated as highly understandable and relevant, with moderate usefulness, low acceptance, bias, inversion, redundancy, and low ability to improve clinical workflow. Therefore, these AI-generated suggestions could be an important complementary part of optimizing CDS alerts, can identify potential improvements to alert logic and support their implementation, and may even be able to assist experts in formulating their own suggestions for CDS improvement. Overall, ChatGPT shows great potential for using large language models and reinforcement learning from human feedback to improve CDS alert logic and potentially other medical areas involving complex, clinical logic, a key step in the development of an advanced learning health system.
Supplementary Material
Contributor Information
Siru Liu, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Aileen P Wright, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Barron L Patterson, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jonathan P Wanderer, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Robert W Turer, Department of Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA; Clinical Informatics Center, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Scott D Nelson, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Allison B McCoy, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Dean F Sittig, School of Biomedical Informatics, University of Texas Health Science Center, Houston, Texas, USA.
Adam Wright, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
FUNDING
This work was supported by NIH grant numbers: K99LM014097-01, R01AG062499-01, and R01LM013995-01.
AUTHOR CONTRIBUTIONS
SL applied ChatGPT to provide AI-generated suggestions and conducted survey development, data extraction, statistical analysis, and drafting the work. ABM, AW, and DFS helped to design experiments. ABM, AW, and APW participated in the pilot test of the survey. APW, BLP, JPW, RWT, and SDN participated in the survey. SL, AW, ABM, DFS, APW, BLP, JPW, RWT, and SDN revise the drafted manuscript. All authors approved the submitted version.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
CONFLICT OF INTEREST STATEMENT
The authors do not have conflicts of interest related to this study.
DATA AVAILABILITY
The input prompts and AI outputs are available in the Supplementary Appendix.
REFERENCES
- 1. Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005; 330 (7494): 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sorace J, Wong H-H, DeLeire T, et al. Quantifying the competitiveness of the electronic health record market and its implications for interoperability. Int J Med Inform 2020; 136: 104037. [DOI] [PubMed] [Google Scholar]
- 3. Clinical Decision Support (CDS) | HealthIT.gov. https://www.healthit.gov/test-method/clinical-decision-support-cds. Accessed September 7, 2021.
- 4. Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision-support systems. Ann Intern Med 2012; 157 (1): 29–43. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell J, Probst J, Brock-Martin A, et al. Association between clinical decision support system use and rural quality disparities in the treatment of pneumonia. J Rural Health 2014; 30 (2): 186–95. [DOI] [PubMed] [Google Scholar]
- 6. Lau BD, Haider AH, Streiff MB, et al. Eliminating health care disparities with mandatory clinical decision support. Med Care 2015; 53 (1): 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas Craig KJ, Fusco N, Lindsley K, et al. Rapid review: identification of digital health interventions in atherosclerotic-related cardiovascular disease populations to address racial, ethnic, and socioeconomic health disparities. Cardiovasc Digit Health J 2020; 1 (3): 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sirajuddin AM, Osheroff JA, Sittig DF, et al. Implementation pearls from a new guidebook on improving medication use and outcomes with clinical decision support. Effective CDS is essential for addressing healthcare performance improvement imperatives. J Healthc Inf Manag 2009; 23: 38–45. [PMC free article] [PubMed] [Google Scholar]
- 9. Seidling HM, Klein U, Schaier M, et al. What, if all alerts were specific – estimating the potential impact on drug interaction alert burden. Int J Med Inform 2014; 83 (4): 285–91. [DOI] [PubMed] [Google Scholar]
- 10. van der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006; 13 (2): 138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weingart SN, Toth M, Sands DZ, et al. Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med 2003; 163 (21): 2625–31. [DOI] [PubMed] [Google Scholar]
- 12. Powers EM, Shiffman RN, Melnick ER, et al. Efficacy and unintended consequences of hard-stop alerts in electronic health record systems: a systematic review. J Am Med Inform Assoc 2018; 25 (11): 1556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horn J, Ueng S.. The effect of patient-specific drug-drug interaction alerting on the frequency of alerts: a pilot study. Ann Pharmacother 2019; 53 (11): 1087–92. [DOI] [PubMed] [Google Scholar]
- 14. Olakotan OO, Yusof MM.. Evaluating the alert appropriateness of clinical decision support systems in supporting clinical workflow. J Biomed Inform 2020; 106: 103453. [DOI] [PubMed] [Google Scholar]
- 15. Paterno MD, Maviglia SM, Gorman PN, et al. Tiering drug-drug interaction alerts by severity increases compliance rates. J Am Med Inform Assoc 2009; 16 (1): 40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniels CC, Burlison JD, Baker DK, et al. Optimizing drug-drug interaction alerts using a multidimensional approach. Pediatrics 2019; 143 (3): e20174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parke C, Santiago E, Zussy B, et al. Reduction of clinical support warnings through recategorization of severity levels. Am J Health Syst Pharm 2015; 72 (2): 144–8. [DOI] [PubMed] [Google Scholar]
- 18. Kane-Gill SL, O’Connor MF, Rothschild JM, et al. Technologic distractions (part 1): summary of approaches to manage alert quantity with intent to reduce alert fatigue and suggestions for alert fatigue metrics. Crit Care Med 2017; 45 (9): 1481–8. [DOI] [PubMed] [Google Scholar]
- 19. McCoy AB, Russo EM, Johnson KB, et al. Clinician collaboration to improve clinical decision support: the Clickbusters initiative. J Am Med Inform Assoc 2022; 29 (6): 1050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Croskerry P. From mindless to mindful practice – cognitive bias and clinical decision making. N Engl J Med 2013; 368 (26): 2445–8. [DOI] [PubMed] [Google Scholar]
- 21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf 2017; 26 (2): 87–9. [DOI] [PubMed] [Google Scholar]
- 22. Liu S, Kawamoto K, Del Fiol G, et al. The potential for leveraging machine learning to filter medication alerts. J Am Med Informatics Assoc 2022; 29 (5): 891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reese T, Wright A, Liu S, et al. Improving the specificity of drug-drug interaction alerts: can it be done? Am J Health Syst Pharm 2022; 79 (13): 1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ChatGPT: Optimizing Language Models for Dialogue. https://openai.com/blog/chatgpt/. Accessed December 25, 2022.
- 25. Kung TH, Cheatham M, Medenilla A, et al. Performance of ChatGPT on USMLE: potential for AI-assisted medical education using large language models. PLOS Digit Health 2023; 2 (2): e0000198. doi: 10.1101/2022.12.19.22283643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeblick K, Schachtner B, Dexl J, et al. ChatGPT makes medicine easy to swallow: an exploratory case study on simplified radiology reports. Published Online First: 30 December 2022. http://commoncrawl.org/. Accessed January 30, 2023. [DOI] [PMC free article] [PubMed]
- 27. Brown TB, Mann B, Ryder N, et al. Language models are few-shot learners. Published Online First: 28 May 2020. http://arxiv.org/abs/2005.14165. Accessed January 30, 2023.
- 28. Friedman CP. A ‘fundamental theorem’ of biomedical informatics. J Am Med Inform Assoc 2009; 16 (2): 169–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ye C, Coco J, Epishova A, et al. A crowdsourcing framework for medical data sets. Proc AMIA Jt Summits Transl Sci 2018; 2017: 273–80. [PMC free article] [PubMed] [Google Scholar]
- 30. Maynez J, Narayan S, Bohnet B, et al. On faithfulness and factuality in abstractive summarization. In: Proceedings of the 58th Annual Meeting of the Association for Computational Linguistics. Stroudsburg, PA: Association for Computational Linguistics; 2020: 1906–19. doi: 10.18653/v1/2020.acl-main.173. [DOI]
- 31. Willits F, Theodori G, Luloff A.. Another look at Likert scales. J Rural Soc Sci 2016; 31: 126–39. https://egrove.olemiss.edu/jrss/vol31/iss3/6. Accessed March 23, 2023. [Google Scholar]
- 32. de Winter JCF, Dodou D.. Five-point Likert items: t test versus Mann-Whitney-Wilcoxon. Pract Assess Res Eval 2010; 15: 11. [Google Scholar]
- 33. Shou Y, Sellbom M, Chen H-F.. Fundamentals of measurement in clinical psychology. In: Asmundson GJG, ed. Comprehensive Clinical Psychology. Amsterdam, The Netherlands: Elsevier; 2022: 13–35. doi: 10.1016/B978-0-12-818697-8.00110-2 [DOI] [Google Scholar]
- 34. Koo TK, Li MY.. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15 (2): 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2006; 27 (2): 237–46. [Google Scholar]
- 36. Yang X, Chen A, PourNejatian N, et al. A large language model for electronic health records. NPJ Digit Med 2022; 5 (1): 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo R, Sun L, Xia Y, et al. BioGPT: generative pre-trained transformer for biomedical text generation and mining. Brief Bioinform 2022; 23 (6): 1–11. [DOI] [PubMed] [Google Scholar]
- 38. Ouyang L, Wu J, Jiang X, et al. Training language models to follow instructions with human feedback. Published Online First: 4 March 2022. http://arxiv.org/abs/2203.02155. Accessed January 30, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The input prompts and AI outputs are available in the Supplementary Appendix.