Dear Editor,
The epicardial adipose tissue (EAT) is a visceral fat deposit that covers up to 80% of the heart surface and can constitute up to 20% of total cardiac mass. The EAT and myocardium both originate from the splanchnic mesoderm, and due to their shared embryology have a common blood supply with no fascia separating the EAT from the myocardium. In terms of blood supply, ontogenetic origin and transcriptome, EAT differs from other visceral and subcutaneous fat depots [1]. As a result of approximation and shared blood supply, the EAT can have a direct (bidirectional) paracrine effect on the myocardium potentially influencing cardiomyocyte function. The EAT is a dynamic and anatomically heterogeneous organ secreting various hormones, growth factors, microRNAs (miRNAs), cytokines, chemokines and extracellular vesicles invoking autocrine, endocrine, and paracrine actions on the neighboring myocardium. In healthy conditions the EAT serves as a local energy source releasing fatty acids to the myocardium, has diverse actions in comparison to subcutaneous adipose tissue and plays an important role during embryogenesis. More than 400 genes have been identified unique to EAT and functional analysis of EAT reveal transcriptomic signatures involving (1) extracellular matrix remodeling, (2) thrombosis and (3) inflammation. In diseases associated with HFpEF, such as diabetes, coronary artery disease, atrial fibrillation, and obesity, EAT volume increase and the precise inflammatory properties of the EAT change. Epicardial fat samples in patients undergoing coronary artery bypass grafting illustrate increased levels of IL-1b, IL-6, monocyte chemotactic protein-1, and TNFa [2]. Similarly epicardial fat derived extracellular vesicles analysis in patients with atrial fibrillation, show elevated levels of pro-inflammatory and pro-fibrotic cytokines such as IL-1α, IL-6, TNF-α and IL-4 in comparison to patients without atrial fibrillation. Injection of extracellular vesicles from patients with atrial fibrillation into the left ventricle of mice induces pro-fibrotic myocardial alterations reminiscent of HFpEF [3]. Numerous studies have shown that HFpEF patients have higher EAT volumes in comparison to weight and body mass index matched individuals [4,5]. These studies underscore that not subcutaneous fat but EAT is associated with the development of HFpEF [6]. Next to altering the loco-regional inflammatory portrait, increased EAT enhances the cardio-mechanical pericardial restrain, hereby aggravating diastolic dysfunction through enhanced diastolic ventricular interaction [7]. Indeed, hemodynamic evaluation during exercise shows that increased EAT is associated with more profound hemodynamic derangements, including greater elevation in cardiac filling pressures, more pronounced elevations in pulmonary pressures, and greater pericardial restraint, culminating in poorer exercise capacity (Fig. 1).
Fig. 1.
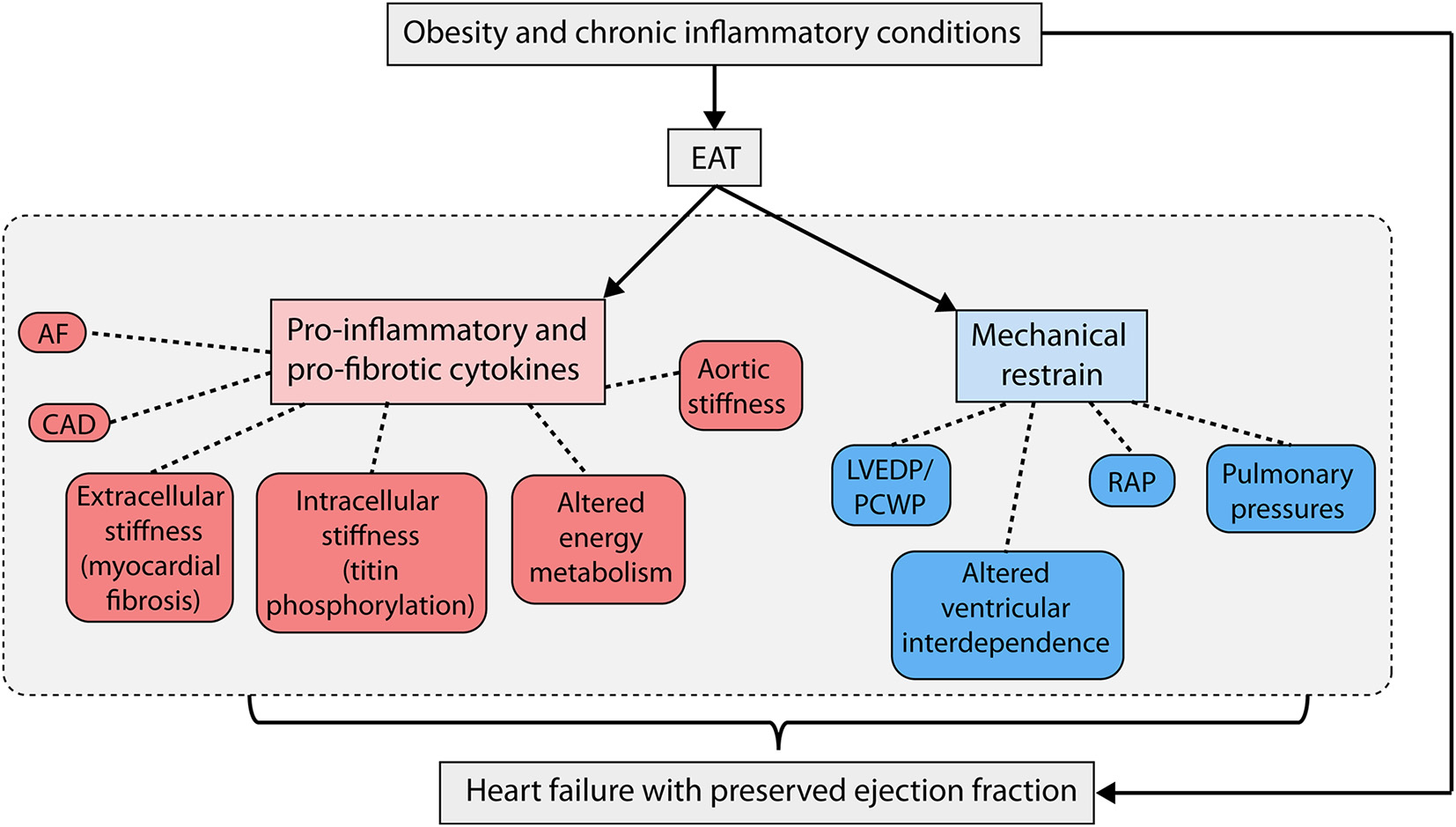
Overview of relation between EAT and HFpEF (Abbreviations: EAT - epicardial adipose tissue; HFpEF = heart failure with preserved ejection fraction; AF = atrial fibrillation; CAD = coronary artery disease; LVEDP = left ventricular end-diastolic pressure; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure.
Given these aggravating metabolic, inflammatory and mechanical properties of EAT, it begs the question whether: (1) Is EAT an independent driver of disease and not just a marker of an overall different metabolic state and (2) Can therapeutically targeting of the EAT potentially benefit patients with HFpEF. While certain inflammatory condition such as nonalcoholic fatty liver disease (NAFLD) might be associated with heart failure, there is currently no direct evidence to support that selective inducement of NAFLD also induces heart failure. Although injections of extracellular vesicles of the pathologic EAT into the myocardium may induce myocardial changes reminiscent of HFpEF, it is unclear as to whether the EAT in that setting is just a barometer of a metabolic state, or if selective intervention on the EAT could also lessen myocardial alterations. Clearly more selective EAT interventional studies are necessary. Interestingly, both exercise training and sodium glucose linked transporter 2 inhibitors (SGLT2-i) can improve the disease severity in HFpEF [8,9], but have also been shown to reduce EAT. The effect of other agents on EAT in HFpEF are unknown. For instance, glucagon-like peptide 1 receptor agonist can induce significant weight reduction and EAT expresses glucagon-like peptide 1 receptor (GLP-1R) to a higher extent than subcutaneous fat. Yet the effect of GLP-1R agonist on EAT remains conflicting. Metabolic surgery including gastric bypass and sleeve gastrectomy can have a profound sustained effect on reducing the EAT volume, which at the level of the myocardium are paralleled by a reduction in left ventricular mass, a reduction in intra-myocardial fat deposits and enhanced cardiac metabolism of branched chain amino acids, suggestive of enhanced TCA-cycle influx and reduced lipotoxicity [10]. Clearly ongoing studies are necessary to determine how certain therapies that affect EAT (such as exercise training, SGLT2-I and metabolic surgery) can affect metabolic and inflammatory alterations in the HFpEF myocardium. Additionally, these studies question whether direct targeting of the EAT secretome can alter the development of HFpEF or associated conditions such as atrial fibrillation.
Footnotes
Disclosure
Dr. Martens has received consultancy fees from AstraZeneca, Abbott, Bayer, Boehringer-Ingelheim, Daiichi Sankyo, Novartis, Novo Nordisk and Vifor pharma – all unrelated to the subject and contents of this paper. Dr. Tang is a consultant for Sequana Medical A.V., Cardiol Therapeutics Inc., Genomics plc, Zehna Therapeutics Inc., and has received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation.
References
- [1].Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, et al. , Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source, Nat. Cell Biol. 16 (4) (2014. Apr) 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. , Human epicardial adipose tissue is a source of inflammatory mediators, Circulation 108 (20) (2003. Nov 18) 2460–2466. [DOI] [PubMed] [Google Scholar]
- [3].Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, et al. , Extracellular vesicles from epicardial fat facilitate atrial fibrillation, Circulation 143 (25) (2021. Jun 22) 2475–2493. [DOI] [PubMed] [Google Scholar]
- [4].Gorter TM van WG, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M, Epicardial fat in heart failure patients with mid-range and preserved ejection fraction, Eur. J. Heart Fail 20 (11) (2018. Nov) 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu CK, Lee JK, Hsu JC, Su MM, Wu YF, Lin TT, et al. , Myocardial adipose deposition and the development of heart failure with preserved ejection fraction, Eur. J. Heart Fail 22 (3) (2020. Mar) 445–454. [DOI] [PubMed] [Google Scholar]
- [6].Lin HH, Lee JK, Yang CY, Lien YC, Huang JW, Wu CK, Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis, Cardiovasc. Diabetol. (12) (2013. Aug 30) 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mullens W, Martens P, Empagliflozin-induced changes in epicardial fat: the centerpiece for myocardial protection? JACC Heart Fail. 9 (8) (2021. Aug) 590–593. [DOI] [PubMed] [Google Scholar]
- [8].Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. , Empagliflozin in heart failure with a preserved ejection fraction, N. Engl. J. Med. 385 (16) (2021. Oct 14) 1451–1461. [DOI] [PubMed] [Google Scholar]
- [9].Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, et al. , Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the ex-DHF (exercise training in diastolic heart failure) pilot study, J. Am. Coll. Cardiol. 58 (17) (2011. Oct 18) 1780–1791. [DOI] [PubMed] [Google Scholar]
- [10].Castagneto-Gissey L, Angelini G, Mingrone G, Cavarretta E, Tenori L, Licari C, et al. , The early reduction of left ventricular mass after sleeve gastrectomy depends on the fall of branched-chain amino acid circulating levels, EBioMedicine 76 (2022. Feb), 103864. [DOI] [PMC free article] [PubMed] [Google Scholar]


