Abstract
Purpose:
To compare the types and dosages of anti-vascular endothelial growth factors (VEGFs) to ascertain whether specific dosages or types of injection were associated with retreatment in clinical practice in the United States.
Design:
Multicenter, retrospective, consecutive series.
Participants:
Patients with retinopathy of prematurity (ROP) treated with anti-VEGF injections from 2007 to 2021.
Methods:
Sixteen sites from the United States participated. Data collected included demographics, birth characteristics, examination findings, type and dose of anti-VEGF treatment, retreatment rates, and time to retreatment. Comparisons of retreatment rates between bevacizumab and ranibizumab intravitreal injections were made.
Main Outcome Measures:
Relative rate of retreatment between varying types of anti-VEGF therapy, including bevacizumab and ranibizumab, and the various dosages used for each.
Results:
Data from 873 eyes of 661 patients (61% male and 39% female) were collected. After exclusion of 40 eyes treated with laser before anti-VEGF injection and 266 eyes re-treated with laser at or beyond 8 weeks after the initial anti-VEGF treatment, 567 eyes of 307 patients (63% male and 37% female) remained and were included in the primary analysis. There was no difference between the no retreatment and retreatment groups in terms of birthweight, gestational age, age at first injection, ROP stages, or number of involved clock hours. The retreatment group had a larger percentage of aggressive ROP (34% vs. 18%, P < 0.001) and greater percentage of zone 1 ROP (49 vs. 34%, P = 0.001) than the no retreatment group. Ranibizumab use was associated with a higher rate of retreatment than bevacizumab use (58% vs. 37%, P < 0.001), whereas the rate of retreatment was not associated with a specific dose of ranibizumab (R2 = 0.67, P = 0.32). Meanwhile, lower doses of bevacizumab were associated with higher rates of retreatment compared with the higher doses (R2 = 0.84, P = 0.01). There was a dose-specific trend with higher doses trending toward lower retreatments for bevacizumab.
Conclusions:
In a multicenter study of ROP patients initially treated with anti-VEGF therapy, ranibizumab and lower-dose bevacizumab use were associated with an increased rate of retreatment when compared with higher-dose bevacizumab.
Financial Disclosure(s):
Proprietary or commercial disclosure may be found after the references.
Keywords: Anti-VEGF, Retinopathy of prematurity, Re-treatment
Retinopathy of prematurity (ROP) is the leading cause of preventable blindness in children in the United States.1 Intravitreal injections of anti-vascular endothelial growth factor (VEGF), specifically bevacizumab and ranibizumab, have recently gained wider applicability in the treatment armamentarium for ROP.2 However, there are limited data regarding whether different types of anti-VEGF therapy or their dosages are associated with retreatments for ROP in the clinical setting.3,4 In this study, we compare the formulations and dosages of anti-VEGFs, particularly in regard to retreatment rates.
Methods
The Institutional Review Board at the University of Texas at Austin approved the study protocol. At the remaining centers, Institutional Review Board approvals and exemptions were given based on individual requirements. This included a data sharing agreement to send de-identified information.
The study was conducted according to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study. This is a multicenter, retrospective, consecutive series of patients with ROP treated with anti-VEGF, gathered from the ROP Injection Consortium (ROPIC). A total of 16 sites from the United States were included between 2007 and 2021. Sites were recruited individually based on a network of pediatric ophthalmologists and pediatric retina specialists. Inclusion criteria were patients diagnosed with ROP who were initially treated with anti-VEGF therapy. The decision to treat was at the discretion of the local physician. Exclusion criteria were patients treated with laser before anti-VEGF therapy. Additionally, for the primary analysis, after data collection and before analysis, patients retreated with laser after 8 weeks after the initial injection were excluded to remove likely prophylactic treatments. The cutoff for 8 weeks was chosen based on a survey of the study group.
Demographic data including gestational age, birth weight, and ROP severity were collected. Primary outcomes measure was the rate of retreatment. Comparison of continuous variables was performed using the Student t test and regression analysis, and categorical data were analyzed using the chi-square test. A P value of < 0.05 was used to determine statistical significance. Analysis and descriptive statistics were calculated using Microsoft Excel software. Secondary analyses were performed. The first entirely excluded laser only retreatments, and the second included all postinjection retreatments, including laser 8 weeks or later postinjection.
Results
Data from 873 eyes of 661 patients (61% male and 39% female) were collected. A total of 40 eyes were excluded for having been treated with laser before anti-VEGF injection. A further 266 eyes were excluded from the primary analysis for having been retreated with laser at or beyond 8 weeks after the initial anti-VEGF treatment. A total of 567 eyes of 307 patients remained (63% male and 37% female) and were included in the primary analysis from 16 sites in the United States. Average follow-up time was 16.4 months (range, 1 day to 7.9 years). The average time interval between first injection and retreatment was 41.4 days (range, 1–191 days). The average birthweight and gestational age were comparable between the no retreatment group (665.7 g and 24.6 weeks) and retreatment group (654.3 g and 24.4 weeks). Likewise, the average post-conceptual age of first injection was similar between groups, with an average of 36.3 weeks in the no retreatment group and 35.8 weeks in the retreatment group (P = 0.09). Both the no-retreatment and retreatment groups included more male participants (62% and 63%, respectively). In terms of ROP severity, the retreatment group had a larger percentage of aggressive ROP (34% vs. 18%, P < 0.001). The percentage of Plus disease was lower in the retreatment group compared with the no-retreatment group (80% vs. 89%, P = 0.01). The retreatment group had a greater percentage of zone 1 ROP compared with the no-retreatment group (49% vs. 34%, P = 0.001), respectively. Both retreatment and no-retreatment groups had similar rates of ROP stages, with stage 3 ROP observed in 85% and 82% of the eyes, respectively (P = 0.36). Likewise, there was no difference in clock hours between the groups, 7.8 versus 8.5 in the no-retreatment and retreatment groups, respectively (P = 0.11).
Ranibizumab use was associated with higher rates of retreatment compared with bevacizumab (58% vs. 37%, P < 0.001). Retreatment rate for ranibizumab was not associated with the specific dose of the therapy (R2 = 0.67, P = 0.32). Meanwhile, lower doses of bevacizumab were associated with higher rates of retreatment compared with higher doses (R2 = .84, P = 0.01). There was a dose-specific trend with higher doses trending toward lower retreatments for bevacizum (Table 1 and Fig 1).
Table 1.
Comparison of Patients’ Characteristics, Disease Severity, and Doses of Bevacizumab and Ranibizumab Injections between the No-Retreatment and Retreatment Groups
| No Retreatments (%) | All Retreatments (%) | P Value | |
|---|---|---|---|
| Eyes | 337 | 230 | |
| Gender | |||
| Male (%) | 153 (62) | 112 (63) | 0.82 |
| Female (%) | 93 (38) | 65 (37) | |
| Mean gestational age at birth (wks) | 24.6 | 24.4 | 0.05 |
| Mean birth weight (g) | 665.7 | 654.3 | 0.53 |
| Mean postconceptual age at first injection (wks) | 36.3 | 35.8 | 0.09 |
| Zone of ROP (most posterior) | |||
| 1 (%) | 114 (34) | 106 (49) | 0.001* |
| 2 (%) | 213 (64) | 110 (51) | 0.002* |
| 3 (%) | 5 (2) | 0 (0) | |
| Stage of ROP at injection | |||
| 1 (%) | 5 (1.5) | 8 (3.7) | 0.1 |
| 2 (%) | 53 (15.9) | 18 (8.4) | 0.01* |
| 3 (%) | 273 (81.7) | 182 (84.7) | 0.36 |
| 4 (%) | 2 (0.6) | 5 (2.3) | 0.08 |
| 5 (%) | 1 (0.3) | 2 (0.9) | |
| Average total clock hours | 7.8 | 8.5 | 0.11 |
| Plus disease (%) | 293 (89) | 170 (80) | 0.01* |
| Aggressive ROP (%) Anti-VEGF injection | 59 (18) | 72 (34) | <0.001* |
| Bevacizumab (%) | 296 (64) | 170 (37) | <0.001* |
| Ranibizumab (%) | 41 (42) | 56 (58) | |
| Bevacizumab (Avastin) dose (mg) | |||
| 1.25 | 5 (83) | 1 (17) | |
| 0.75 | 41 (71) | 17 (29) | |
| 0.625 | 214 (69) | 95 (31) | |
| 0.5 | 25 (68) | 12 (32) | |
| 0.3125 | 0 (0) | 0 (0) | |
| 0.25 | 0 (0) | 0 (0) | |
| 0.125 | 4 (24) | 13 (76) | |
| 0.03 | 7 (18) | 32 (82) | |
| Ranibizumab (Lucentis) dose (mg) | |||
| 0.3 | 2 (100) | 0 (0) | |
| 0.25 | 7 (26) | 20 (74) | |
| 0.2 | 2 (33) | 4 (67) | |
| 0.15 | 30 (48) | 32 (52) |
ROP = retinopathy of prematurity; VEGF = vascular endothelial growth factor.
Statistically significant, P < 0.05.
Figure 1.
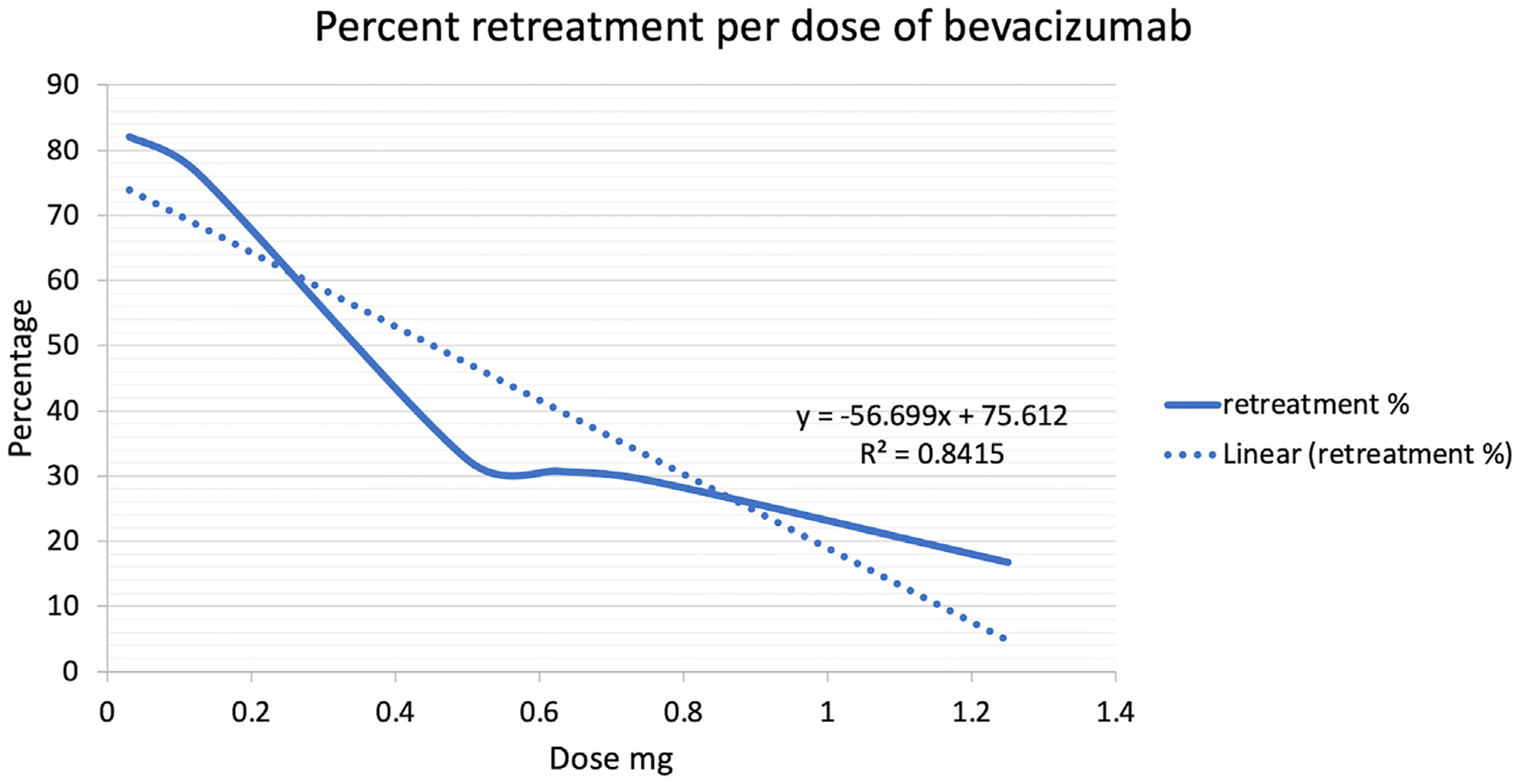
Percentage of retreatment per dose of bevacizumab. There was a dose-specific trend in terms of retreatment, with lower bevacizumab doses associated with higher retreatment rates (R2 = 0.84, P = 0.01).
Secondary Analysis: Injection-Only Retreatments
When excluding laser retreatments, a total of 422 eyes remained. There was no difference between the no treatment and retreatment groups in terms of birth weight (665.7 vs. 673.3 g, P = 0.67), gestational age (24.6 vs. 24.3 weeks, P = 0.07), ROP zones, or presence of Plus disease (89% vs. 85%, P = 0.47), respectively. Aggressive ROP was more prevalent in the retreatment than in the no-retreatment group (36% vs. 18%, P = 0.001). Ranibizumab use was similarly associated with higher rates of retreatment compared with bevacizumab (42% vs. 15%, P = 0.001). There was no difference in terms of retreatment rates between the bevacizumab dosages (R2 = 0.75, P = 0.16). Sufficient data were not available to compare specific ranibizumab dosages. The average time to retreatment was 67.4 days (range, 26–148) with reinjection for eyes primarily treated with ranibizumab and 75 days (range, 5–191) for bevacizumab.
Secondary Analysis: Including All Laser and Injection Retreatments
When including all laser and injection retreatments, a total of 873 eyes of 661 patients were analyzed. There was no difference between the no-treatment and retreatment groups in terms of birth weight (667.6 vs. 664.2 g, P = 0.81), gestational age (24.6 vs. 24.5 weeks, P = 0.21), ROP zones, stages, or presence of Plus disease (88% vs. 89%, P = 0.86). Aggressive ROP was more prevalent in the re-treatment versus the no-retreatment group (31.5% vs. 18.8%, P < 0.001). The number of involved clock hours was higher in the retreatment group (10.1) compared with the no-retreatment group (7.6) (P < 0.001). Ranibizumab use was similarly associated with higher rates of retreatment compared with bevacizumab (83% vs. 47%, P < 0.001). The rates of retreatments in the 3 groups analyzed are summarized in Table 2.
Table 2.
Comparison of the Retreatment Rates between Bevacizumab and Ranibizumab Injections
| Rate of Retreatment | ||||
|---|---|---|---|---|
| Eyes | Ranibizumab | Bevacizumab | P Value | |
| Analysis | ||||
| All laser and injection retreatments | 873 | 83% | 47% | < 0.001 |
| Laser within 8 wks and injection retreatments* | 567 | 58% | 37% | < 0.001 |
| Laser within 8 wks and injection retreatments† | 307 | 58% | 37% | < 0.001 |
| Injection only retreatments | 422 | 42% | 15% | 0.001 |
Primary analysis.
Analysis excluding second eyes of bilateral cases.
Secondary Analysis: Correction for Bilateral Correlations
To correct for correlations across eyes, we performed 2 subgroup analyses: the first one included all eyes of unilateral cases and the right eyes of bilateral cases (307 eyes, 289 right eyes and 18 left eyes), whereas the second one included all eyes of unilateral cases and the left eyes of bilateral cases (307 eyes, 30 right eyes and 277 left eyes). Both subgroup analyses demonstrated a similar statistically higher rate of retreatment with ranibizumab compared with bevacizumab (58% vs. 37%, P < 0.001).
Discussion
The present study is the largest database in the United States on retreatment outcomes after intravitreal anti-VEGF injections in neonates with ROP. We report data of 567 eyes with ROP from 16 eye centers in the United States over a period of 14 years. Our major findings include (1) ranibizumab use was associated with a greater rate of retreatment than bevacizumab; (2) lower dosages of bevacizumab were associated with greater rates of retreatments compared with higher doses; and (3) the specific dose of ranibizumab was not associated with rate of retreatment.
There is known global variation in the demographics of ROP neonates, associated treatment practice patterns, and neonatal care5. Because of this, the study was limited to the United States. Resultingly, the retreatment and no-retreatment groups were comparable with regard to birthweight, gestational age, and postconception age at first injection, thus reducing the potential for confounding bias. No difference was found in ROP stages or clock hours involved between the 2 groups. As might be expected, the retreatment group had a larger percentage of aggressive ROP and greater percentage of zone 1 involvement.
It has been considered previously that ROP patients treated with injection, especially in the United States, may have subsequent laser to the avascular retina with an examination under anesthesia at approximately 60 weeks postconception.5 The cause for this is multifactorial: the patient becomes difficult to examine in clinic, the risk for complications or overnight admission is reduced, and laser treatment alleviates the need for close follow-up or repeated angiograms. A novel finding from the prior ROPIC report was the substantial rate of laser after intravitreal anti-VEGF injection in a clinical setting, particularly in the United States.5 Much of this was found to be prophylactic based on a follow-up survey for the study group.5 Thus, after consultation with the study group, a postinjection 8-week maximum cutoff timeframe was established before which laser photocoagulation would likely be considered a true retreatment for the primary analysis.
Regarding the primary outcome, type of medication, ranibizumab use was associated with higher rates of retreatment than bevacizumab (58% vs. 37%). This was true in both the secondary analyses when investigating injection only retreatments as well as the pooled data with all laser and injection retreatments. There has been literature reporting a trend toward higher, but not significant, reactivation rate after ranibizumab use compared with bevacizumab.6,7 Prior literature on retreatment rate is variable between these 2 agents, ranging from 20.9% to 64% with ranibizumab6,8 and 6% to 18% with bevacizumab.4,9 In a smaller recent study, Süren et al3 similarly demonstrated a higher retreatment rate with ranibizumab than bevacizumab (P < 0.05). Moreover, disease reactivation was found to be more frequent after ranibizumab use compared with bevacizumab (37% vs. 60%, P = 0.023).10 However, these reports were all limited by their small sample size, and none were conducted in the United States. This is the largest study to demonstrate a higher retreatment rate after ranibizumab compared with bevacizumab injection.
In terms of dosages, there has been an effort, due to the concern of systemic absorption, to find the minimal effective quantity. However, in this study, there was a trend for lower doses of bevacizumab to be associated with retreatment. Moreover, it follows a dose—eresponse relationship (Fig 1). These findings are consistent with previous smaller studies of assessing varying amounts of bevacizumab that showed a need for additional treatment in some cases yet concluded the lower doses were effective.4,11,12 Conversely, a lower dose of ranibizumab was not associated with inferior outcomes compared with higher dosing. Recent trials have likewise demonstrated a similar retreatment rate between 0.1 mg and 0.2 mg doses of ranibizumab.13–15 In a recent post hoc analysis of the RAINBOW trial, rates of additional treatment for incomplete disease regression (6% vs. 8%) and for disease reactivation (17% vs. 15%) were similar between 0.1 mg and 0.2 mg doses of ranibizumab, respectively.15
Overall, higher doses of bevacizumab appear to regress ROP and decrease the need for retreatments compared with lower doses of bevacizumab or any dose of ranibizumab. This observation could be explained by the shorter ocular half-life of ranibizumab16 and its more rapid clearing from the vitreous.17 However, it is important to caution that long-term effects of anti-VEGF on organ development are unknown and higher doses of anti-VEGF could potentially lead to altered organ maturation.
Study Limitations
A limitation is the inability to ascertain the intention for subsequent laser treatments. A review of individual records could not determine this accurately in many cases. In some instances, there may be treatment partially for prophylaxis and treatment partially for disease reactivation in the same eye. To account for the variability, 3 separate analyses were performed: one excluding all laser retreatments, one excluding laser retreatments 8 weeks postinjection, and one with no exclusions for laser retreatment. The conclusions were similar in all 3 of these groups. Data from the ROPIC have now identified this practice pattern of prophylactic laser for future prospective studies to consider.
Although a prospective study of this scale may not be feasible, a limitation of the study is its retrospective nature. Additionally, because this was a multicenter study, there was reliance on the individual sites to collect and document the data based on the requirements. There could be deviances made that would not be known to the collecting group. These are the largest data on retreatment outcomes after the use of bevacizumab and ranibizumab, the most frequently anti-VEGF used, yet other anti-VEGF medications, such as aflibercept, were not included in the analysis because of the limited number of corresponding cases in our database.
Given the global variations in neonatal care, the particular rates of retreatments may not be generalizable; however, the medication type and dose effect would be expected to be maintained if the study was repeated internationally.
Conclusions
In a multicenter retrospective study of ROP patients initially treated with anti-VEGF therapy, ranibizumab and lower-dose bevacizumab use were associated with an increased rate of retreatment when compared with higher-dose bevacizumab.
Pictures & Perspectives
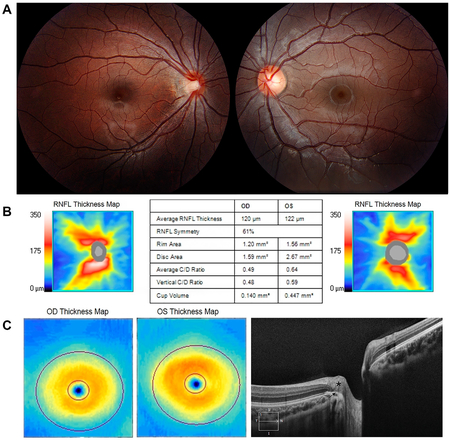
The Pseudo-Hypoplastic Myopic Optic Disc
A 7-year-old girl had 20/25 vision in the right eye (OD) (with correction) and 20/20 vision in the left eye (OS), with no afferent pupillary defect. Retinoscopy showed −4.75 OD and plano OS. Retinal examination showed a small tilted right optic disc, with a temporal crescent of retinal pigment epithelium (RPE) and choroid pierced inferotemporally by a retinal vein (A). OCT showed a smaller right disc with a smaller neuroretinal rim and cup volume (B), but equal retinal nerve fiber layer (RNFL) (B) and ganglion cell thickness (bottom left). The thickened, retruded, temporal RNFL (C, asterisk) abutted the overhanging RPE (C, arrow). The myopic optic disc may simulate optic nerve hypoplasia both clinically and on OCT measurement (Magnified version of Fig A-C is available online at www.aaojournal.org).
Michael C. Brodsky, MD
Departments of Ophthalmology and Neurology, Mayo Clinic, Rochester, Minnesota
Footnotes and Disclosures
Supported in part by Mayo Clinic, Rochester, Minnesota. The funding organization had no role in the design or conduct of this research.
Financial Support:
Retina Innovation Fund. The funding organization had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- ROP
retinopathy of prematurity
- ROPIC
ROP Injection Consortium
- VEGF
vascular endothelial growth factor
Footnotes
Supplemental material available at www.aaojourmal.org.
Presented at: the American Academy of Ophthalmology, September 30 - October 3, 2022, Chicago, Illinois; and the Retina Society annual meetings November 2–5, 2022, Pasadena, California.
* A list of members of the Retinopathy of Prematurity Injection Consortium (ROPIC) Study Group appear online (www.aaojournal.org).
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): N.A.P.: Consultant – Atheneum, Alcon, Allergan, Alimera, Eyepoint, Lifesciences, Guidepoint, Gerson Lehrman Group, Inc.
C.R.B.: Consultant – Genentech, Regeneron; Payment for lectures – Genentech
C.A.H.: Consultant – Regeneron
A.M.B.: Consultant – Alcon, Dutch Ophthalmic Research Center, Allergan, Bayer, Visionex, Oculus, Applied Genetic Technologies Corporation, PROQR Therapeutics, Aerie, Regenxbio, Novartis
HUMAN SUBJECTS: Human subjects were included in this study. The institutional review board from the University of Texas at Austin approved the study protocol. At the remaining centers, Institutional Review Board approvals and exemptions were given based on individual requirements. This included a data sharing agreement to send de-identified information. The study was conducted according to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
No animal subjects were used in this study.
References
- 1.Ludwig CA, Chen TA, Hernandez-Boussard T, et al. The epidemiology of retinopathy of prematurity in the United States. Ophthalmic Surg Lasers Imaging Retina. 2017;48: 553–562. [DOI] [PubMed] [Google Scholar]
- 2.Hartnett ME. Retinopathy of prematurity: evolving treatment with anti-vascular endothelial growth factor. Am J Ophthalmol. 2020;218:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Süren E, Özkaya D, Çetinkaya E, et al. Comparison of bevacizumab, ranibizumab and aflibercept in retinopathy of prematurity treatment. Int Ophthalmol. 2022;42:1905–1913. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DK, Dean TW, Hartnett ME, et al. A dosing study of bevacizumab for retinopathy of prematurity: late recurrences and additional treatments. Ophthalmology. 2018;125:1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel NA, Acaba-Berrocal LA, Hoyek S, et al. Practice patterns and outcomes of intravitreal anti-VEGF injection for retinopathy of prematurity: an international multicenter study. Ophthalmology. 2022;129:1380–1388. [DOI] [PubMed] [Google Scholar]
- 6.Iwahashi C, Utamura S, Kuniyoshi K, et al. Factors associated with reactivation after intravitreal bevacizumab or ranibizumab therapy in infants with retinopathy of prematurity. Retina. 2021;41:2261–2268. [DOI] [PubMed] [Google Scholar]
- 7.Ling KP, Liao PJ, Wang NK, et al. Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina. 2020;40:1793–1803. [DOI] [PubMed] [Google Scholar]
- 8.Lyu J, Zhang Q, Chen CL, et al. Recurrence of retinopathy of prematurity after intravitreal ranibizumab monotherapy: timing and risk factors. Invest Ophthalmol Vis Sci. 2017;58: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 9.Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364: 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alyamaç Sukgen E, Çömez A, Koçluk Y, Cevher S. The process of retinal vascularization after anti-VEGF treatment in retinopathy of prematurity: a comparison study between ranibizumab and bevacizumab. Ophthalmologica. 2016;236:139–147. [DOI] [PubMed] [Google Scholar]
- 11.Hillier RJ, Connor AJ, Shafiq AE. Ultra-low-dose intravitreal bevacizumab for the treatment of retinopathy of prematurity: a case series. Br J Ophthalmol. 2018;102:260–264. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Reynolds AL, Beiter A, et al. Effect of low-dose intravitreal bevacizumab and ranibizumab on regression and late reactivation in retinopathy of prematurity in the treatment-naïve eyes. Ophthalmol Retina. 2022;6:328–330. [DOI] [PubMed] [Google Scholar]
- 13.Stahl A, Krohne TU, Eter N, et al. Comparing alternative ranibizumab dosages for safety and efficacy in retinopathy of prematurity: a randomized clinical trial. JAMA Pediatr. 2018;172:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl A, Lepore D, Fielder A, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label rand-omised controlled trial. Lancet Lond Engl. 2019;394: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 15.Fleck BW, Reynolds JD, Zhu Q, et al. Time course of retinopathy of prematurity regression and reactivation after treatment with ranibizumab or laser in the RAINBOW Trial. Ophthalmol Retina. 2022;6:628–637. [DOI] [PubMed] [Google Scholar]
- 16.Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012;154: 682–686.e2. [DOI] [PubMed] [Google Scholar]
- 17.Wu WC, Shih CP, Lien R, et al. Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina. 2017;37:694–701. [DOI] [PubMed] [Google Scholar]


