Abstract
Background
Diabetic ketoacidosis (DKA) is associated with a high mortality rate, especially if cerebral edema develops during the disease course. It is rarer and more severe in adults than in children. We present cases of two patients with cerebral edema‐related DKA.
Case presentation
The first patient was a 38‐year‐old man with diabetes mellitus who presented with DKA‐related disturbed consciousness. Although glycemic correction was performed slowly, he showed pupil dilation 11 h later. He underwent emergency ventricular drainage, but died of cerebral herniation. The second patient was a 25‐year‐old woman who presented with impaired consciousness secondary to DKA. Head computed tomography showed subarachnoid hemorrhage and cerebral edema. No related intraoperative findings were observed; it was concluded that the first computed tomography scan revealed pseudo‐subarachnoid hemorrhage.
Conclusion
Diabetic ketoacidosis‐related cerebral edema develops despite treatment according to guidelines and is difficult to predict. Therefore, adult patients should be treated cautiously during DKA management.
Keywords: cerebral edema, consciousness, diabetes, diabetic ketoacidosis, DKA
A 38‐year‐old man presented with unconsciousness due to diabetic ketoacidosis. The patient showed bilateral pupil dilation, and computed tomography scan showed prominent cerebral edema 9 h after admission. Ventricular drainage was carried out and spinal fluid was drained 12 h after admission.
INTRODUCTION
Diabetic ketoacidosis (DKA) is characterized by hyperglycemia, metabolic acidosis, and hyperketonemia. Besides hyperosmolar hyperglycemic syndrome, it is the most serious acute metabolic complication of diabetes mellitus. Diabetic ketoacidosis develops mainly in young people with type 1 diabetes and is triggered by infection, insulin deficiency, and new‐onset diabetes. The early symptoms of the disease include polydipsia, polyuria, and weight loss. As the disease progresses, nausea, vomiting, abdominal pain, and hyperventilation develop. Furthermore, as blood glucose levels increase, serum osmolality increases and acidosis progresses, resulting in neurological deterioration, such as mental confusion and coma. Cerebral edema is the most serious complication of DKA, with a mortality rate of 20–40%. 1 It develops in up to 1% of pediatric DKA cases in individuals younger than 20 years of age. However, little is known about DKA in adults. Here, we report two cases of DKA‐induced cerebral edema.
CASE REPORTS
Case 1
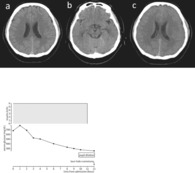
A 38‐year‐old man with type 2 diabetes mellitus presented with fever, vomiting, and dry mouth for several days and was brought to the emergency department due to unconsciousness. On admission, his Glasgow Coma Scale score (level of consciousness) was 3 (E1V1M1), pupil diameter was 2 mm, light reflex was prompt, heart rate was 166 b.p.m., blood pressure was 130/96 mmHg, body temperature was 35.5°C, and peripheral oxygen saturation was 95% on room air. Laboratory examination findings were as follows: pH 7.194, base excess –20 mEq/L, anion gap 22 mEq/L, blood glucose, C‐reactive protein, and procalcitonin levels, 681 mg/dL, 41.9 mg/dL, and >100 ng/mL, respectively, white blood cell count 19,200/μL, and positive urine ketones. Abdominal computed tomography (CT) showed ring enhancement in the S2 region of the liver. These findings led to a diagnosis of DKA due to a liver abscess. An initial head CT scan did not reveal any abnormalities (Figure 1A,B). The patient was on mechanical ventilation and treated with 6 U/h of continuous i.v. insulin. Blood glucose levels decreased slowly; however, 11 h after the start of treatment, the patient's pupils were dilated and blood pressure decreased. Because head CT showed marked cerebral edema (Figure 1C), the patient underwent ventricular drainage and intracranial pressure (ICP) sensor placement urgently, resulting in an initial ICP of 90 mmHg. Postoperative CT showed the disappearance of the cerebral sulcus, slit‐like ventricles, and progressive cerebral edema. Treatment and serum glucose trends are shown in Figure 1D. The patient died on day 3.
FIGURE 1.
Case 1: A 38‐year‐old man presented with unconsciousness. (A) A brain computed tomography (CT) scan obtained on admission shows no brain edema. (B) A brain CT on admission shows no compression to the midbrain. (C) Eleven hours after admission, bilateral pupil dilation was noted. The CT scan shows prominent cerebral edema. (D) Change in serum glucose and insulin dosage.
Case 2
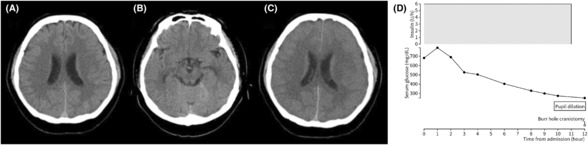
A 25‐year‐old woman without diabetes mellitus or history of trauma presented with vomiting and headache for 10 days prior to admission. The patient was transferred to the emergency department because of decreased consciousness. On admission, her level of consciousness was Glasgow Coma Scale E1V1M1 and both pupils were dilated. Laboratory examination findings were as follows: pH 6.945, blood glucose level 754 mg/dL, blood total ketone body 12649 μmol/L, and positive urine ketones. A head CT scan showed cerebral edema and a high‐density area in the subarachnoid and basal cistern (Figure 2A,B). Because the 3D CT angiogram showed a right middle cerebral artery aneurysm (Figure 2C), emergency craniotomy was performed to clip the aneurysm. No subarachnoid hemorrhage or aneurysm was noted intraoperatively in the middle cerebral artery; therefore, external decompression was carried out and ICP was monitored to treat the cerebral edema (Figure 2D,E). Blood glucose correction was faster than the rate recommended by the guidelines (Figure 2F). In addition, a cerebrospinal fluid examination revealed no findings indicating bacterial or viral meningitis. On postoperative day 7, the patient was extubated because her level of consciousness improved. The patient improved enough to be wheelchair‐bound and moved to another hospital for rehabilitation 28 days after the surgery. A magnetic resonance imaging scan on postoperative day 18 did not show the aneurysm, and the initial head CT showed pseudo‐subarachnoid hemorrhage due to cerebral edema and cerebral ischemia.
FIGURE 2.
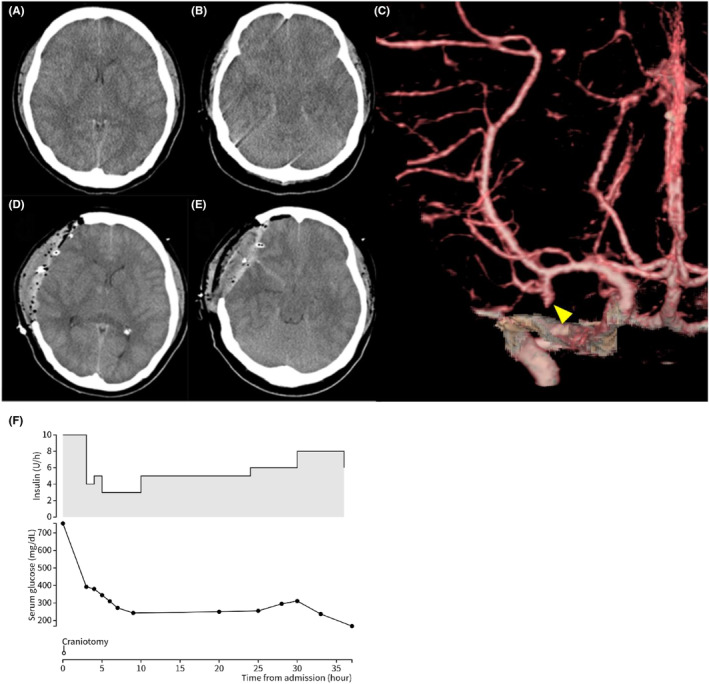
Case 2: A 25‐year‐old woman presented with impaired consciousness. (A, B) A brain computed tomography (CT) scan obtained on admission showing narrowed ventricles and an area of high density in the basal cistern, suggesting subarachnoid hemorrhage. (C) A 3D CT angiogram on admission shows a right middle cerebral artery aneurysm (yellow arrowhead). (D, E) Postoperative findings. Intraoperative brain edema was severe, and external decompression was carried out. (F) Change in serum glucose and insulin dosage.
DISCUSSION
Cerebral edema is a rare complication in adult patients with DKA and is associated with a high mortality rate. In a retrospective observational study of adults with DKA and hyperosmolar hyperglycemic syndrome, the mortality rate was more than 30 times higher in the group with cerebral edema than in the group without cerebral edema (35% vs. 1.1%, P < 0.01), 2 and cerebral edema was a poor prognostic complication. The risk factors for the development of cerebral edema in pediatric patients have been reported to include severe acidosis, high urea nitrogen levels, and hypocapnia. Cerebral edema could reflect the severity of DKA, as metabolic acidosis causes respiratory compensation that results in fast and deep breathing, called Kussmaul breathing, and low carbon dioxide. The osmotic pressure range between intravascular and brain cells during DKA treatment has also been proposed as a cause of cerebral edema. Diabetic ketoacidosis causes an increase in plasma osmolality, whereas osmolytes accumulate in brain cells, resulting in no osmotic pressure gap. Because rapid blood glucose correction causes a rapid decrease in plasma osmolality, time is required for intracellular osmolytes to disappear, allowing water to flow from outside the cell into the cell. Thus, cerebral edema develops. Pediatric guidelines recommend slowing the infusion rate (15‐20 mL/kg/h) in the first few hours for patients with high plasma osmolality and administering a sugar‐containing infusion at less than 200 mg/dL (11.1 mmol/L) of blood sugar to prevent cerebral edema. Although there is no consensus on the rate of glucose correction for adult patients with DKA, it is reasonable that blood glucose should be reduced to 50–75 mg/dL/h or less, in accordance with pediatric guidelines. However, there are reports that the rate of glucose correction, initial blood glucose level, plasma osmolality, and the extent to which plasma osmolality decreases during treatment do not affect cerebral edema. 3
Therefore, relative reductions in cerebral blood flow and inflammatory changes have also been hypothesized as causes of DKA‐related cerebral edema rather than the intravascular and brain cell osmotic pressure gap. Kiabetic ketoacidosis causes relative ischemia due to decreased cerebral blood flow as a result of hyperventilation, regardless of increased cerebral metabolism. The CT finding of pseudo‐subarachnoid hemorrhage can be caused by a variety of etiology including cerebral edema related to hypoxic‐ischemic brain injury, meningeal infection, cerebral/cerebellar infarction, or intra‐arterial contrast administration. Thus, pseudo‐subarachnoid hemorrhage could be seen in case 2 due to cerebral edema related to DKA. 4 Intravenous fluid administration initiated during treatment could cause brain damage due to reperfusion. 5 , 6 It is also thought that inflammatory mediators associated with DKA might damage the vascular endothelium and disrupt the blood–brain barrier and that neuroinflammation could cause brain damage. 7 It is assumed that the adult brain is less vulnerable to hypoxia due to hypoperfusion than the pediatric brain; thus, cerebral edema is less frequent in adults than in children.
A review of published reports on DKA‐related cerebral edema in adults was carried out using MEDLINE, Scopus, and Ichushi (a Japanese bibliographic database) from January 1980 to August 2023, and 11 cases along with the present case were found (Table 1). In five of these cases, the patients developed cerebral edema despite adherence to the guideline‐recommended rate of glycemic correction. Cerebral edema is rarely diagnosed at the time of admission and often develops hours to a day later. In some cases, there is no evidence of cerebral edema on CT at time of admission; however, rapid progression of cerebral edema could occur. There is no specific treatment for diabetes‐related cerebral edema. In our review, mannitol and hypertonic saline were found to be frequently administered. Hyperventilation should also be carefully avoided, because there have been reports of poor prognosis when the blood carbon dioxide level is too low after intubation. 8
TABLE 1.
Summary data about cases of cerebral edema associated with diabetic ketoacidosis
| Author (PMID) | Age (years), sex | Diabetes | pH | Glucose (md/dL) | BUN | Osmolarity | Rate of glucose correction | Time from admission to cerebral edema (h) | Outcomes at discharge (mRS) |
|---|---|---|---|---|---|---|---|---|---|
| Hiller (15915425) | 31, M | No | N/A | 439 | N/A | N/A | N/A | 2.25 | 0 |
| Kanazawa (N/A) | 48, M | Type 2 | 7.281 | 704 | 73.3 | 369.4 | N/A | 0 | 5 |
| Meaden (29755797) | 26, M | No | 7.140 | 650 | 42.0 | 325.0 | 73.40 | 6 | 6 |
| Patrik (16174971) | 27, M | No | 7.290 | 309 | N/A | N/A | 9.25 | N/A | 6 |
| Kabashneh (32742870) | 20, F | No | 6.910 | 2228 | 60.0 | 461.0 | 79.00 | 24–48 | 6 |
| Natarajan (32411486) | N/A, M | No | 6.860 | 694 | N/A | N/A | N/A | N/A | 6 |
| Fisken (10220211) | 21, F | Type 1 | 6.860 | 568 | N/A | 312.0 | 11.70 | 24–48 | 0 |
| Leary (16164879) | 26, F | Type 1 | 7.290 | 378 | 5.0 | 286.7 | 42.30 | 8 | 0 |
| Susman (N/A) | 27, M | N/A | N/A | 500 | N/A | N/A | N/A | N/A | 6 |
| Case 1 | 38, M | Type 2 | 7.194 | 681 | 69.2 | 306.0 | 41.00 | 12 | 6 |
| Case 2 | 25, F | No | 6.945 | 754 | 11.6 | N/A | 126.00 | 0 | 4 |
Abbreviations: BUN, blood urea nitrogen; F, female; M, male; mRS, modified Rankin score; N/A, not applicable.
CONCLUSION
Cerebral edema is a rare and serious complication in adult patients with DKA. Even if blood glucose correction and infusion are slow, cerebral edema can develop rapidly; therefore, care must be taken during the course of treatment.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol: N/A.
Informed consent: Written informed consent for the publication was obtained from the patient's family on behalf of the patient.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Namatame K, Igarashi Y, Nakae R, Suzuki G, Shiota K, Miyake N, et al. Cerebral edema associated with diabetic ketoacidosis: Two case reports. Acute Med Surg. 2023;10:e860. 10.1002/ams2.860
DATA AVAILABILITY STATEMENT
Research data are not shared because this article is case series.
REFERENCES
- 1. Wolfsdorf J, Glaser N, Sperling MA, American Diabetes Association . Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–9. [DOI] [PubMed] [Google Scholar]
- 2. Siwakoti K, Giri S, Kadaria D. Cerebral edema among adults with diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome: incidence, characteristics, and outcomes. J Diabetes. 2017;9:208–9. [DOI] [PubMed] [Google Scholar]
- 3. Platt A, Collins J, Ramos E, Goldenberg FD. Pseudosubarachnoid hemorrhage: a systematic review of causes, diagnostic modalities, and outcomes in patients who present with pseudosubarachnoid hemorrhage. Surg Neurol Int. 2021;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuppermann N, Ghetti S, Schunk JE, Stoner MJ, Rewers A, McManemy J, et al. Clinical trial of fluid infusion rates for pediatric diabetic ketoacidosis. N Engl J Med. 2018;378:2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glaser NS, Marcin JP, Wootton‐Gorges SL, Buonocore MH, Rewers A, Strain J, et al. Correlation of clinical and biochemical findings with diabetic ketoacidosis‐related cerebral edema in children using magnetic resonance diffusion‐weighted imaging. J Pediatr. 2008;153:541–6. [DOI] [PubMed] [Google Scholar]
- 6. Glaser NS, Tancredi DJ, Marcin JP, Caltagirone R, Lee Y, Murphy C, et al. Cerebral hyperemia measured with near infrared spectroscopy during treatment of diabetic ketoacidosis in children. J Pediatr. 2013;163:1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garro A, Chodobski A, Szmydynger‐Chodobska J, Shan R, Bialo SR, Bennett J, et al. Circulating matrix metalloproteinases in children with diabetic ketoacidosis. Pediatr Diabetes. 2017;18:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcin JP, Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis‐related cerebral edema. J Pediatr. 2002;141:793–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared because this article is case series.


