Abstract
Background
Valine-glutamine (VQ) proteins are non-specific plant proteins that have a highly conserved motif: FxxhVQxhTG. These proteins are involved in the development of various plant organs such as seeds, hypocotyls, flowers, leaves and also play a role in response to salt, drought and cold stresses. Despite their importance, there is limited information available on the evolutionary and structural characteristics of VQ family genes in Coix lacryma-jobi.
Results
In this study, a total of 31 VQ genes were identified from the coix genome and classified into seven subgroups (I–VII) based on phylogenetic analysis. These genes were found to be unevenly distributed on 10 chromosomes. Gene structure analysis revealed that these genes had a similar type of structure within each subfamily. Moreover, 27 of ClVQ genes were found to have no introns. Conserved domain and multiple sequence alignment analysis revealed the presence of a highly conserved sequences in the ClVQ protein. This research utilized quantitative real-time PCR (qRT-PCR) and promoter analysis to investigate the expression of ClVQ genes under different stress conditions. Results showed that most ClVQ genes responded to polyethylene glycol, heat treatment, salt, abscisic acid and methyl jasmonate treatment with varying degrees of expression. Furthermore, some ClVQ genes exhibited significant correlation in expression changes under abiotic stress, indicating that these genes may act synergistically in response to adversarial stress. Additionally, yeast dihybrid verification revealed an interaction between ClVQ4, ClVQ12, and ClVQ26.
Conclusions
This study conducted a genome-wide analysis of the VQ gene family in coix, including an examination of phylogenetic relationships, conserved domains, cis-elements and expression patterns. The goal of the study was to identify potential drought resistance candidate genes, providing a theoretical foundation for molecular resistance breeding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-023-04294-9.
Keywords: Coix lacryma-jobi, Valine glutamine (VQ), Phytohormone, Drought stress
Introduction
Coix lacryma-jobi, commonly referred to as Adlay or Job's tears, is a perennial herbaceous plant belonging to the Poaceae family. It is widely cultivated in East and Southeast Asian countries for its nutritional and therapeutic properties [1]. The plant's grain is a rich source of protein, making it the most protein-rich cereal crop. Additionally, extracts from its seeds are used to treat various ailments, further highlighting its medicinal value [2, 3]. Coix is primarily cultivated, produced, and consumed in China where it has been grown for over 6000 years [4]. Guizhou Province, in particular, has emerged as the primary production region for coix in China and Southeast Asia, with a cultivation area of 50,000 hm2 as of 2021 [5].
The VQ protein family is a vital plant transcription regulatory cofactor that plays a crucial role in controlling plant development, growth, and responses to environmental stresses [6]. VQ conserved domain can be categorized based on differences in the last three amino acids of the VQ domain since members of the VQ gene family all have the same core conserved sequence (FxxhVQxhTG) [7, 8]. For instance, grape has only three varieties (LTG, FTG and VTG) while Arabidopsis have six (LTG, FTG, VTG, YTG, LTS and LTD) [9, 10]. Additionally, the majority of VQ genes lack introns and the length of protein sequences are less than 300 aa.
The VQ genes perform a variety of roles during various stages of plant growth and development, including organ development, biotic and abiotic stress response, and defense response [6]. In Arabidopsis, AtVQ20 is expressed specifically in pollen and interacts with AtWRKY2 and AtWRKY34 to regulate pollen development [11]. AtVQ18 and AtVQ26 are negative interacting factors of ABI5 transcription factors, which fine-tune seed germination by antagonizing ABI5 to maintain appropriate ABA signaling levels [12]. Recent research has shown that WRKY75 and SIBs could collaborate to control ABA-mediated leaf senescence and seed germination [13]. It was found that OsVQ25 plays a crucial role in maintaining a balance between disease resistance and plant growth through the interaction of OsVQ25 with OsPUB73 and OsWRKY53 [14]. Numerous studies have found that the expression of VQ gene is induced by salt, drought, and temperature stresses as well as ABA [6, 15, 16]. The expression of most genes in cotton, maize and rice was induced under drought, salt, cold stress, and heat stress [17–19]. Overexpression of PeVQ28 enhances salt tolerance in Arabidopsis by reducing malondialdehyde content and increasing proline content [20]. IbWRKY2 has been found to enhance drought and salt tolerance and interacts with IbVQ4. Additionally, it has been observed that PEG and NaCl treatments lead to a similar increase in IbVQ4 expression, suggesting that IbVQ4 may be an essential factor in sweet potato's ability to tolerate drought and salt stress [21]. The data clearly showed that overexpression of MdVQ37 decreased the tolerance of transgenic apple lines to heat and drought stress [22]. VQ proteins act as transcriptional regulatory cofactors and play a crucial role in regulating various physiological and biochemical processes in plants [23–25]. Among the interacting proteins of VQ, WRKY transcription factors are the most significant [26–28]. Additionally, VQ proteins can also interact with each other, as observed by Wang et al. For instance, AtVQ12 can strongly interact with AtVQ3, AtVQ8, AtVQ10, AtVQ12, AtVQ17, AtVQ18, AtVQ29, and AtVQ32 [22].
The VQ protein family has been identified in several species, but little is known about its members in coix. However, with the complete coix genome now available, researchers have the opportunity to conduct a thorough investigation of VQ genes in coix. This study aims to identify VQ protein family members from the coix genome and analyze their phylogenetic relationships, gene structure, and conserved motifs using bioinformatics tools. Further, chromosome distribution and cis-elements were analyzed. Finally, the expression levels of VQ genes in different tissues and in response to stresses (ABA, MeJA, drought) were analyzed. In addition, the coregulatory networks of ClVQs under abiotic stress were analyzed based on the PCCs of their relative expression levels. The detailed information provided in this study will contribute to further understanding of the VQ gene family. Meanwhile, the basis for further research on the biological functions of VQ genes and screening of candidate genes for resistance in coix.
Results
A total of 31 VQ genes were identified in coix
An HMM search was performed against the coix genome database, a total of 40 VQ-containing sequences were obtained. After manual de-duplication, a total of 31 nonredundant VQ genes were identified, which were named ClVQ1 to ClVQ31 according to their physical location (from top to bottom) on chromosome. In Table 1, these genes encode proteins ranging from 85 to 1408 amino acids (aa), with the majority of ClVQ proteins being less than 300aa in length (87.1% of coix). The molecular weight ranged from 9353.38 Da (ClVQ31) to 155310.57 Da (ClVQ18), and the predicted isoelectric point was 5.06 (ClVQ12) to 10.97 (ClVQ19). Analysis of the cellular localization of ClVQ proteins showed that most ClVQ proteins were localized in the nucleus, some in the chloroplast, and two ClVQ proteins were localized in the mitochondria (Table S1). Additionally, a three-dimensional model of the ClVQ proteins was created using a Swiss-Model web server (Fig. S1). Most homologous pairs of the protein have different three-dimensional structures, suggesting potential functional variety.
Table 1.
Details of the identified ClVQ genes
| Name | Gene Identifier | Location | ORF length (bp) | Protein | |||
|---|---|---|---|---|---|---|---|
| Length (a.a.) | PI | Mol.Wt. (Da) | Exons | ||||
| ClVQ1 | Cl017671_T1 | Chr1:10323082..10323943 | 705 | 234 | 10.4 | 23771.31 | 2 |
| ClVQ2 | Cl019380_T1 | Chr1:49425867..49426730 | 864 | 287 | 8.82 | 30137.97 | 1 |
| ClVQ3 | Cl020396_T1 | Chr1:156501734..156502135 | 402 | 133 | 6.82 | 14353.06 | 1 |
| ClVQ4 | Cl000473_T1 | Chr2:6309585..6310178 | 594 | 197 | 6.43 | 20985.22 | 1 |
| ClVQ5 | Cl000656_T1 | Chr2:8951166..8951897 | 732 | 243 | 10.9 | 25216.57 | 1 |
| ClVQ6 | Cl001487_T1 | Chr2:21666176..21666712 | 537 | 178 | 8.07 | 18197.02 | 1 |
| ClVQ7 | Cl001606_T1 | Chr2:24792544..24793188 | 645 | 214 | 7.84 | 21857.68 | 1 |
| ClVQ8 | Cl002136_T1 | Chr2:34913778..34914668 | 891 | 296 | 9.05 | 30245.5 | 1 |
| ClVQ9 | Cl002879_T1 | Chr2:58387199..58387981 | 783 | 260 | 7.88 | 26524.77 | 1 |
| ClVQ10 | Cl005732_T1 | Chr2:176638900..176639427 | 528 | 175 | 11 | 18163.87 | 1 |
| ClVQ11 | Cl026223_T1 | Chr3:12773846..12774622 | 777 | 258 | 7.35 | 26181.84 | 1 |
| ClVQ12 | Cl026639_T1 | Chr3:32524660..32525238 | 579 | 192 | 5.06 | 20878.53 | 1 |
| ClVQ13 | Cl026809_T1 | Chr3:44946616..44947272 | 657 | 218 | 10.5 | 22032.86 | 1 |
| ClVQ14 | Cl027282_T1 | Chr3:91338266..91338640 | 375 | 124 | 7.82 | 13364.03 | 1 |
| ClVQ15 | Cl006467_T1 | Chr4:1557742..1558497 | 756 | 251 | 6.33 | 25881.22 | 1 |
| ClVQ16 | Cl006939_T1 | Chr4:7821656..7822342 | 687 | 228 | 6.86 | 23254.79 | 1 |
| ClVQ17 | Cl009284_T1 | Chr4:58154166..58155515 | 1350 | 449 | 6.48 | 44285.14 | 1 |
| ClVQ18 | Cl010380_T1 | Chr4:139422371..139433003 | 4227 | 1408 | 7.45 | 155310.6 | 9 |
| ClVQ19 | Cl029546_T1 | Chr5:5018347..5019111 | 765 | 254 | 11 | 26171.85 | 1 |
| ClVQ20 | Cl029762_T1 | Chr5:8673329..8673864 | 507 | 168 | 4.44 | 16842.47 | 2 |
| ClVQ21 | Cl013179_T1 | Chr6:19907346..19914547 | 882 | 293 | 7.86 | 31082.46 | 2 |
| ClVQ22 | Cl014167_T1 | Chr6:37112888..37113145 | 258 | 85 | 9.1 | 9390.39 | 1 |
| ClVQ23 | Cl033684_T1 | Chr7:43528212..43529414 | 1203 | 400 | 7.84 | 40598.52 | 1 |
| ClVQ24 | Cl038325_T1 | Chr8:141599917..141600591 | 675 | 224 | 9.13 | 23249.17 | 1 |
| ClVQ25 | Cl038326_T1 | Chr8:141623530..141624261 | 732 | 243 | 9.29 | 24671.47 | 1 |
| ClVQ26 | Cl021872_T1 | Chr9:1099634..1100230 | 597 | 198 | 10.3 | 21147.02 | 1 |
| ClVQ27 | Cl022088_T1 | Chr9:3558783..3559259 | 477 | 158 | 6.51 | 16369.4 | 1 |
| ClVQ28 | Cl022372_T1 | Chr9:7243171..7243860 | 690 | 229 | 9.51 | 24141.31 | 1 |
| ClVQ29 | Cl041459_T1 | Chr10:133307856..133308575 | 720 | 239 | 10.1 | 24140.92 | 1 |
| ClVQ30 | Cl043095_T1 | Unplaced_contig5:752346..753404 | 1059 | 352 | 7.26 | 35749.07 | 1 |
| ClVQ31 | Cl042147_T1 | Unplaced_contig315:31568..31840 | 273 | 90 | 9.22 | 9353.38 | 1 |
Phylogenetic tree of VQ domains in Arabidopsis, rice, maize, and coix
To understand the evolutionary relationships of VQ genes, a phylogenetic tree was constructed using 166 VQ proteins from Arabidopsis, rice, maize and coix (Fig. 1). Also, Table 1 and Table S2 contain comprehensive information about VQ genes. The 31 ClVQ genes were classified into seven subgroups (I-VII) based on the classification of Arabidopsis, rice, and maize VQ gene families. Subgroup VI had the highest number of members (11), followed by subgroup II (7), while subgroup VII had the lowest number of members with only one. This distribution pattern was similar to that of VQ genes in rice and maize [17, 18]. Moreover, coix, rice, and maize all belong to the gramineae family, resulting in a relatively even distribution of VQ genes among the phylogenetic tree of these three species.
Fig. 1.
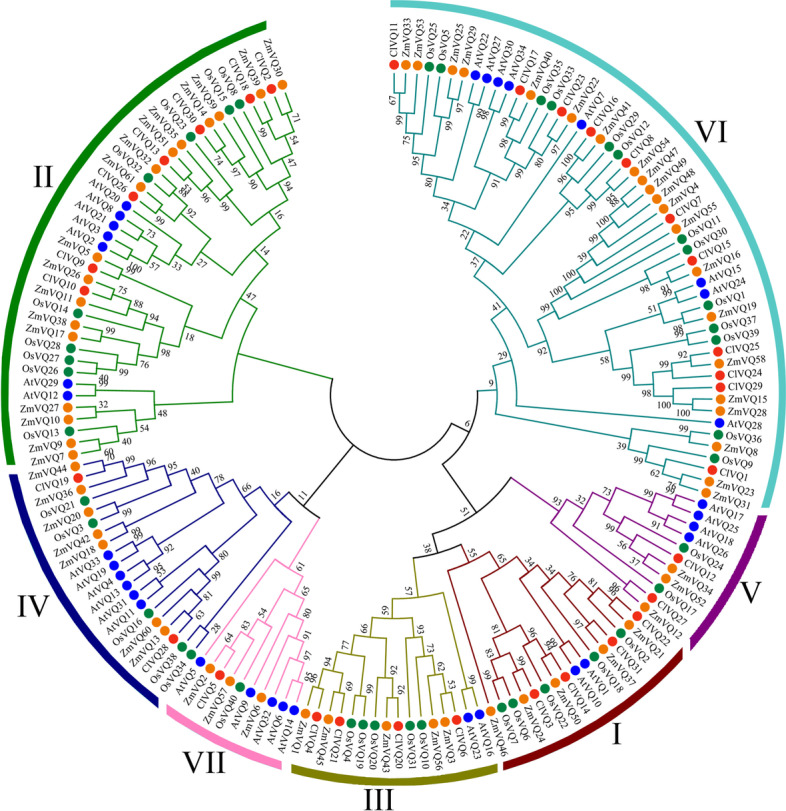
Phylogenetic tree of VQ genes from coix, Arabidopsis, rice and maize. 31 ClVQ genes, 34 AtVQ genes, 40 OsVQ genes and 61 ZmVQ genes are clustered into 7 subgroups (I-VII). VQ genes from coix, Arabidopsis, rice and maize are denote by red, blue, green and yellow shape, respectively. Details of the VQ genes from four species are listed in Table S2. The tree was generated with the Clustal X 2.0 software using the neighbor-joining (N-J) method
Conserved motifs, multiple alignment, and gene structural analysis
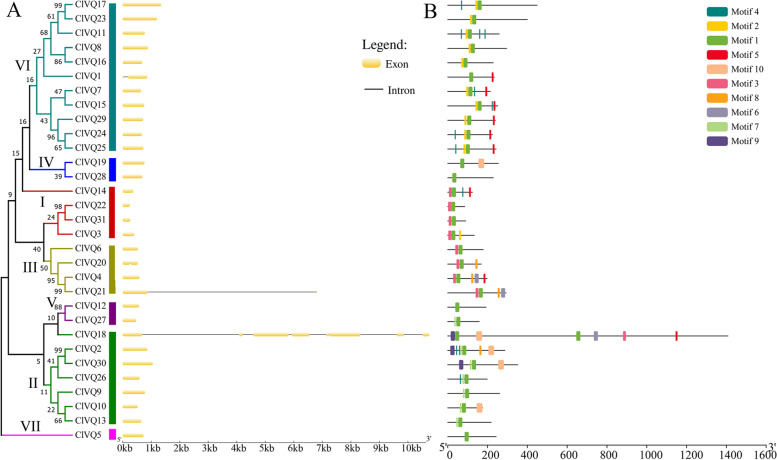
Exon/intron structures were created based on the coding sequences of each ClVQ gene to gain a better understanding of the structural diversity of ClVQ genes. As shown in Fig. 2A, subgroup I members had shorter coding regions ranging from 273 bp to 402 bp compared to the other subgroups. The results revealed that 27 out of 31 ClVQ genes (87.1%) had no introns, which was consistent with previous studies on AtVQs, ZmVQs, and OsVQs. Only ClVQ1, ClVQ18, ClVQ20, and ClVQ21 were found to contain introns. In particular, the number of introns in ClVQ18 was much higher than in other genes. Similarly, there is one such gene in Chinese cabbage and Moso bamboo [29, 30].
Fig. 2.
Phylogenetic relationships, gene structure and Conserved motifs of VQ genes in coix. A Phylogenetic relationships and gene structure of VQ genes in coix. Exons are indicated by yellow rectangles. Gray lines connecting two exons represent introns. B Conserved motifs of VQ genes in coix. Distribution of the 10 conserved motifs in the ClVQ genes following analysis by MEME tool. The different-colored boxes represent different motifs and their position in each protein sequence of ClVQ
The conserved motifs in VQ proteins of coix were analyzed by Motif Elicitation tools. Table S3 displays the length and conserved amino acid sequences of the 10 unique motifs that were discovered. The potential motif sequences identified from MEME were annotated by scanning Pfam. The study found that Motif 1 encodes the VQ domain, while the other motifs lacked functional annotation. Fig. 2B illustrates that each ClVQ protein had 1-6 conserved motifs, with Motif 1 present in all VQ proteins. Subgroup VII members contained both Motif 1 and 2, while subgroup III members contained both Motif 1 and 3. The results indicated that VQ proteins within the same subgroup exhibited similar motifs, which aligned with the findings of phylogenetic analysis.
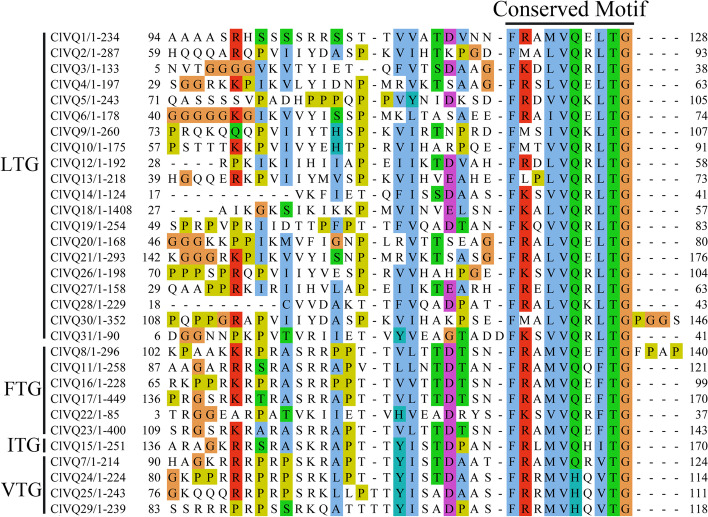
To better understand the characteristics of coix VQ domain, a multiple sequence alignment was constructed. As depicted in Fig. 3, ClVQ proteins were found to have four types of VQ domains: FxxxVQxLTG (20/31), FxxxVQxFTG (6/31), FxxxVQxITG (1/31), and FxxxVQ/HxVTG (4/31). In contrast to AtVQs and ZmVQs, ClVQ proteins lacked the FxxxVQxLTD, FxxxVQxATG, and FxxxVQxYTG domains [9, 18].
Fig. 3.
Multiple sequence alignment of VQ genes in coix. Sequences were aligned using Jalview software
Chromosomal location and gene pairs analysis in coix
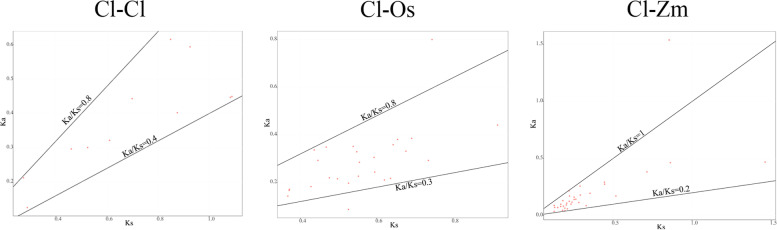
Based on the coix database, there are 29 ClVQ genes located unevenly across 10 chromosomes, with the exception of ClVQ30 and ClVQ31 (Fig. S2). Chromosome 2 contains the highest number of ClVQ genes with 7, while chromosomes 7 and 10 have the lowest number with only 1. The remaining chromosomes have 2-4 ClVQ genes. Additionally, 11 paralogues were identified in coix using BLASTN methods (Table S4). We found 26 orthologues between ClVQ and OsVQ genes, and 35 orthologues between ClVQ and ZmVQ genes. We calculated Ks values, Ka values, and Ka/Ks ratios of both paralogues and orthologues to examine the influence of selection pressure on the evolution of the ClVQ gene family (Table S4 and Fig. 4). In general, Ka/Ks ratios below 1, larger than 1, and equal to 1 suggest purifying selection, positive selection, and neutral selection, respectively. The Ka/Ks ratio for all paralogues ranged from 0.4 to 0.8, while for most orthologues it was between 0.2 and 1.0 (Fig. 4). These findings suggest that purifying selection may have played a significant role in the evolution of VQ genes in coix.
Fig. 4.
Ka/Ks ratios of paralogs and orthologs. The black lines indicated Ka/Ks equal to 0.2, 0.3, 0.4, 0.8 and 1. The y-axis indicates the value of Ka and the x-axis indicates the value of Ks
Identification of cis-elements in the promoter regions of ClVQs
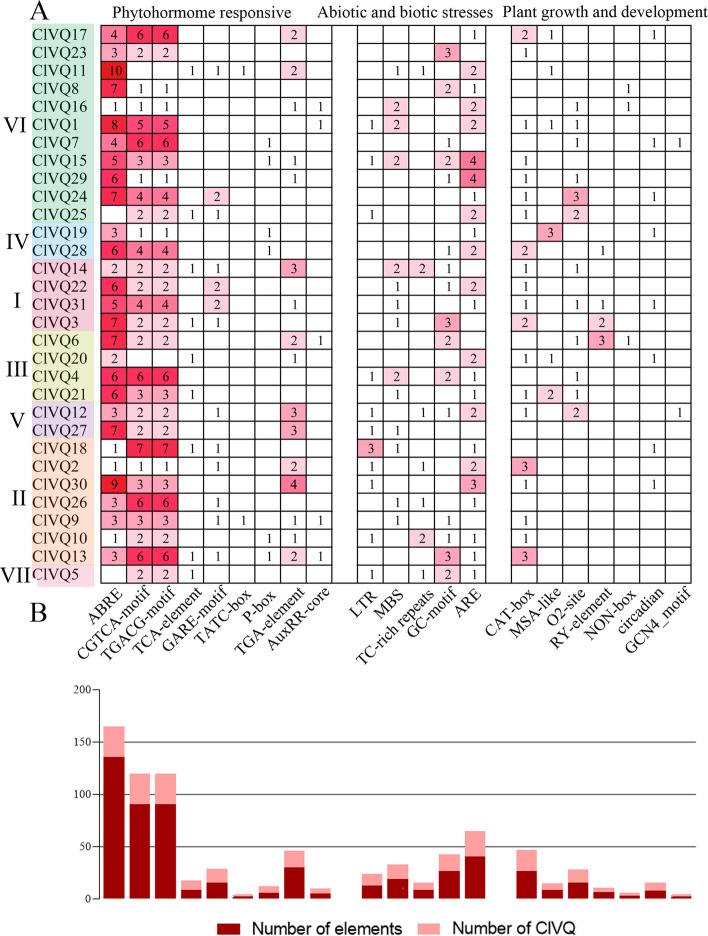
To investigate the regulatory mechanism of the ClVQ gene, we conducted cis-acting element analysis on a 2000bp sequence of its promoter region. Our analysis also revealed the presence of 386 hormone response elements, 109 stress-related response elements, and 72 growth and development elements in the promoter sequences of 31 VQ genes. Fig. 5 shows that most ClVQ genes are involved in the ABA signaling pathway, with 136 ABA-responsive elements (ABRE) found in the promoters of 29 ClVQs (excluding ClVQ25 and ClVQ5). The promoters of these 29 ClVQs also contain MeJA-responsive elements (CGTCA-motif and TGACG-motif), as well as SA-responsive elements (TCA-element) and gibberellin-responsive elements (P-box, TATC-box and GARE-motif), with 9 and 24 of each, respectively, found in the promoter region of the gene. In the promoters of 14, 11, 25, and 7 ClVQs, various cis-elements related to abiotic and biotic stresses were identified. These include MBS (drought induced response element), LTR (low temperature response element), ARE (anaerobic induced response element), and TC-rich (defense and stress response element). In addition, 72 elements related to plant growth and development were identified in the promoter regions. Among these, 27 elements (CAT-box) were found in the promoters of 20 ClVQs, which are associated with meristem expression.
Fig. 5.
Cis-acting elements analysis of VQ genes in promoter region of coix. A Number of each cis-acting element in the promoter region (2000 bp) of ClVQs. B Statistics for the total number of ClVQs
Expression pattern of the ClVQs in different tissues
The expression patterns of ClVQ genes were analyzed in root, stem, leaf, and flower using qRT-PCR. As shown in Fig. 6, the expression patterns of various VQ genes differed among tissues, with members of subgroups III and VI exhibiting high expression in all tested tissues. Over 50% of ClVQ genes were up-regulated in leaves, while 9 and 5 ClVQ genes were found to be highly expressed in roots and flowers, respectively.The study found that out of 11 paralogous genes, 6 had similar expression patterns and were highly expressed in the same tissue. For instance, ClVQ29/ClVQ24 and ClVQ2/ClVQ30 were up-regulated in the root. Moreover, ClVQ11, ClVQ21, and ClVQ22 showed increased expression levels in roots, leaves, and flowers, but decreased expression in stems.
Fig.6.
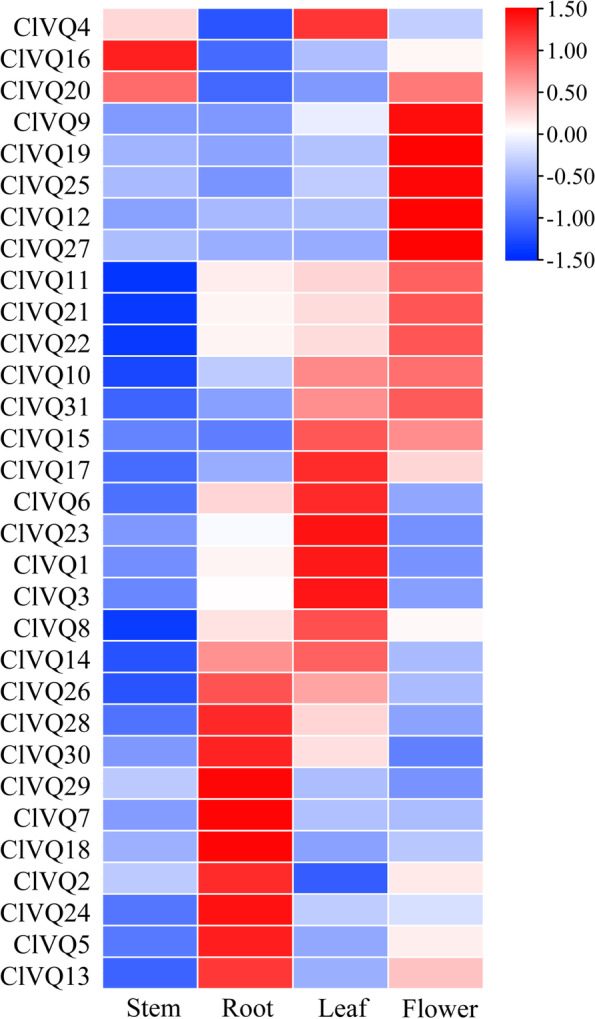
Tissue-specific expression patterns of 31 ClVQ genes in coix by qRT-PCR. The heatmap shows the hierarchical clustering of 31 ClVQ genes at different time points. The color scale: Blue represents low expression and red indicates a high expression level
ClVQ genes expression following various stresses
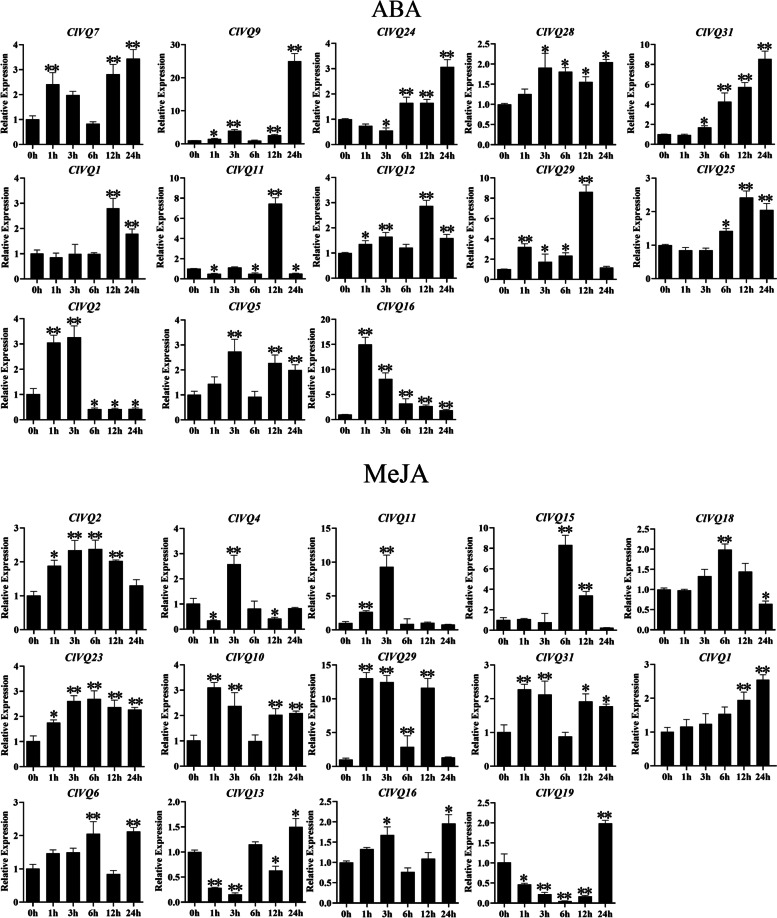
Promoter analysis revealed that ClVQ genes play a crucial role in hormone response. To investigate the effects of hormone treatment on the expression pattern of ClVQ genes, the expression levels of 31 ClVQ genes were quantified using qRT-PCR following ABA and MeJA administration. The study found that treatment with ABA resulted in the up-regulation of 24 out of the 29 ClVQ genes studied, as shown in Fig. S3. Notably, ClVQ7, ClVQ9, ClVQ24, ClVQ28, and ClVQ31 exhibited significant increases in expression levels, with ClVQ9 showing a particularly high increase of over 20 times that of the control group (Fig. 7). However, the expression levels of ClVQ17, ClVQ18, and ClVQ30 did not show significant changes. Three ClVQ genes (ClVQ2, 5, and 16) were significantly up-regulated at early time points, but their expression levels decreased later on. On the other hand, expression of ClVQ4, ClVQ6, ClVQ15 and ClVQ27 was down-regulated during ABA treatment, with ClVQ4 being consistently suppressed. These results suggest that ABA treatment has a selective effect on the expression of ClVQ genes.
Fig. 7.
Expression analysis of ClVQ genes following hormone treatments by qRT-PCR. A Expression patterns of 13 ClVQs under ABA treatment. B Expression patterns of 15 ClVQs under MeJA treatment.The Y-axis and X-axis indicates relative expression levels and the time courses of stress treatments, respectively. Mean values and standard deviations (SDs) were obtained from three biological and three technical replicates. The error bars indicate standard deviation
After treatment with MeJA, the expression of 31 ClVQ genes showed changes when compared to the untreated control (Fig. S4). Among these genes, ClVQ2, 4, 11, 15, 18 and 23 exhibited similar expression patterns, as depicted in Fig. 7. For instance, ClVQ15 was upregulated and reached its peak at 6 h, followed by a decline. During MeJA treatment, ClVQ23 and ClVQ29 showed consistent up-regulation at all time points, while ClVQ7, ClVQ21, and ClVQ24 were consistently down-regulated. ClVQ13 and ClVQ19 were significantly down-regulated at early time points (1 h, 3 h, 6 h, and 12 h), but showed a considerable 1.5-fold up-regulation at 24 h, which differs from the expression patterns of other VQ genes.
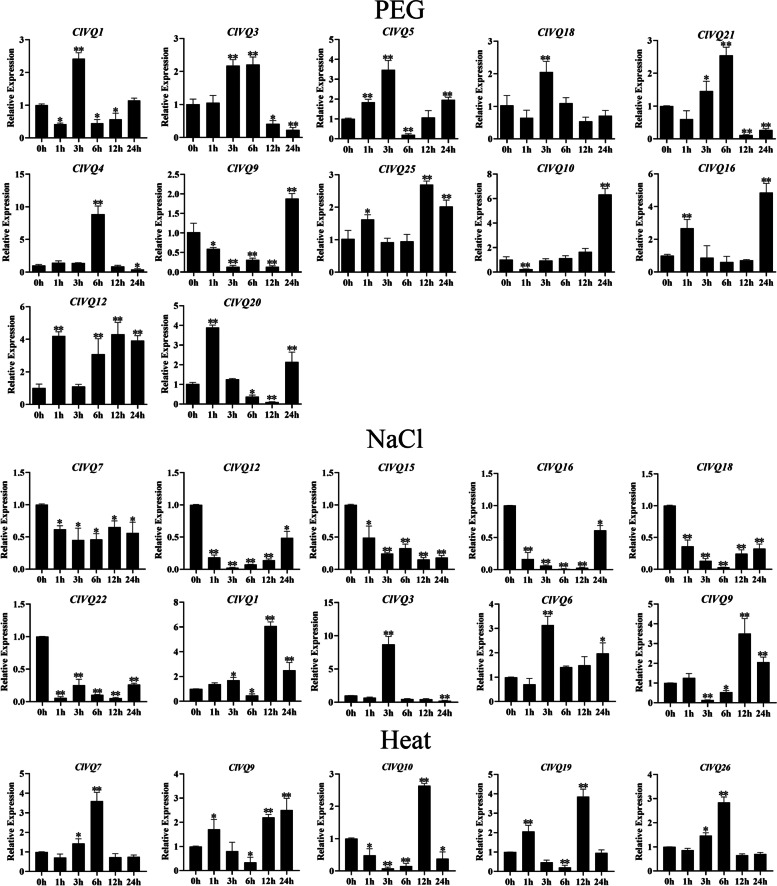
This study aimed to investigate the impact of adverse environmental conditions on the growth and development of plants, specifically focusing on the expression of ClVQ genes. The study utilized qRT-PCR to analyze the expression patterns of these genes under drought, salt, and heat stress (Figs. S5, S6 and S7). After drought treatment (Fig. 8), it was observed that the expressions of 14 ClVQ genes were up-regulated at varying time intervals. Specifically, after 24 hours of drought treatment, the expression levels of eight ClVQ genes were found to be up-regulated. Notably, the expression levels of ClVQ10 and ClVQ16 were significantly up-regulated to 6-fold and 4-fold of the control after 24 hours, respectively. During salt stress (Fig. 8), it was found that the six VQ genes (ClVQ7, ClVQ12, ClVQ15, ClVQ16, ClVQ18, and ClVQ22) were consisitently down-regulated at all times.Conversely, ClVQ1, ClVQ3, ClVQ6, and ClVQ9 showed obvious upregulation during stress periods. Furthermore, certain genes such as ClVQ8 and ClVQ30 exhibited consistent expression levels throughout the duration of the stress. As for heat stress, 15 of the 31 ClVQ genes were down-regulated apparently at any time, while ClVQ6 and ClVQ23 were also express stably. Specifically, expression of ClVQ7, ClVQ9, ClVQ10, ClVQ19 and ClVQ26 were strongly up-regulated more than twofold during heat stress.
Fig. 8.
Expression analysis of 20 ClVQ genes following drought treatments by qRT-PCR. The Y-axis and X-axis indicates relative expression levels and the time courses of stress treatments, respectively. Mean values and standard deviations (SDs) were obtained from three biological and three technical replicates. The error bars indicate standard deviation
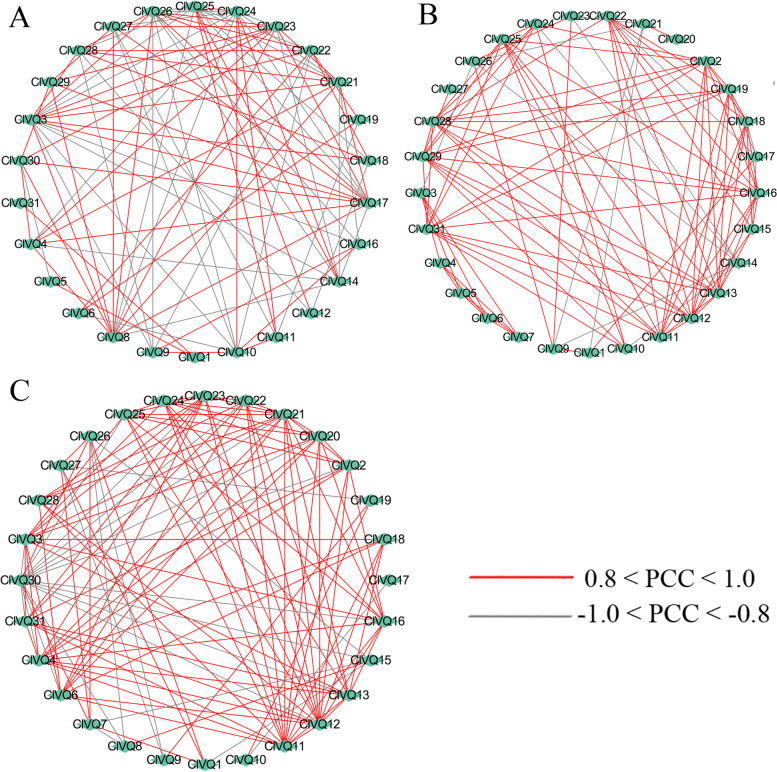
Coregulatory networks of ClVQs under abiotic stress
To investigate the relationships among genes in response to PEG, NaCl, and heat treatment, correlation and coregulatory networks were established based on the PCCs of their relative expression levels. The coregulatory network was created by gathering and displaying 31 ClVQ genes with PCC absolute values larger than 0.8 and significant at the 0.05 significance level (Tables S6 and S7). All ClVQ genes appeared to be correlated with each other to varying degrees of positive or negative correlation (Fig. 9). Most ClVQs had positive significant correlations between them and were greater than 0.8 under these stresses. The paralogues (ClVQ24/-25) had positive correlations under three treatment, and ClVQ26/-14 ClVQ14/-23 and ClVQ16/-17 had negative correlations under PEG and NaCl treatment.
Fig. 9.
Correlations among ClVQ genes under stress treatment. A The co-regulatory networks of ClVQ genes under PEG treatment. B The co-regulatory networks of ClVQ genes under NaCl treatment. C The co-regulatory networks of ClVQ genes under heat treatment were established based on the Pearson correlation coefficients (PCCs) of these gene pairs using transformed qPCR data
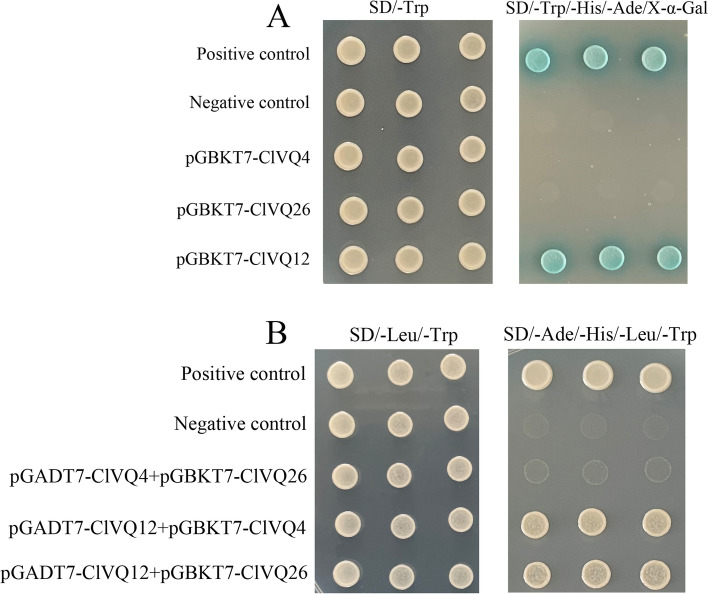
Physical interaction between VQs
The results of the experiment showed that the positive control and pGBKT7-ClVQ12 transformants grew normally and turned blue on D/-Ura/-His/-Trp/-Leu/X-α-Gal medium. On the other hand, the negative control, pGBKT7-ClVQ4 transformants, and pGBKT7-ClVQ26 transformants did not grow normally on the plate (Fig. 10A). These findings suggest that ClVQ12 has self-activating activity, whereas ClVQ26 and ClVQ4 do not. Yeast two-hybrid confirmation was performed by observing the growth of transformants from both control and experimental groups on SD/-Trp/-Leu medium. Both positive control and experimental groups grew on SD/-Ura/-His/-Trp/-Leu, while the negative control did not show any growth (Fig. 10B). The results indicate that ClVQ12, ClVQ4, and ClVQ26 can interact with each other, with the interaction between ClVQ4 and ClVQ26 being relatively weaker.
Fig. 10.
Physical interaction between VQs. A Transcriptional activation validation assays. B Yeast-two-hybrid assays. ClVQ12, ClVQ4, and ClVQ26 can interact with each other. Sequences of full-length ClVQ4, ClVQ12 and ClVQ26 were fused to the pGBKT7 binding domain (BD, bait), sequences of full-length ClVQ4 Aand ClVQ12 were fused to the pGADT7 activation domain (AD, prey)
Discussion
VQ proteins have been identified as transcriptional regulatory cofactors in various plants. They have also been found in fungi, lower animals, and bacteria, with single to several VQ proteins present [31]. Research on VQ gene function has shown that it is not only involved in plant responses to biotic and abiotic stresses but also plays a role in regulating plant growth and developmental processes [12, 16]. However, little is known about VQ genes in coix. Therefore, bioinformatic analysis of the ClVQ genes and their patterns of expression under various stress treatments may help us better understand the mechanisms that affect plant stress resistance, which could be applied to coix molecular breeding.
A total of 31 VQ genes were identified in coix, whereas Arabidopsis had 34 VQ genes despite having a much smaller genome size of 135 Mb. This trend was also observed in rice and bamboo, indicating a possible loss of VQ genes during genome expansion in these species [17, 30, 32]. A comprehensive phylogenetic tree was used to divide the 31 ClVQ genes into seven subgroups. The tree showed that ClVQs, OsVQs, and ZmVQs were consistently clustered together, likely due to the fact that all three species belong to the gramineae family. Additionally, comparative genomics analysis suggested that coix was more closely related to maize than rice [33]. The study found that ZmVQs and ClVQs had a higher number of orthologous compared to OsVQs and ClVQs (Table S4). Additionally, the Ka/Ks analysis revealed that ClVQ underwent purifying selection. Most ClVQ proteins (87.1%) had an amino acid length of less than 300 aa. Similarly, Arabidopsis, rice, C. pepo, and maize had a high percentage of VQ protein length less than 300 aa, ranging from 81.8% to 90.3% [7, 9, 16, 18]. The subcellular localization of VQ proteins was analyzed and it was found that most ClVQ proteins were present in the nucleus and chloroplasts. A few ClVQ and AtVQ proteins were found in mitochondria (Table S1), while some AtVQ and OsVQ proteins were found in the cytoplasm [17]. These results suggest that the VQ protein may have diverse functions in different cellular locations.The distribution of ClVQs on chromosomes was found to be non-uniform. Chromosome 2 contained 7 ClVQ genes, while chromosome 10 contained only 1 ClVQ gene (Fig. S2). This pattern is consistent with previous studies on VQ genes, for instance, Arabidopsis chromosomes 1 to 5 have 11, 7, 8, 4, and 4 VQ genes, respectively [9].
In this study, 28 ClVQ proteins were found to contain the conserved FxxxVQxhTG motif, while the other three ClVQ proteins contained the FxxxVHxhTG motif (Fig. 3). The FxxxVHxhTG motif has previously been observed in the VQ gene of maize, rice, and moso bamboo [17, 18, 30]. Further analysis revealed that the conserved domain of 20 ClVQ proteins had LTG as the last three amino acid residues, with only a few members having FTG, ITG, and VTG. This classification based on the differences in the last three amino acids resulted in six types in both Arabidopsis and maize [9, 18]. The VQ family members exhibit functional diversity, and in addition to the highly conserved VQ structural domain, there is also abundant amino acid sequence diversity at other positions. Studies have shown that during long-term evolution, most VQ genes have lost introns, which is consistent with the gene structure analysis of 87.1% (27/31) VQ genes in coix without introns (Fig. 2).
The promoter sequences of ClVQ genes were found to contain several cis-acting elements, with hormone response elements accounting for 68% of them (Fig. 5). The results were similar to wheat and Brassica juncea [34, 35], with the highest number of members responding to ABRE elements. This suggests that the expression of most VQ genes is regulated by ABA. ClVQ11 contained the largest number of ABRE elements, with up to 10 identified. The expression of ClVQ11 increased to more than 6-fold of the control after ABA treatment for 12 hours. The study also identified several stress-related response elements, as well as growth and development elements. A recent study found that OsVQ13 positively regulates JA signaling and increases grain size in transgenic rice [36]. AtVQ8 in Arabidopsis was found to be involved in plant growth and development. The majority of ClVQs are expressed in roots and leaves, while five ClVQs (ClVQ9, 19, 26, 12 and 27) were highly expressed in flowers (Fig. 6). Similarly, almost all CsVQs in tea were expressed in the root, stem, and leaf, with four VQs highly expressed in flowers of M. truncatula [8, 37]. The document demonstrated that OsVQ1 interacts with OsMPK6 and enhances the expression of genes that promote flowering [38]. Therefore, it could be concluded that the ClVQs is not only involved in hormone signaling and abiotic stress processes, but also plays a crucial role in regulating growth and development.
Recent research has shown that VQ genes play a crucial role in responding to different hormones and stresses such as ABA, MeJA, drought, NaCl, and heat. For instance, after ABA or MeJA treatment in wheat, 12 TaVQ genes were found to be induced. In sugarcane, seven genes were found to be affected by JA and ABA treatments [35, 39]. In our study, we found that 29 out of 31 ClVQs contained both ABA response elements and MeJA response elements. Additionally, most of these genes were regulated by treatments of ABA or MeJA. Notably, six of these genes (ClVQ1, 2, 5, 11, 29 and 31) were significantly up-regulated after exposure to ABA and SA treatments (Fig. 7). The VQ genes is known to have a significant impact on abiotic stress. Overexpression of PeVQ28 can enhance the salt tolerance of Arabidopsis, while overexpression of MdVQ37 in transgenic apple can reduce the drought resistance of Arabidopsis [20, 40]. Additionally, the VQ genes is sensitive to temperature changes, with the majority of Chinese cabbage VQ genes responding to heat [29]. In this work, the expression of ClVQ genes (ClVQ1, 9, 10 and 27) was significantly up-regulated during NaCl, drought and heat treatment at certain time points. ClVQ12, ClVQ13, ClVQ16, ClVQ18, and ClVQ22 expression was found to be highly up-regulated with drought treatment, but the expression was suppressed under NaCl and heat treatment. Recent studies have shown that AtVQ12 and AtVQ29 have the ability to form both heterodimers and homologous dimers through physical interaction [22]. Additionally, the yeast dihybrid assay has confirmed the interaction of ClVQ4, ClVQ12, and ClVQ26.
Conclusion
This work identified 31 VQ genes in the genome of coix. A systematic bioinformatics analysis of the VQ gene family of coix was performed, including phylogenetic relationships, conserved domain, exon-intron structure and so forth. Through the integration of promoter analysis and expression pattern, it was observed that ClVQ genes exhibited positive responses to various stressors, including ABA, MeJA, drought, NaCl, and heat. Notably, ClVQ1, ClVQ9, ClVQ10, ClVQ26, and ClVQ29 displayed significant increases in expression levels in response to various abiotic stresses. Additionally, yeast dihybrid verification revealed an interaction between ClVQ4, ClVQ12, and ClVQ26. In a word, the stress response candidate genes of coix were screened in this study, providing a foundation for further research on the function of VQ family members in abiotic stresses.
Materials and methods
Plant materials, growth conditions, and stress treatments
The Wanyi 2 variety of coix is extensively cultivated in Anhui, China (Breeding by the cotton research institute of Anhui academy of agricultural). To conduct the experiment, the seeds were planted in pots filled with a mixture of vermiculite and black soil and were grown in a greenhouse at a temperature of 25°C with a light/dark cycle of 16/8 hours. After three weeks of growth, seedlings of uniform size were selected for studying the expression level of the VQ gene of coix under stress treatment.
To conduct stress and hormone treatments, we poured a 200 Mm NaCl, a 20% PEG-6000 solution, a 100 µM ABA solution and a 100 µM MeJA solution over the culture medium vermiculite and black soil, respectively. Heat stress treatments were conducted by controlling the temperature in the plant climate incubator at 40±1°C. We harvested plant leaves at 0, 1, 3, 6, 12, and 24 hours after treatment and immediately froze them in liquid nitrogen. Samples of roots, stems, leaves, and inflorescence tissues were collected from coix plants that had been cultivated for at least three months.
Database search for VQs in coix
The whole genome data of coix was downloaded from Coge (https://genomevolution.org/coge/). The VQ domain Hidden Markov Model (HMM) information with the number PF05678 was obtained from the Pfam database (http://pfam.xfam.org/). The Linux version of HMMER software was used to identify the VQ protein. The coix VQ protein sequence was obtained by using HMMER software for identification with the E-value set to less than 10^-5 and redundant sequences were removed. The candidate VQ protein sequences underwent verification through the CDD database on the NCBI website. The protein sequence that contained the VQ domain was ultimately retained. The identified ClVQ proteins had their predicted sequence length, molecular weight, isoelectric point, and subcellular localization determined using the ExPasy and PSORT websites.The 3D structure of each ClVQ protein was determined using SWISS-MODEL (https://swissmodel.expasy.org/interactive) [41].
Phylogenetic analysis and multiple alignment
To align the VQ amino acid sequences, we utilized MEGA 7.0 software and constructed a phylogenetic tree using the neighbor-joining method (NJ) with a Bootstrap value of 1000 and default parameters [42]. We obtained VQ proteins from A. thaliana, rice, and maize from Phytozome v13 and aligned them with 31 ClVQ protein sequences using Jalview software.
Motif prediction and gene structure analysis
To predict the gene structure, we uploaded the GFF file of 31 ClVQ genes to the Gene Structure Display Server 2.0 website. We also used the MEME online tool to query the protein domain [43]. The criteria for the MEME analysis included a site distribution of zero or one occurrence per sequence, a maximum number of motifs of 10, and an optimum motif width of 6-200.
Chromosomal distribution and Ka/Ks analysis
In order to locate the ClVQ genes, we retrieved their location information from the GFF annotation file from the Coge database. We then used TBtools software to map their distribution on the chromosome [44]. To identify paralogs and orthologs, we ran a BLASTN [45] for the nucleotide sequences of all VQ genes, following the same method described by Blanc & Wolfe [46]. We calculated non-synonymous (ka) and synonymous (ks) substitutions using TBtools [44].
Analysis of ClVQ genes regulatory elements
The 2 kb sequences upstream of the start codon for ClVQ genes were obtained in FASTA format from the Coge database. These sequences were then uploaded to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) for identification and analysis of Cis-elements.
RNA extraction and quantitative real-time PCR (qRT-PCR)
RNA from each sample was extracted using the Aidlab plant RNA kit (Aidlab Biotech, Beijing, China) following specific protocols. To ensure quality, the concentration and integrity of all RNAs were assessed using electrophoresis and NanoDrop™ One/OneC (ThermoFisher SClentific, USA). The EF1α gene was used as the reference gene, and gene-specific primers were designed and checked for specificity using Primer Premier 5.0 and TBtools, respectively (Table S8). The first strand of cDNA was synthesized using the Prime ScriptTMRT reagent Kit (TaKaRa, Dalian, China). Real-time PCR was performed on a CFX96TM Real-Time System (BIO-RAD, California, USA) using TB Green Premix Ex Taq II (Tli RNaseH Plus; TaKaRa Biotechnology) with a sample volume of 10 µL. The standard 2−∆∆CT method was used to calculate the relative expression levels of each gene [47].
Y2H assays
The ClVQ4, ClVQ12 and ClVQ26 CDSs were cloned into the decoy vector pGBKT7, and then transformed into yeast strain Y2HGold (Weidi Biotechnology, shanghai, China) to verify the self-activation. Transformants were screened and verified by SD/-Trp and SD/-Ura/-His/-Trp/-Leu/X-α-Gal. To confirm protein-protein interactions, full-length CDSs of three VQ proteins were cloned into the prey vector pGADT7. Transformants were screened and verified by SD/-Trp/-Leu and SD/-Ura/-His/-Trp/-Leu. Primers used for amplifying these fragments for yeast two-hybrid assays are listed in Supplementary Table S8.
Statistical and Pearson correlation analysis
Statistical analyses were conducted using Dunnett's two-tailed t test. Mean values and standard deviations (SD) of three replicates were presented, with significant differences relative to controls denoted as ∗P ≤ 0.05 and ∗∗P ≤ 0.01. Pearson correlation coefficients (PCCs) and p-values of stress-induced ClVQ gene pairs were obtained and plotted using the R package based on the qRT-PCR results. The coexpression network was constructed using Cytoscape by gathering all gene pairings with PCC values greater than 0.5 and significant at the 0.05 significance level (P-value).
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
Conceived and designed the experiments: YJW, JBZ and BLJ. Performed the experiments: YJW, CY and XYL Analyzed the data: YJW, HJW, YHF and JSC. Wrote the paper: YJW. Participated in the design of this study and revised manuscript: YJW, JBZ and BLJ. The authors read and approved the final manuscript.
Authors’ information
Not applicable.
Funding
This work was supported by a grant from the Innovation team for the development and utilization of medicinal food and plant resources of Anhui academy of agricultural sciences (2022YL019), and cotton germplasm creation and utilization innovation team (2023YL005).
Availability of data and materials
The genome sequences of coix were downloaded from Coge database (https://genomevolution.org/coge/OrganismView.pl?dsgid=54616). The genome sequences of A. thaliana were downloaded from Phytozome database (https://phytozome-next.jgi.doe.gov/info/Athaliana_TAIR10). The genome sequences of rice were downloaded from Phytozome database (https://phytozome-next.jgi.doe.gov/info/Osativa_v7_0). The genome sequences of maize were downloaded from Phytozome database (https://phytozome-next.jgi.doe.gov/info/Zmays_RefGen_V4). The datasets supporting the results of this article are included in the article and Additional files.
Declarations
Ethics approval and consent to participate
Experimental research and field studies on plants including the collection of plant material are comply with relevant guidelines and regulation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yujiao Wang and Xianyong Lu contributed equally to this work.
Contributor Information
Benli Jiang, Email: 543806899@qq.com.
Jiabao Zhu, Email: 13955611798@139.com.
References
- 1.Arora RK. Job’s-tears (coix lacryma-jobi) - a minor food and fodder crop of northeastern India. Econ Bot. 1977;31(3):358–366. doi: 10.1007/BF02866887. [DOI] [Google Scholar]
- 2.Woo JH, Li D Fau-Wilsbach K, Wilsbach K Fau-Orita H, Orita H Fau-Coulter J, Coulter J Fau-Tully E, Tully E Fau-Kwon TK, et al. Coix seed extract, a commonly used treatment for cancer in China. Cancer Biol Ther. 2007;6(12):2005–2011. [DOI] [PubMed]
- 3.Cai Z, Liu H, He Q, Pu M, Chen J, Lai J, et al. Differential genome evolution and speciation of Coix lacryma-jobi L. and Coix aquatica Roxb. hybrid guangxi revealed by repetitive sequence analysis and fine karyotyping. BMC Genomics. 2014;15(1):1025. [DOI] [PMC free article] [PubMed]
- 4.Fu YH, Yang C, Meng Q, Liu F, Shen G, Zhou M, et al. Genetic diversity and structure of Coix lacryma-jobi L. from its world secondary diversity center, southwest China. Int J Genomics. 2019:9815697. [DOI] [PMC free article] [PubMed]
- 5.Yu J, Wang X, Yao XA-O, Wu X. Safety Evaluation of heavy metal contamination and pesticide residues in coix seeds in Guizhou province, China. Foods. 2022;11(15):2286. [DOI] [PMC free article] [PubMed]
- 6.Yuan G, Qian Y, Ren Y, Guan Y, Wu X, Ge C, et al. The role of plant-specific VQ motif-containing proteins: an ever-thickening plot. Plant Physiol Bioch. 2021;159:12–16. doi: 10.1016/j.plaphy.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Xu K, Wang P. Genome-wide identification and expression analysis of the VQ gene family in Cucurbita pepo L. Peerj. 2022;10:e12827. doi: 10.7717/peerj.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Chen J, Yang J, Yu Y, Yang Y, Wang W. Identification, characterization and expression analysis of the VQ motif-containing gene family in tea plant (Camellia sinensis) BMC Genomics. 2018;19(1):710. doi: 10.1186/s12864-018-5107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Zhou Y Fau-Yang Y, Yang Y Fau-Chi Y-J, Chi Yj Fau-Zhou J, Zhou J Fau-Chen J-Y, Chen Jy Fau-Wang F, et al. Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol. 2012;159(2):810–825. [DOI] [PMC free article] [PubMed]
- 10.Wang M, Vannozzi A, Wang G, Zhong Y, Corso M, Cavallini E, et al. A comprehensive survey of the grapevine VQ gene family and its transcriptional correlation with WRKY proteins. Front Plant Sci. 2015;6:417. doi: 10.3389/fpls.2015.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei R, Li X, Ma Z, Lv Y, Hu Y, Yu DJPJ. Arabidopsis WRKY2 and WRKY34 transcription factors interact with VQ20 protein to modulate pollen development and function. Plant J. 2017;91(6):962–976. doi: 10.1111/tpj.13619. [DOI] [PubMed] [Google Scholar]
- 12.Pan J, Wang H, Hu Y, Yu D. Arabidopsis VQ18 and VQ26 proteins interact with ABI5 transcription factor to negatively modulate ABA response during seed germination. Plant J. 2018;95(3):529–544. doi: 10.1111/tpj.13969. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Zhang L, Ji Y, Jing Y, Li L, Chen Y, et al. Arabidopsis SIGMA FACTOR BINDING PROTEIN1 (SIB1) and SIB2 inhibit WRKY75 function in abscisic acid-mediated leaf senescence and seed germination. J Exp Bot. 2021;73(1):182–196. doi: 10.1093/jxb/erab391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Z, Tian J, Fang H, Fang L, Xu X, He F, et al. A VQ-motif-containing protein fine-tunes rice immunity and growth by a hierarchical regulatory mechanism. Cell Rep. 2022;40(7):111235. doi: 10.1016/j.celrep.2022.111235. [DOI] [PubMed] [Google Scholar]
- 15.Ding H, Yuan G, Mo S, Qian Y, Ge C. Genome-wide analysis of the plant-specific VQ motif-containing proteins in tomato (Solanum lycopersicum) and characterization of SlVQ6 in thermotolerance. Plant Physiol Bioch. 2019;143:29–39. doi: 10.1016/j.plaphy.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Chen L, Wang H, Zhang L, Wang F, Yu D. Transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013;74(3):730–745. doi: 10.1111/tpj.12159. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Kwon SI, Choi C, Lee H, Ahn I, Park SR, et al. expression analysis of rice VQ genes in response to biotic and abiotic stresses. Gene. 2013;529(2):208–214. doi: 10.1016/j.gene.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Weibin S, Haiming Z, Xiangbo Z, Lei L, Jinsheng L. Genome-wide identification of VQ motif-containing proteins and their expression profiles under abiotic stresses in maize. Front Plant Sci. 2016;6(281):1177. doi: 10.3389/fpls.2015.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Wei F, Cheng S, Ma L, Wang H, Zhang M, Mao G, Lu J, Hao P, Ahmad A, Gu L, Ma Q, Wu A, Wei H, Yu S. A comprehensive analysis of cotton VQ gene superfamily reveals their potential and extensive roles in regulating cotton abiotic stress. BMC Genomics. 2020;21(1):795. doi: 10.1186/s12864-020-07171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng X, Wang Y, Xiong R, Gao Y, Yan H, Xiang Y. A Moso bamboo gene VQ28 confers salt tolerance to transgenic Arabidopsis plants. Planta. 2020;251(5):99. doi: 10.1007/s00425-020-03391-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Zhou Y, Zhai H, He S, Liu Q. A novel sweetpotato WRKY transcription factor, IbWRKY2, positively regulates drought and salt tolerance in transgenic Arabidopsis. Biomolecules. 2020;10(4):506. doi: 10.3390/biom10040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Hu Y, Pan J, Yu DJR. Arabidopsis VQ motif-containing proteins VQ12 and VQ29 negatively modulate basal defense against Botrytis cinerea. Science Report. 2015;5(1):14185. doi: 10.1038/srep14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pecher P, Eschen-Lippold L, Herklotz S, Kuhle K, Naumann K, Bethke G, et al. The Arabidopsis thaliana mitogen-activated protein kinases MPK3 and MPK6 target a subclass of 'VQ-motif'-containing proteins to regulate immune responses. New Phytol. 2014;203(4):592–606. doi: 10.1111/nph.12817. [DOI] [PubMed] [Google Scholar]
- 24.Lai Z, Li Y, Wang F, Cheng Y, Fan B, Yu JQ, et al. Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell. 2011;23(10):3824–3841. doi: 10.1105/tpc.111.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Jing BY, Li AJ, Xu CG, Lina BR. Arabidopsis VQ-motif-containing protein 29 represses seedling de-etiolation by interacting with PIF1. Plant Physiol. 2014;164(4):2068–2080. doi: 10.1104/pp.113.234492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing Y, Lin R. The VQ motif-containing protein family of plant-specific transcriptional regulators. Plant Physiol. 2015;169(1):371–378. doi: 10.1104/pp.15.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong QL, Duan DY, Zheng WQ, Huang D, Wang Q, Yang J, et al. Overexpression of MdVQ37 reduces drought tolerance by altering leaf anatomy and SA homeostasis in transgenic apple. Tree Physiol. 2022;42(1):60–174. doi: 10.1093/treephys/tpab098. [DOI] [PubMed] [Google Scholar]
- 28.Ye YJ, Xiao YY, Han YC, Shan W, Fan ZQ, Xu QG, et al. Banana fruit VQ motif-containing protein5 represses cold-responsive transcription factor MaWRKY26 involved in the regulation of JA biosynthetic genes. Science Report. 2016;6(1):23632. doi: 10.1038/srep23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaoyuan Z, Fengde W, Jingjuan L, Qian D, Yihui Z, Huayin L, et al. Genome-wide identification and analysis of the VQ motif-containing protein family in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Int J Mol Sci. 2015;16(12):28683–28704. [DOI] [PMC free article] [PubMed]
- 30.Wang Y, Liu H, Zhu D, Gao Y, Yan H, Y X. Genome-wide analysis of VQ motif-containing proteins in Moso bamboo (Phyllostachys edulis). Planta. 2017;246(1):165–181. [DOI] [PubMed]
- 31.Jiang SY, Sevugan M, Ramachandran S. Valine-glutamine (VQ) motif coding genes are ancient and non-plant-specific with comprehensive expression regulation by various biotic and abiotic stresses. BMC Genomics. 2018;19(1):342. doi: 10.1186/s12864-018-4733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Sun P, Li Y, Liu Y, Yu J, Ma X, et al. Hierarchically aligning 10 legume genomes establishes a family-level genomics platform. Plant Physiol. 2017;174(1):284–300. doi: 10.1104/pp.16.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Shi J, Cai Z, Huang Y, Jin W. Evolution and domestication footprints uncovered from the genomes of coix. Mol Plant. 2019;13(2):295–308. doi: 10.1016/j.molp.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Zheng J, Li H, Guo Z, Zhuang X, Huang W, Mao C, et al. Comprehensive identification and expression profiling of the VQ motif-containing gene family in Brassica juncea. Biology. 2022;11(12):1814. doi: 10.3390/biology11121814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Wang K, Han Y, Yan L, Zheng Y, Bi Z, et al. Genome-wide analysis of the VQ motif-containing gene family and expression profiles during phytohormones and abiotic stresses in wheat (Triticum aestivum L.). BMC Genomics. 2022;23(1):292. [DOI] [PMC free article] [PubMed]
- 36.Uji Y, Kashihara K, Kiyama H, Mochizuki S, Gomi K. Jasmonic Acid-Induced VQ-Motif-Containing Protein OsVQ13 influences the OsWRKY45 signaling pathway and grain size by associating with OsMPK6 in rice. Int J Mol Sci. 2019;20(12):2917. doi: 10.3390/ijms20122917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling L, Qu Y, Zhu J, Wang D, Guo C. Genome-wide identification and expression analysis of the VQ gene family in Cicer arietinum and Medicago truncatula. PeerJ. 2020;8(2):e8471. doi: 10.7717/peerj.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Li J, Zhang Z, Zhang Q, Li X, Xiao J, et al. OsVQ1 links rice immunity and flowering via interaction with a mitogen-activated protein kinase OsMPK6. Plant Cell Rep. 2021;40(10):1989–1999. doi: 10.1007/s00299-021-02766-6. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Liu X, Yang D, Yin Z, Jiang Y, Ling H, et al. A comprehensive identification and expression analysis of VQ motif-containing proteins in sugarcane (Saccharum spontaneum L.) under phytohormone treatment and cold stress. Int J Mol Sci. 2022; 23(11):6334 [DOI] [PMC free article] [PubMed]
- 40.Cheng X, Yao H, Cheng Z, Tian B, Gao C, Gao W, et al. The wheat gene TaVQ14 confers salt and drought tolerance in transgenic Arabidopsis thaliana plants. Front Plant Sci. 2022;13:870586. doi: 10.3389/fpls.2022.870586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang XY, Guo T, Li J, Chen Z, Guo B, An XM. Genome-wide analysis of the MYB-related transcription factor family and associated responses to abiotic stressors in populus. Int J Biol Macromol. 2021;191:359–76. doi: 10.1016/j.ijbiomac.2021.09.042. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. [DOI] [PMC free article] [PubMed]
- 43.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C, Chen H, Zhang Y, Thomas HR, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Altschul S, Madden T, Schäffer A, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST (Basic Local Alignment Search Tool) and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanc G, Wolfe KH. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 2004;16(7):1667–1678. doi: 10.1105/tpc.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequences of coix were downloaded from Coge database (https://genomevolution.org/coge/OrganismView.pl?dsgid=54616). The genome sequences of A. thaliana were downloaded from Phytozome database (https://phytozome-next.jgi.doe.gov/info/Athaliana_TAIR10). The genome sequences of rice were downloaded from Phytozome database (https://phytozome-next.jgi.doe.gov/info/Osativa_v7_0). The genome sequences of maize were downloaded from Phytozome database (https://phytozome-next.jgi.doe.gov/info/Zmays_RefGen_V4). The datasets supporting the results of this article are included in the article and Additional files.