SUMMARY:
A microencapsulated, cell-based IL-2 cytokine factory was recently developed, and the safety and efficacy of this platform in a mouse model of mesothelioma was demonstrated. This platform has the potential to overcome current challenges in the delivery of therapeutic cytokines for cancer immunotherapy.
In this issue of Clinical Cancer Research, Nash and colleagues engineered a cell-based interleukin-2 (IL-2) cytokine factory (RPE-mIL2) and demonstrated pre-clinical safety and efficacy of this immunotherapy in a mouse model of an aggressive mesothelioma.1 Current treatment options for mesothelioma include chemotherapy, radiation, and surgical resection. However, the efficacies of these therapies are limited, given that localized malignant pleural mesothelioma possesses a 5-year survival rate of only 20%.2 Recently, clinical trials using immune checkpoint inhibitors for malignant mesothelioma have demonstrated encouraging results, yet safe and effective delivery of these therapeutics remains a challenge due to the adverse immune events associated with their systemic administration.
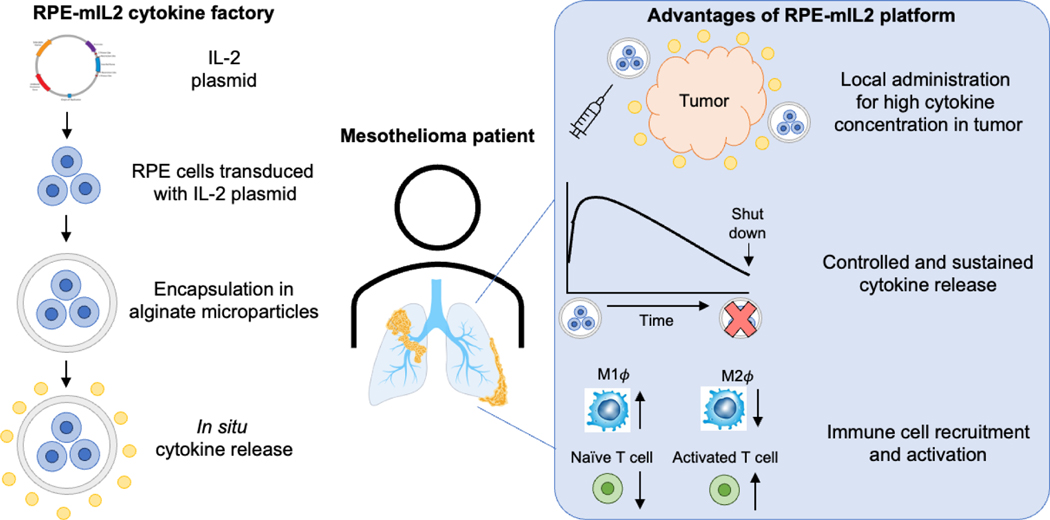
To address this therapeutic gap, Nash et al. developed the RPE-mIL2 platform (Figure 1), which consists of alginate microparticle-encapsulated retinal pigmented epithelial cells designed to stably express IL-2. In this study, treatment with RPE-mIL2 monotherapy significantly reduced tumor burden in a malignant mesothelioma mouse model, while complete eradication of tumor burden and protection against recurrence was observed in mice treated with a combination of RPE-mIL2 and an FDA-approved anti-PD1 checkpoint therapy. Immune profiling of mice treated with RPE-mIL2 demonstrated activation of both the innate and adaptive immune responses against tumors. Additional pharmacokinetic and safety studies in rats exhibited that a modified cytokine factory that secretes human IL-2 (RPE-hIL2) can be safely administered to the intraperitoneal or pleural cavity and is well-tolerated without adverse clinical events, aberrant histopathological features, or divergence from normal serum chemistries.
Figure 1.
The cytokine factories developed by Nash and colleagues consist of polymer encapsulated human retinal pigmented epithelial cells engineered to stably express mouse IL-2 (RPE-mIL2) or human IL-2 (RPE-hIL2). Xenogeneic engineered cells are protected from the host immune system by alginate hydrogel microencapsulation. In vivo, engineered cells secrete IL-2 and cytokine molecules escape from the pores of the hydrogel via diffusion. As demonstrated in a mouse model of mesothelioma, these cytokine factories enable controlled IL-2 secretion in a local compartment with limited leakage into the systemic circulation. Through recruitment and activation of the innate and adaptive immune systems against tumor cells, these cytokine factories were shown to be safe and effective as either a monotherapy or as an enhancer of novel checkpoint inhibitor potency. In the future, these cytokine factories have the potential to be translated to the clinic for the treatment of mesothelioma and other localized solid tumors.
Local delivery of pro-inflammatory cytokines – like IL-2 – enables the use of cytokines in cancer immunotherapy for several reasons. First, spatiotemporal control of cytokine delivery facilitates preferential therapeutic activity within the tumor microenvironment. Indeed, several cytokine immunotherapy clinical trials have failed as a result of narrow therapeutic windows due to dose-limiting systemic toxicity, which the RPE-mIL2 platform appears to avoid.3 Second, there is increasing evidence that a locally-initiated cytokine immune response can generate systemic antitumor immunity.4 In a rechallenge experiment, Nash et al. observed this effect, whereby animals treated with RPE-mIL2 and anti-PD1 combination therapy were protected from tumor recurrence, suggesting the generation of systemic, anti-cancer immunologic memory. Third, cytokine delivery directly to solid tumors can overcome tumor-supporting immunosuppression, including a physically dense stroma and a milieu of pro-tumor chemokines and cells.5 In the present study, IL-2 concentration in the tumor compartment was 150-fold higher in comparison to control animals after combination therapy. The authors also observed both a significant activation of tumor-specific CD4+ and CD8+ T cells and a phenotypic shift from anti-inflammatory M2 macrophages to pro-inflammatory M1 macrophages. Taken together, these results suggest that RPE-mIL2 successfully delivers a therapeutically relevant dose of IL-2 to the tumor microenvironment and subsequently generates an effective anti-tumor immune response.
Delivery of cytokines can be divided into three general strategies. Immunocytokine conjugates are the most common delivery platform consisting of a cytokine fused to a tumor-binding antibody fragment, which enhances tumor localization. However, these modified cytokines are typically less potent, have altered bioactivity, and may still induce systemic toxicity through interaction with circulating immune cells.6 Intra-tumoral delivery of genetic material encoding for cytokines allows for their expression by transduced cells within the tumor microenvironment. Major pharmaceutical companies, including Moderna and BioNTech, have taken this approach to clinical trials, utilizing non-viral lipid nanoparticle (LNPs) platforms to encapsulate messenger RNA (mRNA) encoding IL-12 for intra-tumoral cancer immunotherapy.7,8 Despite this, transfection of mammalian host cells remains a challenge, and off-target toxicities from both the delivery carrier and the cargo are a possibility.9 The third major approach involves controlled release of a cytokine from a sustained delivery system. These delivery platforms can be engineered to control the release of cytokines directly to the tumor without reduction in bioactivity, systemic reaction, or required production by host cells. For example, Schukur et al. previously designed microencapsulated designer cells that dynamically interface with metabolic cytokine levels to trigger the release of anti-inflammatory cytokines.10 In contrast, the delivery platform produced by Nash and colleagues does not depend on receiving a stimulus from the host and secretes an appropriate dose of de novo cytokines for a controlled time period. Unlike recombinant cytokines that can be easily administered intravascularly, a limitation of controlled release systems is their relatively large size, which necessitates an invasive procedure for therapeutic introduction. Notably, the authors of the present study demonstrate successful administration of RPE-hIL2 via intrapleural catheters in porcine cadavers. Since many patients with mesothelioma develop pleural effusions that require intrapleural catheter drainage, introduction of cytokine factories via this route limits morbidity associated with introducing an additional invasive procedure, further justifying the clinical translatability of RPE-hIL2 for this disease.
The cytokine factory engineered by Nash and colleagues has high potential for the clinical translation of cytokine-based immunotherapy for patients with mesothelioma. Current standard-of-care for these patients involves chemotherapy, radiation, and surgical debulking; however, the overall recurrence rate for these patients remains high. In fact, in a study published in the European Journal of Cardiothoracic Surgery, 78% of mesothelioma patients treated with induction chemotherapy followed by extra-pleural pneumonectomy were found to have recurrent disease.11 For these patients and those that are platinum-resistant, IL-2 cytokine factories have the potential to act as either a monotherapy through induction of tumor cytotoxicity or as an enhancer for novel checkpoint inhibitors by mediating T cell exhaustion.
More broadly, the cytokine factories developed in the present study can be applied to treat a wide range of cancers. The authors’ work builds upon a previous study where the RPE-mIL2 platform was used to deliver IL-2 intraperitoneally and eradicate tumors in ovarian and colorectal cancer mouse models.12 Based on this preclinical data, AVB-001 developed by the biotechnology company Avenge Bio recently received FDA clearance as an Investigational New Drug (IND) with human clinical trials beginning with a cohort of ovarian cancer patients in late 2022 (NCT05538624). An additional clinical application of this platform includes the treatment of appendiceal cancer. These tumors are most commonly neuroendocrine in origin but can penetrate through the appendicular wall and spread into the peritoneal cavity in the form of mucinous depositions, resulting in the often-lethal clinical condition pseudomyxoma peritonei. Since this disease process occupies a single compartment, like the other tumor models in which RPE-hIL2 demonstrated success, it has the potential to be treated with cytokine factory-mediated IL-2 monotherapy or in combination with the current standard-of-care therapies, hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery.
The high promise of cytokine-based immunotherapies has never been realized, primarily due to the inability to deliver a therapeutic dose of cytokine without systemic toxicity. Although many cytokine therapies are currently being studied in clinical trials, for this reason, only two cytokine immunotherapies have been FDA-approved.13 To overcome this challenge, Nash et al. elegantly engineer an IL-2 cytokine factory and provide significant pre-clinical burden of proof for its safety and efficacy in the treatment of mesothelioma. This highly modular, clinically translatable system unlocks the possibility to deliver a diverse array of cytokines in a similar manner, providing a new therapeutic avenue for patients with difficult to treat cancer.
ACKNOWLEDGMENTS:
R.P. was supported by a National Heart, Lung, and Blood Institute (NHLBI) Ruth L. Kirschstein Pre-Doctoral National Research Service Award (1F30HL162465-01A1). K.L.S. was supported by a National Science Foundation (NSF) Graduate Research Fellowship (Award 1845298). R.P., K.L.S., and M.J.M. were supported by a National Institutes of Health (NIH) Director’s New Innovator Award (DP2 TR002776), a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), additional funding from the NIH (NCI R01 CA241661, NCI R37 CA244911, NIDDK R01 DK123049), and an NSF CAREER Award (CBET-2145491).
Footnotes
Conflict of Interest/Disclosures: The authors declare no conflict of interests or disclosures.
REFERENCES:
- 1.Nash AM, Aghlara-Fotovat S, Castillo B, Hernandez A, Pugazenthi A, Lee HS, et al. Activation of adaptive and innate immune cells via localized Interleukin-2 cytokine factories eradicates mesothelioma tumors. Clin. Cancer Res. CCR-22-1493 (2022) doi: 10.1158/1078-0432.CCR-22-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries (2000–2018), National Cancer Institute, DCCPS, Surveillance Research Program, 2021. [Google Scholar]
- 3.Berraondo P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiam-Galvez KJ, Allen BM & Spitzer MH Systemic immunity in cancer. Nat. Rev. Cancer 21, 345–359 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valkenburg KC, de Groot AE & Pienta KC Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol 15, 366–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neri D. Antibody-cytokine fusions: versatile products for the modulation of anti-cancer immunity. Cancer Immunol. Res 7, 348–354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotz C, Wagenaar TR, Gieseke F, Bangari DS, Callahan M, Cao H, et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 13, eabc7804 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Hewitt SL, Bailey D, Zielinski J, Apte A, Musenge F, Karp R, et al. Intratumoral IL12 mRNA Therapy Promotes TH1 Transformation of the Tumor Microenvironment. Clin. Cancer Res. 26, 6284–6298 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Wu J, Zhou H, Yuan A, Zhang X, Xu F, et al. Local Gene Delivery for Cancer Therapy. Curr. Gene Ther. 11, 423–432 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Schukur L, Geering B, Charpin-El Hamri G. & Fussenegger M. Implantable synthetic cytokine converter cells with AND-gate logic treat experimental psoriasis. Sci. Transl. Med. 7, 318ra201–318ra201 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Kostron A, Friess M, Crameri O, Inci I, Schneiter D, Hillinger S, et al. Relapse pattern and second-line treatment following multimodality treatment for malignant pleural mesothelioma. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 49, 1516–1523 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Nash AM, Jarvis MI, Aghlara-Fotovat S, Mukherjee S, Hernandez A, Hecht AD, et al. Clinically translatable cytokine delivery platform for eradication of intraperitoneal tumors. Sci. Adv. 8, eabm1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S. & Margolin K. Cytokines in Cancer Immunotherapy. Cancers 3, 3856–3893 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]