Abstract
Background
Anti-melanoma differentiation-associated protein 5-positive dermatomyositis (MDA5+ DM) is characterized by a life-threatening complication of rapidly progressive interstitial lung disease (RP-ILD). Early prediction of RP-ILD can enhance diagnostic accuracy and therapeutic efficacy. This study was conducted to develop a nomogram model for predicting RP-ILD in patients with MDA5+ DM.
Material/Methods
We retrospectively analyzed 53 patients with MDA5+ DM, of whom 21 patients were diagnosed with RP-ILD between January 2018 and January 2021. Univariate analysis (t test, Mann-Whitney U test, chi-squared test, or Fisher’s exact test) and receiver operating characteristic (ROC) analysis were used to select candidate variables. Multivariate logistic regression analysis was conducted to construct a prediction model, which was subsequently transformed into a nomogram. ROC analysis, calibration curve and decision curve analysis were performed to evaluate the model’s performance. The bootstrapping method (resampling=500) was used for internal validation.
Results
We successfully established a nomogram, called the CRAFT model, to predict RP-ILD in MDA5+ DM patients. The model included 4 variables, namely C-reactive protein-to-albumin ratio, red blood cell distribution width-coefficient of variation, fever status, and CD3+ T cells. The model presented high predictive power and a good performance in calibration curve and decision curve analysis. In addition, the model had a good predictive ability in internal validation.
Conclusions
The CRAFT model could help to predict RP-ILD in patients with MDA5+ DM.
Keywords: Dermatomyositis; IFIH1 Protein, Human; Lung Diseases, Interstitial; Nomograms
Background
Anti-melanoma differentiation-associated protein 5-positive dermatomyositis (MDA5+ DM) is a unique subgroup of idiopathic inflammatory myopathy, commonly presenting hallmark DM skin lesions and relatively rare muscle involvement, namely clinically amyopathic DM (CADM) [1]. Rapidly progressive interstitial lung disease (RP-ILD), the most important pulmonary complication in patients with MDA5+ DM, has a rapidly progressive clinical course and poor responsiveness to immunosuppressive treatment, which result in a high 6-month mortality rate [2–4]. Multiple survival curves for RP-ILD descend sharply within the first 6 months after disease onset, while stabilizing in the following years, implying that patients with RP-ILD could achieve long-term survival as long as they got through the crisis period of the first 6 months [2–4]. Hence, it is crucial to predict high-risk patients who are likely to develop RP-ILD before serious symptoms appear, in order to pursue appropriate management and improve prognosis. When a patient with MDA5+ DM is rated as high-risk for developing RP-ILD by multiple risk factors at admission, more frequent high-resolution computed tomography (HRCT) should be performed and more intensive immunosuppressive therapy should be initiated.
In recent years, multiple studies have identified a variety of risk factors for predicting RP-ILD in patients with MDA5+ DM. Some literature focused on the clinical characteristics and found that MDA5+ DM patients with high age [5,6], male sex [5], and fever [6,7] had a greater risk for developing RP-ILD, whereas arthralgia decreased that risk [7]. Others pay attention to serum biomarkers, and the following were identified as predictive risk factors: C-reactive protein (CRP) [5], ferritin [5,7,8], neutrophil-to-lymphocyte ratio [6], lactate dehydrogenase [6,7,9], alanine aminotransferase [7], carcinoembryonic antigen [7–9], carbohydrate antigen 153 [7], cytokeratin 19 fragment [10], lymphopenia [7,10], elevated B cells [7], Krebs von den Lungen-6 [11], interluekin-15 [12], higher titers of anti-MDA5 antibody [5,13], and anti-Ro 52 antibody [5,13–15]. The genetic marker of theWDFY4 variant was also found to be an independent risk factor for RP-ILD in patients with MDA5+ DM [16].
A single indicator fails to accurately predict a particular outcome, owing to limited predictive value; however, a prediction model incorporating several relevant risk factors may serve as a useful tool to enhance predictive power. To date, many prediction models associated with MDA5+ DM or RP-ILD have been established [6,17–21]. In their study, So et al proposed a prediction model in MDA5+ DM patients consisting of fever, lactate dehydrogenase, age, and neutrophil-to-lymphocyte ratio to stratify the risk of the RP-ILD complication [6]. However, the model presented low predictive power.
In the present study, we aimed to develop a prediction model based on baseline clinical characteristics and laboratory examinations by retrospectively using a group of patients with MDA5+ DM to early predict RP-ILD in MDA5+ DM patients. Subsequently, a nomogram was formulated for better visualization of intricate regression equations.
Material and Methods
Ethics Statement
This study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (protocol code UHCT221032). Written informed consent and assent was obtained from patients or their guardians.
Patients and Study Design
This was a single-center retrospective study aimed to develop a prediction model for predicting the risk possibility of RP-ILD in patients with MDA5+ DM using baseline clinical characteristics and laboratory test data. The study reviewed all first-diagnosed DM patients at the Department of Rheumatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan Union Hospital) between January 2019 and January 2022.
We included all patients who (1) were diagnosed with probable/definite DM based on the Bohan and Peter criteria [22] or CADM based on the Sontheimer’s criteria [23]; and (2) were positive for anti-MDA5 antibody. Included patients were excluded if (1) other connective tissue disorders, paraneoplastic dermatomyositis, active infection, or drug-induced ILD were confirmed; (2) inadequate demographic, clinical, and laboratory test information were retrieved; and (3) HRCT was not performed at admission or reexamined within 3 months from respiratory symptoms onset.
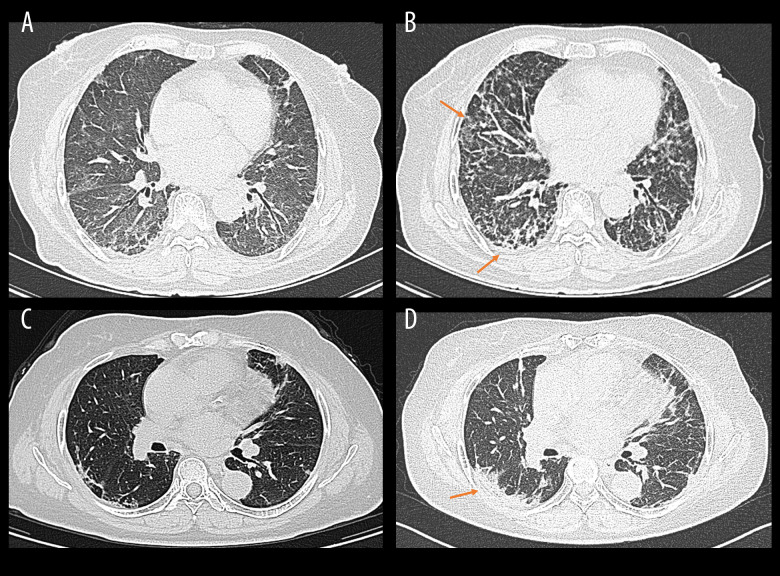
A total of 53 patients with MDA5+ DM were analyzed in this study according to the above inclusion and exclusion criteria. They were followed up for at least 3 months, and the primary outcome of interest was the occurrence of RP-ILD. HRCT was performed for all the included patients in this study when the DM/CADM was first diagnosed and reexamined at least once within 3 months after respiratory symptoms onset. ILD was diagnosed based on respiratory manifestations (dry cough, dyspnea, and Velcro rales) and characteristic findings on HRCT such as ground-glass opacity, consolidations, reticular pattern, or honeycombing [14]. RP-ILD was considered as progressive dyspnea and hypoxemia and a rapid worsening of characteristic HRCT findings within 3 months from respiratory symptoms onset. Representative HRCT images for RP-ILD in patients with MDA5+ DM is illustrated in Figure 1.
Figure 1.
Representative high-resolution computed tomography (HRCT) images for rapidly progressive interstitial lung disease (RP-ILD) in patients with anti-melanoma differentiation-associated protein 5-positive dermatomyositis (MDA5+ DM). (Image A and B were from the same patient, and image C and D were from another patient). (A, C) HRCT images when ILD was first diagnosed. (B) Rapidly aggravated ground-glass opacity (upper right) and reticular patterns (subpleural area) within 3 months compared to image A. (D) Rapidly aggravated consolidation within 3 months compared to image B (orange arrows). (Created by RStudio software, version 4.2.0.).
Data Collection
Clinical and laboratory test data of patients with MDA5+ DM at admission before initiating standardized treatment were defined as baseline data. Demographic information included patients’ age at onset and sex. Clinical data included fever (body temperature ≥38°C), heliotrope rash, Gottron’s sign/papules, erythematous facial rash, V sign, shawl sign, skin ulcers, periungual erythema, and proximal muscle weakness. Laboratory examination results included routine blood tests, biochemical and coagulation function, CRP, T lymphocyte subsets, and cytokines. The combined marker of CRP-to-albumin ratio (CAR) was calculated by dividing the value of CRP by the value of albumin. All patients’ baseline data were collected through the electronic medical record system.
CRP was measured by rate nephelometric assay using a specific protein analyzer (BN II, Siemens, Germany). Albumin was determined by ultraviolet-visible absorption spectroscopy using a fully automatic biochemical analyzer (AU5831, Beckman Coulter, USA). Red blood cell distribution width-coefficient of variation (RDW-CV) was estimated by the impedance method using an automated hematology analyzer (XN-9000, SYSMEX, Japan). The lymphocyte subsets were analyzed by flow cytometry assay on a flow cytometer (DxFLEX, Beckman Coulter, USA).
Anti-MDA5 antibody was identified by enzyme-linked immunosorbent assay (ELISA) using a commercial human MDA-5 Ab ELISA kit (Euroimmun, Germany). According to the analyzer’s interpretation of the results, (-) stands for negativity, (+) for weak positivity, (++) for moderate positivity, and (+++) for strong positivity.
Statistical Analyses
Division of Groups
All 53 patients with MDA5+ DM were divided into an RP-ILD group and non-RPILD group based on the occurrence of RP-ILD during follow-up.
Missing Value Processing
Variables with missing data larger than 10% were removed. The missing values of the remaining variables were multiply imputed by the missForest algorithm.
Variable Selection
Between-group differences of continuous variables with normal distribution were assessed by the t test, and between-group differences of continuous variables with non-normal distribution were analyzed using the Mann-Whitney U test. All continuous variable data were transformed into standardized values ranging from 0 to 1 by the min-max normalization according to the following algorithm: standardized value of continuous variable = [continuous variable – min (continuous variable)] / [max (continuous variable) – min (continuous variable)]. Categorical variables were expressed as number (percentage), and their between-group differences were measured using the chi-squared test without continuity correction (theoretical frequency ≥5), chi-squared test with continuity correction (1≤ theoretical frequency <5), or Fisher’s exact test (theoretical frequency <1). Statistically significant variables in univariate analysis were selected for ROC analysis, in which variables with an area under the ROC curve (AUC) >0.6 were regarded as candidate variables for model construction. Candidate continuous variables were converted to binary variables according to the cut-off values corresponding to the highest Youden index.
Construction of the Prediction Model
Stepwise multivariable logistic regression analysis was used to develop a prediction model for predicting RP-ILD in patients with MDA5+ DM. Then, the prediction model was converted to a nomogram.
Assessment and Internal Validation of the Prediction Model
The ROC analysis, calibration curve, and decision curve analysis were performed to evaluate the discriminatory capacity of the model. The bootstrapping method was used for internal validation. In the process of repeated model selection and AUC calculation, 500 bootstrapping samples were extracted. The corrected AUC was obtained by subtracting the average difference between the original AUC and the recalculated AUC from the original AUC.
All statistical analyses were conducted using SPSS software 22.0 (IBM Corp., Armonk, NY, USA), and RStudio software (version 4.2.0). Standard error, 95% CI, and P values of the AUC were generated automatically by SPSS software. The R packages used included “rms”, “mstate”, “ROCR”, “rmada”, “ggplot2”, “dplyr”, “grid”, “showtext”, “Cairo”, “rio”, “boot”, and “missForest”.
Results
Patient Characteristics
The study recruited 53 patients with MDA5+ DM, including 32 non-RPILD patients and 21 RP-ILD patients. Our data showed that the prevalence of RP-ILD reached 40% in MDA5+ DM patients. The baseline demographic and clinical characteristics of MDA5+ DM patients are shown in Table 1.
Table 1.
The baseline demographic and clinical characteristics of patients with MDA5+ DM (n=53).
| Characteristic | Non-RP-ILD Group (n=32) | RP-ILD Group (n=21) | Statistical methods | P value |
|---|---|---|---|---|
| Age, years, median (range) | 50 (42–57) | 57 (52–63) | Mann-Whitney U test | 0.011 |
| Sex, n (%) | ||||
| Female | 21 (66) | 13 (62) | ||
| Male | 11 (34) | 8 (38) | Chi-squared test | 0.78 |
| ILD, n (%) | 28 (86) | 21 (100) | Fisher’s exact test | 1 |
| CADM, n (%) | 10 (31) | 10 (48) | Chi-squared test | 0.23 |
| Anti-MDA5 titer, n (%) | ||||
| + | 11 (34) | 2 (10) | ||
| ++ | 4 (13) | 7 (33) | ||
| +++ | 17 (53) | 12 (57) | Chi-squared test | 0.32 |
| Clinical features, n (%) | ||||
| Heliotrope rash | 14 (44) | 12 (57) | Chi-squared test | 0.34 |
| Gottron’s sign/papules | 25 (78) | 15 (71) | Chi-squared test | 0.58 |
| Erythematous facial rash | 24 (75) | 9 (43) | Chi-squared test | 0.018 |
| Periungual erythema | 5 (06) | 8 (38) | Chi-squared test | 0.063 |
| Mechanic’s hands | 2 (6) | 6 (29) | Fisher’s exact test | 0.047 |
| V sign | 11 (34) | 5 (24) | Chi-squared test | 0.41 |
| Shawl Sign | 1 (3) | 5 (24) | Chi-squared test | 0.41 |
| Skin ulcers | 11 (34) | 3 (14) | Chi-squared test | 0.105 |
| Pruritus | 4 (13) | 4 (19) | Fisher’s exact test | 0.70 |
| Dry mouth | 5 (16) | 4 (19) | Fisher’s exact test | 1 |
| Oral ulcer | 5 (16) | 3 (14) | Fisher’s exact test | 1 |
| Raynaud’s phenomenon | 7 (22) | 2 (10) | Fisher’s exact test | 0.29 |
| Arthritis | 25 (78) | 13 (62) | Chi-square test | 0.2 |
| Morning stiffness | 6 (19) | 4 (19) | Fisher’s exact test | 1 |
| Myalgia | 13 (41) | 6 (29) | Chi-squared test | 0.37 |
| Proximal weakness | 22 (69) | 11 (52) | Chi-squared test | 0.23 |
| Fever | 4 (13) | 13 (62) | Chi-squared test | <0.001 |
| Dysphagia | 2 (6) | 3 (14) | Fisher’s exact test | 0.37 |
| Alopecia | 8 (25) | 1 (5) | Fisher’s exact test | 0.071 |
| Weight loss | 1 (3) | 2 (10) | Fisher’s exact test | 0.56 |
MDA5 – anti-melanoma differentiation-associated gene 5; RP-ILD – rapidly progressive interstitial lung disease; ILD – interstitial lung disease; CADM – clinically amyopathic dermatomyositis.
Variable Selection
A total of 99 variables (25 clinical features and 74 laboratory variables) were analyzed in this study. As Table 1 shows, 4 of the 25 clinical features (age, erythematous facial rash, mechanic’s hands, and fever) had differences between groups by univariate analysis. Subsequently, ROC analysis suggested that age and fever presented a good ability for early prediction of RP-ILD, and the AUCs were 0.71 (95% CI: 0.56–0.85, P=0.011) and 0.75 (95% CI: 0.60–0.89, P=0.003), respectively. However, mechanic’s hands and erythematous facial rash had no predictive value for RP-ILD in MDA5+ DM patients (P≥0.05; Table 2).
Table 2.
Receiver operating characteristic analysis of significant variables.
| Variables | AUC | Standard error | P values | 95% CI | |
|---|---|---|---|---|---|
| Upper | Lower | ||||
| Up variables/risk factors | |||||
| Mechanic’s hands | 0.612 | 0.082 | 0.173 | 0.45 | 0.773 |
| Fever | 0.747 | 0.074 | 0.003 | 0.603 | 0.891 |
| Age | 0.708 | 0.073 | 0.011 | 0.565 | 0.852 |
| CAR | 0.775 | 0.067 | 0.001 | 0.645 | 0.906 |
| FIB | 0.675 | 0.076 | 0.033 | 0.527 | 0.823 |
| TT | 0.679 | 0.074 | 0.029 | 0.533 | 0.824 |
| CRP | 0.769 | 0.069 | 0.001 | 0.635 | 0.904 |
| C4 | 0.694 | 0.076 | 0.018 | 0.546 | 0.842 |
| Down variables/protective factors | |||||
| Erythematous facial rash | 0.661 | 0.078 | 0.05 | 0.507 | 0.815 |
| PCT | 0.719 | 0.071 | 0.007 | 0.58 | 0.859 |
| RDW-SD | 0.732 | 0.072 | 0.005 | 0.592 | 0.872 |
| RDW-CV | 0.746 | 0.069 | 0.003 | 0.611 | 0.88 |
| ALB | 0.696 | 0.075 | 0.017 | 0.55 | 0.842 |
| Calcium | 0.641 | 0.077 | 0.084 | 0.49 | 0.793 |
| CD3+ T cells | 0.665 | 0.083 | 0.044 | 0.503 | 0.828 |
| Ts cells | 0.722 | 0.079 | 0.007 | 0.567 | 0.876 |
ROC – receiver operating characteristic; AUC – area under the ROC curve; CAR – C-reactive protein to albumin ratio; FIB – fibrinogen; TT – thrombin time; CRP – C-reactive protein; C4 – complement 4; PCT – procalcitonin; RDW-SD – red blood cell distribution width-standard deviation; RDW-CV – red blood cell distribution width-coefficient of variation; ALB – albumin.
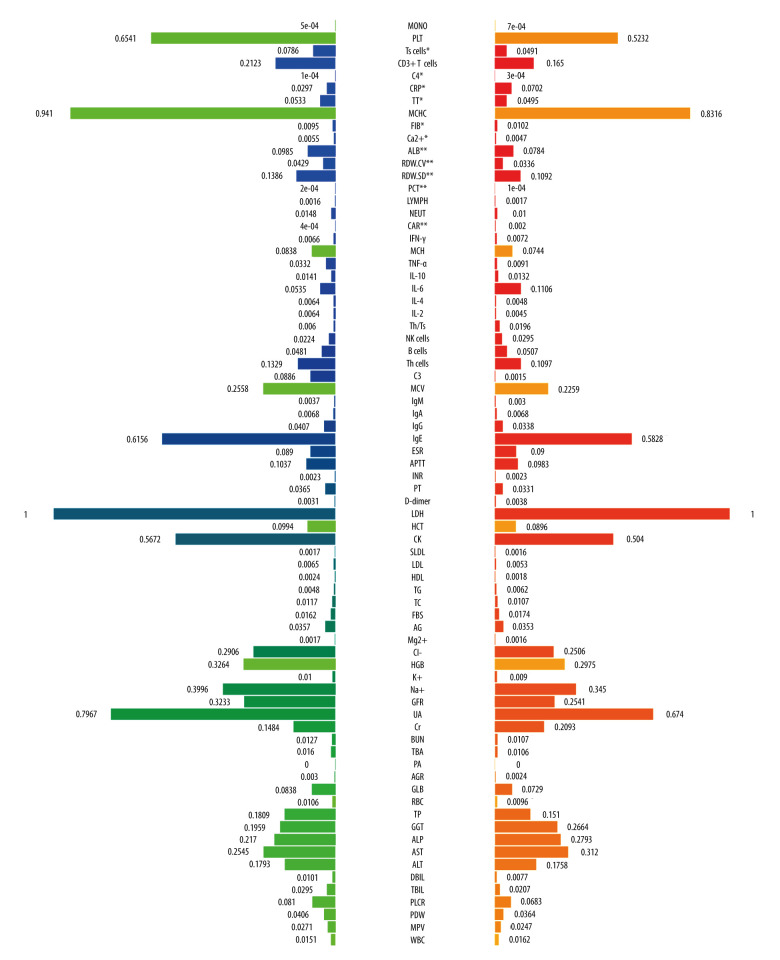
A total of 180 laboratory variables were collected in the study, of which 106 parameters with missing data greater than 10% were removed. Finally, 74 continuous variables of laboratory examination were used for subsequent analysis. Twelve variables had differences between the RP-ILD group and non-RPILD group by the t test or Mann-Whitney U test. Specifically, the levels of complement 4, CRP, fibrinogen, and CAR in the RP-ILD group were significantly higher than those in the non-RPILD group, while the levels of suppressor T cells, CD3+ T cells, thrombin time, calcium, albumin, RDW-CV, red blood cell distribution width-standard deviation (RDW-SD), and procalcitonin were significantly lower (Figure 2). Calcium level had no predictive value for RP-ILD in subsequent ROC analysis, while the remaining 11 continuous variables showed a good identification ability between RP-ILD and non-RPILD patients. CAR had the highest AUC, at 0.78 (95% CI: 0.65–0.91, P=0.001; Table 2).
Figure 2.
The comparison of baseline laboratory examinations between rapidly progressive interstitial lung disease (RP-ILD) and non-RP-ILD patients. The values represented standardized values ranging from 0 to 1 by the min-max normalization. * P<0.05 and ** P<0.001. (Created by RStudio software, version 4.2.0.). MONO – monocytes; PLT – platelet; Ts cells – suppressor T cells; C4 – complement 4; CRP – C-reactive protein; TT – thrombin time; MCHC – mean corpuscular hemoglobin concentration; FIB – fibrinogen; ALB – albumin; RDW-CV – red blood cell distribution width-coefficient of variation; RDW-SD – red blood cell distribution width-standard deviation; PCT – procalcitonin; LYMPH – lymphocytes; NEUT – neutrophils; CAR – C-reactive protein to albumin ratio; IFN-γ – interferon-γ; MCH – mean corpuscular hemoglobin; TNF-α – tumor necrosis factor-α; IL-10 – interleukin-10; IL-6 – interleukin-6; IL-4 – interleukin-4; IL-2 – interleukin-2; Th/Ts, helper T cells/suppressor T cells; NK cells, natural killer cells; Th cells, helper T cells; C3 – complement 3; MCV – mean corpuscular volume; IgM – immunoglobulin M; IgG – immunoglobulin G; IgA – immunoglobulin A; IgE – immunoglobulin E; ESR – erythrocyte sedimentation rate; APTT – activated partial thromboplastin time; INR – international normalized ratio; PT – prothrombin time; LDH – lactate dehydrogenase; HCT – hematocrit; CK – creatine kinase; SLDL – small dense low-density lipoprotein; LDL – low density lipoprotein; HDL – high density lipoprotein; TG – triglyceride; TC – total cholesterol; FBS – fasting blood sugar; AG – anion gap; HGB – hemoglobin; GFR – glomerular filtration rate; UA – uric acid; Cr – creatinine; BUN – blood urea nitrogen; TBA – total bile acid; PA – prothrombin activity; AGR – albumin-to-globulin ratio; GLB – globulin; RBC – red blood cell; TP – total protein; GGT – gamma-glutamyl transpeptidase; ALP – alkaline phosphatase; AST – aspartate transaminase; ALT – alanine transaminase; DBIL – direct bilirubin; TBIL – total bilirubin; PLCR – platelet large cell ratio; PDW – platelet distribution width; MPV – mean platelet volume; WBC – white blood cells. Units: monocytes, 109/L; PLT, 109/L; Ts cells,%; CD3+ T cells,%; C4, g/L; CRP, mg/L; TT, s; MCHC, g/L; FIB, g/L; ALB, g/L; RDW-CV,%; RDW-SD, fl; PCT, ng/mL; lymphocytes, 109/L; neutrophils, 109/L; IFN-γ, pg/mL; MCH, pg; TNF-α, pg/mL; IL-10, pg/mL; IL-6, pg/mL; IL-4, pg/mL; IL-2, pg/mL; Th/Ts,%; NK cells,%; B cells,%; Th cells,%; C3, g/L; MCV, fl; IgM, g/L; IgA, g/L; IgG, g/L; IgE, IU/mL; ESR, mm/h;APTT, s; PT, s; D-Dimer, mg/L; LDH, U/L; HCT,%; CK, U/L; SLDL, mmol/L; LDL, mmol/L; HDL, mmol/L; TG, mmol/L; TC, mmol/L; FBS, mmol/L; AG, mmol/L; Mg2+, mmol/L; Cl–, mmol/L; HGB, g/L; K+, mmol/L; Na+, mmol/L; GFR, ml/min; UA, μmol/L; Cr, μmol/L; BUN, mmol/L; TBA, μmol/L;; AGR,%; GLB, g/L; RBC, 1012/L; TP, g/L; GGT, U/L; ALP, U/L; AST, U/L; ALT, U/L; DBIL, μmol/L; TBIL, μmol/L; PLCR,%; PDW,%; MPV, fl; WBC, 109/L.
Construction of the Prediction Model for Predicting RP-ILD in MDA5+ DM Patients
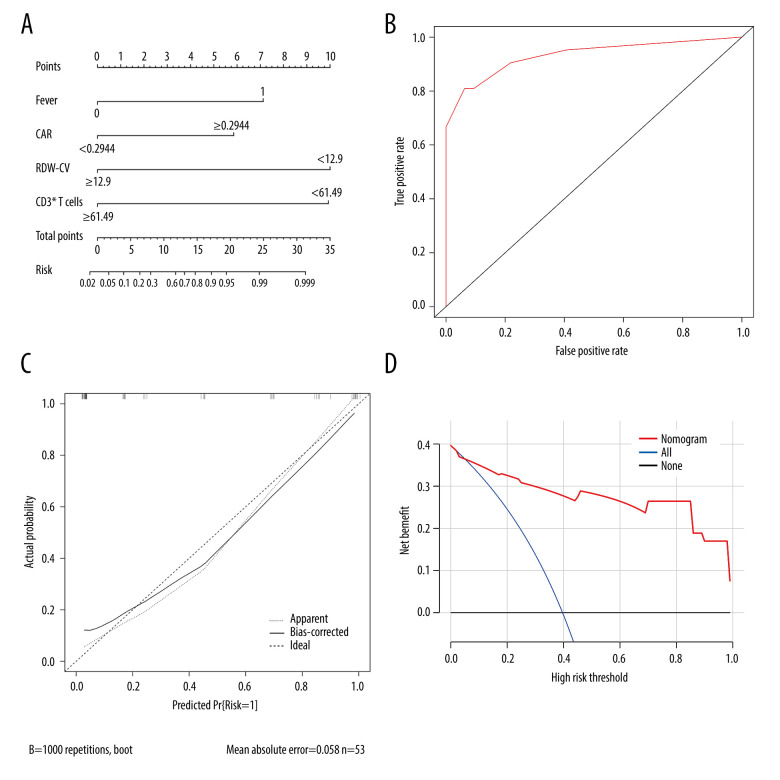
Based on the analysis of variable selection, we confirmed 13 candidate variables (2 clinical features and 11 laboratory examinations) for model construction, including fever, age, complement 4, CRP, fibrinogen, CAR, suppressor T cells, CD3+ T cells, thrombin time, albumin, RDW-CV, RDW-SD, and procalcitonin. Then, all the continuous variables were converted into binary variables based on their calculated cut-off values for subsequent analysis. The cut-off values of each variable are shown in Table 3. Multivariate stepwise logistic regression analysis was used to determine variables in the final model. According to the results, 4 important parameters (fever, CAR, RDW-CV, and CD3+ T cells) were identified (Table 4). Then, we integrated the 4 parameters to construct a nomogram to predict MDA5+ DM-RPILD (Figure 3A). After statistical evaluation, we found that the diagnostic nomogram possessed high predictive power (AUC=0.93; 95% CI: 0.86–0.99; sensitivity, 0.81; specificity, 0.94; P<0.05; Figure 3B). A good performance in calibration curve (Figure 3C) and decision curve analysis (Figure 3D) showed that the nomogram in this study had an advantage in identifying RP-ILD risk in patients with MDA5+ DM. The threshold probability was 0.42, which means that the nomogram might be useful in clinical practice.
Table 3.
The cut-off values of candidate variables.
| Variables | Values | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|
| Age | 50.5 | 0.857 | 0.438 | 0.419 |
| CAR | 0.2944 | 0.714 | 0.219 | 0.495 |
| FIB | 3.18 | 0.905 | 0.613 | 0.292 |
| TT | 20.35 | 0.476 | 0.161 | 0.315 |
| CRP | 10.6 | 0.714 | 0.188 | 0.526 |
| C4 | 0.255 | 0.600 | 0.226 | 0.374 |
| PCT | 0.265 | 0.556 | 0.105 | 0.451 |
| RDW-SD | 43.25 | 0.806 | 0.450 | 0.356 |
| RDW-CV | 12.9 | 0.969 | 0.524 | 0.445 |
| ALB | 28.5 | 0.969 | 0.667 | 0.302 |
| Calcium | 2.25 | 0.219 | 0.000 | 0.219 |
| CD3+ T cells | 61.485 | 0.929 | 0.550 | 0.379 |
| Ts cells | 23.78 | 0.679 | 0.200 | 0.479 |
CAR – C-reactive protein to albumin ratio; FIB – fibrinogen; TT – thrombin time; CRP – C-reactive protein; PCT – procalcitonin; RDW-SD – red blood cell distribution width-standard deviation; RDW-CV – red blood cell distribution width-coefficient of variation; ALB – albumin.
Table 4.
Significant indexes in the multivariable logistic regression analysis.
| Variables | β coefficient | Standard error | HR | 95% CI | P values | |
|---|---|---|---|---|---|---|
| Upper | Lower | |||||
| Fever | 2.371 | 1.027 | 10.706 | 1.430 | 80.178 | 0.021 |
| CAR | 1.949 | 0.956 | 7.020 | 1.078 | 45.735 | 0.042 |
| RDW-CV | −3.328 | 1.393 | 0.036 | 0.002 | 0.551 | 0.017 |
| CD3+ T cells | −3.302 | 1.266 | 0.037 | 0.003 | 0.440 | 0.009 |
CAR – C-reactive protein to albumin ratio; RDW-CV – red blood cell distribution width-coefficient of variation.
Figure 3.
Calibration and clinical utility of the nomogram for the prediction of rapidly progressive interstitial lung disease (RP-ILD) in patients with anti-melanoma differentiation-associated protein 5-positive dermatomyositis (MDA5+ DM). (A) Diagnostic nomogram for predicting RP-ILD in MDA5+ DM. (B) Receiver operating characteristic curve of the nomogram. (C) Calibration curve of the nomogram. (D) Decision curve analysis of the nomogram. CAR – C-reactive protein-to-albumin ratio; RDW-CV – red blood cell distribution width-coefficient of variation. (Created by RStudio software, version 4.2.0.).
Internal Validation of the Nomogram Prediction Model for MDA5+ DM-RPILD
To internally validate the predictive power of the nomogram model, the bootstrapping method (resampling=500) was used for internal validation. Figure 4 shows that the nomogram presented a good predictive ability for RP-ILD, and the AUC was 0.93 (95% CI: 0.85–0.99, P<0.05).
Figure 4.
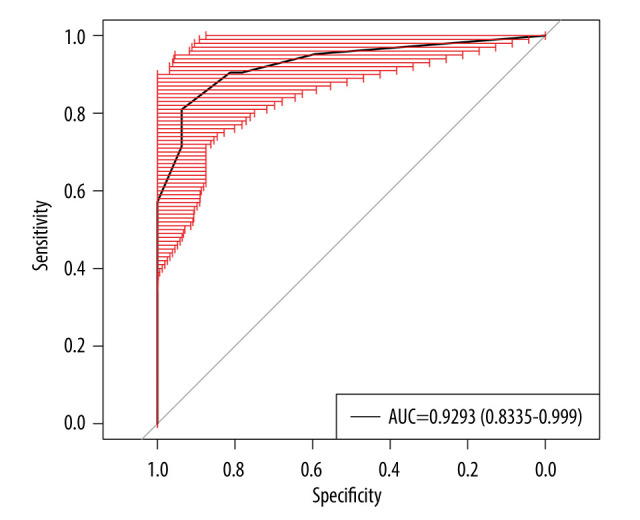
Internal validation of the prediction model using bootstrapping method (resampling=500). AUC – area under the ROC curve. (Created by RStudio software, version 4.2.0.).
Discussion
RP-ILD is a common and life-threatening complication of MDA5+ DM, with a reported prevalence of 39.8% to 63.6% [2,5–7]. Early diagnosis seems rather important, owing to the refractory nature of RP-ILD. Recently, with the growth of clinical data and advanced machine learning methodologies, an increasing number of studies have developed mathematical models based on several markers to improve the predictive performance for severe diseases. In the present study, we successfully developed a model for predicting RP-ILD in MDA5+ DM patients based on baseline clinical features and laboratory examinations. We named it the “CRAFT” model because it includes the CRP-to-albumin ratio, RDW-CV, fever status, and CD3+ T cells. The CRAFT model had a good performance in internal validation, with sensitivity of 0.81, specificity of 0.94, and AUC of 0.93 (95% CI: 0.86–0.99), suggesting its potential efficacy in clinical settings. Excitingly, the bootstrapping method (resampling=500) also showed a good predictive power in predicting RP-ILD. In addition, indicators in the CRAFT model are readily available, especially in primary hospitals or economically underdeveloped areas. When patients with MDA5+ DM are at greater risk of developing RP-ILD, early referral to senior hospitals for further treatment can reduce their mortality.
The model includes not only clinical characteristics and single laboratory indexes but also combined indexes such as CAR. CRP is a well-known inflammatory biomarker, and albumin is a visceral protein valuable for assessing status of nutrition and inflammation [24]. CAR is a better marker for inflammation and nutritional status than CRP or albumin alone. Qi et al found that CAR is a useful marker to predict diabetes mellitus in patients with pulmonary tuberculosis [25]. Interestingly, there have been no studies that investigated the correlation between CAR and RP-ILD, to the best of our knowledge. Our findings proved that CAR was an independent risk factor for RP-ILD in MDA5+ patients and had a good diagnostic value, with an AUC value of 0.78 (95% CI: 0.65–0.91).
The red blood cell distribution width (RDW), a simple index in peripheral blood circulation, reflects the size heterogeneity of red blood cells, commonly used for differential diagnosis of anemia [26]. Several studies have demonstrated that elevated RDW is correlated with inflammatory status in various diseases [27,28]. However, we found a downregulated RDW level in RP-ILD patients, similar to the results of Alparslan Bekir et al, in which lower RDW values were observed in patients with moderate and severe acute exacerbation of chronic obstructive pulmonary disease [29]. However, these conflicting results still need further studies. Furthermore, we demonstrated that decreased CD3+ T cells was an independent risk factor for the development of RP-ILD in patients with MDA5+ DM, consistent with a previous study [7]. Although the exact role of T cells in this serious complication remains unclear, Huang et al hypothesized that lymphocytes can escape from blood circulation to local lung tissue to participate in an immune response, rendering blood lymphopenia in MDA5+ DM-ILD [30]. Taken together, RP-ILD in MDA5+ DM is an intractable disease, with hyperinflammation being a key role in the disease pathogenesis. Our CRAFT model might indirectly predict RP-ILD incidence risk through immune inflammation status.
A few previous studies have demonstrated that elderly age at disease onset increased the risk for developing RP-ILD in patients with MDA5+ DM [5,6]. Consistent with past results, we found elderly patients were more prone to develop RP-ILD with MDA5+ DM (AUC: 0.71, 95% CI: 0.57–0.85, P=0.011). Nevertheless, we did not further include elderly age into our model, because no statistical significance was drawn in the multivariate logistic analysis. In addition, MDA5+ DM patients with fever reportedly easily developed RP-ILD, while the presence of arthralgia was negatively associated with RP-ILD incidence [7]. Our results showed that patients with RP-ILD manifested more fever and less erythematous facial rash than did non-RPILD patients, and fever was identified as an independent risk factor for RP-ILD in subsequent logistic regression analysis (AUC 0.75, 95% CI: 0.60–0.89, P=0.003). Yet, no more evidence of any difference was reported regarding the appearance of other cutaneous lesions, muscular involvement, and systemic symptoms between RP-ILD and non-RP-ILD patients; perhaps the reason lies in the small number of patients with this rare disease. Nevertheless, risk relevance of clinical manifestations still warrant investigation in future studies, since clinical features are the easiest to obtain in clinical practice.
Additionally, the CRAFT model might contribute to the therapeutic field. According to a previously described evidence-based study, the treatment of the first choice for RP-ILD associated with MDA5+ DM is a combination therapy of high-dose glucocorticoids and a calcineurin inhibitor (cyclosporine A or tacrolimus) with or without intravenouscyclophosphamide [31]. Some reports suggest that up-front administration of the combined triple therapy might significantly improve prognosis [32,33]. However, opportunistic infection of CMV reactivation can emerge during the treatment course, which may aggravate the severity of ILD [32,33]. Therefore, the pros and cons must be weighed before giving potent drugs. The CRAFT model might act as a suitable stratification tool for predicting patients with MDA5+ DM at higher risk of the complication RP-ILD, thus guiding clinical decision-making and improving prognosis.
Nevertheless, our study had several limitations. First and foremost, our study was retrospectively conducted at a Chinese single tertiary referral center, and the referral bias can make our findings less generalizable. Second, this study has a small sample size owing to the rarity of MDA5+ DM. The limited sample size and the lack of external validation can decrease the credibility of the nomogram and therefore limit its clinical application. Although the CRAFT model had a relatively good performance in internal validation, it is imperative to conduct multi-center external validation on a large scale before taking the CRAFT model into clinical application. Third, some previously identified predictors, such as ferritin, did not exhibit statistical significance in our study, owing to missing data. In addition, nearly one-third of patients with MDA5+ DM in our institution failed to perform pulmonary function tests, and we speculated that critical patients were not able to complete the examination, and patients with mild cases refused it for the lack of clinical symptoms. Overall, large-scale prospective studies are needed in the future to further explore more practical risk factors predicting the development of RP-ILD in patients with MDA5+ DM.
Conclusions
We successfully created the CRAFT model using a combination of baseline parameters in patients with MDA5+ DM to predict RP-ILD. The CRAFT model could potentially facilitate early recognition and management of this troublesome and death-threatening complication, and insights from our findings might be beneficial for better understanding of its complex pathogenesis.
Acknowledgements
We would like to thank all the anti-MDA5-positive patients and the researchers who took part in this clinical study.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Name of Department and Institution Where Work Was Done
Department of Rheumatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Nombel A, Fabien N, Coutant F. Dermatomyositis with anti-MDA5 antibodies: Bioclinical features, pathogenesis and emerging therapies. Front Immunol. 2021;12:773352. doi: 10.3389/fimmu.2021.773352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Li Y, Wu J, et al. Predictors of poor outcome of anti-MDA5-associated rapidly progressive interstitial lung disease in a Chinese cohort with dermatomyositis. J Immunol Res. 2020;2020:2024869. doi: 10.1155/2020/2024869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Gao X, Li Y, et al. Predictors and mortality of rapidly progressive interstitial lung disease in patients with idiopathic inflammatory myopathy: A series of 474 patients. Front Med (Lausanne) 2020;7:363. doi: 10.3389/fmed.2020.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 2012;51(9):1563–70. doi: 10.1093/rheumatology/kes102. [DOI] [PubMed] [Google Scholar]
- 5.Lv C, You H, Xu L, et al. Coexistence of anti-Ro52 antibodies in anti-MDA5 antibody-positive dermatomyositis is highly associated with rapidly progressive interstitial lung disease and mortality risk. J Rheumatol. 2023;50(2):219–26. doi: 10.3899/jrheum.220139. [DOI] [PubMed] [Google Scholar]
- 6.So J, So H, Wong VT, et al. Predictors of rapidly progressive interstitial lung disease and mortality in patients with autoantibodies against melanoma differentiation-associated protein 5 dermatomyositis. Rheumatology (Oxford) 2022;61(11):4437–44. doi: 10.1093/rheumatology/keac094. [DOI] [PubMed] [Google Scholar]
- 7.Zuo Y, Ye L, Chen F, et al. Different multivariable risk factors for rapid progressive interstitial lung disease in anti-MDA5 positive dermatomyositis and anti-synthetase syndrome. Front Immunol. 2022;13:845988. doi: 10.3389/fimmu.2022.845988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Gao C, Zhang C, et al. Tumor markers are associated with rapidly progressive interstitial lung disease in adult-dermatomyositis. Clin Rheumatol. 2022;41(6):1731–39. doi: 10.1007/s10067-022-06089-z. [DOI] [PubMed] [Google Scholar]
- 9.Han YY, Jiang T, Zhang ZH, et al. [risk factors of rapidly progressive interstitial lung disease in patients with anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023;54(2):422–25. doi: 10.12182/20230360201. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gui X, Ma M, Ding J, et al. Cytokeratin 19 fragment is associated with severity and poor prognosis of interstitial lung disease in anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 2021;60(8):3913–22. doi: 10.1093/rheumatology/keaa843. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Wang L, Sun Y, et al. Serum Krebs von den Lungen-6 concentrations reflect severity of anti-melanoma differentiation-associated protein 5 antibody positive dermatomyositis associated interstitial lung disease. Clin Exp Rheumatol. 2022;40(2):292–97. doi: 10.55563/clinexprheumatol/zmn18h. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Koga T, Furukawa K, et al. IL-15 is a biomarker involved in the development of rapidly progressive interstitial lung disease complicated with polymyositis/dermatomyositis. J Intern Med. 2021;289(2):206–20. doi: 10.1111/joim.13154. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, You H, Wang L, et al. Identification of three different phenotypes in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis patients: Implications for prediction of rapidly progressive interstitial lung disease. Arthritis Rheumatol. 2023;75(4):609–19. doi: 10.1002/art.42308. [DOI] [PubMed] [Google Scholar]
- 14.Xu A, Ye Y, Fu Q, et al. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology (Oxford) 2021;60(7):3343–51. doi: 10.1093/rheumatology/keaa786. [DOI] [PubMed] [Google Scholar]
- 15.Gui X, Shenyun S, Ding H, et al. Anti-Ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatology (Oxford) 2022;61(11):4570–78. doi: 10.1093/rheumatology/keac090. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Zhang X, Pu W, et al. WDFY4 polymorphisms in Chinese patients with anti-MDA5 dermatomyositis is associated with rapid progressive interstitial lung disease. Rheumatology (Oxford) 2023;62(6):2320–24. doi: 10.1093/rheumatology/kead006. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Tian Y, Liu S, et al. Risk factors and predictive model for dermatomyositis associated with rapidly progressive interstitial lung disease. Pharmgenomics Pers Med. 2022;15:775–83. doi: 10.2147/PGPM.S369556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Li Y, Wang Y, et al. A clinical risk model to predict rapidly progressive interstitial lung disease incidence in dermatomyositis. Front Med (Lausanne) 2021;8:733599. doi: 10.3389/fmed.2021.733599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gui X, Li W, Yu Y, et al. Prediction model for the pretreatment evaluation of mortality risk in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with interstitial lung disease. Front Immunol. 2022;13:978708. doi: 10.3389/fimmu.2022.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang ZM, Lin JZ, Tang AJ, et al. A matrix prediction model for the 6-month mortality risk in patients with anti-melanoma differentiation-associated protein-5-positive dermatomyositis. Front Med (Lausanne) 2022;9:860798. doi: 10.3389/fmed.2022.860798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Wu W, Zheng Y, et al. A computed tomography radiomics-based prediction model on interstitial lung disease in anti-MDA5-positive dermatomyositis. Front Med (Lausanne) 2021;8:768052. doi: 10.3389/fmed.2021.768052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–47. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 23.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. 2002;46(4):626–36. doi: 10.1067/mjd.2002.120621. [Erratum in: J Am Acad Dermatol. 2002;46(5):699] [DOI] [PubMed] [Google Scholar]
- 24.Evans DC, Corkins MR, Malone A, et al. ASPEN Malnutrition Committee. The use of visceral proteins as nutrition markers: An ASPEN position paper. Nutr Clin Pract. 2021;36(1):22–28. doi: 10.1002/ncp.10588. [Erratum in: Nutr Clin Pract. 2021;36(4):909] [DOI] [PubMed] [Google Scholar]
- 25.Yu Q, Weng W, Luo H, et al. The novel predictive biomarkers for type 2 diabetes mellitus in active pulmonary tuberculosis patients. Infect Drug Resist. 2022;15:4529–39. doi: 10.2147/IDR.S377465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q, Weng W, Luo H, et al. The novel predictive biomarkers for type 2 diabetes mellitus in active pulmonary tuberculosis patients. Infect Drug Resist. 2022;15:4529–39. doi: 10.2147/IDR.S377465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herraez I, Bento L, Del Campo R, et al. Prognostic role of the red blood cell distribution width (RDW) in Hodgkin lymphoma. Cancers (Basel) 2020;12(11):3262. doi: 10.3390/cancers12113262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Öztürk ZA, Ünal A, Yiğiter R, et al. Is increased red cell distribution width (RDW) indicating the inflammation in Alzheimer’s disease (AD)? Arch Gerontol Geriatr. 2013;56(1):50–54. doi: 10.1016/j.archger.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Alparslan Bekir S, Tuncay E, Gungor S, et al. Can red blood cell distribution width (RDW) level predict the severity of acute exacerbation of chronic obstructive pulmonary disease (AECOPD)? Int J Clin Pract. 2021;75(11):e14730. doi: 10.1111/ijcp.14730. [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Ren F, Luo L, et al. The characteristics of lymphocytes in patients positive for anti-MDA5 antibodies in interstitial lung disease. Rheumatology (Oxford) 2020;59(12):3886–91. doi: 10.1093/rheumatology/keaa266. [DOI] [PubMed] [Google Scholar]
- 31.Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, et al. MEDRA5 (Spanish MDA5 Register) group (listed contributors at the end of the article) Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum. 2020;50(4):776–90. doi: 10.1016/j.semarthrit.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–98. doi: 10.1002/art.41105. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda KM, Yoshizaki A, Kuzumi A, et al. Combined immunosuppressive therapy provides favorable prognosis and increased risk of cytomegalovirus reactivation in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. J Dermatol. 2020;47(5):483–89. doi: 10.1111/1346-8138.15274. [DOI] [PubMed] [Google Scholar]