ABSTRACT
Surveillance helps us identify and monitor strains with zoonotic potential. A tracheal swab from a pelican on a Peruvian beach was H5N1 positive (clade 2.3.4.4b) using Oxford Nanopore’s MinION platform. The near-complete genome sequence of strain VFAR-140 will aid us in understanding avian influenza epidemiology and spread.
ANNOUNCEMENT
Highly pathogenic avian influenza (HPAI) caused by the H5N1 virus, a member of the family Orthomyxoviridae and genus Influenza, is an epidemic disease that causes significant economic losses (1). Seabird colonies, with their high population density, are particularly vulnerable to HPAI (2).
Tracheal swabs were collected from a dead pelican displaying respiratory symptoms consistent with avian influenza virus (AIV) in December 2022 in Tambo de Mora District (Chincha Province, Ica, Peru). The bird exhibited gasping, sneezing, and neurological symptoms such as opisthotonus. The swabs were analyzed at FARVET’s biosecurity level III (BSL-3) laboratory. A reverse transcription-quantitative PCR (qRT-PCR) assay targeting the M gene (3) was used to confirm the presence of AIV, while also screening for other respiratory viruses (4–6).
To isolate AIV H5N1 (VFAR-140), one positive tracheal swab sample was centrifuged (4,500 rpm); the supernatant was filtered (0.22 μm) and inoculated into 10-day-old specific pathogen-free (SPF) embryonated eggs. The allantoic fluid (AF) was collected, and the M (3), HA (7), and NA (8) genes were amplified for typing. A hemagglutination assay (9) was performed on the AF, and a titer of 1:512 was obtained. VFAR-140 was concentrated and purified from 200 mL of infected AF using ultracentrifugation (18,000 rpm for 16 h at 4°C), followed by 25% sucrose gradient ultracentrifugation (27,000 rpm for 6 h). VFAR-140 was then resuspended in 200 μL of 1× Dulbecco’s phosphate buffered saline (DPBS), and the RNA was extracted using the RNeasy Plus microkit (Qiagen). A cDNA library was generated using the direct cDNA sequencing kit (SQK-DCS109; Oxford Nanopore Technologies) and sequenced on the MinION Mk1b instrument (Oxford Nanopore Technologies) using the FLO-MIN106 flow cell (Oxford Nanopore Technologies).
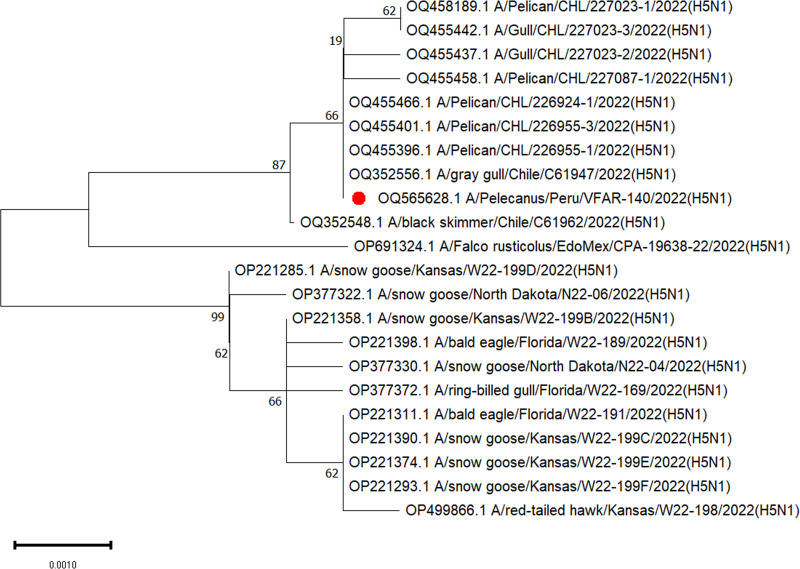
Default parameters were used for all software unless otherwise specified. Base calling was performed using Guppy v.6.3.7 (HAC model) (10). Fastq files were taxonomically assigned using the Fastq WIMP pipeline (11) with Kraken2 using the k2_standard_20210517 database (Galaxy v.2.0.8_beta+galaxy0) (12, 13) and visualized using Krona (Galaxy v.2.6.1.1) (12, 14). Adapters were trimmed using Porechop (Galaxy v.0.2.4+galaxy0) (12, 15). De novo assembly of all reads was performed using Raven (Galaxy v.1.8.0+galaxy0) (12, 16). A BLAST (17) analysis was used to identify four segments of AIV (segments 1, 2, 4, 5), and we selected a genome with complete coding sequences (A/gray gull/Chile/C61947/2022[H5N1]) as the reference genome. We subsequently mapped all reads against the reference sequence using BWA-MEM (Galaxy v.0.7.17.2) (12, 18) to obtain the final VFAR-140 genome sequences. The depth and coverage were determined using SAMtools (Galaxy v.1.15.1+galaxy0) (12, 19) and visualized using weeSAM v.1.6 (20). The identified positions were confirmed using BLASTn (17) analysis (Table 1). We obtained a total of 10,136 reads (N50, 3,353 bp; >Q5, 6,586 reads) from the isolate and successfully recovered eight segments of the VFAR-140 genome. Phylogenetic analysis of HA gene segment 4 was performed using MEGA v.11 (21) (Fig. 1).
TABLE 1.
BLAST comparison results of nucleotide sequences of all segments of isolate VFAR-140 with those of closely related strains
| Segment (gene[s]) | Length (bp) | %GC | Depth of coverage (×) | Most closely related strain | Identity (%) | Reference sequence GenBank accession no. |
|---|---|---|---|---|---|---|
| 1 (PB2) | 2,338 | 45 | 71.96 | A/pelican/CHL/226958-1/2022(H5N1) | 99.23 | OQ455420.1 |
| 2 (PB1; PB1-F2) | 2,341 | 43 | 108.75 | A/gray gull/Chile/C61947/2022(H5N1) | 99.62 | OQ352554.1 |
| 3 (PA; PA-X) | 2,231 | 44 | 357.49 | A/gray gull/Chile/C61947/2022(H5N1) | 99.86 | OQ352555.1 |
| 4 (HA) | 1,754 | 41 | 63.23 | A/gray gull/Chile/C61947/2022(H5N1) | 99.66 | OQ352556.1 |
| 5 (NP) | 1,552 | 47 | 85.25 | A/pelican/CHL/227023-1/2022(H5N1) | 98.65 | OQ455407.1 |
| 6 (NAa) | 1,411 | 44 | 136.43 | A/gull/CHL/227023-3/2022(H5N1) | 99.72 | OQ455402.1 |
| 7 (M2 and M1) | 1,006 | 49 | 50.28 | A/pelican/CHL/226955-1/2022(H5N1) | 99.70 | OQ455399.1 |
| 8 (NEPa; NS1) | 814 | 46 | 62.51 | A/Peruvian pelican/Chile/C61740/2022(H5N1) | 99.75 | OQ352544.1 |
Partial coding sequence; all others listed are complete coding sequences.
FIG 1.
Phylogenetic tree based on coding-complete sequences (CDS) of the HA gene. The tree was obtained using the neighbor-joining method (TN93+G), with 1,000 bootstrap replicates (complete deletion). The analysis included 22 nucleotide sequences. Isolate VFAR-140 is marked with a red dot.
The viral isolate VFAR-140 belongs to clade 2.3.4.4b H5N1 AIV, with the HPAI pathotype confirmed by the PLREKRRKRGLF cleavage site in HA (22). Molecular markers associated with increased polymerase activity in mice (23) were found in PB2 (L89V, G309D, T339K), and those associated with increased virulence in birds and mammals were found in PA (A515T) (24) and NS1 (P42S, V149A) (25, 26). However, no markers associated with mammalian adaptation were detected.
Data availability.
The eight obtained segments were deposited in GenBank (accession numbers OQ565625–OQ565632). The raw sequence reads were deposited under SRA accession number SRR23852495. The sequences were also deposited in EpiFlu at GISAID (EPI_ISL_17099964: EPI2441726 to EPI2441733).
ACKNOWLEDGMENTS
We thank Julio Ticona for their outstanding technical support. This research was conducted with funds from Farmacológicos Veterinarios SAC (FARVET SAC).
Contributor Information
Luis Tataje-Lavanda, Email: luis.tatajel@upsjb.edu.pe.
John J. Dennehy, Queens College Department of Biology
REFERENCES
- 1.Zhou J-Y, Shen H-G, Chen H-X, Tong G-Z, Liao M, Yang H-C, Liu J-X. 2006. Characterization of a highly pathogenic H5N1 influenza virus derived from bar-headed geese in China. J Gen Virol 87:1823–1833. doi: 10.1099/vir.0.81800-0. [DOI] [PubMed] [Google Scholar]
- 2.Boulinier T. 2023. Avian influenza spread and seabird movements between colonies. Trends Ecol Evol 38:391–395. doi: 10.1016/j.tree.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Spackman E. 2014. Avian influenza virus detection and quantitation by real-time RT-PCR. Methods Mol Biol 1161:105–118. doi: 10.1007/978-1-4939-0758-8_10. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Kong C, Cui X, Cui H, Shi X, Zhang X, Hu S, Hao L, Wang Y. 2013. Detection of infectious laryngotracheitis virus by real-time PCR in naturally and experimentally infected chickens. PLoS One 8:e67598. doi: 10.1371/journal.pone.0067598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seal BS, King DJ, Bennett JD. 1995. Characterization of Newcastle disease virus isolates by reverse transcription PCR coupled to direct nucleotide sequencing and development of sequence database for pathotype prediction and molecular epidemiological analysis. J Clin Microbiol 33:2624–2630. doi: 10.1128/jcm.33.10.2624-2630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meir R, Maharat O, Farnushi Y, Simanov L. 2010. Development of a real-time TaqMan RT-PCR assay for the detection of infectious bronchitis virus in chickens, and comparison of RT-PCR and virus isolation. J Virol Methods 163:190–194. doi: 10.1016/j.jviromet.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Yao L, Zhai F, Chen Y, Lei J, Bi Z, Hu J, Xiao Q, Song S, Yan L, Zhou J. 2018. Development and application of a triplex real-time PCR assay for the simultaneous detection of avian influenza virus subtype H5, H7 and H9. J Virol Methods 252:49–56. doi: 10.1016/j.jviromet.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Khan M, Măndoiu II. 2013. Neuraminidase subtyping of avian influenza viruses with PrimerHunter-designed primers and quadruplicate primer pools. PLoS One 8:e81842. doi: 10.1371/journal.pone.0081842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Organization for Animal Health. 2021. OIE terrestrial manual 2021.
- 10.Wick RR, Judd LM, Holt KE. 2019. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol 20:129. doi: 10.1186/s13059-019-1727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juul S, Izquierdo F, Hurst A, Dai X, Wright A, Kulesha E, Pettett R, Turner DJ. 2015. What’s in my pot? Real-time species identification on the MinION. bioRxiv. doi: 10.1101/030742. [DOI]
- 12.de Koning W, Miladi M, Hiltemann S, Heikema A, Hays JP, Flemming S, van den Beek M, Mustafa DA, Backofen R, Grüning B, Stubbs AP. 2020. NanoGalaxy: Nanopore long-read sequencing data analysis in Galaxy. Gigascience 9:giaa105. doi: 10.1093/gigascience/giaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood DE, Salzberg SLS, Venter C, Remington K, Heidelberg J, Halpern A, Rusch D, Eisen J, Wu D, Paulsen I, Nelson K, Nelson W, Fouts D, Levy S, Knap A, Lomas M, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers Y-H, Smith H, Tyson G, Chapman J, Hugenholtz P, Allen E, Ram R, Richardson P, Solovyev V, Rubin E, Rokhsar D, Banfield J, Huttenhower C, Gevers D, Knight R, Abubucker S, Badger J, Chinwalla A, Creasy H, Earl A, FitzGerald M, Fulton R, Giglio M, Hallsworth-Pepin K, Lobos E, Madupu R, Magrini V, et al. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ondov BD, Bergman NH, Phillippy AM. 2011. Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 12:385. doi: 10.1186/1471-2105-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wick RR. 2018. Porechop. Github. https://github.com/rrwick/Porechop/. Retrieved 28 February 2023.
- 16.Vaser R, Šikić M. 2021. Time- and memory-efficient genome assembly with Raven. Nat Comput Sci 1:332–336. doi: 10.1038/s43588-021-00073-4. [DOI] [PubMed] [Google Scholar]
- 17.Boratyn GM, Camacho C, Cooper PS, Coulouris G, Fong A, Ma N, Madden TL, Matten WT, McGinnis SD, Merezhuk Y, Raytselis Y, Sayers EW, Tao T, Ye J, Zaretskaya I. 2013. BLAST: a more efficient report with usability improvements. Nucleic Acids Res 41:W29–W33. doi: 10.1093/nar/gkt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H. 2017. BWA-MEM. GitHub. https://github.com/lh3/bwa. Accessed 28 February 2023.
- 19.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centre for Virus Research. 2023. weeSAM. https://github.com/centre-for-virus-research/weeSAM. Accessed 31 January 2023.
- 21.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Shesheny R, Moatasim Y, Mahmoud SH, Song Y, El Taweel A, Gomaa M, Kamel MN, Sayes ME, Kandeil A, Lam TTY, McKenzie PP, Webby RJ, Kayali G, Ali MA. 2022. Highly pathogenic avian influenza A(H5N1) virus clade 2.3.4.4b in wild birds and live bird markets, Egypt. Pathogens 12:36. doi: 10.3390/pathogens12010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Ishaq M, Prudence M, Xi X, Hu T, Liu Q, Guo D. 2009. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res 144:123–129. doi: 10.1016/j.virusres.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Hulse-Post DJ, Franks J, Boyd K, Salomon R, Hoffmann E, Yen HL, Webby RJ, Walker D, Nguyen TD, Webster RG. 2007. Molecular changes in the polymerase genes (PA and PB1) associated with high pathogenicity of H5N1 influenza virus in mallard ducks. J Virol 81:8515–8524. doi: 10.1128/JVI.00435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao P, Tian G, Li Y, Deng G, Jiang Y, Liu C, Liu W, Bu Z, Kawaoka Y, Chen H. 2008. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J Virol 82:1146–1154. doi: 10.1128/JVI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Jiang Y, Jiao P, Wang A, Zhao F, Tian G, Wang X, Yu K, Bu Z, Chen H. 2006. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J Virol 80:11115–11123. doi: 10.1128/JVI.00993-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The eight obtained segments were deposited in GenBank (accession numbers OQ565625–OQ565632). The raw sequence reads were deposited under SRA accession number SRR23852495. The sequences were also deposited in EpiFlu at GISAID (EPI_ISL_17099964: EPI2441726 to EPI2441733).