ABSTRACT
The quantitative detection of drug-resistance mutations in Mycobacterium tuberculosis (MTB) is critical for determining the drug resistance status of a sample. We developed a drop-off droplet digital PCR (ddPCR) assay targeting all major isoniazid (INH)-resistant mutations. The ddPCR assay consisted of three reactions: reaction A detects mutations at katG S315; reaction B detects inhA promoter mutations; and reaction C detects ahpC promoter mutations. All reactions could quantify 1%–50% of mutants in the presence of the wild-type, ranging from 100 to 50,000 copies/reaction. Clinical evaluation with 338 clinical isolates yielded clinical sensitivity of 94.5% (95% confidence interval [CI] = 89.1%–97.3%) and clinical specificity of 97.6% (95% CI = 94.6%–99.0%) compared with the traditional drug susceptibility testing (DST). Further clinical evaluation using 194 nucleic acid-positive MTB sputum samples revealed clinical sensitivity of 87.8% (95% CI = 75.8%–94.3%) and clinical specificity of 96.5% (95% CI = 92.2%–98.5%) in comparison with DST. All the mutant and heteroresistant samples detected by the ddPCR assay but susceptible by DST were confirmed by combined molecular assays, including Sanger sequencing, mutant-enriched Sanger sequencing and a commercial melting curve analysis-based assay. Finally, the ddPCR assay was used to monitor longitudinally the INH-resistance status and the bacterial load in nine patients undergoing treatment. Overall, the developed ddPCR assay could be an indispensable tool for quantification of INH-resistant mutations in MTB and bacterial loads in patients.
KEYWORDS: Mycobacterium tuberculosis, isoniazid resistance, quantification, heteroresistant, droplet digital PCR
INTRODUCTION
Isoniazid (INH) is a central component of combined first-line antituberculosis (TB) drug therapy. However, resistance to INH hinders its efficacy. Drug-resistant TB can occur at the onset of the disease or during disease progression owing to inadequate treatment (1). Heteroresistance represents a state when drug-resistant and -susceptible strains coexist, reflecting mixed infections or an intermediate process from “unfixed” to “full-blown” drug resistance in the Mycobacterium tuberculosis (MTB) population (2–5). De facto recognition of heteroresistance is embodied in the classical proportion method to determine the drug resistance status of TB. The proportion method relies on the ratio of the number of colonies obtained on drug-containing medium to the number of colonies obtained on drug-free medium. For INH, a critical proportion of 1% was used to differentiate between susceptible and resistant strains (6, 7). In this context, an alternative method for IHN-resistance determination should be able to detect minor resistant bacilli down to 1% in a sample; otherwise, heteroresistant samples could be misclassified as susceptible. Ideally, such an alternative should quantify the exact portion of the resistant bacilli in the sample to monitor the dynamic change in the resistant proportion for early warning before resistance occurs.
Although conventional drug susceptibility testing (DST) can meet the above-mentioned requirements, its use in clinical settings is severely impeded by its long turnaround time, which may last for months. Molecular assays can significantly shorten the turnaround time to hours; however, these assays are limited by their inability to detect resistant bacilli of low abundance in heteroresistant samples. For example, it has been reported that ≥10% of resistant organisms is required to be reliably detected by the line probe-based Genotype MTBDRplus assay (8). Sanger sequencing requires the presence of approximately 15%–20% of mutant bacilli to be detected as being heteroresistant (9, 10). Accordingly, both assays showed lower sensitivity in the detection of INH-resistant TB than conventional DST (11). In another example, the MeltPro TB/INH assay, an officially approved qualitative diagnostic assay for INH-resistant TB, could only detect 20% to 40% INH hetererosistence (12). Our laboratory developed an improved melting curve analysis assay, DeepMelt TB/INH, that can detect 1% INH heteroresistance in 104 MTB genomes/μL (13). However, this high sensitivity varied with the overall template concentrations. On the other hand, quantitative detection of heteroresistant TB remains largely unavailable for current molecular assays (11, 14, 15).
Droplet digital PCR (ddPCR) is a method that can achieve absolute quantification of the template and is particularly useful for the detection of rare mutations in the background of excess wild-type DNA (16). For example, Shallom et al. developed ddPCR assays for rapid detection of clarithromycin resistance in M. abscessus whereby both clarithromycin-susceptible and -resistant mixed populations could be quantified in clinical samples (17). However, a standard ddPCR assay usually detects one or few mutations in one reaction, and it is difficult to detect dozens of IHN-resistant mutations. Here, we developed a novel ddPCR approach to target multiple INH-resistant mutations using a drop-off probe design (18). The three ddPCR reaction-based assay could detect all major mutations located in katG S315, inhA promoter, and ahpC promoter, respectively. Meanwhile, this assay could detect INH heteroresistance down to 1% and reliably quantify both wild-type and mutant in a sample. Clinical evaluation with both clinical isolates and sputum samples demonstrated that the ddPCR assay had improved clinical sensitivity compared with the commercial MeltPro TB/INH assay. Moreover, its quantitative nature enabled longitudinal monitoring of INH resistance status and bacterial load in patients undergoing treatment.
MATERIALS AND METHODS
Reference strains.
One H37RV reference strain (ATCC 27294) and six phenotypically INH-resistant MTB strains of different mutations were used in the analytical study. All strains were stored at −80°C in a restricted access facility in our laboratory. Sanger sequencing was used to determine the mutation types of the six resistant strains: katG S315T (AGC→ACC), katG S315N (AGC→AAC), katG S315I (AGC→ATC), inhA promoter −15 C→T, ahpC promoter −30 C→T, and ahpC promoter −10 C→T.
The MTB genomic DNA (gDNA) of these isolates was extracted using the AxyPrep bacterial genomic DNA miniprep kit according to the manufacturer’s instructions (Axygen Scientific, Union City, CA, USA). The concentrations of the extracted gDNA were measured using an ND-2000 UV−VIS spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE). The concentration of each gDNA sample was adjusted to 5 ng/μL (~106 genomes/μL) using 10 mM Tris-HCl containing 1 mM EDTA, pH 8.5 (TE buffer) before detection.
Sample collections.
Two sample collections were used in the clinical study. The first collection comprised 338 archived MTB gDNA samples extracted from clinical isolates stored in our laboratory. DST of these clinical isolates was performed according to the standard proportion method using Lowenstein-Jensen (L-J) solid medium (19). The INH-resistant mutations in the gDNA samples were detected using the MeltPro TB/INH assay kit (Zeesan Biotech, Xiamen, China), as previously described (12). The second collection comprised 194 sputum samples from patients with positive MTB determined by nucleic acid testing (NAT) using a real-time PCR-based MTB diagnostic kit (Da’an Gene, Guangzhou, China). These archived sputum samples were obtained from Guangzhou Chest Hospital (Guangzhou, Guangdong, China) and use of them was reviewed and approved by the Institutional Review Board of Guangzhou Chest Hospital. A waiver of informed consent was obtained given the retrospective nature of the study. The sputum samples were subjected to the ddPCR assay and traditional DST.
For ddPCR assay, sputum MTB DNA was extracted using a Lab-Aid 824 MTB DNA Extraction kit (Zeesan Biotech) according to the instruction manual. Briefly, 500 μL of sputum was transferred to a 2-mL centrifuge tube. Thereafter, 1 mL of buffer T was added to the tube, which was briefly vortexed and left at room temperature for 15 min for sputum liquefaction. The liquefied sputum was centrifuged at 12 000 × g for 5 min. The precipitate was resuspended in 300 μL buffer F5 and incubated at 99°C for 10 min. The dissolved sample solution was then transferred to a Lab-Aid 824 cartridge (Zeesan Biotech) for automated nucleic acid extraction. The purified DNA was finally eluted in 120 μL of the eluent buffer and stored at −20°C before use.
For DST, the remaining sputum was decontaminated using the N-acetyl-l-cysteine/NaOH method for 15 min, and then neutralized with sterile phosphate-buffered saline (PBS, pH 6.8). After centrifugation at 3 000 × g for 15 min, the pellet was resuspended in 2 mL of PBS buffer. A 0.5-mL portion of the decontaminated samples was subjected to liquid culture using the MGIT 960 system (Becton, Dickinson, MD, USA). Drug susceptibility of the culture-positive isolates was determined using the standard proportion protocol on L-J solid medium. INH resistance was defined as the growth of more than 1% colonies on L-J medium containing the critical concentration of 0.2 μg/mL of INH compared to growth on the drug-free control medium (6).
Study cohort.
A longitudinal study was conducted from September 2019 to December 2021 at the Fuzhou Pulmonary Hospital (Fujian, China). The use of patient samples was reviewed and approved by the Institutional Review Board of Fuzhou Pulmonary Hospital. Nine patients diagnosed as pulmonary TB both clinically and experimentally (smear, culture, and NAT) were enrolled for treatment. According to the treatment guidelines established by the World Health Organization, patients received a combination of at least four effective antibiotics. Anti-TB therapy was administered for 6 to 12 months, including an intensive period of 2 to 3 months. Time-serial sputum samples were collected at baseline and 2, 4, 6, 8, 12, 16, 18, 24, 26, and 32 weeks after treatment. Fresh sputum samples were smeared and stained by acid-fast staining, and then examined for the presence of acid-fast bacilli under a light microscope, according to the Laboratory Science Procedure of Diagnostic Bacteriology in Tuberculosis (19). NAT was performed using the real-time PCR-based MTB diagnostic kit (Da’an Gene). The remaining sputum samples were subjected to liquid culture using the MGIT 960 system to determine the presence of MTB, and ddPCR assay to quantitatively detect the INH-resistant mutations and bacterial loads.
ddPCR assay.
The ddPCR assay was composed of three reactions. The primers and probes (see Table S1) were designed using Primer Premier software (version 5.0; Premier Biosoft, Palo Alto, CA, USA) and OligoAnalyzer Tool (https://sg.idtdna.com/pages/tools/oligoanalyzer), respectively, and synthesized by Sangon Biotech (Shanghai, China). ddPCR was conducted on a QX100 platform (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions. Briefly, a 20-μL ddPCR mix was prepared with 10 μL of 2×ddPCR Supermix for Probes (no dUTP), 900 nmol/L forward and reverse primers, 250 nmol/L probes, and 5 μL of DNA template. After droplet generation with 70 μL of droplet-generating oil, 40 μL of the emulsified PCR mixture (approximately 12,000 to 15,000 droplets) was transferred to a 96-well PCR plate and heat-sealed with foil sheets. PCR (PCR) was performed using an A300 Fast Thermal Cycler (Longgene, Hangzhou, China). The following thermal cycling program was employed: denaturation at 95°C for 10 min, followed by 40 cycles of 94°C for 30 s and 63°C for 1 min (ramp rate of 2.5°C per second), and a final 10-min hold at 98°C to deactivate the enzyme and stabilize the droplets. The endpoint fluorescence of each droplet was measured using a QX100 droplet reader (Bio-Rad Laboratories). The QuantaSoft v.1.7.4 software was used for quality control and preliminary analysis of the raw data. Cluster thresholding and quantification were performed with QuantaSoft v.1.7.4. For the drop-off assays, droplets were manually assigned as wild-type or mutant based on their fluorescence amplitude. The overall bacterial load in a sample was calculated by all the positive droplets. The mutant fraction (MF), as exemplified by the FAM-labeled probe, was calculated using the following equation: [HEXhighFAMlow copies/(HEXhighFAMhigh copies + HEXhighFAMlow copies)], wherein HEXhighFAMlow represents the mutant droplets and HEXhighFAMhigh represents the wild-type droplets.
Limit of blank (LOB) and limit of detection (LOD).
The template copy number ranges used in LOB determination were determined by referring to the estimated MTB number recorded in sputum samples and the need for robust detection of 1% heteroresistance as well as the droplet number in each ddPCR reaction provided by the instrument. The LOB was determined as previously described (18, 20–22). Briefly, the false-positive mean and associated standard deviation of the four drop-off probes were obtained by analyzing 20 replicates at 10,000, 5,000, and 1,000 copies/reaction of 100% H37RV gDNA, respectively. The 95% CI was calculated and used to define LOB as follows: LOB = mean false-positive + 95% CI (11). The LOD was determined by testing five replicates of samples in a series of mixtures containing 32%, 16%, 8%, 4%, 2%, 1%, 0.5%, 0.25%, and 0.125% mutations for the katG 315 codon (AGC→ACC), inhA promoter (-15 C→T), and ahpC promoter (-30 C→T, -10 C→T), respectively. The LOD was the lowest analyte concentration that could be reliably distinguished from the LOB.
DNA sequencing.
For DNA sequencing, three fragments of the katG, inhA promoter, and ahpC promoter were amplified by PCR, and the recovered amplicons were sent for Sanger sequencing (12). For mutant-enriched Sanger sequencing, the DeepMelt protocol was used for PCR amplification to produce the amplicons for Sanger sequencing (13). Briefly, during PCR amplification of both wild-type and mutant templates, a clamping probe was used to specifically block chain elongation of the wild-type template, but it did not interfere with chain elongation of the wild-type template. Therefore, the mutant template was enriched after PCR.
Statistical analysis.
The results of the clinical evaluation were analyzed using OpenEpi (v.3.01; A. G. Dean, K. M. Sullivan, and M. M. Soe) at 95% CI (23). Graph drawing was performed using GraphPad Software Prism 8.0 (GraphPad Software, CA, USA).
RESULTS
Design of the ddPCR assay.
The ddPCR INH resistance assays consisted of three reactions to detect all major mutations that confer INH resistance using a drop-off probe strategy. This strategy relies on a reference probe (REF) to quantify the total number of targets and a drop-off probe (detection probe), complementary to the wild-type, to discriminate between the wild-type and the mutant via suboptimal hybridization to the mutant (18). Reaction A contained a FAM-labeled drop-off probe targeting katG 315 and a HEX-labeled reference probe spanning an adjacent invariable region (Fig. 1A). Mutations in the katG 315 were detected as FAMlow/HEXhigh droplets with a vertical shift, which could be distinguished from the FAMhigh/HEXhigh double positive droplets of the wild-type sequence. The ΔTm of the drop-off katG probe for each mutation was > 5.5°C, resulting in a clear separation between wild-type and mutations droplets. Reaction B contained a HEX-labeled drop-off probe targeting the clustered mutations at positions -17 to -8 in the inhA promoter, and a FAM-labeled REF probe located 10 bp downstream of the drop-off probe within the same amplicon (Fig. 1B). Mutations in the inhA promoter were detected as FAMhigh/HEXlow droplets with a horizontal shift, which could be distinguished from the double positive droplets of the wild type. Reaction C contained a pair of drop-off probes to cover the scattered mutations in the ahpC promoter (24). The FAM-labeled probe was complementary to the wild-type sequence at positions -39 to -30, and the HEX-labeled probe was complementary to the wild-type at positions -15 to 4 (Fig. 1C). Unlike the standard drop-off probe design where one probe was used as the reference and another was used for the detection, the two probes in this reaction both served as the detection probe.
FIG 1.
Schematic illustration of the ddPCR assay for the detection of INH-resistant mutations. (A) Reaction A detects the hot spot mutations located in codon 315 of katG. (B) Reaction B detects the mutations located in the promoter region from -17 to -8 of inhA. (C) Reaction C detects the mutations located in the promoter region from -39 to -30 and -15 to 4 of ahpC.
The feasibility of the assay was assessed using gDNA of the reference strain H37Rv and strains with different mutation types at a concentration of 10 000 copies/reaction. Based on the results, all mutant signals could be clearly distinguished from wild-type signals (Fig. 1, right panels). The large Tm difference enabled some mutation types to be differentiated from other mutations according to the fluorescence amplitude. As shown in Fig. 1C, the mutations in the ahpC promoter region (-10 C→T, -9G→A, -32G→A, -39C→T, -30C→T) could be distinguished from each other due to the large Tm difference.
Analytical evaluation of the ddPCR assay.
First, we determined the ability of the ddPCR assays to quantify the MF of different mutations. A series of mixtures containing 50%, 10%, 5%, 1%, and 0% mutant DNA with a total template concentration of 10 000 copies/reaction was employed for this assessment. The mutations studied were katG 315 codon AGC→ACC, AGC→ATC, and AGC→AAC in reaction A, inhA promoter mutation -15 C→T in reaction B, and ahpC promoter mutations -30 C→T and -10 C→T in reaction C. All MFs were reliably quantified regardless of the mutation type (see Fig. S1 and S2).
To assess the quantitative performance of the ddPCR assay, we detected three replicates of six series of mixture composed of H37Rv (wild-type) and katG S315T (mutant) gDNA with overall concentrations of 100, 500, 1,000, 5,000, 10,000, and 50,000 copies/reaction, respectively, and each containing 50%, 25%, 10%, 5%, 2.5%, 1%, and 0.1% mutant, respectively. Excellent linear correlations were obtained between the observed and prepared MFs for all the six mixtures (R2 = 0.999 to 0.992) (see Fig. S3). These results further supported the reliability of the ddPCR assay for mutation quantification.
The LOB for each reaction was determined for each probe using the wild-type template. The false-positive signals at three different template concentrations, including 10,000, 5,000, and 1,000 copies/reaction, were detected. Based on the results, the LOBs obtained from the four probes in the three reactions ranged from 0.061% to 0.067% at 10,000 copies/reaction, 0.059% to 0.093% at 5,000 copies/reaction, and 0.184% to 0.21% at 1,000 copies/reaction (see Table S2).
We proceeded to determine the LOD of each reaction using a series of mixtures containing different MFs (32%, 16%, 8%, 4%, 2%, 1%, 0.5%, 0.25%, and 0.125%) with a template concentration of 10,000 copies/reaction. A linear relationship was observed for all studied mutations in the three reactions (Fig. 2). By referring to the corresponding LOB, the LOD was 0.25% for katG 315 codon (AGC→ACC) in reaction A, 0.25% for inhA promoter (-15 C→T) in reaction B, 0.25% for ahpC promoter (-10 C→T) in reaction B, and 0.5% for ahpC promoter (-30 C→T) in reaction C. Collectively, the ddPCR assay could detect INH heteroresistance lower than 1%, which is the threshold value for drug resistance defined by the traditional DST.
FIG 2.
Observed versus expected MFs in serial dilutions (32%, 16%, 8%, 4%, 2%, 1%, 0.5%, 0.25%, 0.125%) for the katG 315 codon (AGC→ACC), inhA promoter (-15 C→T), and ahpC promoter (-30 C→T, -10 C→T). Gray line, false-positive mean; Dashed line, limit of blank (LOB).
Clinical performance of the ddPCR assay.
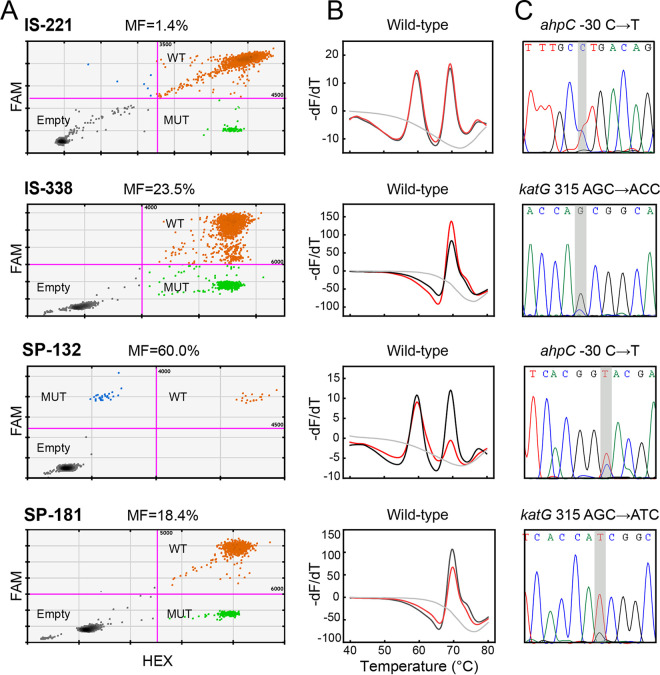
Clinical evaluation of the ddPCR assay using the first collection of 338 archived precharacterized DNA samples revealed that 94.5% (120/127) of the phenotypically resistant isolates were mutant, 97.6% (206/211) of the phenotypically susceptible isolates were wild type. Subsequent Sanger sequencing or mutant-enriched Sanger sequencing confirmed the results of the ddPCR assay, which yielded MFs ranging from 1.4% to 89.2% (Fig. 3 and Table S3). The ddPCR assay detected five more heteroresistant samples in addition to the 10 strains detected by MeltPro assay from the phenotypically resistant isolates and one more heteroresistant sample from the phenotypically susceptible isolates (Table 1). Therefore, the ddPCR assay had an overall increased sensitivity (90.6% versus 94.5%) with a marginally decreased specificity (98.1% versus 97.6%) compared with the MeltPro assay.
FIG 3.
Representative results of four heteroresistant samples. IS-221 and IS-338 were isolates. SP-132 and SP-181 were sputa. The result of each method is presented at the top of the plot. (A) ddPCR assay results. MF, mutant fraction; WT, wild type; MUT, mutant type. (B) MeltPro TB/INH assay results. Red lines and black lines represent the melting curves of the samples and WT control, respectively, and the gray line represents the no template control. (C) Mutant-enriched Sanger sequencing. The mutated bases are shaded in the sequencing results.
TABLE 1.
Clinical performance of the ddPCR assay for the detection of 338 clinical isolatesa
| Pilot | DST |
Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Accuracy (%) (95% CI) | Kappa value (95% CI) | ||
|---|---|---|---|---|---|---|---|
| R | S | Total | |||||
| ddPCR | |||||||
| MT | 106 | 4 | 110 | 94.5 (89.1–97.3) | 97.6 (94.6–99.0) | 96.4 (93.9–98.0) | 0.924 (0.818–1.031) |
| HET | 14 | 1 | 15 | ||||
| WT | 7 | 206 | 213 | ||||
| Total | 127 | 211 | 338 | ||||
| MeltPro | |||||||
| MT | 106 | 4 | 110 | 90.6 (84.2–94.5) | 98.1 (95.2–99.3) | 95.3 (92.4–97.1) | 0.898 (0.791–1.004) |
| HET | 9 | 0 | 9 | ||||
| WT | 12 | 207 | 219 | ||||
| Total | 127 | 211 | 338 | ||||
WT, wild type; MT, mutant type; HET, heteroresistance; R, resistance; S, sensitive.
Clinical evaluation with the second collection of 194 sputum samples revealed that 87.8% (43/49) of the INH-resistant samples were mutant, including four heteroresistant samples, and 96.6% (140/145) of the INH-susceptible samples were wild type. Compared with the MeltPro assay, the ddPCR assay detected two more heteroresistant samples from the INH-resistant samples and had a full concordance in the detection of the INH-susceptible samples. The two heteroresistant samples (SP-132 and SP-181) were confirmed using Sanger sequencing (Fig. 3 and Table S3). Thus, the ddPCR assay had an overall higher sensitivity than the MeltPro assay (87.8% versus 83.8%) with identical specificity (96.6%) (Table 2).
TABLE 2.
Clinical performance of the ddPCR assay for the detection of 194 sputum samplesa
| Pilot | DST |
Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Accuracy (%) (95% CI) | Kappa value (95% CI) | ||
|---|---|---|---|---|---|---|---|
| R | S | Total | |||||
| ddPCR | |||||||
| MT | 39 | 4 | 43 | 87.8 (75.8–94.3) | 96.5 (92.2–98.5) | 94.3 (90.1–96.8) | 0.849 (0.708–0.989) |
| HET | 4 | 1 | 5 | ||||
| WT | 6 | 140 | 146 | ||||
| Total | 49 | 145 | 194 | ||||
| MeltPro | |||||||
| MT | 39 | 4 | 43 | 83.7 (71.0–91.5) | 96.5 (92.2–98.5) | 93.3 (88.9–96.0) | 0.819 (0.678–0.959) |
| HET | 2 | 1 | 3 | ||||
| WT | 8 | 140 | 148 | ||||
| Total | 49 | 145 | 194 | ||||
WT, wild type; MT, mutant type; HET, heteroresistance; R, resistance; S, sensitive.
As ddPCR assay provided quantitative results for the target genes, the bacterial load in a sample could be easily calculated from all the positive droplets, including the wild-type and mutant. Assuming that katG, inhA promoter, ahpC promoter each presents as a single copy in the MTB genome, the bacterial loads of the 194 sputum samples were obtained using each gene. As expected, the three bacterial loads obtained closely correlated pairwise among the 194 samples (Fig. S4). The consistent bacterial loads obtained across three independent reactions with different targets confirmed the quantitative accuracy of the ddPCR assay. Using the katG results from Reaction A as an example, the median value of the bacterial load of the 194 sputum samples was 4.3 log 10 copies/mL, ranging from 1.0 to 6 log 10 copies/mL (Fig. S5). Strikingly, 42.3% (92/194) of the sputum samples had no more than 4 log 10 copies/mL, which is the minimum threshold for MTB smear microscopy testing (25).
Monitoring antituberculosis treatment responses.
The ability of the ddPCR assay to determine both the bacterial load and MF in a sample prompted us to monitor the antituberculosis treatment responses. Nine patients undergoing anti-TB treatment were recruited. Their bacterial loads were measured at baseline and specific time points after treatment initiation. For comparison, the results from the smear microscopy testing and culture method were also given.
The three drug-sensitive TB cases (patients number 1, number 2, and number 3) were all negative for INH-resistance mutations, showed a continuous decline in bacterial loads during the intensive phase, became MTB DNA-negative within 6 to 8 weeks, and remained negative throughout the consolidation phase (Fig. 4), indicating the efficacy of the treatment (Table S4). Symptom improvement was observed during the treatment. The above dynamic change in bacterial loads was also confirmed by the smear and culture methods, which however gave the negative result much earlier than the ddPCR assay.
FIG 4.
Longitudinal monitoring of the bacterial loads in the sputum samples from patients undergoing treatment. The blue shades represent the intensive phase of anti-TB treatment. PTB, pulmonary tuberculosis; EBTB, endobronchial tuberculosis; TBP, tuberculous pleurisy. S, sensitive tuberculosis; R, resistant tuberculosis; MDR, multidrug-resistant tuberculosis. Culture results: “+,” positive; and “-,” negative. Smear results of “1+/2+/3+/4+” represent the strength of positive results. ND indicates that no detection was performed.
Patient number 4 had tuberculous pleurisy, being negative for INH-resistance mutations, and showed a rapid decrease of bacterial load to negative within 3 weeks (Fig. 4). However, after 3 weeks, the bacterial load increased to 2.56 log 10 copies/mL (beyond the baseline), indicating poor response to the treatment. In the meantime, the patient had shortness of breath and chest pain, showing an obvious relation between the bacterial loads and clinical symptoms. Notably, neither the culture nor smear indicated an increased bacterial load during this period.
Patients number 5 and number 6 had diabetes (Fig. 4 and Table S4) and both were negative for INH-resistance mutations. A common feature of the two patients was the relatively high bacterial load at baseline (patient number 5: 5.34 log 10 copies/mL; patient number 6: 4.50 log 10 copies/mL). In week 3, the bacterial load of patient number 5 further increased to the maximal level of 5.41 log 10 copies/mL and patient number 6 increased to 4.91 log 10 copies/mL. Both patients exhibited TB DNA-positivity despite an overall decrease after treatment, indicating a poor response to the therapy during the entire treatment procedure that lasted 28 to 32 weeks.
Patients number 7, number 8, and number 9 were all diagnosed as drug-resistant TB (Fig. 4 and Table S4) and all had a katG S315T mutation according to the ddPCR assay. Patient number 7 was INH mono-resistant and became MTB-negative at week 8 after treatment with levofloxacin/rifampicin/pyrazinamide/ethambutol, obtaining a conversion time similar to that of drug-sensitive TB patients (number 1, number 2, and number 3). Patient number 8 had multidrug-resistant TB, but denied therapy switch from a sensitive TB regimen (isoniazide/rifampicin/pyrazinamide/ethambutol) to an alternative therapy for MDR patients for economic reasons. However, the bacterial load decreased to negative at week 12 with the standard regimen. Unfortunately, the patient was lost after week 16. Patient number 9 was also an MDR-TB patient who failed to respond to the standard treatment and then switched to advanced antibiotics (amikacin/moxifloxacin/pyrazinamide/ethambutol/clofazimine/cycloserine). The dynamic change of the bacterial load for this patient was similar to that for patients number 5 and number 6. After reaching the highest level in week 2, the bacterial load progressively decreased and finally became negative in week 16. This patient had the longest conversion time among all patients. Fortunately, the bacterial load remained negative after week 16.
DISCUSSION
We developed a ddPCR assay for INH-resistant mutations using a drop-off probe strategy. To the best of our knowledge, this is the first assay so far developed to detect all the major INH-resistant mutations. The ddPCR assay overcomes a common drawback of current molecular assays based on analog signal detection, as such detection might underestimate the heteroresistance rate owing to its qualitative nature and low sensitivity in detecting minor mutant bacilli (11, 14, 26). The established assay enabled the detection of less than 1% minor mutants and could accurately quantify MFs over a wide range. Clinical evaluations with both clinical isolates and sputum samples demonstrated that the ddPCR assay had clinical sensitivity higher than the MeltPro TB/INH assay with closely identical specificity.
Digital PCR used to be a low-throughput method for mutation detection because one allele-specific fluorogenic probe is required to identify one target allele, rendering it difficult to detect multiple alleles in one reaction (27). The drop-off strategy alleviates this problem by allowing one probe to detect multiple mutations within its binding region (18). In our assay, INH-resistant mutations located in katG and inhA promoter each could be detected using a pair of detection and reference probes. However, mutations in the ahpC promoter were scattered and could not be interrogated by a single probe. Owing to the low frequency of these mutations and the extremely low chance of having compound mutations at positions -39 to -30 and -15 to 4 (12, 13), two detection probes were designed without a reference probe. To obtain a large temperature difference (ΔTm) between the wild-type and mutant necessary to distinguish their droplets, all the detection probes were modified with locked nucleic acid, a synthetic nucleotide chemistry characterized by enhanced hybridization specificity via inclusion of a methylene bridge connecting the 2-oxygen and the 4-carbon atoms in the furanose ring (28). This modification allowed all target mutations to be clearly distinguished from the wild type.
One distinct advantage of ddPCR is its absolute quantitative capability, which provides an accurate mutant percentage from 1% to 50% over a wide template copy-number range (from 100 to 50 000) as exemplified in the detection of katG S315T. The ddPCR assay also had LODs for MF ranging from 0.25% to 0.5%, thus enabling reliable detection of the 1% mutant. The quantitative ability for heteroresistance of ddPCR assay is comparable to the proportion method for DST, which relies on the ratio of the number of colonies obtained on the drug-containing medium to the number of colonies obtained on the drug-free medium; this is in sharp contrast with current molecular assays based on analog signal detection, which markedly vary in their ability to detect heteroresistance (12, 13, 29). In fact, none of the current molecular assays could achieve the critical level of 1% (8, 12, 13, 29). The DeepMelt assay could detect 1% mutants; however, it varied with the overall amount of bacilli (13). Moreover, DeepMelt could not quantify resistant mutations. In this context, the ddPCR assay represents an upgraded molecular approach that is closest to the original concept for the determination of drug-resistant TB by the proportion method.
The increased sensitivity of ddPCR assay in mutation detection was observed in the clinical evaluation using two collections of samples. In the first collection of 338 bacterial isolates, 5 additional heteroresistant samples were identified by the ddPCR assay from the MeltPro assay. In the second collection of 194 sputa samples, the ddPCR assay identified two additional heteroresistant samples. The additionally detected heteroresistant samples were all confirmed by direct or mutant-enriched sequencing, demonstrating that heteroresistant samples containing low levels of mutant DNA missed by the MeltPro assay could be detected by the ddPCR assay. As previously observed, heteroresistant mutations could increase in frequency over the course of treatment and finally led to fixed resistance (3, 30–32), including cases originally identified at <1% frequency (33). However, due to the low sensitivity of the current methods for heteroresistance detection and the lack of molecular tools for absolute quantification, the prevalence of heteroresistant MTB varies among studies and the heteroresistant frequency remain largely unknown. For example, the prevalence of heteroresistance to INH varied from < 1% to > 5% (26). Our study revealed an overall heteroresistant prevalence of 10.2%, which is markedly larger than that previously reported (10, 34). In clinical settings, the increased sensitivity of ddPCR in heteroresistance detection may help to find emerging resistance early during treatment, improve cure rates and reduce risks of drug resistance development.
The ddPCR assay also provide information about the bacterial load in a sample by knowing the amount of mutant and wild type. Bacterial load is a direct biomarker of disease progression in patients and may reflect the severity of TB (35). We observed a wide range of MTB bacterial loads in patients’ sputa, spanning 6 orders of magnitude, indicating the wide diversity of the disease severity among patients with TB. As such, monitoring the bacterial load in patients during treatment could provide a direct measure of disease progression. Our longitudinal study uncovered many facts regarding TB patients during treatment. First, a correlation was found between the output bacterial load of ddPCR and disease symptoms. For example, patient number 4 had an elevated bacterial load in the later weeks of treatment, experienced shortness of breath and chest pain, suggesting poor response to treatment. For such patients with extrapulmonary TB, longitudinal monitoring and timely intervention of chemotherapy regimens must be strengthened during treatment. Second, hyperglycemic conditions severely interfered with the efficacy of anti-TB drugs (36). The bacterial load in such patients (patients number 5 and number 6) decreased slowly. Accordingly, the review and monitoring of the status of drug-resistant mutations must be increased to prevent the emergence of drug resistance. Finally, the bacterial load of INH mono-resistant TB patients (e.g., patient number 7) decreased more rapidly than that of MDR-TB patients (patients number 8 and number 9), further emphasizing the importance of INH-resistance testing. Owing to the limited number of patients recruited in this study, whether the above observations could be applicable to the general population warrants further validation. Despite this limitation, we anticipate that the adoption of a bacterial load detection provided by the ddPCR assay for longitudinal monitoring would improve the management of TB patients.
We admitted that, as a targeted detection method, the ddPCR INH resistance assay could only detect mutations in the probe binding region. In contrast, next generation sequencing (NGS) has the potential to detect simultaneously all possible variants at once and thus enables comprehensive evidence-based treatment plans (37). Moreover, the targeted deep sequencing is able to estimate the proportion of resistance-to-wild-type alleles ≤1% (38, 39). However, NGS assays are costly and time-consuming, preventing its routine use for rapid drug-resistance screening and longitudinal monitoring of response to treatment. In comparison, the ddPCR assay quantitatively reached the critical 1% proportion of the mutants, representing a conceptual return of molecular assays to the classical proportion method that defines drug-resistant TB. Considering the original DST and current analog signal-based molecular assays, the ddPCR assay could be regarded as the third generation of assay for TB resistance and an indispensable tool for the improvement of TB treatment.
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
This work was supported by the Xiamen Major Science and Technology Project (3502Z20191007), Basic Research Project of Shenzhen Basic Research Program (JCYJ20180306170526435), Xiamen Industry-University Research Project Subsidy Project (2022CXY0113), Major Science and Technology Projects of Inner Mongolia Autonomous Region in 2021 (2021ZD0006), and Fujian University Industry-University-Research Joint Innovation Project (2021Y4001).
Footnotes
Supplemental material is available online only.
Contributor Information
Ye Xu, Email: xuye@xmu.edu.cn.
Qingge Li, Email: qgli@xmu.edu.cn.
Christine Y. Turenne, University of Manitoba
REFERENCES
- 1.Dean AS, Tosas Auguet O, Glaziou P, Zignol M, Ismail N, Kasaeva T, Floyd K. 2022. 25 years of surveillance of drug-resistant tuberculosis: achievements, challenges, and way forward. Lancet Infect Dis 22:e191–e196. doi: 10.1016/S1473-3099(21)00808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro RAD, Borrell S, Gagneux S. 2021. The within-host evolution of antimicrobial resistance in Mycobacterium tuberculosis. FEMS Microbiol Rev 45:fuaa071. doi: 10.1093/femsre/fuaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trauner A, Liu Q, Via LE, Liu X, Ruan X, Liang L, Shi H, Chen Y, Wang Z, Liang R, Zhang W, Wei W, Gao J, Sun G, Brites D, England K, Zhang G, Gagneux S, Barry CE, 3rd, Gao Q. 2017. The within-host population dynamics of Mycobacterium tuberculosis vary with treatment efficacy. Genome Biol 18:71. doi: 10.1186/s13059-017-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson DI, Nicoloff H, Hjort K. 2019. Mechanisms and clinical relevance of bacterial heteroresistance. Nat Rev Microbiol 17:479–496. doi: 10.1038/s41579-019-0218-1. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Zhao X, Drlica K. 2022. Fluoroquinolone heteroresistance, antimicrobial tolerance, and lethality enhancement. Front Cell Infect Microbiol 12:938032. doi: 10.3389/fcimb.2022.938032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ 41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. 2018. Technical Report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. https://www.who.int/publications/i/item/WHO-CDS-TB-2018.5.
- 8.Hofmann-Thiel S, van Ingen J, Feldmann K, Turaev L, Uzakova GT, Murmusaeva G, van Soolingen D, Hoffmann H. 2009. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur Respir J 33:368–374. doi: 10.1183/09031936.00089808. [DOI] [PubMed] [Google Scholar]
- 9.Eilertson B, Maruri F, Blackman A, Herrera M, Samuels DC, Sterling TR. 2014. High proportion of heteroresistance in gyrA and gyrB in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother 58:3270–3275. doi: 10.1128/AAC.02066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravorty S, Roh SS, Glass J, Smith LE, Simmons AM, Lund K, Lokhov S, Liu X, Xu P, Zhang G, Via LE, Shen Q, Ruan X, Yuan X, Zhu HZ, Viazovkina E, Shenai S, Rowneki M, Lee JS, Barry CE, 3rd, Gao Q, Persing D, Kwiatkawoski R, Jones M, Gall A, Alland D. 2017. Detection of isoniazid-, fluoroquinolone-, amikacin-, and kanamycin-resistant tuberculosis in an automated, multiplexed 10-color assay suitable for point-of-care use. J Clin Microbiol 55:183–198. doi: 10.1128/JCM.01771-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkvardsen DB, Svensson E, Thomsen VO, Rasmussen EM, Bang D, Werngren J, Hoffner S, Hillemann D, Rigouts L. 2013. Can molecular methods detect 1% isoniazid resistance in Mycobacterium tuberculosis? J Clin Microbiol 51:1596–1599. doi: 10.1128/JCM.00472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S, Li G, Li H, Liu X, Niu J, Quan S, Wang F, Wen H, Xu Y, Li Q. 2014. Rapid detection of isoniazid resistance in Mycobacterium tuberculosis isolates by use of real-time-PCR-based melting curve analysis. J Clin Microbiol 52:1644–1652. doi: 10.1128/JCM.03395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang B, Tan Y, Li Z, Tian X, Du C, Li H, Li G, Yao X, Wang Z, Xu Y, Li Q. 2018. Highly sensitive detection of isoniazid heteroresistance in Mycobacterium tuberculosis by DeepMelt assay. J Clin Microbiol 56:e01239-17. doi: 10.1128/JCM.01239-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng KCS, Supply P, Cobelens FGJ, Gaudin C, Gonzalez-Martin J, de Jong BC, Rigouts L. 2019. How well do routine molecular diagnostics detect rifampin heteroresistance in Mycobacterium tuberculosis? J Clin Microbiol 57:e00717-19. doi: 10.1128/JCM.00717-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai M, Nicol MP, Boehme CC. 2016. Tuberculosis diagnostics: state of the art and future directions. Microbiol Spectr 4:361–378. doi: 10.1128/microbiolspec.TBTB2-0019-2016. [DOI] [PubMed] [Google Scholar]
- 16.Stieglitz E, Troup CB, Gelston LC, Haliburton J, Chow ED, Yu KB, Akutagawa J, Taylor-Weiner AN, Liu YL, Wang YD, Beckman K, Emanuel PD, Braun BS, Abate A, Gerbing RB, Alonzo TA, Loh ML. 2015. Subclonal mutations in SETBP1 confer a poor prognosis in juvenile myelomonocytic leukemia. Blood 125:516–524. doi: 10.1182/blood-2014-09-601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shallom SJ, Zelazny AM. 2022. Detection of mixed populations of clarithromycin-susceptible and -resistant Mycobacterium abscessus strains. J Clin Microbiol 60:e0169421. doi: 10.1128/jcm.01694-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decraene C, Silveira AB, Bidard FC, Vallee A, Michel M, Melaabi S, Vincent-Salomon A, Saliou A, Houy A, Milder M, Lantz O, Ychou M, Denis MG, Pierga JY, Stern MH, Proudhon C. 2018. Multiple hotspot mutations scanning by single droplet digital PCR. Clin Chem 64:317–328. doi: 10.1373/clinchem.2017.272518. [DOI] [PubMed] [Google Scholar]
- 19.Chinese Antituberculosis Association. 2006. The laboratory science procedure of diagnostic bacteriology in tuberculosis, p 46–51. China Education and Culture Press, Beijing, China. [Google Scholar]
- 20.Bidshahri R, Attali D, Fakhfakh K, McNeil K, Karsan A, Won JR, Wolber R, Bryan J, Hughesman C, Haynes C. 2016. Quantitative detection and resolution of BRAF V600 status in colorectal cancer using droplet digital PCR and a novel wild-type negative assay. J Mol Diagn 18:190–204. doi: 10.1016/j.jmoldx.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Milbury CA, Zhong Q, Lin J, Williams M, Olson J, Link DR, Hutchison BJBD, Quantification . 2014. Determining lower limits of detection of digital PCR assays for cancer-related gene mutations. Biomol Detect Quantif 1:8–22. doi: 10.1016/j.bdq.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silveira AB, Bidard FC, Kasperek A, Melaabi S, Tanguy ML, Rodrigues M, Bataillon G, Cabel L, Buecher B, Pierga JY, Proudhon C, Stern MH. 2020. High-accuracy determination of microsatellite instability compatible with liquid biopsies. Clin Chem 66:606–613. doi: 10.1093/clinchem/hvaa013. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan KM, Dean A, Soe MM. 2009. OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep 124:471–474. doi: 10.1177/003335490912400320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman DR, Mdluli K, Hickey MJ, Arain TM, Morris SL, Barry CE, 3rd, Stover CK. 1996. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science 272:1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. 2000. Diagnostic standards and classification of tuberculosis in adults and children. this official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 26.Ye M, Yuan W, Molaeipour L, Azizian K, Ahmadi A, Kouhsari E. 2021. Antibiotic heteroresistance in Mycobacterium tuberculosis isolates: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 20:1–9. doi: 10.1186/s12941-021-00478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taly V, Pekin D, Benhaim L, Kotsopoulos SK, Le Corre D, Li X, Atochin I, Link DR, Griffiths AD, Pallier K, Blons H, Bouché O, Landi B, Hutchison JB, Laurent-Puig P. 2013. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin Chem 59:1722–1731. doi: 10.1373/clinchem.2013.206359. [DOI] [PubMed] [Google Scholar]
- 28.Koshkin AA, Singh SK, Nielsen P, Rajwanshi VK, Kumar R, Meldgaard M, Olsen CE, Wengel J. 1998. LNA (Locked Nucleic Acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 54:3607–3630. doi: 10.1016/S0040-4020(98)00094-5. [DOI] [Google Scholar]
- 29.Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, Banada PP, Deshpande S, Shenai S, Gall A, Glass J, Krieswirth B, Schumacher SG, Nabeta P, Tukvadze N, Rodrigues C, Skrahina A, Tagliani E, Cirillo DM, Davidow A, Denkinger CM, Persing D, Kwiatkowski R, Jones M, Alland D. 2017. The New Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 8:e00812-17. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun G, Luo T, Yang C, Dong X, Li J, Zhu Y, Zheng H, Tian W, Wang S, Barry CE, 3rd, Mei J, Gao Q. 2012. Dynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patients. J Infect Dis 206:1724–1733. doi: 10.1093/infdis/jis601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelthaler DM, Streicher EM, Kelley EJ, Allender CJ, Wiggins K, Jimenez D, Lemmer D, Vittinghoff E, Theron G, Sirgel FA, Warren RM, Metcalfe JZ. 2019. Minority Mycobacterium tuberculosis genotypic populations as an indicator of subsequent phenotypic resistance. Am J Respir Cell Mol Biol 61:789–791. doi: 10.1165/rcmb.2019-0178LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmo C, Brien K, Millard J, Grant AD, Padayatchi N, Pym AS, O'Donnell M, Goldstein R, Breuer J, Balloux F. 2020. Dynamics of within-host Mycobacterium tuberculosis diversity and heteroresistance during treatment. EBioMedicine 55:102747. doi: 10.1016/j.ebiom.2020.102747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vos M, Ley SD, Wiggins KB, Derendinger B, Dippenaar A, Grobbelaar M, Reuter A, Dolby T, Burns S, Schito M, Engelthaler DM, Metcalfe J, Theron G, Van Rie A, Posey J, Warren R, Cox H. 2019. Bedaquiline Microheteroresistance after cessation of tuberculosis treatment. N Engl J Med 380:2178–2180. doi: 10.1056/NEJMc1815121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daum LT, Konstantynovska OS, Solodiankin OS, Liashenko OO, Poteiko PI, Bolotin VI, Hrek II, Rohozhyn AV, Rodriguez JD, Fischer GW, Chambers JP, Gerilovych AP. 2018. Next-generation sequencing for characterizing drug resistance-conferring Mycobacterium tuberculosis genes from clinical isolates in the Ukraine. J Clin Microbiol 56:e00009-18. doi: 10.1128/JCM.00009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabiiti W, Azam K, Farmer ECW, Kuchaka D, Mtafya B, Bowness R, Oravcova K, Honeyborne I, Evangelopoulos D, McHugh TD, Khosa C, Rachow A, Heinrich N, Kampira E, Davies G, Bhatt N, Ntinginya EN, Viegas S, Jani I, Kamdolozi M, Mdolo A, Khonga M, Boeree MJ, Phillips PPJ, Sloan D, Hoelscher M, Kibiki G, Gillespie SH. 2020. Tuberculosis bacillary load, an early marker of disease severity: the utility of tuberculosis molecular bacterial load assay. Thorax 75:606–608. doi: 10.1136/thoraxjnl-2019-214238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dooley KE, Chiasson RE. 2009. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker TM, Kohl TA, Omar SV, Hedge J, Del Ojo Elias C, Bradley P, Iqbal Z, Feuer Riegel S, Niehaus KE, Wilson DJ, Clifton DA, Kapadia G, Ip CLC, Bowden R, Dronies FA, Allix-Béguec C, Gaudin C, Parkhill J, Diel R, Supply P, Crook DW, Smith EG, Walker AS, Ismail N, Niemann S, Peta TEA, Modernizing Medical Microbiology (MMM) Informatics Group . 2015. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect Dis 15:1193–1202. doi: 10.1016/S1473-3099(15)00062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colman RE, Anderson J, Lemmer D, Lemuel E, Georghiou SB, Heaton H, Wiggins K, Gallice JD, Schupp JM, Catanzaro DG, Crudup V, Cohen T, Rodwell TC, Engelthaler DM. 2016. Rapid drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates directly from clinical samples by use of amplicon sequencing: a proof-of-concept study. J Clin Microbiol 54:2058–2067. doi: 10.1128/JCM.00535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigouts L, Motto P, Schats M, Lamperns P, Cabibbe AM, Galbiati S, Lampasona V, de Rijk P, Cirillo DM, de Jong BC. 2019. Fluoroquinolone heteroresistance in Mycobacterium tuberculosis: detection by genotypic and phenotypic assays in experimentally mixed populations. Sci Rep 9:11760. doi: 10.1038/s41598-019-48289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download jcm.01884-22-s0001.pdf, PDF file, 1.4 MB (1.4MB, pdf)