PURPOSE
An international meta-analysis identified a group of patients with advanced epithelial ovarian cancer (EOC) with a very poor survival because of two unfavorable features: (1) a poor chemosensitivity defined by an unfavorable modeled CA-125 ELIMination rate constant K (KELIM) score <1.0 with the online calculator CA-125—Biomarker Kinetics, and (2) an incomplete debulking surgery. We assumed that patients belonging to this poor prognostic group would benefit from a fractionated densified chemotherapy regimen.
METHODS
The data set of ICON-8 phase III trial (ClinicalTrials.gov identifier: NCT01654146), where patients with EOC were treated with the standard three-weekly, or the weekly dose-dense, carboplatin-paclitaxel regimens and debulking primary surgery (immediate primary surgery [IPS] or delayed primary [or interval] surgery [DPS]), was investigated. The association between treatment arm efficacy, standardized KELIM (scored as favorable ≥1.0, or unfavorable <1.0), and surgery completeness was assessed by univariate/multivariate analyses in IPS and DPS cohorts.
RESULTS
Of 1,566 enrolled patients, KELIM was calculated with the online model in 1,334 with ≥3 CA-125 available values (85%). As previously reported, both KELIM and surgery completeness were complementary prognostic covariates, and could be combined into three prognostic groups with large OS differences: (1) good if favorable KELIM and complete surgery; (2) intermediate if either unfavorable KELIM or incomplete surgery; and (3) poor if unfavorable KELIM and incomplete surgery. Weekly dose-dense chemotherapy was associated with PFS/OS improvement in the poor prognostic group in both the IPS cohort (PFS: hazard ratio [HR], 0.50; 95% CI, 0.31 to 0.79; OS: HR, 0.58; 95% CI, 0.35 to 0.95) and the DPS cohort (PFS: HR, 0.53; 95% CI, 0.37 to 0.76; OS: HR, 0.57; 95% CI, 0.39 to 0.82).
CONCLUSION
Fractionated dose-dense chemotherapy might be beneficial for patients belonging to the poor prognostic group characterized by lower tumor chemosensitivity assessed with the online calculator CA-125—Biomarker Kinetics and incomplete debulking surgery. Further investigation in the future SALVOVAR trial is warranted.
INTRODUCTION
The standard management of advanced stage epithelial ovarian carcinoma (EOC) relies on combined medical and surgical treatment.1 Debulking surgery can be performed either as immediate primary surgery (IPS), or as delayed primary (or interval) surgery (DPS) after three or four cycles of neoadjuvant chemotherapy, depending on the probability of achieving complete debulking without any macroscopic residual disease.2,3 The standard systemic chemotherapy consists of six to eight cycles of carboplatin-paclitaxel given every 3 weeks.2,3
CONTEXT
Key Objective
To assess if the chemotherapy densification with the weekly dose-dense carboplatin-paclitaxel would be associated with an improved survival compared with the standard once-every-three-weeks regimen in patients with a poor prognostic advanced ovarian cancer.
Knowledge Generated
The retrospective analysis of the randomized phase III ICON-8 trial confirmed the very poor survival of patients whose disease was poorly chemosensitive (unfavorable ELIMination rate constant K [KELIM] score <1.0 on the online calculator CA-125—Biomarker Kinetics) and who could not benefit from complete debulking surgery. In these patients, the weekly dose-dense regimen was associated with significantly better progression-free survival and overall survival compared with the standard 3-weekly regimen.
Relevance
The utility of chemotherapy densification in patients with a poor prognostic ovarian cancer, characterized by a lower chemosensitivity (unfavorable KELIM score >1.0, calculable on CA-125—Biomarker Kinetics) and a disease not amenable to complete interval debulking surgery, after three cycles of neoadjuvant chemotherapy, will be assessed in the future European SALVOVAR trial.
The chemotherapy efficacy can now be characterized early, using the online calculator of the modeled CA-125 kinetic parameter ELIMination rate constant K (KELIM) estimated within the first 100 days of neoadjuvant or adjuvant chemotherapy (CA-125—Biomarker Kinetics4). Analyses of data from more than 13,000 patients treated in 13 clinical phase II and III trials, along with a national cancer registry, reproducibly showed that KELIM is a pragmatic effective indicator of tumor chemosensitivity.5-9
These data also suggested that both the chemotherapy efficacy and completeness of the debulking surgery are two complementary major prognostic factors for overall survival (OS).7 More specifically, the patients with a poorly chemosensitive disease (unfavorable KELIM score <1.0) that cannot be completely cytoreduced belong to a poor prognostic group characterized by short survival, as shown in the Gynecology Cancer InterGroup (GCIG) meta-analysis data set and the Netherlands Cancer Registry, along with several retrospective studies of clinical trials.5-7,10,11 These patients should be prioritized for innovative approaches designed to increase tumor chemosensitivity.
In an attempt to enhance chemotherapy efficacy in EOC, alternative fractionated and dose-dense regimens of carboplatin-paclitaxel were developed. These strategies were found to be effective in different solid cancers with high-risk features, such as nonseminomatous germ cell tumors, breast cancers, and bladder cancer.12-14
In ovarian cancer, several preclinical studies suggested that metronomic taxane administration would be associated with improved drug delivery, increased tumor cell apoptosis, and antiangiogenic effects.15,16 Corroborating these hypotheses, the Japanese JGOG-3016 phase III trial demonstrated that weekly dose-dense 80 mg/m2 paclitaxel given with three-weekly carboplatin was superior to the standard three-weekly regimen in terms of progression-free survival (PFS) and OS.17 In that context, the ICON-8 trial was designed to compare the efficacy of the standard three-weekly carboplatin-paclitaxel regimen, with that of two fractionated dose-dense regimens in a predominantly European population.15 However, the trial was negative with no benefit in PFS or OS found with the fractionated dose-dense arms in the whole population.18
The objective of the present retrospective analysis of ICON-8 trial data set was to assess if the weekly dose-dense chemotherapy was associated with longer PFS and OS compared with the standard three-weekly regimen in patients with ovarian cancer belonging to the poor prognostic group identified in the GCIG meta-analysis database (defined as the patients with poorly chemosensitive disease with KELIM score <1.0, and who could not benefit from complete surgical cytoreduction).5
METHODS
Patients and Data
In this international multicenter trial, patients with FIGO stage IC-IV EOC were randomly assigned (1:1:1) to one of three treatment arms: arm 1 (control) to three-weekly carboplatin AUC5 or AUC6 and three-weekly paclitaxel 175 mg/m2; arm 2 to three-weekly carboplatin AUC5 or AUC6 and weekly paclitaxel 80 mg/m2; and arm 3 to weekly carboplatin AUC2 and weekly paclitaxel 80 mg/m2, given in all arms for six cycles. Patients could have been operated with upfront (immediate) primary cytoreductive surgery (IPS, equivalent to primary debulking surgery), or could have received neoadjuvant chemotherapy for three cycles with a plan for delayed primary cytoreductive surgery (DPS, equivalent to interval debulking surgery), followed by three postoperative cycles. The concentrations of CA-125 were measured at baseline, on day 1 of each treatment cycle, and at each follow-up visit. The details of the trial were previously reported (ClinicalTrials.gov identifier: NCT01654146).19
Ethical approval was granted in the United Kingdom by the London-Chelsea Research Ethics Committee. Ethical approval was also granted by the appropriate national or local institutional review boards in other jurisdictions. All Protocol amendments were approved by relevant ethics committees and regulatory bodies and are explained in the protocol.
Estimation of Patient KELIM and KELIM Score
KELIM score was used for assessing the tumor intrinsic chemosensitivity.7 In the case of neo-adjuvant chemotherapy, the CA-125 kinetic analysis was limited to the first three cycles of chemotherapy, before surgery. Our analyses have shown that the addition of a third drug (either chemotherapy or an antiangiogenic drug) to carboplatin-paclitaxel, or changes to the administration schedule (weekly v every 3 weeks), did not alter the CA-125 KELIM values, and that KELIM was prognostic and strongly related to OS regardless of the received regimen.6,7,9,10,20-23
At least three available CA-125 values within the first 100 days of neoadjuvant or adjuvant chemotherapy were required to ensure an accurate assessment of KELIM by the model. The mathematical modeling of early CA-125 kinetics with a nonlinear mixed-effect model was described previously.20,22 Basic details about the semimechanistic kinetic-pharmacodynamic (K-PD) model adjustment and qualification are presented in the Data Supplement.24
Individual KELIM values were estimated with the model implemented of the online calculator CA-125—Biomarker Kinetics25 for patients treated with IPS and adjuvant chemotherapy; or CA-125-neo—Biomarker Kinetics26 for patients treated with neoadjuvant chemotherapy potentially followed by DPS. As previously assessed,6,10 KELIM values of patients were standardized by the prespecified optimized cutoffs (0.07/days for patients treated with IPS and adjuvant chemotherapy; 0.05/days for patients treated with neoadjuvant chemotherapy potentially followed by DPS) as a way of providing an easy reading of patient KELIM outcome, with following equation: Standardized (std) KELIM = KELIM estimated by the model/cutoff. As a consequence, std KELIM was a continuous covariate centered by 1.0. To help the interpretation of KELIM for prognostic analyses, KELIM was dichotomized with a KELIM score: std KELIM <1.0 was considered as unfavorable, while std KELIM ≥1.0 was considered as favorable.
Prognostic Value of Combined Tumor Intrinsic Chemosensitivity and Completeness of Debulking Surgery
In both the IPS and DPS cohorts, the discriminatory predictive ability, along with the prognostic values of both the KELIM score (favorable ≥1.0 v unfavorable <1.0) and the completeness of debulking surgery (complete R0 with no residual visible lesions v incomplete with visible residual lesions ≤1 cm (R1), or >1 cm (R2), or not performed for DPS), regarding PFS and OS were assessed using univariate/multivariate C-index, Kaplan-Meier method, log-rank, and Cox proportional hazards regression tests. The other covariates tested in the multivariate analyses included pathologic subtype (low-grade serous, high-grade serous, or others); disease stage (I-II, III, or IV); radiologic tumor response according to RECIST 1.1 at the end of neoadjuvant chemotherapy (complete response, or partial response, v stable disease, or progressive disease); CA-125 response criterion according to GCIG (yes or no); and treatment arm (arm 1, 2, or 3). The final C-index and Cox models were obtained using backward selections.
Benefit From the Weekly Dose-Dense Chemotherapy in the Patients Belonging to the Poor Prognostic Group
As previously done on the GCIG meta-analysis data set and CHIVA trial,5,10 both the completeness of debulking surgery and KELIM score were combined into prognostic groups regarding PFS and OS. The efficacy of the weekly dose-dense regimens was compared with those of the standard three-weekly regimen in the poor prognostic group (composed of patients operated with incomplete debulking surgery and unfavorable KELIM score <1.0) in the IPS and DPS cohorts. Moreover, interaction tests between treatment arms and KELIM score regarding PFS and OS benefit were performed in patients operated with incomplete or complete debulking surgery, using Cox models.
Statistics and Computing Process
All survival analyses were implemented with a landmark time point set at 100 days after the start of neoadjuvant chemotherapy or at the surgery date, since CA-125 was modeled from day 0 to 100.27 All tests were implemented using a two-sided .05 α risk. NONMEM 7.5 (ICON Development Solutions, Ellicott City, MD) software program was used to fit the semimechanistic model to CA-125 kinetic data.28 XPOSE4 program was used for graphical evaluation of model fits.29 Logistic analyses, survival analyses, and concordance probability (C-index) were obtained using R version 4.1.1.
RESULTS
Patient Characteristics
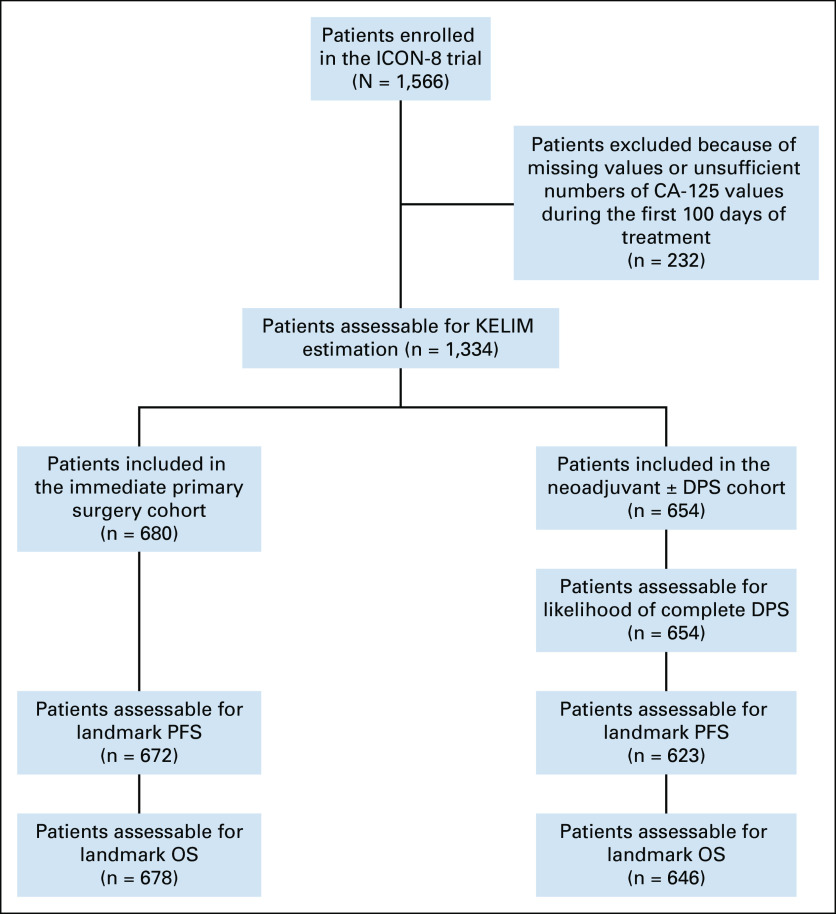
Of 1,566 patients enrolled in ICON-8, 1,334 (85%) were assessable for KELIM score because they had at least three CA-125 evaluations during the first 100 days (Fig 1). The patient characteristics are presented in Data Supplement (Table 1). Six hundred eighty (51%) assessable patients were treated with IPS followed by adjuvant chemotherapy (IPS cohort; complete surgery [R0], n = 453 [67%]; incomplete surgery [R1-R2], n = 227 [33%]). The remaining 654 patients (49%) were treated with neoadjuvant chemotherapy with a view to DPS (DPS cohort), including 483 patients (74%) who underwent surgery, and 171 patients (26%) judged not suitable for delayed surgery. Of 483 patients who underwent DPS, complete (R0) and incomplete (R1-R2) cytoreductions were achieved in 267 (41%), and 368 patients (59%), respectively.
FIG 1.
CONSORT diagram. DPS, delayed primary (or interval) surgery; KELIM, ELIMination rate constant K; OS, overall survival; PFS, progression-free survival.
Model Qualification
Typical parameter estimates, along with the qualification analyses from the final semimechanistic models, are presented in the Data Supplement (Text, Table 2, Figure 1). The median values of KELIM were 0.066/days (95% CI, 0.062 to 0.068) and 0.051/days (95% CI, 0.049 to 0.052) in the IPS and DPS cohorts, respectively. Std KELIM was not different across treatment arms, as already seen in previous studies (Data Supplement, Figure 2).
Prognostic of KELIM Score and Completeness of Debulking Surgery Regarding PFS and OS
The median follow-up for OS was 64.1 months (95% CI, 63.2 to 65.0) in the IPS cohort, and 65.2 months (95% CI, 64.0 to 68.0) in the DPS cohort. Higher KELIM value during neoadjuvant chemotherapy was associated with higher likelihood of complete DPS (Data Supplement, Table 3 and Figure 3).
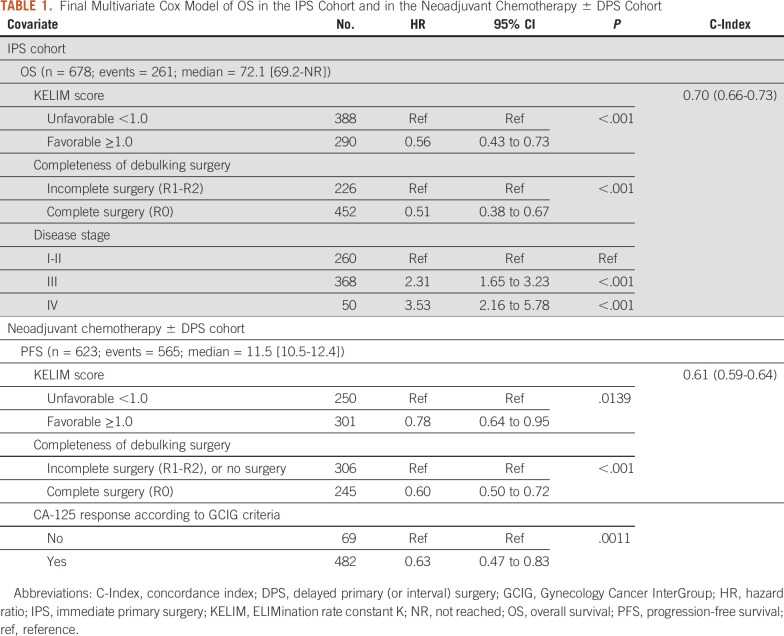
The results of the univariate C-index and log-rank tests for PFS and OS, followed by multivariate C-index and Cox survival models, are presented in Table 1 and Data Supplement (Tables 4-9). They confirm the significant and independent prognostic values of the KELIM score (favorable ≥1.0 v unfavorable <1.0) and the completeness of debulking (complete R0 v incomplete R1-R2).
TABLE 1.
Final Multivariate Cox Model of OS in the IPS Cohort and in the Neoadjuvant Chemotherapy ± DPS Cohort
KELIM score and completeness of surgery were combined into the three prognostic groups (Fig 2): (1) a good prognostic group with patients operated with complete surgery and having favorable KELIM score ≥1.0 (IPS cohort: median PFS: not reached; median OS: not reached; DPS cohort: median PFS: 15.2 months; median OS: 42.3 months); (2) an intermediate prognostic group composed of patients with either incomplete surgery or unfavorable KELIM <1.0 (IPS cohort: median PFS: 44.5 months; median OS: 72.1 months; DPS cohort: median PFS: 12.3 months; median OS: 35.5 months); and (3) a poor prognostic group with patients operated with incomplete surgery and unfavorable KELIM <1.0 (IPS cohort: median PFS: 12.1 months; median OS: 37.6 months; DPS cohort: median PFS: 7.9 months; median OS: 24.0 months).
FIG 2.
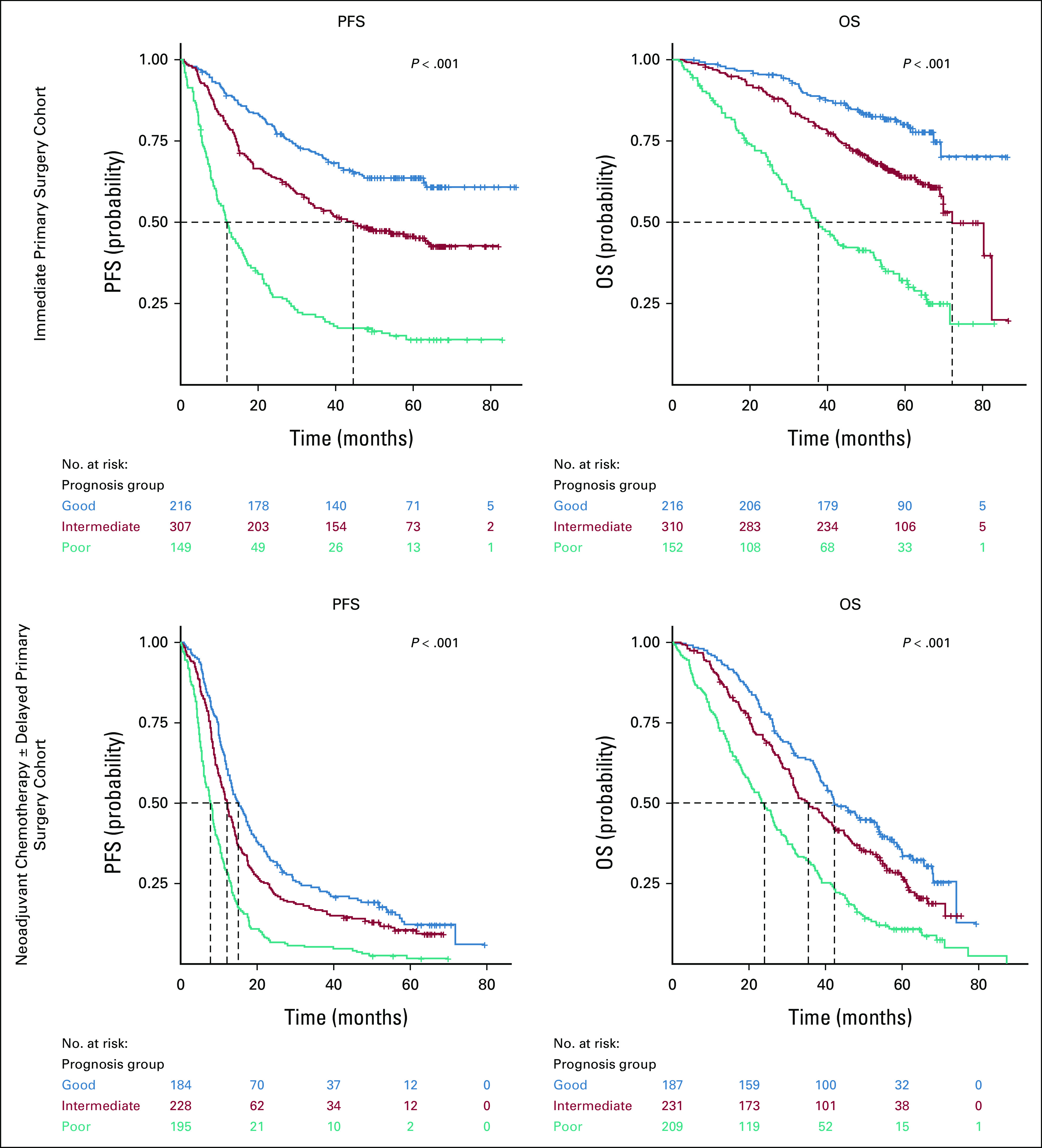
PFS and OS Kaplan-Meier curves of the three prognostic groups, according to the tumor primary chemosensitivity and the completeness of debulking surgery, in the two cohorts of patients. Good prognostic group: favorable KELIM ≥1.0, and complete debulking surgery; intermediate prognostic group: unfavorable KELIM <1.0, or incomplete debulking surgery; poor prognostic group: unfavorable KELIM <1.0, and incomplete debulking surgery. KELIM, ELIMination rate constant K; OS, overall survival; PFS, progression-free survival.
Benefit From Fractionated Dose-Dense Regimens
In the whole population.
An association was found between unfavorable KELIM score and higher efficacy of weekly dose-dense chemotherapy. For example, in the IPS cohort, there was a nonsignificant trend toward improved PFS with the weekly dose-dense carboplatin-paclitaxel compared with the standard arm (median PFS, arm 1: 21.5 months; arm 2: 23.5 months; and arm 3: 29.3 months), as well as improved OS (median OS, arm 1: 65.6 months; arm 2: 61.0 months; arm 3: 69.2 months). A similar association was observed with PFS in the DPS cohort (median PFS, arm 1: 7.6 months; arm 2: 9.2 months; and arm 3: 10.8 months). By contrast, the median PFS and OS of patients with favorable KELIM were not different across to treatment arms. No association between efficacy of weekly dose-dense chemotherapy and completeness of surgery was found (except in the DPS cohort, where higher PFS and OS were noted with arm 2 compared with arm 1).
In the poor prognostic group.
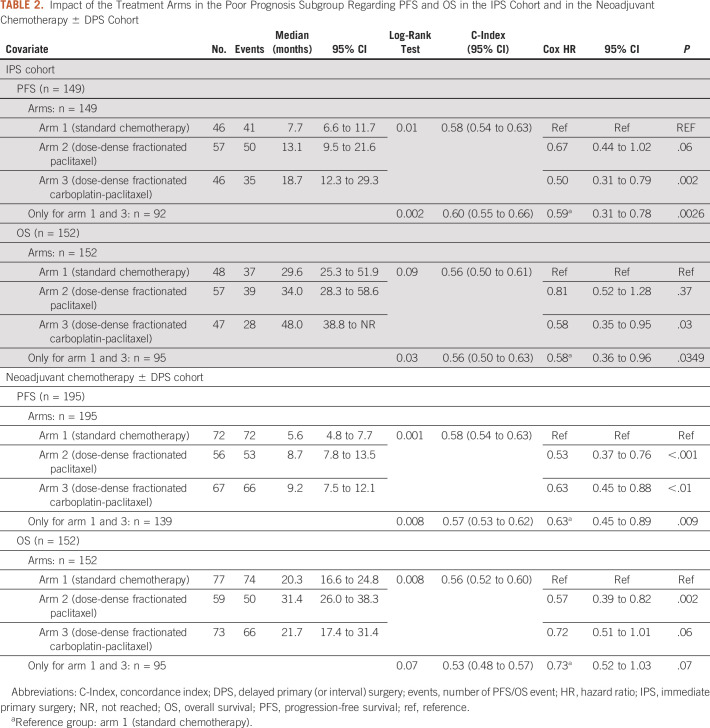
In the IPS cohort, the patients from the poor prognostic group experienced significantly improved PFS and OS when treated with the weekly dose-dense carboplatin and paclitaxel arm 3 compared with the standard arm 1 (Table 2, Fig 3).
PFS: median, arm 1, 7.7 months; arm 2, 13.1 months (arm 2 v arm 1; HR, 0.67; 95% CI, 0.44 to 1.02); arm 3, 18.7 months (arm 3 v arm 1; HR, 0.50; 95% CI, 0.31 to 0.79)
OS: median, arm 1, 29.6 months; arm 2, 34.0 months (arm 2 v arm 1; HR, 0.81; 95% CI, 0.52 to 1.28); arm 3, 48.0 months (arm 3 v arm 1; HR, 0.58; 95% CI, 0.35 to 0.95)
TABLE 2.
Impact of the Treatment Arms in the Poor Prognosis Subgroup Regarding PFS and OS in the IPS Cohort and in the Neoadjuvant Chemotherapy ± DPS Cohort
FIG 3.
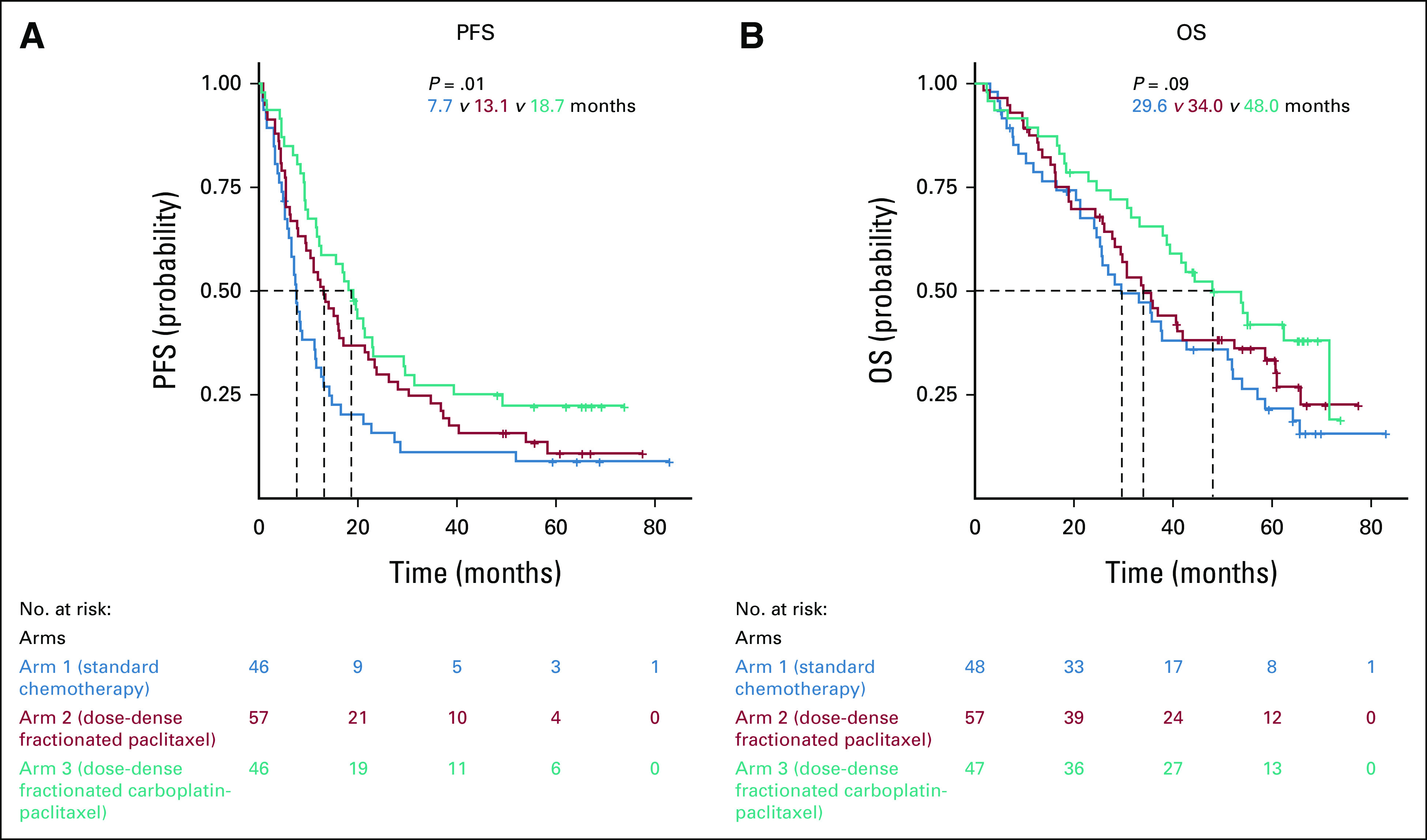
Impact of the treatment arms in the three prognostic groups regarding (A) PFS and (B) OS in the immediate primary surgery cohort. OS, overall survival; PFS, progression-free survival.
In the DPS cohort, the patients from poor prognostic group experienced higher PFS and OS when treated with the weekly dose-dense regimens compared with the standard arm 1 (Table 2, Fig 4).
PFS: median, arm 1, 5.6 months; arm 2, 8.7 months (arm 2 v arm 1; HR, 0.53; 95% CI, 0.37 to 0.76), arm 3, 9.2 months (arm 3 v arm 1; HR, 0.63; 95%, 0.45 to 0.88)
OS: median, arm 1, 20.3 months; arm 2, 31.4 months (arm 2 v arm 1; HR, 0.57; 95% CI, 0.39 to 0.82); arm 3, 21.7 months (arm 3 v arm 1; HR, 0.72; 95% CI, 0.51 to 1.01)
FIG 4.
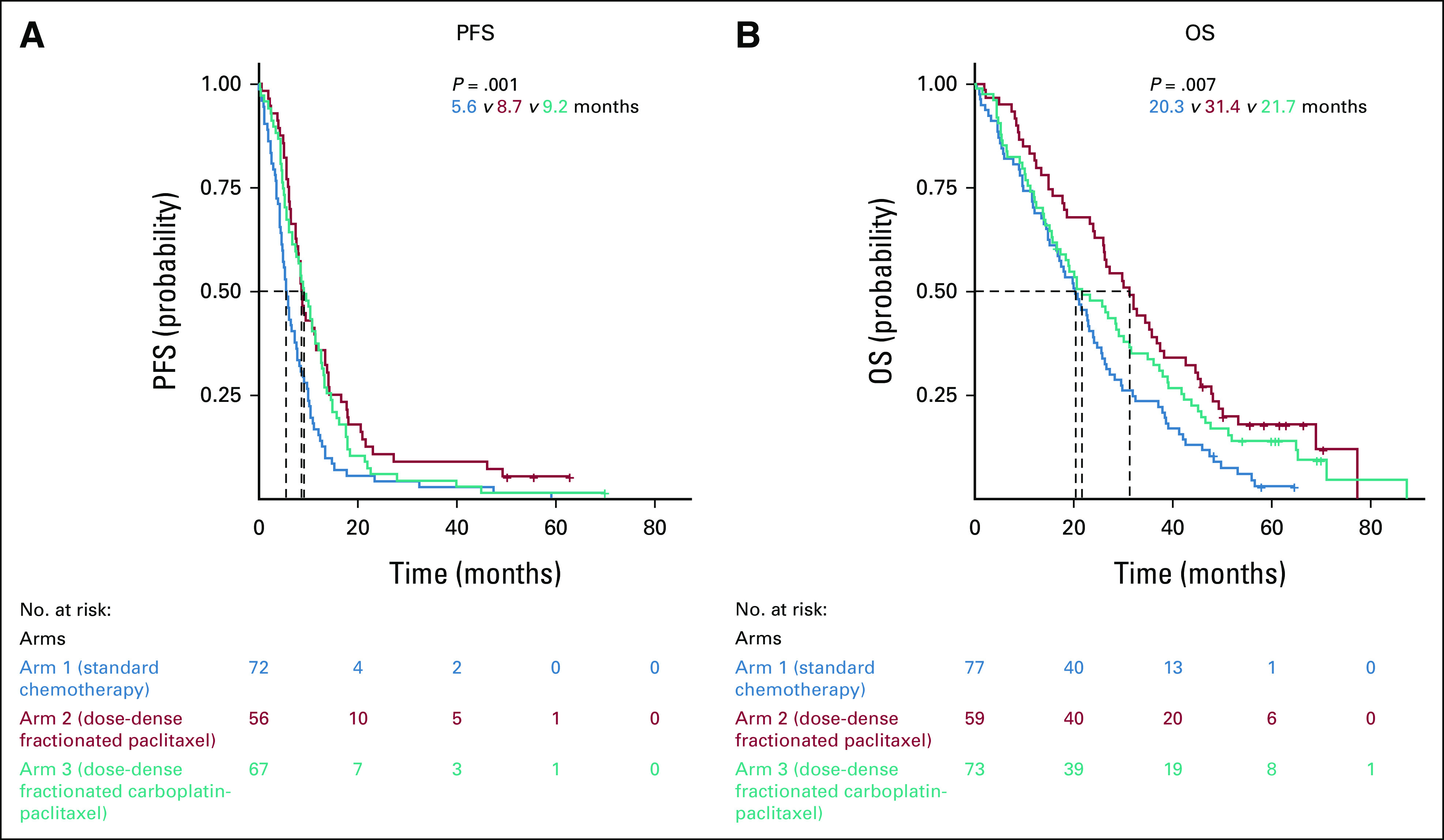
Impact of the treatment arms in the three prognostic groups regarding (A) PFS and (B) OS in the neoadjuvant chemotherapy ± delayed primary surgery cohort. OS, overall survival; PFS, progression-free survival.
The interaction tests between treatment arms (arm 3 v arm 1) and KELIM score were significant in patients operated with incomplete surgery (interaction terms: IPS cohort, 0.47 for PFS, P < .01, 0.58 for OS, P = .03; DPS cohort, 0.62 for PFS, P < .01, 0.72 for OS, P = .05).
DISCUSSION
Consistent with previous studies about the determinants of the success of first-line medical and surgical treatment, the present analysis confirms the complementary prognostic values of both the completeness of debulking surgery and the tumor chemosensitivity assessed by KELIM with the online numeric tool Biomarker Kinetics.5,8-10,20,23 These parameters could be combined to generate three prognostic groups with clinically relevant PFS and OS differences. In the ICON-8 trial population, the poor prognostic group (including patients with a poorly chemosensitive disease and operated with incomplete debulking surgery [or not operated]) was characterized by a short median OS of 37.6 months and 24.0 months in the IPS and DPS cohorts, respectively. These findings are consistent with the 22.1-month median OS in patients with unfavorable KELIM score and suboptimal surgery with postoperative residual disease ≥1 cm reported in the GCIG meta-analysis.5
This group should be prioritized for innovative treatment adjustments hypothesized to increase chemosensitivity, as a way of improving their prognosis. Indeed, it is likely these patients will not benefit from the progress related to the wider prescription of poly (ADP-ribose) polymerase (PARP) inhibitors as maintenance treatment in the future, since these drugs were found to be more effective in the case of highly chemosensitive disease. If no effort is made to improve the chemosensitivity of this poor prognostic group, the OS gap between the patients with highly chemosensitive disease and those with poorly chemosensitive disease will increase, thereby contributing to exacerbating this inequity.
The present evaluation of ICON-8 trial suggests that weekly dose-dense carboplatin and paclitaxel might produce improved PFS and OS compared with the standard three-weekly regimen in patients with poorly chemosensitive disease characterized by unfavorable KELIM <1.0. A recent post hoc analysis of VELIA trial (ClinicalTrials.gov identifier: NCT02470585), where patients with EOC were treated with carboplatin-paclitaxel ± veliparib in first-line setting, suggested that those with homologous recombination proficient disease (known to be less platinum-sensitive) treated with the weekly dose-dense regimen experienced better PFS compared with those treated with the three-weekly standard regimen.9,30 This would be consistent with the hypothesis of the improved efficacy of metronomic treatment administration in chemoresistant disease as seen in platinum-resistant recurrent ovarian cancers.19,31,32
The most clinically relevant outcome relates to the significant higher PFS and OS associated with weekly dose-dense chemotherapy in patients belonging to the poor prognostic group (unfavorable KELIM score and incomplete debulking surgery), representing about 27% of all patients in ICON-8. This outcome is consistent with the reproducible benefit from the addition of bevacizumab observed in patients with a poorly chemosensitive and high-risk disease in ICON-7 and GOG-0218 phase trials.8,21 Given the antiangiogenic effects of fractionated metronomic chemotherapy, efficacy of the weekly dose-dense carboplatin-paclitaxel regimen was anticipated in the same population of patients.
This study had several limitations. First, this was a post hoc analysis of ICON-8 trial, and only hypotheses could be extracted from this investigation because of limited statistical power, especially for subgroup analyses with reduced number of patients. If we found a benefit with the weekly dose-dense paclitaxel arms compared with the standard regimen arm, no difference between arm 2 (three-weekly carboplatin) or arm 3 (weekly carboplatin) was clearly observed. ICON-8 was conducted before the emergence of PARP inhibitors, which have revolutionized the prognosis of patients with EOC. As a consequence, the differential prognosis findings according to chemosensitivity may not be applicable to the current patients frequently treated with PARP inhibitors.2,3,33 Similarly, no patients in ICON-8 received bevacizumab as part of their first-line therapy. It is therefore possible that the prognostic value of KELIM score regarding the benefit from dose-dense fractionated chemotherapy would have been different if bevacizumab had been incorporated into trial treatment. Another limitation of this study relates to the lack of genomic biomarkers. ICON-8 did not integrate data about the homologous recombination status of enrolled patients, although BRCA1/2 mutations and to a lesser extent genomic instability have been shown to be associated with platinum sensitivity.7
Despite these limitations, the outcomes of this study are consistent with those of other investigations in the first-line setting and suggest that the poor prognosis of patients with poorly chemosensitive (assessed by the online calculator Biomarker Kinetics4) and incompletely resected disease (whether performed as a primary or delayed procedure) warrants innovative approaches to increase the chemosensitivity. In line with the outcomes seen with bevacizumab in ICON-7 and GOG-0218 trials, the fractionated dose-dense chemotherapy, thought to induce antiangiogenic effects, was associated with significantly improved OS in patients belonging to the poorest prognostic group.8,21 Of note, patients with poorly chemosensitive disease can be easily identified, since patient KELIM score is assessable in routine using an online numeric tool.
The present retrospective exploratory analysis of ICON-8 cannot assert that the chemotherapy adjustment to weekly dose-dense chemotherapy administration schedule in patients belonging to the poor prognostic group will prolong survival. However, it does provide a strong rationale for the European pragmatic randomized phase III trial SALVOVAR, which will compare the efficacy of the standard chemotherapy with those of a SALVage regimen composed of the weekly dose-dense chemotherapy in OVARian patients belonging to the poor prognostic group after three cycles of neoadjuvant chemotherapy.
ACKNOWLEDGMENT
The authors acknowledge Experimental Cancer Medicine Centres and National Institute for Health Research Biomedical Research Centres for support at ICON-8 centers in the United Kingdom. The authors thank all the women who participated in ICON-8 and their families.
Andrew Clamp
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline, Immunogen
Speakers' Bureau: Clovis Oncology, GlaxoSmithKline, MSD/AstraZeneca
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Pfizer (Inst), Immunogen (Inst), Merck (Inst), Verastem (Inst), Eisai (Inst), Advenchen Laboratories (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Iain A. McNeish
Honoraria: Clovis Oncology, AstraZeneca, Roche, OncoC4, GlaxoSmithKline, Transgene, Theolytics
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, Carrick Therapeutics, Roche, BeiGene, Scancell Ltd, GlaxoSmithKline, Epsila Bio
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Benoit You
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Novartis, LEK, TESARO, Bayer, Amgen, Clovis Oncology, GlaxoSmithKline, ECS Progastrin, Immunomedics, Daiichi Sankyo Europe GmbH, Myriad Genetics, MSD Oncology, Seattle Genetics
Research Funding: Merck Serono (Inst), Roche/Genentech (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, AstraZeneca, BMS, MSD Oncology, Bayer, Seattle Genetics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting (abstr 5530), Chicago, IL, June 4-8, 2019.
SUPPORT
The trial ICON-8 was publicly funded by Cancer Research UK (CRUK) through the CRUK Clinical Trials Awards and Advisory Committee (C1489/A12127 and CA1489/A17092) and was supported by UK Medical Research Council core funding. The trial was also funded by the Irish Health Research Board, Irish Cancer Society, and Cancer Australia. The Medical Research Council was the trial sponsor and has delegated responsibility for the overall management of the ICON-8 Trials Programme to the Medical Research Council Clinical Trials Unit at University College London (UK). The trial was part of the UK National Cancer Research Network portfolio. O.C. is employed by Lyon University (Université Claude Bernard Lyon 1, Lyon, France).
AUTHOR CONTRIBUTIONS
Conception and design: Olivier Colomban, Benoit You
Financial support: Benoit You
Administrative support: Benoit You
Provision of study materials or patients: Andrew Clamp, Adrian Cook, Iain A. McNeish, Benoit You
Collection and assembly of data: Olivier Colomban, Andrew Clamp, Adrian Cook, Benoit You
Data analysis and interpretation: Olivier Colomban, Andrew Clamp, Iain A. McNeish, Benoit You
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
DATA SHARING STATEMENT
ICON-8 trial and KELIM data: ICON-8 trial data will be shared according to the Medical Research Council Clinical Trials Unit controlled access approach, on the basis of the following principles: no data should be released that would compromise an ongoing trial or study; there must be a strong scientific or other legitimate rationale for the data to be used for the requested purpose; investigators who have invested time and effort into developing a trial or study should have a period of exclusivity in which to pursue their aims with the data, before key trial data are made available to other researchers; the resources required to process requests should not be underestimated, particularly successful requests that lead to preparing data for release, thus adequate resources must be available to comply in a timely manner or at all, and the scientific aims of the study must justify the use of such resources; and data exchange complies with Information Governance and Data Security Policies in all the relevant countries. Researchers wishing to access data from the ICON-8 should contact mrcctu.icon8and8b@ucl.ac.uk in the first instance. Researchers wishing to access KELIM data should contact benoit.you@chu-lyon.fr in the first instance.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew Clamp
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline, Immunogen
Speakers' Bureau: Clovis Oncology, GlaxoSmithKline, MSD/AstraZeneca
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Pfizer (Inst), Immunogen (Inst), Merck (Inst), Verastem (Inst), Eisai (Inst), Advenchen Laboratories (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Iain A. McNeish
Honoraria: Clovis Oncology, AstraZeneca, Roche, OncoC4, GlaxoSmithKline, Transgene, Theolytics
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, Carrick Therapeutics, Roche, BeiGene, Scancell Ltd, GlaxoSmithKline, Epsila Bio
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Benoit You
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Novartis, LEK, TESARO, Bayer, Amgen, Clovis Oncology, GlaxoSmithKline, ECS Progastrin, Immunomedics, Daiichi Sankyo Europe GmbH, Myriad Genetics, MSD Oncology, Seattle Genetics
Research Funding: Merck Serono (Inst), Roche/Genentech (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, AstraZeneca, BMS, MSD Oncology, Bayer, Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Torre LA, Trabert B, DeSantis CE, et al. : Ovarian cancer statistics, 2018. CA Cancer J Clin 68:284-296, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo N, Sessa C, Bois AD, et al. : ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 29:728-760, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Wright AA, Bohlke K, Armstrong DK, et al. : Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 34:3460-3473, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.biomarker-kinetics.org/presentation Biomarker Kinetics: Biomarkers Kinetics: Is it a favorable decrease?
- 5.Corbaux P, You B, Glasspool R, et al. : Survival prognostic and surrogate values of the early modeled CA-125 KELIM in newly diagnosed advanced ovarian cancer: Data from the GCIG meta-analysis group. Ann Oncol 32:S744, 2021. (abstr 751P) [Google Scholar]
- 6.Van Wagensveld L, Colomban O, Van der AA M, et al. : The prognostic value of chemosensitivity, estimated by the modeled CA-125 KELIM, in ovarian cancer patients treated with neo-adjuvant chemotherapy in the Netherlands. Proceedings of ESMO 2020 Virtual Meeting, 2020 (abstr 847P)
- 7.You B, Freyer G, Gonzalez-Martin A, et al. : The role of the tumor primary chemosensitivity relative to the success of the medical-surgical management in patients with advanced ovarian carcinomas. Cancer Treat Rev 100:102294, 2021 [DOI] [PubMed] [Google Scholar]
- 8.You B, Purdy C, Copeland LJ, et al. : Identification of patients with ovarian cancer experiencing the highest benefit from bevacizumab in the first-line setting on the basis of their tumor-intrinsic chemosensitivity (KELIM): The GOG-0218 validation study. J Clin Oncol 40:3965-3974, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You B, Sehgal V, Hosmane B, et al. : CA-125 KELIM as a potential complementary tool for predicting veliparib benefit: An exploratory analysis from the VELIA/GOG-3005 study. J Clin Oncol 41:107-116, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You B, Robelin P, Tod M, et al. : CA-125 ELIMination rate constant K (KELIM) is a marker of chemosensitivity in patients with ovarian cancer: Results from the phase II CHIVA trial. Clin Cancer Res 26:4625-4632, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Adams SF, Levine DA, Cadungog MG, et al. : Intraepithelial T cells and tumor proliferation: Impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer 115:2891-2902, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citron ML, Berry DA, Cirrincione C, et al. : Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431-1439, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fizazi K, Pagliaro L, Laplanche A, et al. : Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): A phase 3, multicentre, randomised trial. Lancet Oncol 15:1442-1450, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. : Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32:1895-1901, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clamp AR, James EC, McNeish IA, et al. : Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 394:2084-2095, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Printezi MI, Kilgallen AB, Bond MJG, et al. : Toxicity and efficacy of chronomodulated chemotherapy: A systematic review. Lancet Oncol 23:e129-e143, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Katsumata N, Yasuda M, Isonishi S, et al. : Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol 14:1020-1026, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Clamp AR, James EC, McNeish IA, et al. : Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal cancer treatment (ICON8): Overall survival results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol 23:919-930, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clamp AR, McNeish I, Dean A, et al. : ICON8: A GCIG phase III randomised trial evaluating weekly dose-dense chemotherapy integration in first-line epithelial ovarian/fallopian tube/primary peritoneal carcinoma (EOC) treatment: Results of primary progression-free survival (PFS) analysis. Ann Oncol 28:v627, 2017 [Google Scholar]
- 20.Colomban O, Tod M, Leary A, et al. : Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: A GINECO AGO MRC CTU study. Clin Cancer Res 25:5342-5350, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Colomban O, Tod M, Peron J, et al. : Bevacizumab for newly diagnosed ovarian cancers: Best candidates among high-risk disease patients (ICON-7). JNCI Cancer Spectr 4:pkaa026, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You B, Colomban O, Heywood M, et al. : The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: Data from CALYPSO trial (a GINECO-GCIG study). Gynecol Oncol 130:289-294, 2013 [DOI] [PubMed] [Google Scholar]
- 23.You B, Van Wagensveld L, Tod M, et al. : Low probability of disease cure in advanced ovarian carcinomas before the PARP inhibitor era. Br J Cancer 127:79-83, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dartois C, Brendel K, Comets E, et al. : Overview of model-building strategies in population PK/PD analyses: 2002-2004 literature survey. Br J Clin Pharmacol 64:603-612, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biomarker Kinetics: Modeled CA-125 KELIM™ in patients with stage III-IV high grade serous ovarian carcinomas treated with first line adjuvant chemotherapy. https://www.biomarker-kinetics.org/CA-125
- 26.Biomarker Kinetics: Modeled CA-125 KELIM™ in patients with stage III-IV high grade serous ovarian carcinomas treated with first line neo-adjuvant chemotherapy. https://www.biomarker-kinetics.org/CA-125-neo
- 27.Heller G, McCormack R, Kheoh T, et al. : Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol 36:572-580, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beal S, Boeckmann L, Bauer R, Sheiner L: NONMEM User’s Guides (1989–2009). Ellicott City, MD, Icon, 2009 [Google Scholar]
- 29.Jonsson EN, Karlsson MO: Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51-64, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Aghajanian C, Swisher EM, Okamoto A, et al. : Impact of veliparib, paclitaxel dosing regimen, and germline BRCA status on the primary treatment of serous ovarian cancer—An ancillary data analysis of the VELIA trial. Gynecol Oncol 164:278-287, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baird RD, Tan DSP, Kaye SB: Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev Clin Oncol 7:575-582, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Pujade-Lauraine E, Hilpert F, Weber B, et al. : Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302-1308, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Tew WP, Lacchetti C, Ellis A, et al. : PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol 38:3468-3493, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ICON-8 trial and KELIM data: ICON-8 trial data will be shared according to the Medical Research Council Clinical Trials Unit controlled access approach, on the basis of the following principles: no data should be released that would compromise an ongoing trial or study; there must be a strong scientific or other legitimate rationale for the data to be used for the requested purpose; investigators who have invested time and effort into developing a trial or study should have a period of exclusivity in which to pursue their aims with the data, before key trial data are made available to other researchers; the resources required to process requests should not be underestimated, particularly successful requests that lead to preparing data for release, thus adequate resources must be available to comply in a timely manner or at all, and the scientific aims of the study must justify the use of such resources; and data exchange complies with Information Governance and Data Security Policies in all the relevant countries. Researchers wishing to access data from the ICON-8 should contact mrcctu.icon8and8b@ucl.ac.uk in the first instance. Researchers wishing to access KELIM data should contact benoit.you@chu-lyon.fr in the first instance.