ABSTRACT
Long COVID hinders people from normal life and work, posing significant medical and economic challenges. Nevertheless, comprehensive studies assessing its impact on large populations in Asia are still lacking. We tracked over 20,000 patients infected with COVID-19 for the first time during the Omicron BA.2 outbreak in Shanghai from March-June 2022 for one year. Of the 21,799 COVID-19 patients who participated in the 6-month telephone follow-up, 1939 (8.89%) had self-reported long COVID symptoms. 450 long COVID patients participated in the 6-month outpatient follow-up. Participants underwent healthy physical examinations and questionnaires focused on long-COVID-related symptoms and mental health. Mobility problem (P < 0.001), personal care problem (P = 0.003), usual activity problem (P < 0.001), pain/discomfort (P < 0.001), anxiety/depression (P = 0.001) and PTSD (P = 0.001) were more prevalent in long COVID patients than in healthy individuals, but no significant differences were found between the two groups on chest CT and laboratory examinations. Of the 856 long COVID patients who participated in the 12-month follow-up, 587 (68.5%) had their symptoms resolved. In the multivariable logistic analysis, females (P < 0.001), youth (age <40 years) (P < 0.001), ≥ 2 comorbidities (P = 0.009), and severe infection in the acute phase (P = 0.006) were risk factors for developing long COVID. Middle age (40–60 years) was a risk factor for persistent long COVID one year after hospital discharge (P = 0.013). The study found that long COVID mainly manifested as subjective symptoms and impacts partial patients’ quality of life and mental status. After one year, most (68.5%) of the patients recovered from long COVID with no impairment of organ function observed.
KEYWORDS: COVID-19, long COVID, omicron variant, follow-up study, post-acute COVID-19 syndrome
Introduction
As of the April 2023, there have been 760 million confirmed cases of COVID-19, including 6,887,000 deaths, reported to WHO. Some of the patients developed signs and symptoms that persisted for months to years after SARS-CoV-2 infection including fatigue/weakness, anxiety, breathlessness, memory loss, concentration difficulties and insomnia, etc. The population is at high risk of persisting health impairments associated with reduced physical function and health-related quality of life. WHO had established technical working groups to provide a clinical case definition for this condition and amplified the calls of patient groups for recognition, research, and rehabilitation. WHO stated that long COVID occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis [1].
The incidence of the long COVID was different globally. An estimated 2.0 million (3.1% of the population) people living in the UK experienced long COVID infections [2]. According to CDC's analyses of data, 18–19% of US adults had symptoms of long COVID [3]. The world's largest observational meta-analysis enrolled 1.2 million individuals reported 6.2% of symptomatic SARS-CoV-2 infection patients suffered from long COVID, including 3.7% for ongoing respiratory problems, 3.2% for persistent fatigue with bodily pain or mood swings, and 2.2% for cognitive problems after adjusting for health status before COVID-19 [4]. What's more, who is at risk of developing long-COVID and how can this be prevented is still unanswered. Some factors seemed to be associated with the occurrence of long COVID conditions: severity of initial infection, female sex, pre-existing conditions and older age, and different SARS-CoV-2 variants and vaccination state [5–7].
It is worth noting that most of the current data on long COVID are from Europe and the United States, reporting the long COVID symptoms and the influencing factors of developing long COVID such as gender, race, vaccination, and different SARS-CoV-2 variants infection [5–10], while large-scale population studies in Asia are missing. Perlis and colleagues reported on the differences in the prevalence of long COVID among US adults of different races and ethnicities as Asians showed relatively lower risk factors for experiencing long COVID than White [9]. Subramanian et al. claimed the same perspective based on data from the UK, and they also hold the view that the mainly reported long-COVID-related symptoms are slightly different among the ethnicities [10]. Moreover, there are still few studies covering a comprehensive assessment of asymptomatic and symptomatic infected individuals, especially focusing on the long COVID symptoms after the Omicron pandemic. What's more, whether such symptoms would persist is a question urgently awaiting more answers.
Long COVID seems to be a formidable challenge for healthcare systems, however, whether it would cause major long-term health issues among populations is still under debate. On one side, numerous studies have reported a 3%–20% incidence of long COVID, but symptoms vary widely from mild discomfort to severe conditions. As the trend of COVID pandemic is slowly flattened, never more urgently had health and workforce planners needed information on the impact of long-COVID to appropriately scale future medical resource allocation. It is essential to understand both the longer-term trajectory of recovery to identify ongoing healthcare needs and the required response by healthcare systems and policymakers for this already large and ever-increasing population.
We have established a longitudinal cohort of more than 20,000 COVID-19 patients and prospectively observed the incidence and dynamic patterns of long COVID for one year since the outbreak of Omicron BA.2 in Shanghai, March, 2022. Most of the patients experienced the COVID-19 infection for the first time and the population represented similar composition of asymptomatic, mild and severe cases as reported by Shanghai CDC, therefore, the data from this cohort can objectively reflect the overall short and long-term influence of long COVID among populations post Omicron BA.2 outbreak.
Methods
Study design and participants
We conducted follow-ups with 28,051 patients who were laboratory confirmed with or clinician diagnosed with COVID-19 at Huashan Hospital from March 24 to June 7, 2022. All discharged patients met the same discharge criteria according to the Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment published by the National Health Commission [11]. The first follow-up was conducted 6 months after the patient's discharge from Huashan Hospital, by telephone, for the patients who met the enrollment criteria (Supplementary Table 1). They were interviewed with the survey questions on long COVID symptoms, also their demographic and clinical characteristics would be retrieved from electronic medical records with consent. Then those reported with physical or mental symptoms indicating that the participant might have long COVID would be further invited to undergo a comprehensive healthy physical examination and a more detailed questionnaire via face-to-face interview.
Those with long COVID according to the survey and healthy physical examination would enter the second follow-up, which was conducted 12 months after discharge. In the 12-month follow-up, participants were investigated whether they had experienced reinfection with SARS-CoV-2. Those who had not been reinfected were required to complete the survey on long COVID symptoms and undergo a healthy physical examination, to assess whether their long COVID symptoms had resolved.
Meanwhile, we recruited 979 healthy individuals without a history of COVID-19 diagnosis according to the enrollment criteria (Supplementary Table 1). They were asked to complete the online questionnaires regarding whether they recently had experienced any symptoms that last for more than two months (Supplementary Table 3), and their demographic information was also recorded with consent. Besides, based on the healthy participants’ wiliness, they would undergo a healthy physical examination in December 2022.
Written informed consent was obtained from all study participants. The study was approved by the Huashan Ethics Committee (KY2022-721).
Definitions of long COVID
Long COVID was defined as conditions that occur in individuals with a history of probable or confirmed SARS-CoV-2 infection 3 months from the onset of COVID-19 that last for 2 months and cannot be explained by an alternative diagnosis [1]. Symptoms suggestive of long COVID were evaluated through questionnaires (Supplementary Tables 2, 4–9), and individuals with one or more long COVID symptoms would be considered as having “long COVID.”
Telephone follow-up survey questions and questionnaire of psychological and somatic symptom
The follow-ups were conducted by trained medical staff via telephone. The consultations made to those discharged patients included self-reported symptoms, COVID-19 vaccination status, previous medical history, and the intention to undergo a health examination and a detailed questionnaire.
Follow-up patients who completed the offline questionnaire were interviewed face-to-face by experienced physicians, while those healthy participants completed another online survey. Both questionnaires collected demographic information (age, gender, height, weight, place of usual residence, education, career, cigarette smoking, and COVID-19 vaccination status). Additionally, the questionnaires included the modified British Medical Research Council (mMRC) dyspnoea scale, the EuroQol five-dimension five-level (EQ-5D-5L) questionnaire, the Generalized Anxiety Disorder-7 (GAD-7) questionnaire, the EuroQol Visual Analogue Scale (EQ-VAS), the Patients Health Questionnaire (PHQ-9), the PTSD Checklist-Civilian Version (PCL-C). Physical symptoms such as hair loss, taste or smell abnormalities, somatic pain, and respiratory symptoms were also recorded. We also collected data on the length of isolation for healthy individuals from 24 March to 7 June 2022.
The mMRC dyspnoea scale classifies the severity of dyspnoea in respiratory diseases into 5 grades, ranging from “Dyspnoea only with strenuous exercise” to “Too dyspneic to leave house or breathless when dressing.” The EQ-5D-5L is a validated questionnaire that assesses subjects across five dimensions including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [12]. Each dimension has 5 levels, ranging from no problems to extreme problems. Furthermore, the patients would be asked to tick the item closest to his/her health status. The GAD-7 questionnaire is used to screen or measure the severity of generalized anxiety disorder [13, 14]. The interviewer would calculate the subject's GAD-7 score by assigning scores of 0, 1, 2, and 3, from “not at all” to “nearly every day,” according to the patients’ symptoms frequency within 2 weeks, and adding together the scores for the seven questions. The EQ-VAS aims to record a patient's self-rated health status on a vertical visual analogue scale which could reflect the self-judgment of the patient's health outcome [15]. The PHQ-9 is used to monitor the severity of depression [16, 17]. It scores each of the items as “0” (not at all) to “3” (nearly every day). The PHQ-9 score is the sum of the scores for the nine questions. The PCL-C questionnaire screens individuals for PTSD [18]. The respondents would be asked to rate how frequently the items in the survey bothered them during the past month. The scale range from 0 to 4, as 0 stands for “Not at all” and 4 stands for “Extremely.”
Healthy physical examination
The healthy physical examination conducted during the two follow-up visits for patients who recovered from COVID-19 included routine laboratory tests and imaging examinations. These tests were mostly consistent with the health examination items arranged for the healthy participants. Venous blood samples were collected for complete blood count, haemoglobin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total serum bilirubin, and erythrocyte sedimentation rate (ESR), serum creatinine. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation [19]. Chest computerized tomography (CT) was used to evaluate the participant's lung lesion. The laboratory tests conducted during the follow-up for the patients recovered from COVID-19 included additional indicators of B-type natriuretic peptide (BNP), C-reactive protein (CRP).
Statistical analysis
The recruited convalescent patients were categorized into two groups, according to their self-report symptoms in telephone follow-up and the symptom questionnaire. Those reported positive symptoms in either telephone survey questions or face-to-face symptom questionnaires would be classed as the long COVID Group, and others would be considered the non-long COVID Group. The healthy individuals with no history of COVID-19 diagnosis would be classified into the healthy control Group.
Baseline characteristics are presented as mean (SD) for continuous variables. We used Mann–Whitney U to compare the demographic data for continuous variables. Differences in proportions were evaluated using χ2 tests. Univariable and multivariable logistic analysis were used to estimate the odds ratios (ORs) and 95% CIs to explore risk factors associated with long COVID. Variables included in the multivariable model based on clinical plausibility were age, sex, COVID-19 vaccination status, number of comorbididities and severity during acute infection. Variables with P < 0.2 in the univariable analysis were included in the multivariable analysis. All tests were two-sided, and a p value less than 0.05 was considered statistically significant. Without imputed missing data, we included all individuals for whom the relevant variables were available in the final analysis. All statistical analyses were done with SPSS statistical package 25.0 software.
Results
Baseline of participants
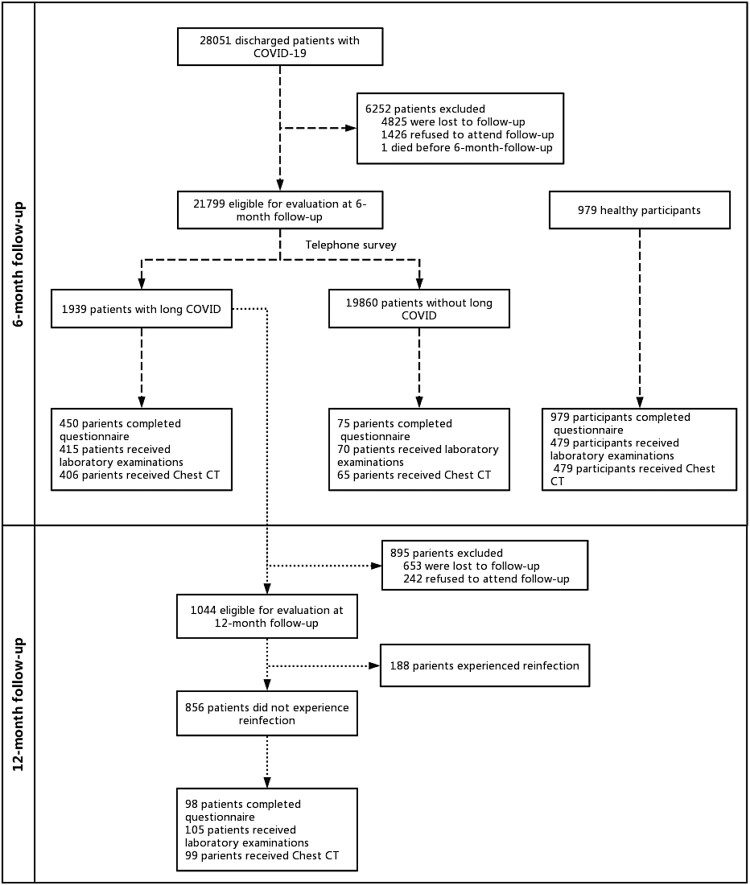
A total of 28,051 patients with COVID-19 were discharged from Huashan Hospital in March–June. The 6-month follow-up visit was done between 17 September and 19 November 2022, and the 12-month follow-up visit between 1 March and 1 April 2023 (Figure 1). By excluding the individuals who did not meet our inclusion and exclusion criteria, 21,799 patients were finally enrolled at 6-month telephone follow-up. The demographic and clinical characteristics of the 21,799 COVID-19 patients at the 6-month follow-up are shown in Table 1. The mean age of the participants was 43.89 ± 13.95 years, with 14,052 (64.4%) male; 4384 (20.1%) had at least one pre-existing health condition; 1152 (5.28%) had received one dose of vaccination and 18,546 (85.08%) had received two or more doses of vaccination; 21,662 (99.4%) patients were diagnosed with non-severe cases and 135 (0.6%) patients were diagnosed with severe-critical cases during acute infection phase.
Figure 1.
Flow chart of the study.
Table 1.
Demographic and clinical characteristics of participants at the 6-month follow-up.
| Omicron infection n = 21799 |
Non-severe n = 21,664 |
Severe-critical n = 135 |
Healthy control n = 979 |
P value Non-severe vs severe-critical |
P value Omicron infection vs healthy control |
|
|---|---|---|---|---|---|---|
| Gender(male) | 14,053/21,799 (64.47) | 13,958/21,664 (64.43) | 95/135 (70.37) | 624/979(63.74) | 0.151 | 0.642 |
| Age (years)‡ | 43.89 ± 13.64 | 43.75 ± 13.53 | 65.21 ± 14.04 | 43.51 ± 10.70 | <0.001 | 0.391 |
| Vaccination | <0.001 | <0.001 | ||||
| Unvaccinated | 2101/2,1799 (9.64) | 2007/21,664 (9.26) | 94/135 (69.63) | 93/979 (9.50) | ||
| One dose | 1152/21,799 (5.28) | 1148/21,664 (5.30) | 4/135 (2.96) | 21/979 (2.15) | ||
| Two or more dose | 18,546/21,799 (85.08) | 18,509/21,664 (85.44) | 37/135 (27.41) | 865/979 (88.35) | ||
| Number of comorbidities | <0.001 | NA | ||||
| 0 | 17,415/21,799 (79.89) | 17,395/21,664 (80.29) | 20/135 (14.81) | NA | ||
| 1 | 3286/21,799 (15.07) | 3254/21,664 (15.02) | 32/135 (23.70) | NA | ||
| ≥2 | 1098/21,799 (5.04) | 1015/21,664 (4.69) | 83/135 (61.48) | NA | ||
| Comorbidities, n (%) | ||||||
| Hypertension | 3296/21,799 (15.12) | 3204/21,664 (14.79) | 92/135 (68.15) | NA | <0.001 | NA |
| Diabetes miletus | 1097/21,799 (5.03) | 1053/21,664 (4.86) | 44/135 (32.59) | NA | <0.001 | NA |
| Chronic pulmonary disease | 264/21,799 (1.21) | 248/21,664 (1.14) | 16/135 (11.85) | NA | <0.001 | NA |
| Cardio-cerebral vascular disease | 407/21,799 (1.87) | 378/21,664 (1.74) | 29/135 (21.48) | NA | <0.001 | NA |
| Chronic liver disease | 187/21,799 (0.86) | 187/21,664 (0.86) | 0/135 (0) | NA | 0.278 | NA |
| Chronic kidney disease | 170/21,799 (0.78) | 129/21,664 (0.60) | 41/135 (30.37) | NA | <0.001 | NA |
| Chronic neurologic disease | 106/21,799 (0.49) | 96/21,664 (0.44) | 10/135 (7.41) | NA | <0.001 | NA |
| Rheumatic disease | 101/21,799 (0.46) | 99/21,664 (0.46) | 2/135 (1.48) | NA | 0.130 | NA |
| Cancer | 144/21,799 (0.66) | 131/21,664 (0.60) | 13/135 (9.63) | NA | <0.001 | NA |
| Number of symptoms | 0.041 | <0.001 | ||||
| 0 | 19,860/21,799 (91.11) | 19,744/21,664 (91.14) | 116/135 (85.93) | 894/979 (91.32) | ||
| 1 | 1303/21,799 (5.98) | 1288/21,664 (5.95) | 15/135 (11.11) | 36/979 (3.68) | ||
| ≥2 | 636/21,799 (2.92) | 632/21,664 (2.92) | 4/135 (2.96) | 49/979 (5.00) | ||
| Long covid symptoms, n (%) | ||||||
| At least one symptom | 1939/21,799 (8.89) | 1920/21,664 (8.86) | 19/135 (14.07) | 85/979 (8.68) | 0.034 | 0.819 |
| Fatigue | 736/21,799 (3.38) | 732/21,664 (3.38) | 4/135 (2.96) | 22/979 (2.25) | 0.790 | 0.054 |
| Sleep difficulties | 480/21,799 (2.20) | 477/21,664 (2.20) | 3/135 (2.22) | 41/979 (4.19) | 0.987 | <0.001 |
| Hair loss | 450/21,799 (2.06) | 449/21,664 (2.07) | 1/135 (0.74) | 17/979 (1.74) | 0.278 | 0.479 |
| Cough | 380/21,799 (1.74) | 374/21,664 (1.73) | 6/135 (4.44) | 5/979(0.51) | 0.016 | 0.003 |
| Sore throat | 277/21,799 (1.27) | 275/21,664 (1.27) | 2/135 (1.48) | 9/979 (0.92) | 0.826 | 0.334 |
| Chest tightness/Chest pain | 199/21,799 (0.91) | 196/21,664 (0.90) | 3/135 (2.22) | 7/979 (0.72) | 0.109 | 0.522 |
| Breathing difficulties | 43/21,799 (0.20) | 42/21,664 (0.19) | 1/135 (0.74) | 0/979 (0) | 0.235 | 0.164 |
| Smell disorder | 107/21,799 (0.49) | 107/21,664 (0.49) | 0/135 (0) | 0/979 (0) | 1.000 | 0.028 |
| Taste disorder | 94/21,799 (0.43) | 94/21,664 (0.43) | 0/135 (0) | 0/979 (0) | 1.000 | 0.040 |
| Headache | 145/21,799 (0.67) | 145/21,664 (0.67) | 0/135 (0) | 16/979 (1.63) | 1.000 | <0.001 |
| Palpitations | 181/21,799 (0.83) | 181/21,664 (0.84) | 0/135 (0) | 7/979 (0.92) | 0.286 | 0.696 |
| Skin rash | 96/21,799 (0.44) | 95/21,664 (0.44) | 1/135 (0.74) | 9/979 (0.92) | 0.450 | 0.030 |
| Dizziness | 165/21,799 (0.76) | 164/21,664 (0.76) | 1/135 (0.74) | 7/979 (0.72) | 0.983 | 0.882 |
| Muscle pain | 106/21,799 (0.49) | 105/21,664 (0.48) | 1/135 (0.74) | 4/975 (0.41) | 0.483 | 0.732 |
| Joint pain | 180/21,799 (0.83) | 179/21,664 (0.83) | 1/135 (0.74) | 17/975 (1.74) | 0.913 | 0.003 |
| Inappetence | 76/21.799 (0.35) | 76/21,664 (0.35) | 0/135 (0) | 10/975 (1.03) | 1.000 | 0.001 |
| Brain fog | 72/21,799 (0.33) | 72/21,664 (0.33) | 0/135 (0) | 0/975 (0) | 1.000 | 0.072 |
| Impairied hearing | 13/21,799 (0.06) | 13/21,664 (0.06) | 0/135 (0) | 0/975 (0) | 1.000 | 1.000 |
| Impaired vision | 6/21,799 (0.03) | 6/21,664 (0.03) | 0/135 (0) | 0/975 (0) | 1.000 | 1.000 |
| Abdominal pain | 8/21,799 (0.03) | 8/21,664 (0.04) | 0/135 (0) | 0/975 (0) | 1.000 | 1.000 |
Long COVID symptoms, functional scales, and laboratory examinations of participants at 6-month follow-up
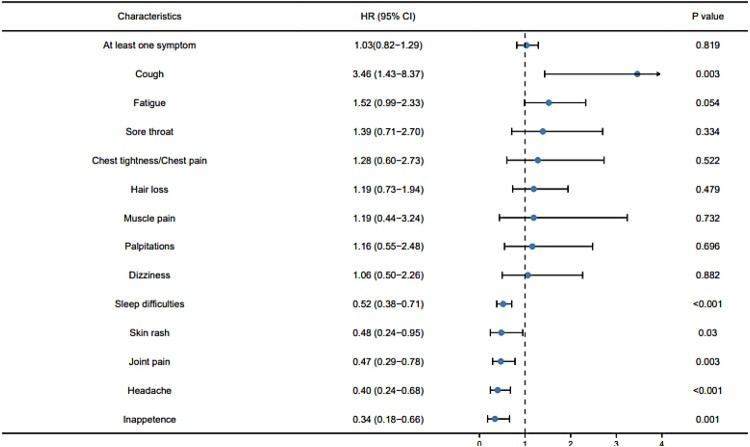
Of the 21,797 patients, 1939 (1939/21,799, 8.89%) self-reported the presence of long COVID symptoms and 636 (636/21,799, 2.92%) reported two or more symptoms. The most common symptom was fatigue (736/21,799, 3.38%), followed by sleep difficulties (480/21,799, 2.20%), hair loss (437/21,797, 2.06%), cough (437/21,797, 1.74%), and sore throat (268/21,797, 1.27%). Compared with non-severe cases, severe-critical cases were more likely to report one long COVID symptoms: cough (4.44% vs 1.73%, P = 0.016). The proportion of COVID-19 patients reporting symptoms was not significantly different from healthy control (9.24% vs 8.68%, P = 0.562), but COVID-19 patients were more likely to report cough, smell disturbance and taste disturbance, and the healthy control were more likely to report sleep difficulties, headache, skin rash and inappetence (Figure 2).
Figure 2.
Forest plot of long COVID symptoms in patients infected with COVID-19, with the healthy control as reference.
To assess whether long COVID would impact patients’ quality of life and mental status, 450 long COVID patients and 75 non-long COVIDs patients participated in our 6-month outpatient follow-up. All patients attended the outpatient follow-up completed questionnaires (Table 2). For the EQ-5D-5L questionnaires, long COVID people were significantly more likely to report pain/discomfort (45.11% vs 2.67%, P < 0.001) and anxiety/depression (53.33% vs 10.67%, P < 0.001) than non-long COVID people, and were significantly more likely to report mobility problem (6.44% vs 1.33%, P < 0.001), personal care problem (2.00% vs 0.41%, P = 0.003), usual activity problem (4.89% vs 1.12%, P < 0.001), pain/discomfort (45.11% vs 13.89%, P < 0.001) and anxiety/depression (53.33% vs 43.51%, P = 0.001) than healthy control. On the psychiatric questionnaire, long COVID patients were more likely to report anxiety (40.67% vs 8.00%, P < 0.001), depression (46.00% vs 6.67%, P < 0.001) and PTSD (19.33% vs 1.33%, P < 0.001) than non-long COVID patients, and were more likely to report depression (46.00% vs 31.97%, P < 0.001) and PTSD (19.33% vs 11.34%, P < 0.001) than healthy control.
Table 2.
Quality of life, mental health, imaging examination and laboratory examinations of participants at the 6-month follow-up.
| Long COVID n = 450 |
Non-long COVID n = 75 |
Health control n = 979 |
P Long COVID vs non-long COVID |
P Long COVID vs health control |
|
|---|---|---|---|---|---|
| Gender(male) | 255/450 (56.67) | 47/75 (62.67) | 624/979(63.74) | 0.330 | 0.011 |
| Age (years)‡ | 42.32 ± 12.67 | 39.77 ± 14.83 | 43.51 ± 10.70 | 0.117 | 0.007 |
| Vaccination | 0.003 | <0.001 | |||
| Unvaccinated | 56/450 (12.44) | 19/75 (25.33) | 93/979 (9.50) | ||
| One dose | 29/450 (6.44) | 8/75 (10.67) | 21/979 (2.15) | ||
| Two or more doses | 365/450 (81.11) | 48/75 (64.00) | 865/979 (88.35) | ||
| mMRC score ≥1 | 167/450 (37.11) | 5/75 (6.67) | 64/979 (6.54) | < 0.001 | <0.001 |
| EQ-5D-5L questionnaire | |||||
| Mobility problem | 29/450 (6.44) | 1/75 (1.33) | 13/979 (1.33) | 0.077 | <0.001 |
| Personal care problem | 9/450 (2.00) | 1/75 (1.33) | 4/979 (0.41) | 0.695 | 0.003 |
| Usual activity problem | 22/450 (4.89) | 0/75 (0) | 11/979 (1.12) | 0.057 | <0.001 |
| Pain or discomfort | 203/450 (45.11) | 2/75 (2.67) | 136/979 (13.89) | < 0.001 | <0.001 |
| Anxiety or depression | 240/450 (53.33) | 8/75 (10.67) | 426/979 (43.51) | < 0.001 | 0.001 |
| EQ-VAS score | 76.08 ± 18.83 | 89.48 ± 13.95 | 84.03 ± 28.72 | <0.001 | <0.001 |
| Anxiety symptom (GAD-7 ≥ 5) |
183/450 (40.67) | 6/75 (8.00) | 370/979 (37.79) | <0.001 | 0.300 |
| Depression symptom (PHQ-9 ≥ 5) |
207/450 (46.00) | 5/75 (6.67) | 313/979 (31.97) | <0.001 | <0.001 |
| PTSD symptom (PCL-C ≥ 38) |
87/450 (19.33) | 1/75 (1.33) | 111/979 (11.34) | <0.001 | <0.001 |
| Chest CT | |||||
| Normal CT pattern | 170/406 (41.87) | 29/65 (44.62) | 160/479 (33.40) | 0.678 | 0.009 |
| Pleural thickening | 21/406 (5.17) | 4/65 (6.15) | 50/479 (10.44) | 0.743 | 0.004 |
| Chronic slight inflammation | 43/406 (10.59) | 5/65 (7.69) | 58/479 (12.11) | 0.473 | 0.479 |
| Interstitial change | 10/406 (2.46) | 2/65 (3.08) | 6/479 (1.25) | 0.771 | 0.178 |
| Nodule | 94/406 (23.15) | 9/65 (13.85) | 248/479 (51.77) | 0.092 | <0.001 |
| Old lesions | 103/406 (25.37) | 19/65 (29.23) | 85/479 (17.75) | 0.509 | 0.006 |
| Laboratory examinations | |||||
| White blood cell count >9.5, 10^9/L | 11/415 (2.65) | 1/70 (1.43) | 10/479 (2.09) | 0.543 | 0.579 |
| Haemoglobin <90, g/L | 22/415 (5.30) | 5/70 (7.14) | 1/479 (0.21) | 0.534 | <0.001 |
| Neutrophils count >6.3, 10^9/L | 15/415 (3.61) | 1/70 (1.43) | 12/479 (2.51) | 0.344 | 0.334 |
| Lymphocyte count <1.1, 10^9/L | 12/415 (2.89) | 0/70 (0) | 8/479 (1.67) | 0.150 | 0.218 |
| Platelet <125, 10^9/L | 2/415 (0.48) | 0/70 (0) | 0/479 (0) | 1.000 | 0.215 |
| NT-Pro-BNP >125, pg/ml | 4/415 (0.96) | 1/70 (1.43) | NA | 0.543 | NA |
| C-reactive ptotein >5, mg/L | 17/415 (4.10) | 5/70 (7.14) | NA | 0.257 | NA |
| Alanine aminotransferase >2UL, U/L | 18/415 (4.34) | 3/70 (4.29) | 14/479 (2.92) | 0.984 | 0.256 |
| Aspartate aminotransferase >2UL, U/L | 4/415 (0.96) | 2/70 (2.86) | 2/479 (0.42) | 0.210 | 0.424 |
| Total bilirubin >2UL, μmol/L | 0/415 (0) | 0/70 (0) | 0 (0) | NA | NA |
| Glomeruar filtration rate <90, ml/min | 89/415 (21.45) | 11/70 (15.71) | 99/479 (20.67) | <0.001 | 0.776 |
To find out whether Omicron infection impairs patients’ organ function, participants attending the 6-month outpatient follow-up received imaging and laboratory examination. 406 of 450 long COVID patients and 65 of 75 non-long COVID patients completed chest CT at follow-up (Table 2). 170 (170/406, 41.87%) long COVID patients and 47 (29/65, 44.62%) non-long COVID patients had normal CT pattern, with no significant difference between them. Compared to healthy control, long COVID patients had higher proportion of obsolescence change on chest CT, but lower proportion of pleural thickening and nodule on chest CT. 415 of 450 long COVID patients and 70 of 75 non-long COVID patients completed laboratory examinations at follow-up. In the univariate analysis model, long COVID patients and non-long COVID patients were statistically different only in the proportion of eGFR abnormalities, and long COVID patients and healthy control were statistically different only in the proportion of haemoglobin abnormalities.
Comparation of 6-month follow-up and 12-month follow-up
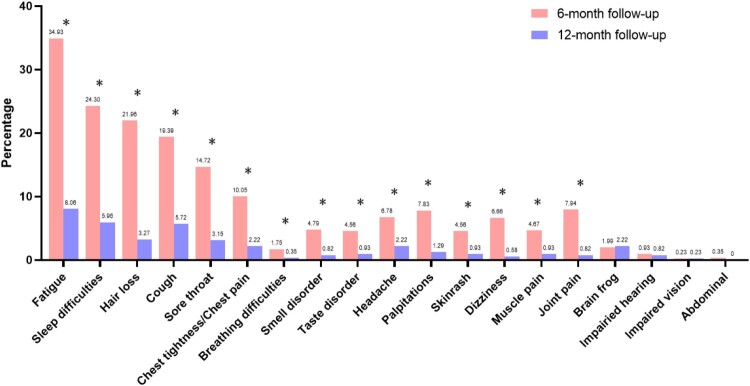
The 12-month follow-up was conducted for the 1939 patients who reported symptoms of long COVID at the 6-month follow-up. A total of 856 patients attended the 12-month follow-up after excluding those who refused to attend and those who had reinfection between 6-month and 12-month follow-up. At 12-month telephone follow-up, 587 (68.5%, 587/856) patients’ symptoms of long COVID had disappeared. A significant decrease was observed in fatigue, sleep difficulties, hair loss, cough, sore throat, chest tightness/chest pain, breathing difficulties, smell disorder, taste disorder, headache, palpitations, skin rash, dizziness, muscle pain and joint pain (Figure 3). 105 participated in the 12-month outpatient follow-up, with 93 questionnaires, 98 chest CT reports and 105 laboratory examinations collected. Compared to the data of 6-month follow-up, there was a decrease in the proportion of patients reporting pain/discomfort on the EQ-5D-5L questionnaire (Table 3). There was no significant change in the proportion of abnormal mental status, the proportion of abnormal chest CT and the proportion of abnormal laboratory examinations (Table 3).
Figure 3.
Dynamic change of long COVID symptoms of participants who completed 6-month, and 12-month follow-up. *P < 0.05.
Table 3.
Quality of life, mental health, imaging examination and laboratory examinations of participants who completed 6-month, and 12-month follow-up.
| 6-month follow-up n = 105 |
12-month follow-up n = 105 |
P value | |
|---|---|---|---|
| mMRC score ≥1 | 38/93 (40.86) | 36/93 (38.71) | 0.764 |
| EQ-5D-5L questionnaire | |||
| Mobility problem | 8/93 (8.60) | 16/93 (17.20) | 0.080 |
| Personal care problem | 2/93 (2.15) | 5/93 (5.38) | 0.444 |
| Usual activity problem | 5/93 (5.38) | 7/93 (7.53) | 0.551 |
| Pain or discomfort | 48/93 (51.61) | 34/93 (35.56) | 0.039 |
| Anxiety or depression | 48/93 (51.61) | 43/93 (46.24) | 0.463 |
| EQ-VAS score | 75.65 ± 18.90 | 79.34 ± 19.01 | 0.187 |
| Anxiety symptom (GAD-7 ≥ 5) |
36/93 (38.71) | 32/93 (34.41) | 0.543 |
| Depression symptom (PHQ-9 ≥ 5) |
45/93 (48.39) | 39/93 (41.94) | 0.377 |
| PTSD symptom (PCL-C ≥ 38) |
18/93 (19.35) | 16/93 (17.20) | 0.704 |
| Chest CT | |||
| Normal CT pattern | 53/98 (54.08) | 51/98 (52.04) | 0.775 |
| Pleural thickening | 6/98 (6.12) | 5/98 (5.10) | 0.756 |
| Chronic slight inflammation | 19/98 (19.39) | 20/98 (20.41) | 0.858 |
| Interstitial change | 2/98 (2.04) | 2/98 (2.04) | 1.000 |
| Nodule | 19/98 (19.39) | 23/98 (23.47) | 0.486 |
| Old fibro lesions | 26/98(26.53) | 31/98 (31.63) | 0.432 |
| Laboratory examinations | |||
| White blood cell count >9.5, 10^9/L | 2/105 (1.90) | 4/105 (3.81) | 0.683 |
| Haemoglobin <90, g/L | 5/105 (4.76) | 1/105 (0.95) | 0.212 |
| Neutrophils count >6.3, 10^9/L | 4/105 (3.81) | 3/105 (2.86) | 0.701 |
| Lymphocyte count <1.1, 10^9/L | 3/105 (2.86) | 2/105 (1.90) | 0.651 |
| Platelet <125, 10^9/L | 2/105 (1.90) | 1/105 (0.95) | 0.561 |
| NT-Pro-BNP >125, pg/ml | 1/105 (0.95) | 5/105 (4.76) | 0.212 |
| C-reactive ptotein >5, mg/L | 4/105 (3.81) | 9/105 (8.57) | 0.152 |
| Alanine aminotransferase >2UL, U/L | 4/105 (15.24) | 3/105 (13.33) | 0.701 |
| Aspartate aminotransferase >2UL, U/L | 1/105 (8.57) | 3/105 (6.67) | 0.621 |
| Total bilirubin >2UL, μmol/L | 0/105 (5.71) | 0/105 (3.81) | NA |
| Glomeruar filtration rate <90, ml/min | 23/105 (21.90) | 32/105 (30.48) | 0.158 |
Logistic regression for development of long COVID and persistent long COVID
In the multivariable logistic regression, people aged equal to or more than 60 years were less likely to develop long COVID than those under 40 years old. Female, pre-existing medical condition and severe-critical infection during the acute infection were risk factors for developing long COVID (Table 4). At 12-month follow-up, patients aged 40–59 were at higher risk for persistent symptoms of long COVID than those under 40 years old (Table 5).
Table 4.
Univariate and multivariate logistic regression model for development of long COVID at the 6-month follow-up.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Characteristics | OR (95%CI) | P values | aORs(95%CI) | P values |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.79 (0.72–0.87) | <0.001 | 0.77 (0.70–0.85) | <0.001 |
| Age | ||||
| <40 | Reference | Reference | ||
| 40–59 | 0.94 (0.85–1.04) | 0.211 | 0.91 (0.82–1.01) | 0.058 |
| ≥60 | 0.74 (0.63–0.87) | <0.001 | 0.63 (0.53–0.75) | <0.001 |
| Vaccination | ||||
| Unvaccinated | Reference | – | ||
| One dose | 1.12 (0.87–1.43) | 0.394 | – | – |
| Two or more doses | 1.06 (0.90–1.24) | 0.512 | – | – |
| Number of comorbidities | ||||
| 0 | Reference | Reference | ||
| 1 | 1.02 (0.90–1.16) | 0.765 | 1.13 (0.98–1.29) | 0.086 |
| ≥2 | 1.15 (0.94–1.41) | 0.175 | 1.33 (1.06–1.66) | 0.013 |
| Severity | ||||
| Non-severe | Reference | Reference | ||
| Severe-critica | 1.79 (1.11–2.88) | 0.017 | 1,95 (1.18–3.21) | 0.009 |
Table 5.
Univariate and multivariate logistic regression model for persistent long COVID at the 12-month follow-up.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Characteristics | OR (95%CI) | P values | aORs(95%CI) | P values |
| Sex | ||||
| Female | Reference | – | ||
| Male | 1.07 (0.80–1.45) | 0.641 | – | – |
| Age | ||||
| <40 | Reference | Reference | ||
| 40–59 | 1.54 (1.13–2.11) | 0.006 | 1.50 (1.09–2.07) | 0.014 |
| ≥60 | 1.57 (0.98–2.51) | 0.064 | 1.60 (0.95–2.67) | 0.075 |
| Vaccination | ||||
| Unvaccinated | Reference | Reference | ||
| One dose | 1.33 (0.57–3.11) | 0.506 | 1.51 (0.64–3.59) | 0.349 |
| Two or more doses | 1.59 (0.88–2.90) | 0.126 | 1.78 (0.96–3.28) | 0.067 |
| Number of comorbidities | ||||
| 0 | Reference | Reference | ||
| 1 | 1.42 (0.97–2.07) | 0.071 | 1.29 (0.86–1.91) | 0.216 |
| ≥2 | 1.09 (0.56–2.09) | 0.806 | 0.99 (0.49–2.03) | 0.997 |
| Severity | ||||
| Non-severe | Reference | – | ||
| Severe-critica | 0.97 (0.30–3.18) | 0.959 | – | – |
Discussion
Current studies have been limited to the incidence and specific symptoms of long COVID in patients with Omicron infection, but it is still unclear whether the long COVID in Omicron-infected patients are accompanied by impairment of organ function. After China lifted the dynamic Zero policy, we hope to systematically and comprehensively assess the health status of first-time COVID infected patients during Omicron pandemic by describing their physical symptoms, mental status and organ function at 6 and 12 months after discharge from hospital.
In our cohort, we found a reduced proportion of long COVID symptoms occurred in Omicron-infected individuals compared to the previous strains reported in other studies, and most symptoms disappeared one year after discharge from hospital. Previous studies have shown that the proportion of Chinese patients with wild-type strains who had at least one clinical sequelae 3 months to 24 months after discharge was 29.9% to 76% [20–23] and the proportion of patients with Delta strains who had at least one clinical sequelae two months after discharge was 40.4% [24], whereas in the present study only 8.89% of patients reported symptoms of long COVID six months after discharge, a much lower proportion than in previous studies. The same downward trend was seen in other countries [25, 26]. The difference in strains might be the main reason for the change in the incidence of long COVID. It has been suggested that the clinical severity of the acute phase is closely related to the development of long COVID [27], and that the Omicron strain causes fewer severe clinical events compared to previous strains [28, 29], which may lead to a lower incidence of long COVID, but the exact mechanism remains to be further investigated. In our study, the most common long COVID symptoms were fatigue, sleep difficulties, hair loss and cough. The symptoms were self-reported during the telephonic interview and no objective assessment was done. This findings is consistent with previous studies, where fatigue has been the most long COVID symptom from wild variant to Omicron, in addition to sleep difficulties, hair loss, and cough were also widely reported long COVID symptoms [23, 25, 30, 31]. Notably, these symptoms are also prevalent in healthy control. In our study, most long COVID symptomshad resolved within one year, which is similar to previous studies. An Israeli study showed that the majority of symptoms in patients infected with the wild-type, Alpha or Delta strains peaked 2–8 months after infection, and then they began to decline and resolved within one year. Our study further shows that the long COVID symptoms caused by the current prevalent strain Omicron, like the earlier strains, also mostly disappear within one year.
The risk factors for developing long COVID are still under exploration. Our research revealed that pre-existing comorbidity was one of the risk factors for developing long COVID, similar to the phenomenon observed in a previous observational cohort study conducted in Italy [32]. The study reported that having four or more comorbidities (OR = 1.32) resulted in a higher risk of developing long COVID. Another prospective cohort study corroborated this finding, with patients having comorbidities such as asthma or COPD more likely to experience long COVID symptoms [27]. Moreover, our results showed that severe-critical infection of COVID-19, female and young adults were also risk factors for developing long COVID, which was consistent with former studies. Blomberg et al. declared that long COVID was independently associated with the severity of initial Illness (RR = 1.28) [27]. In a study that included more than 480,000 COVID-19 patients, female was a risk factor for long COVID (aHRs = 1.52), and the risk of developing long COVID was increased along a gradient of decreasing age [10].
Previous studies have reported vaccination as a protective factor for preventing the occurrence of long COVID. As reported by Azzolini et al., an entire course of vaccination (OR = 0.25) and booster vaccination (OR = 0.16) may be associated with a lower risk of long COVID [32]. Data from the UK Office for National Statistics show that adults aged 18–69 years, receiving two doses of a coronavirus (COVID-19) vaccine at least two weeks before a first test-confirmed COVID-19 infection was associated with a 41.1% decrease in the odds of self-reported long COVID at least 12 weeks later [33]. A risk-benefit analysis from England also estimates that vaccination protects adolescents from long COVID outcomes [34]. Nevertheless, our study did not confirm that the vaccine is a protective factor against the development of long COVID, which might be due to various confounding factors, such as the interval between vaccination and breakthrough infection and the vaccine types. Therefore, further research is needed to be conducted to fully understand the relationship between COVID-19 vaccination and the development of long COVID.
We did not find direct evidence of impairment of organ function in long COVID patients. In our study, patients with long COVID reported lower quality of life than healthy controls, as well as a higher proportion of abnormal mental status than the healthy population, consistent with previous studies. In a meta-analysis of 12 studies covering 4828 COVID-19 infected patients, long COVID was associated with poor quality of life and worse mental health [30]. In our study, although long COVID affected quality of life and mental health, we did not find significant differences in chest CT or laboratory examinations between long COVID patients and healthy individuals. In patients with persistent long COVID at 12-month follow-up, chest CT and laboratory examinations did not reveal a trend towards deterioration, which indicated that the patients with persistent long COVID did not occur impairment of organ function between the 6-month follow-up and 12-month follow-up. Our findings differ from previous studies in that more than half of the patients in the two studies with long-term follow-up of COVID-19 patients had single-organ damage and nearly one third had multi-organ damage [35, 36]. The difference in strains was an important reason for the difference in results, with the populations in both studies originating from the period of COVID-19 waves 1 and 2 in UK, and the patients were infected with either wild-type or alpha strains. Compared to earlier strains, the Omicron strain was associated with a reduced risk of severe infection [37], and the severity of acute infection phase was strongly associated with patient recovery [23, 38–40]. In our study, long COVID in Omicron infected patients was more likely to be subjective symptoms rather than impairment of organ function. A larger sample size, more detailed laboratory examinations and longer follow-up are still needed in the future to determine whether substantial damage will occur in the Omicron infected patients.
In our study, although we did not find impairment of organ function in patients with long COVID, it is undeniable that long COVID are still present in some patients for a long time and have a bad impact on their quality of life and mental status, so there is still a need to explore the mechanisms underlying the development of long COVID from multiple and comprehensive perspectives in the future.
There were several limitations in our study. First, this is a single centre study that included only COVID-19 patients in Shanghai, which limits the representativeness of this cohort. Second, we investigated long COVID symptoms by telephone follow-up, therefore all symptoms were self-reported by patients and could not be objectively assessed, which may have led to some mild symptoms, particularly mild psychiatric symptoms, not being recorded. Moreover, we collected information about symptoms, quality of life and mental status of healthy individuals by online questionnaires, and the different information collection method might have biased the results between healthy individuals and COVID-19 patients. Third, only one patient with severe infection attended the outpatient follow-up, which limits the generalizability of the study findings to this particular population. In the future, larger sample sizes are still needed to evaluate the systemic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection, especially in patients post severe infection.
Conclusion
Female, young adults (age <40 years), ≥ 2 comorbidities and severe infection in the acute phase were risk factors for the development of long COVID. Long COVID mainly manifested as subjective symptoms and partial impacts on the quality of life and mental status. Approximately 70% of long COVID symptoms would resolute after 1 year. No significant impairment of organ function was found among long COVID patients after BA.2 Omicron infection.
Supplementary Material
Acknowledgement
We thank all the patients who participated in this study and the study site personnel for their contributions.
Funding Statement
This study was funded by project supported by National Natural Science Foundation of China (82341033), Shanghai Municipal Science and Technology Major Project (HS2021SHZX001), Shanghai Science and Technology Committee (20dz2260100, 21NL2600100).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. PubMed PMID: 34951953; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matt Bosworth PPaDA . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 2 February 2023 [cited 2023 May 10]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2february2023.
- 3.National Center for Health Statistics USCB . Household Pulse Survey. Long COVID; 2023 [cited 2023 May 10]. Available from: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm.
- 4.Wulf HS, Abbafati C, Aerts JG, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–1615. doi: 10.1001/jama.2022.18931. PubMed PMID: 36215063; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosworth D. Self-reported long COVID after infection with the Omicron variant in the UK: 6 May 2022 [cited 2023 May 10]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidafterinfectionwiththeomicronvariant/6may2022.
- 6.Hastie CE, Lowe DJ, McAuley A, et al. Outcomes among confirmed cases and a matched comparison group in the long-COVID in Scotland study. Nat Commun. 2022;13(1):5663. doi: 10.1038/s41467-022-33415-5. PubMed PMID: 36224173; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taquet M, Dercon Q, Harrison PJ.. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun. 2022;103:154–162. doi: 10.1016/j.bbi.2022.04.013. PubMed PMID: 35447302; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayoubkhani D, Bosworth ML, King S, et al. Risk of long COVID in people infected with severe acute respiratory syndrome coronavirus 2 after 2 doses of a coronavirus disease 2019 vaccine: community-based, matched cohort study. Open Forum Infect Dis. 2022;9(9):ofac464. doi: 10.1093/ofid/ofac464. PubMed PMID: 36168555; PubMed Central PMCID: PMCQ2. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open. 2022;5(10):e2238804. doi: 10.1001/jamanetworkopen.2022.38804. PubMed PMID: 36301542; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–1714. doi: 10.1038/s41591-022-01909-w. PubMed PMID: 35879616; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commission CNH . Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment 2020 [cited 2023 Apr 18]. Available from: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html.
- 12.EuroQol . EQ-5D-5L 2021 Nov 30 [cited 2023 Apr 18]. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/.
- 13.Swinson RP. The GAD-7 scale was accurate for diagnosing generalised anxiety disorder. Evid Based Med. 2006;11(6):184), PubMed PMID: 17213178; eng. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. PubMed PMID: 16717171; eng. [DOI] [PubMed] [Google Scholar]
- 15.Rabin R, de Charro F.. EQ-5D: a measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–343. PubMed PMID: 11491192; eng. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed PMID: 11556941; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron IM, Crawford JR, Lawton K, et al. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br J Gen Pract. 2008;58(546):32–36. doi: 10.3399/bjgp08X263794. PubMed PMID: 18186994; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. PubMed PMID: 26606250; eng. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. PubMed PMID: 19414839; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Li Y, Shao T-R, et al. Some characteristics of clinical sequelae of COVID-19 survivors from Wuhan, China: a multi-center longitudinal study. Influenza Other Respir Viruses. 2022;16(3):395–401. doi: 10.1111/irv.12943. PubMed PMID: 34796652; PubMed Central PMCID: PMCQ2. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. PubMed PMID: 32979574; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Hou C, Shen Y, et al. Two-year health outcomes in hospitalized COVID-19 survivors in China. JAMA Netw Open. 2022;5(9):e2231790. doi: 10.1001/jamanetworkopen.2022.31790. PubMed PMID: 36107425; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. PubMed PMID: 33428867; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q, Wang C, Jing Q, et al. Follow-up of patients with COVID-19 by the delta variant after hospital discharge in Guangzhou, guandong, China. Rev Inst Med Trop Sao Paulo. 2022;64:e31. doi: 10.1590/S1678-9946202264031. PubMed PMID: 35544909; PubMed Central PMCID: PMCQ3. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arjun MC, Singh AK, Roy P, et al. Long COVID following omicron wave in eastern India-a retrospective cohort study. J Med Virol. 2023;95(1):e28214. doi: 10.1002/jmv.28214. PubMed PMID: 36224705; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonelli M, Pujol JC, Spector TD, et al. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. doi: 10.1016/S0140-6736(22)00941-2. PubMed PMID: 35717982; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. PubMed PMID: 34163090; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. PubMed PMID: 35305296; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. Br Med J. 2022;376:e069761. doi: 10.1136/bmj-2021-069761. PubMed PMID: 35264324; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-a systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. PubMed PMID: 34463956; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. PubMed PMID: 34024217; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676–678. doi: 10.1001/jama.2022.11691. PubMed PMID: 35796131; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Statistics OfN . Self-reported long COVID after two doses of a coronavirus (COVID-19) vaccine in the UK: 26 January 2022; 2022 [cited 2023 Apr 18]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidaftertwodosesofacoronaviruscovid19vaccineintheuk/26january2022.
- 34.Gurdasani D, Bhatt S, Costello A, et al. Vaccinating adolescents against SARS-CoV-2 in England: a risk-benefit analysis. J R Soc Med. 2021;114(11):513–524. doi: 10.1177/01410768211052589. PubMed PMID: 34723680; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11(3):e048391. doi: 10.1136/bmjopen-2020-048391. PubMed PMID: 33785495; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis A, Cuthbertson DJ, Wootton D, et al. Multi-organ impairment and long COVID: a 1-year prospective, longitudinal cohort study. J R Soc Med. 2023;116(3). doi: 10.1177/01410768231154703. PubMed PMID: 36787802; eng.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Y, Li X, Zhang L, et al. SARS-CoV-2 omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7(1):141. doi: 10.1038/s41392-022-00997-x. PubMed PMID: 35484110; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Liao X, Ma Z, et al. Clinical status of patients 1 year after hospital discharge following recovery from COVID-19: a prospective cohort study. Ann Intensive Care. 2022;12(1):64. doi: 10.1186/s13613-022-01034-4. PubMed PMID: 35816225; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taboada M, Cariñena A, Moreno E, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect. 2021;82(4):e31–e33. doi: 10.1016/j.jinf.2020.12.022. PubMed PMID: 33373650; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. PubMed PMID: 33490928; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.