PURPOSE
When combined with radiotherapy, limb salvage surgery is an alternative to amputation. This study sought to determine the limb-sparing treatment outcomes in patients diagnosed with soft tissue extremity sarcomas treated at our institution.
MATERIALS AND METHODS
All adult patients with extremity soft tissue sarcoma treated with the radical limb salvage strategy at Shaukat Khanum Memorial Cancer Hospital and Research Canter, Lahore, Pakistan, between January 2017 and December 2019 were retrospectively assessed.
RESULTS
A total of 122 patients were included in the study. The mean age was 42 years (range 19-82), and 64 (52.5%) were males. The majority of patients, 65 (53.3%), were diagnosed with stage III and grade III disease according to American Joint Committee on Cancer TNM classification (Eighth edition). The most common surgical modality was wide local excision that was performed in 106 (86.9%) patients. Adjuvant radiation treatment was given in 111 (91%) patients, whereas 11 (9%) patients received neoadjuvant radiation treatment. The mean dose was 58 Gy (range: 46-66 Gy). Eighty-two (67.2%) of the patients were disease-free on post-treatment radiologic scans with disease recurrence observed in 40 (32.8%) patients. The median disease-free survival was 8 months (95% CI, 5.45 to 10.55). Local recurrence and distant metastases developed in 16 (13%) and 24 (20%) patients, respectively.
CONCLUSION
About two thirds of patients with extremity soft tissue sarcoma were successfully treated with limb salvage strategy, surgery, and radiation therapy. However, high rate of relapse warrants further novel strategies in this patient population.
INTRODUCTION
Soft tissue sarcomas (STSs) are a diverse group of malignant tumors that are mesenchymal in origin arising throughout the body,1 constituting 50%-60%, majority of STS effect extremities and hence the term extremity STSs (ESTSs), which are a rare group of tumors that are challenging to treat. Surgery with negative margins, which most of the times involved amputation, has been the mainspring treatment modality.2 Functional disabilities and impairment after treatment of ESTS by amputation have been a point of major concern as they led to decreased patient functioning.3 Rosenberg et al4 in a randomized prospective study showed no difference in the overall survival (OS) and disease-free survival (DFS) in patients with ESTS undergoing amputation compared with patients undergoing limb-sparing surgery followed by radiotherapy.1 To conserve the optimal structure and function of limbs and joints, the integration of radiotherapy (pre-, intra- or postoperative) with surgery for the management of ESTS is considered the standard of care.5-7 Using this approach of limb salvage therapy, the recurrence rates remain around 10% varying between the institutions. Because of the limitations of enough evidence in providing invariable protocols of treatment for these patients, further research is needed in this context. Here, we present our experience of treating such patients at a tertiary care cancer center of a developing country.
CONTEXT
Key Objective
To investigate the outcomes and toxicity of the limb-sparing approach (wide local excision and radiotherapy) for extremity soft tissue sarcoma from Pakistan, which is a rarely reported subject from low- and middle-income countries.
Knowledge Generated
Wide local excision with pre- or postoperative radiation treatment results in local control in >80% of patients with acceptable toxicity, albeit in a relatively younger population predisposing to higher recurrence rates seen in this population.
Relevance
Presentation in younger population and higher recurrence rate warrant more aggressive surgical and conformal radiotherapy techniques to address this potentially curable disease along with consideration of novel systemic or targeted agents.
MATERIALS AND METHODS
A retrospective review of the data of all the patients diagnosed with ESTS was performed after obtaining formal approval from the Institutional Review Board. Between January 2017 and December 2019, a total of 122 patients with ESTS were treated with curative intent (surgery, radiation therapy or both) at the Clinical and Radiation Oncology Department of Shaukat Khanum Memorial Cancer Hospital and Research Center, Lahore. Other patients who had non-ESTS, those with other treatment options, and those who were treated with palliative intent were excluded from this study.
Basic clinical history, physical examination, serum chemistries, and histopathologic and radiologic testing were performed in all the patients at the in-house facilities. Histopathologic reports were reviewed for type and size of tumor, histologic grade, and surgical margins status. Clinical notes and radiologic reports were reviewed for confirming tumor size and staging.
WHO classification 2017 was used for histologic classification. On the basis of scoring the degree of differentiation, number of mitoses, and tumor necrosis, histologically, ESTSs were classified as grade I, II, and III. Margins were documented as either positive (gross/microscopic) or negative. The size of the tumor was classified as < 5, 5-10, or > 10 cm. Response assessment was performed by interim imaging when needed and post-treatment imaging. Clinical or radiologic recurrence (localized v distant, in-field v out-of-field) was confirmed with histopathologic testing.
Treatment intent for all patients in this study was radical after discussion in the multidisciplinary sarcoma tumor board. One hundred six of 122 patients were treated with wide local excision of tumor with an objective of obtaining negative margins while preserving limb function, and 15 patients were treated with non–limb-sparing surgery with either amputation or disarticulation. One patient did not undergo surgical intervention. All 122 patients received radiation therapy, 11 in the neoadjuvant setting and 111 in the adjuvant setting. Indications for radiotherapy included positive histologic margins, size more than 5 cm, and high histologic grade.
A linear accelerator with 6 MV photon external beam radiotherapy was used to treat all the patients. Computed tomography–based radiation planning was performed. The most common radiotherapy technique was three-dimensional conventional radiotherapy performed in 95 patients followed by volumetric-modulated arc therapy used in 12 patients. Two-dimensional conventional and intensity-modulated radiation therapies were used in nine and six patients, respectively. The mean dose was 58 Gy with a range of 46-66 Gy. Most of the patients received 60 Gy at 2 Gy fractions from Monday to Friday with Saturday and Sunday as off days.
Thirty-five (28.7%) of the patients received chemotherapy, and use of chemotherapy was particularized to individual patients. Ifosfamide and doxorubicin were the most commonly used agents. Select patients received neoadjuvant chemotherapy with concurrent radiotherapy for large tumor size to make resection possible after therapeutic response. Others were offered chemotherapy in the adjuvant setting on the basis of histologic type and high-risk groups (size > 5 cm, histology with high metastatic potential, chemosensitivity, high grade) and for systemic disease.
RESULTS
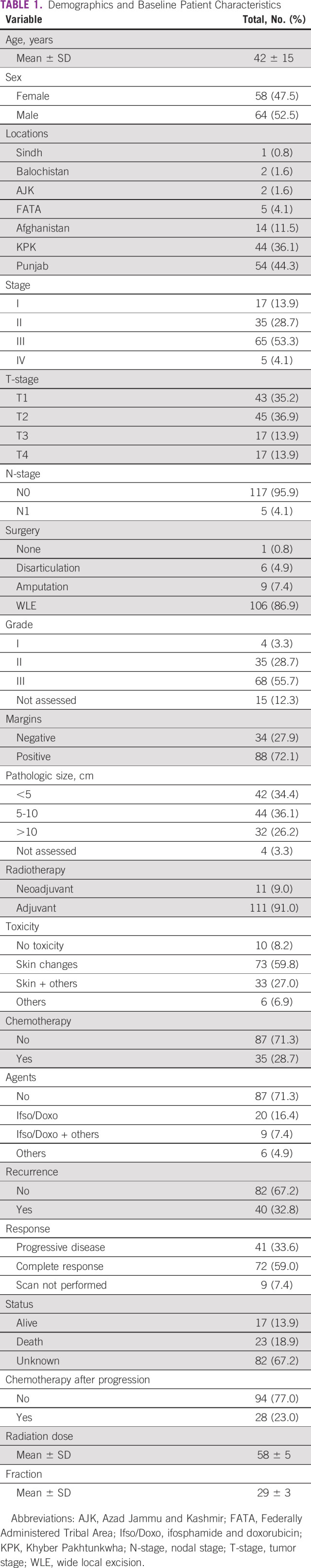
A total of 122 patients were included in this study. The mean age of presentation for patients was 42 years; youngest of them was age 19 years, and oldest was age 82 years. Sixty-four (52.5%) of patients were males, and 58 (47.5%) were females. A majority of patients, 65 (53.3%), had stage III disease, and 68 (55.7%) had grade III disease at the time of diagnosis. Table 1 shows characteristics of patients and tumors included in our study.
TABLE 1.
Demographics and Baseline Patient Characteristics
All the patients in this study received radiation therapy. Of the patients who received adjuvant radiation therapy, most were given 60 Gy at 2 Gy per fraction in 30 fractions, and of those in the preoperative setting, most were treated with a total dose of 46 Gy at 2 Gy per fraction in 23 fractions. All the patients completed their radiation fractions without any significant delays in the treatment.
Radiotherapy was fairly tolerated among all the patients. Ten (8.2%) patients did not experience any side effects of radiations during the whole course of treatment and afterward. The most common acute toxicity observed was grade 1-2 local skin reaction, observed in 106 (86.8%) of the patients. Thirty-three (27%) patients experienced other acute toxicities including grade 1 fatigue, edema, or cellulitis. Because of short follow-up time, late toxicity profile could not be assessed at the time of analysis.
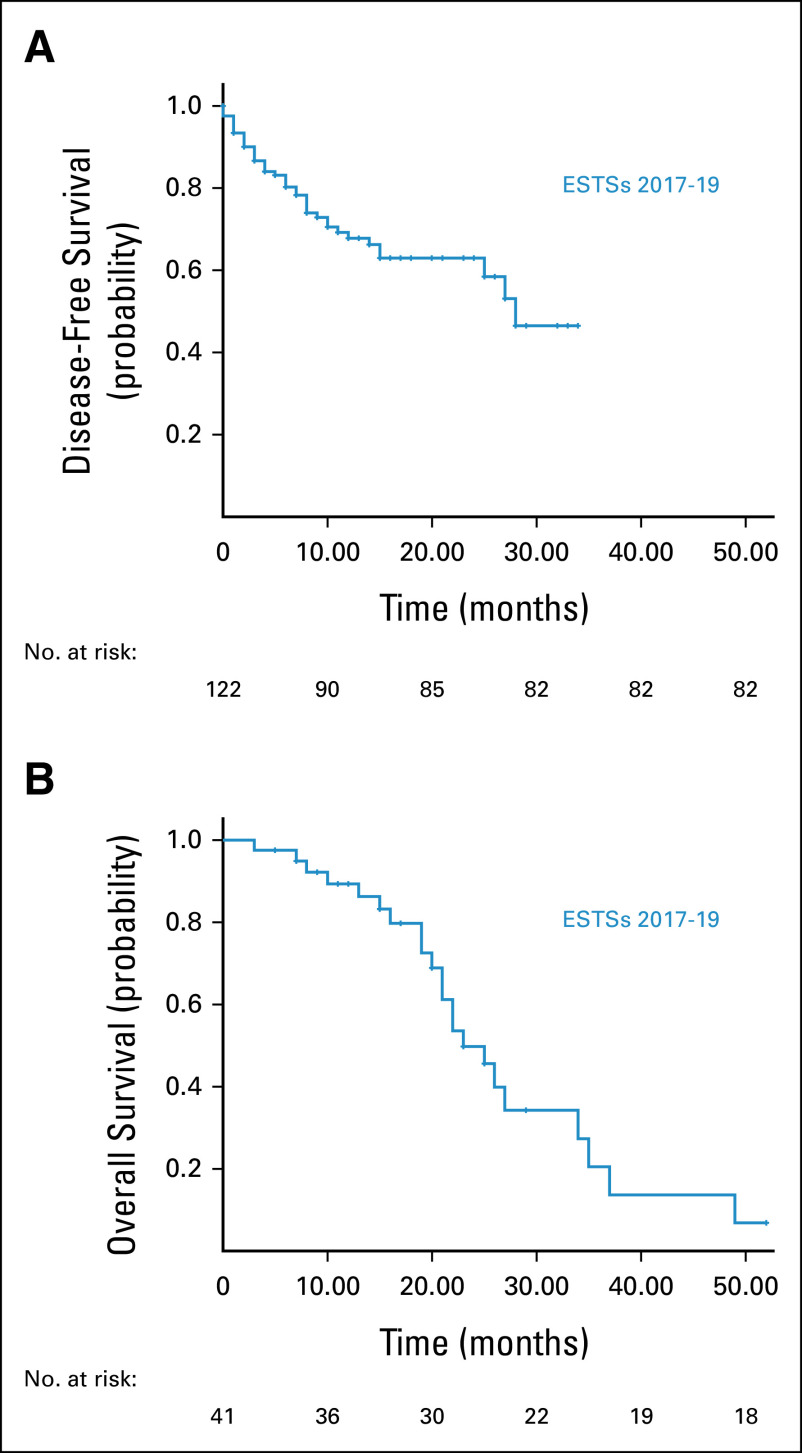
The mean dose was 58 Gy (range: 46-66 Gy). Eighty-two (67.2%) of the patients showed excellent response to radical treatment and did not develop any disease recurrence. Forty patients (32.7%) had disease recurrence despite optimal treatment. Sixteen patients (13.1%) had local recurrence, seven with in-field recurrence and nine with out-of-field recurrence. Twenty-four (19.6%) patients developed distant metastasis mostly to the lungs. Table 2 shows factors associated with disease recurrence. Figure 1 shows the DFS and OS. The DFS at 2-year post-treatment was 63% with a median DFS of 8 months (95% CI, 5.45 to 10.55). The OS was 50% at 2-year post-treatment.
TABLE 2.
Factors Associated With Disease Recurrence
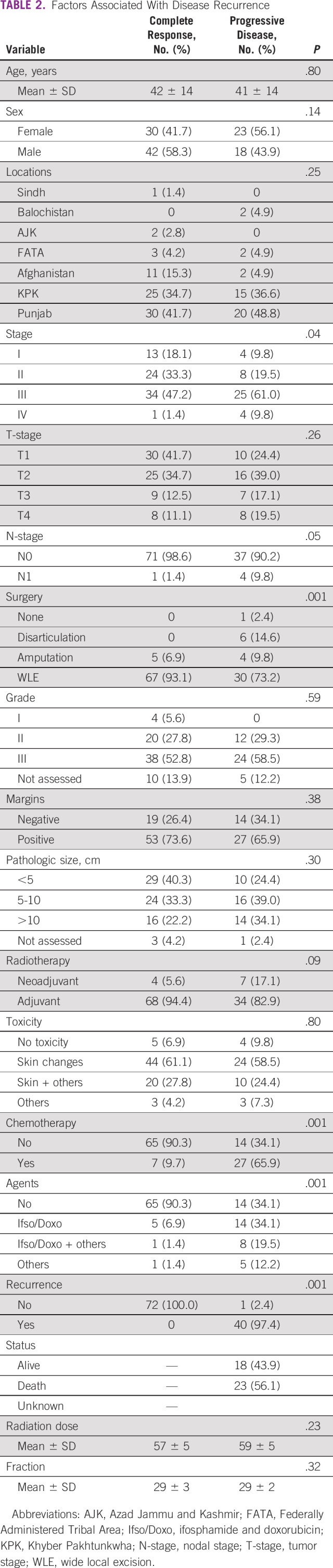
FIG 1.
(A) Disease-free survival and (B) overall survival. ESTS, extremity soft tissue sarcomas.
DISCUSSION
As historically ESTSs were thought of being radio-resistant, surgery has been considered the main modality of management for these tumors.8 Initially, surgical attempts with limited excision were made but led to poor outcomes because of the local disease recurrences. Radical amputation thus became a uniformly accepted management option. Over the time, to avoid total limb function loss, more conservative surgical techniques in lieu of amputation evolved.4
In this study, 122 patients with ESTS treated with the radical limb salvage strategy including radiation therapy in addition to wide surgical resection were analyzed. The mean age was 42 years, which is very low in the study population compared with similar studies in other parts of the world. Two thirds of the patients showed complete response to the treatment despite being diagnosed at a late stage and with high-grade disease. Radiation dosage plays an important role in determining the risk of local recurrence. Patients with ESTS receiving radiation therapy at doses >64 Gy had better local control compared with those receiving <64 Gy.9 The extent of local excision also had a significant impact on the radiation dosage and local control. Patients who have had wide local excision can be given radiation therapy at a dose of 60 Gy without any significant difference in local control and recurrence, with better long-term side effect profiles.10 In our analysis, the mean dose of radiation therapy was 58 Gy. Preoperatively, radiation dose was 46-50 Gy. Because of the wide local excision surgery, postoperative radiotherapy at 60 Gy was used for a majority of patients instead of >64 Gy, providing the same local control with a better toxicity profile.
Unlike other tumor types, many factors dictate the unique radiotherapy technique that is to be used for individualized ESTS. These factors include the normal anatomy, surgical techniques and details, tumor biologic characteristics, and toxicity profiles.11 A majority of patients were treated with a three-dimensional conformal radiotherapy technique owing to better sparing of lymphatic corridor with mild to moderate skin toxicity. Other radiation therapy techniques used were volumetric-modulated arc therapy and two-dimensional conventional and intensity-modulated radiotherapies in a minority of patients. In our study, 40 patients (33%) had local disease recurrence. The OS was about 50% with DFS (63%) at 2-year post-therapy. The median DFS was only 8 months. Our higher rates of local recurrence can be attributed to no use of neoadjuvant, concurrent, or adjuvant chemotherapy.
In this study, 15 (12.3%) of 122 patients had either amputation or disarticulation instead of wide local excision. The reason behind this higher number can be explained by the fact that many patients in Pakistan present at relatively advanced stages and prefer to exercise different alternative medicine options (spiritual healers, hakeems, homeopathy, etc) before actually seeking proper medical advice. The same reason of presenting at later stages and thus advanced local disease might also explain higher than normal number of positive margin cases compared with other studies.
Radiation therapy poses acute toxicities like skin reactions along with late effects like joint stiffness, tissue fibrosis, osteonecrosis, and fracture.12 Lower dose of radiotherapy in our study may be the reason for fewer and milder acute and late toxicities compared with what was observed in other studies.
In conclusion, about two thirds of patients were successfully treated using the limb salvage strategy that includes surgery plus pre- or postoperative radiation therapy providing good local control and survival outcomes, with tolerable toxicity. However, there was a high rate of relapse that warrants further novel strategies to be considered.
AUTHOR CONTRIBUTIONS
Conception and design: Muhammad A. Mansha, Kashif Siddiqui, Ilyas Rafi, Ahmed Shoaib, Muhammad Abu Bakar, Muhammad M. Fareed
Administrative support: Muhammad M. Fareed
Provision of study materials or patients: Syed M. Raza, Muhammad A. Mansha, Umme Kalsoom Awan, Ilyas Rafi, Muhammad M. Fareed
Collection and assembly of data: Syed M. Raza, Adeel Riaz, Muhammad A. Mansha, Umme Kalsoom Awan, Kashif Siddiqui, Ahmed Shoaib, Muhammad Abu Bakar, Muhammad M. Fareed
Data analysis and interpretation: Adeel Riaz, Muhammad A. Mansha, Samir Fasih, Ahmed Shoaib, Muhammad Abu Bakar, Muhammad M. Fareed
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1. Felderhof JM, Creutzberg CL, Putter H, et al. Long-term clinical outcome of patients with soft tissue sarcomas treated with limb-sparing surgery and postoperative radiotherapy. Acta Oncologica. 2013;52:745–752. doi: 10.3109/0284186X.2012.709947. [DOI] [PubMed] [Google Scholar]
- 2. Herbert SH, Corn BW, Solin LJ, et al. Limb-preserving treatment for soft tissue sarcomas of the extremities: The significance of surgical margins. Cancer. 1993;72:1230–1238. doi: 10.1002/1097-0142(19930815)72:4<1230::aid-cncr2820720416>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3. Jacobs AJ, Michels R, Stein J, et al. Improvement in overall survival from extremity soft tissue sarcoma over twenty years. Sarcoma. 2015;2015:279601–279609. doi: 10.1155/2015/279601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kask G, Barner-Rasmussen I, Repo JP, et al. Functional outcome measurement in patients with lower-extremity soft tissue sarcoma: A systematic literature review. Ann Surg Oncol. 2019;26:4707–4722. doi: 10.1245/s10434-019-07698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kjäldman M, Repo J, Sampo M, et al. Functional assessment after treatment of upper extremity soft tissue sarcomas using structured outcome measures: A systematic review. Value Health. 2017;20:A764. [Google Scholar]
- 7. Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: A SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77:203–209. doi: 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sze Tiong S, Dickie C, Haas RL, et al. The role of radiotherapy in the management of localized soft tissue sarcomas. Cancer Biol Med. 2016;13:373–383. doi: 10.20892/j.issn.2095-3941.2016.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 10. Mundt AJ, Awan A, Sibley GS, et al. Conservative surgery and adjuvant radiation therapy in the management of adult soft tissue sarcoma of the extremities: Clinical and radiobiological results. Int J Radiat Oncol Biol Phys. 1995;32:977–985. doi: 10.1016/0360-3016(95)00111-b. [DOI] [PubMed] [Google Scholar]
- 11. Tepper J, Rosenberg SA, Glatstein E. Radiation therapy technique in soft tissue sarcomas of the extremity—Policies of treatment at the National Cancer Institute. Int J Radiat Oncol Biol Phys. 1982;8:263–273. doi: 10.1016/0360-3016(82)90526-0. [DOI] [PubMed] [Google Scholar]
- 12. Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]