Abstract
Elevated expression of lysyl oxidase-like 2 (LOXL2) contributes to the malignant tumor progression in multiple cancers. However, the role of LOXL2 in the 5-fluorouracil (5-FU) resistance of colorectal cancer (CRC) remains unclear. This study aimed to explore the effects of LOXL2 on 5-FU sensitivity in CRC. The mRNA and protein levels of LOXL2 were explored in public databases by bioinformatics, validated in clinical tissues using immunohistochemistry, and detected in 5-FU treated cell lines. The 50% inhibitory concentrations (IC50) values were quantified based on the cell viability at different concentrations of 5-FU with CCK-8 assays. Colony formation and flow cytometry assays were performed to measure the proliferation and apoptosis rates. Gene set enrichment and correlation analyses were conducted to identify the probable mechanism of LOXL2 in TCGA samples. Critical molecules of the Hedgehog signaling pathway and anti-apoptotic BCL2 in protein levels were detected with Western blotting. It concluded that LOXL2 was up-regulated and positively linked to the unfavorable prognosis of CRC patients. The LOXL2 expression increased with the rising 5-FU concentrations, especially at 20 and 40 μM. Elevated LOXL2 promoted the resistance to 5-FU, augmented the proliferation, and inhibited 5-FU-induced apoptosis of CRC cells. LOXL2 activated the Hedgehog signaling pathway by promoting the expression of SMO, GLI1, and GLI2, leading to the upregulation of downstream target gene BCL2 in CRC cells. Moreover, the Hedgehog signaling pathway inhibitor cyclopamine blocked the BCL2 upregulation mediated by LOXL2. This study has demonstrated that LOXL2 can reduce 5-FU sensitivity through the Hedgehog/BCL2 signaling pathway in CRC.
Keywords: LOXL2, 5-FU resistance, Hedgehog pathway, BCL2, SMO, CRC
Impact Statement
Our findings indicated the elevated LOXL2 expression significantly activated the Hedgehog signaling pathway and upregulated the expression of anti-apoptotic BCL2, leading to reduced 5-FU sensitivity in colorectal cancer.
Introduction
Colorectal carcinoma (CRC) is common cancer with high mortality, especially in developed countries. 1 The overall 5-year survival rate for CRC patients with different pathological stages varies, ranging from as low as 8% for stage IV to about 93% for stage I. 2 Surgery and chemotherapy are the main treatments for CRC.
One of the most effective chemotherapeutics used in treating CRC is 5-fluorouracil (5-FU), which has been used in clinics for more than 40 years. 3 5-FU is a cell cycle-specific drug, which is transformed into its active metabolite in the human body to inhibit thymine thymidine synthase, block thymine deoxynucleoside formation, interfere with DNA formation, and mainly kill tumor cells in the S phase of the proliferation cycle. 4 Drug resistance has been the main reason for treatment failure in CRC patients. 5 As a result, it is vital to investigate targets related to drug resistance and clarify their mechanisms of action.
Lysyl oxidase-like protein 2 (LOXL2) belongs to the Lysyl oxidase (LOX) protein family with a highly conserved –COOH terminal sequence, 6 contributing to cell growth, cell adhesion, cell migration and invasion, tissue fibrosis, and many other aspects. 7 LOXL2 is capable of modifying histone tails, reducing cell polarity, and increasing tumor metastasis potential. It can also stabilize Snail protein to promote epithelial-mesenchymal transition in multiple cancers.8,9 Previous research has demonstrated that LOXL2 is highly related to the metastasis and poor prognosis of colon cancer.10,11 However, only a few studies have been performed on exploring the correlation between LOXL2 and 5-FU resistance. One study reported that LINC01347/miR-328/LOXL2 axis may be involved in 5-FU-based chemotherapy resistance in CRC. The LOXL2 can be regulated at the transcriptional level by LINC01347 via sponging miR-328-5p in CRC cells. 12
CRC progression is closely associated with the effectiveness and resistance of chemotherapy drugs. 13 As a vital anti-apoptotic protein, B-cell lymphoma-2 (BCL2) level has been correlated with chemotherapy drug resistance. 14 For example, metformin-mediated BCL2 downregulation was linked to reduced oxaliplatin resistance in diabetes patients with CRC. 15 The Hedgehog signaling pathway has been implicated in acquired chemoresistance 16 and reported to regulate the BCL2 expression through GLI1. 17 This pathway is activated and regulated by two receptors on the target cell membrane: Patched (PTC) and Smoothened (SMO). When PTC interacts with Hedgehog ligands, GLI proteins enter the nucleus, thereby activating downstream genes by relieving the inhibition of SMO. 18
However, no report has been made about the regulatory relationship between LOXL2 and BCL2, nor the underlining mechanism. The elevated LOXL2 expression in our study significantly activated the Hedgehog signaling pathway and up-regulated the expression of anti-apoptotic BCL2, leading to reduced 5-FU sensitivity in CRC.
Materials and methods
Bioinformatic analyses
Gene Expression Profiling Interactive Analysis (GEPIA2) (http://gepia2.cancer-pku.cn) was used to analyze the mRNA expression levels of LOXL2 in TCGA-COAD and TCGA-READ cohorts. 19 The University of ALabama at Birmingham CANcer data analysis Portal (UALCAN) (http://ualcan.path.uab.edu) was applied to analyze the protein expression levels in Clinical Proteomic Tumor Analysis Consortium (CPTAC) colon cancer samples. 20 The mRNA expression data of LOXL2 in multiple cell lines were derived from the Cancer Cell Line Encyclopedia (CCLE) database (http://sites.broadinstitute.org/ccle). 21 Functional enrichment analyses were performed with the GSEA software based on the RNA-sequencing profile of TCGA samples. 22
Clinical tissues and ethical statement
Primary CRC tissues and the paired normal tissues (n = 82) were collected from Zhuhai Hospital. Pathologically, all the patients had primary colon adenocarcinoma, and no chemotherapy or radiotherapy was administered before surgery. Corresponding clinical data were also collected. The study was performed in compliance with the Declaration of Helsinki. Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine’s Ethics Committee approved the experiment. All patients signed informed consent forms.
Cell lines and reagents
HCT116 and SW48 were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). 5-FU and cyclopamine were obtained from MedChemExpress (MCE). Cell Counting Kit-8 and Annexin V-FITC apoptosis detection kits were made by Dojindo (Kyushu, Japan). Anti-LOXL2 antibody (SAB5701556, Sigma-Aldrich), anti-SMO antibody (#92981, CST), anti-SMO antibody (sc-13943, Santa Cruz, San Diego, CA), anti-GLI1 antibody (#3538, CST), anti-GLI2 antibody (#66670, CST), anti-BCL-2 antibody (#4223, CST), anti-GAPDH antibody (#60004-1-Ig, Proteintech), Trizol reagent (B610409, Sangon, Shanghai, China), PrimeScript RT Reagent Kit (#RR037A, TaKaRa, Japan), and SYBR Premix Ex Taq (#RR820A, TaKaRa, Japan) were obtained from appropriate corporations.
Immunohistochemistry
Tissue sections were deparaffinized and rehydrated, and then antigens were extracted using a 10 mM sodium citrate buffer (pH = 6.0). 0.3% H2O2 was added for 15 min to inhibit the endogenous peroxidase. The primary antibodies of LOXL2 and SMO were used for staining after blocking for 30 min with 10% goat serum in PBS. Each section was photographed at 100× and 400× magnifications. The quantitative analyses were conducted with Image Pro Plus 6.0 for calculating the mean optical density (MOD). The differential analyses were performed with the t-test.
Cell counting kit assay
HCT116 and SW48 cells were seeded into 96-well plates and cultured at 37°C and 5% CO2. After indicated treatments for a certain duration, cell viability was determined based on the densities measured with the CCK-8 assay.
Quantitative RT-PCR (qRT-PCR)
qRT-PCR experiments were conducted with the PrimeScript RT Reagent Kit and SYBR Premix Ex Taq as directed by the manufacturer’s instructions. GAPDH was applied as the internal control. The primer sequence for LOXL2 was: Forward AGGACATTCGGATTCGAGCC, Reverse CTTCCTCCGTGAGGCAAAC. The Ct values were calculated with the 2–ΔΔCt method.
Plasmids and lentivirus construction and cell transfection
We generated LOXL2 over-expressed HCT116 cell lines by cloning full-length LOXL2 (NM_002318) into the expression vector pLV-EGFP: T2A:Puro-EF1A (Yunzhou, Guangzhou, China) containing RNA interfering with the endogenous LOXL2 gene. To knock down LOXL2, two shRNA target sequences were synthesized by Cyagen Biosciences (Guangzhou, China), and shRNA oligos were cloned into the pLKO.1 vector. The LOXL2 shRNA sequences were shRNA1 CACCCGATTACTCCAACAACATCATCTCGAGATGATGTTGTTGGAGTAATCGAAAA and shRNA2 CACCCCAGATAGAGAACCTGAATATCTCGAG ATATTCAGGTTCTCTATCTGGAAAA. Lentiviral particles were produced using HEK293T cells utilizing lentiviral packaging system comprising pCMV-dR8.2 dvpr and pCMV-VSVG packaging plasmids and transfected into SW48 cell lines. Addition of puromycin to select for cells that stably expressed exogenous and RNA-interfered endogenous LOXL2 cell lines. LOXL2 expression levels were detected by Western blotting to verify overexpression and knockdown effects.
Western blot analysis
The total protein was extracted and electrophoresed on sodium dodecyl sulfate-polyacrylamide gels. Then, the gels were transferred to PVDF membranes. After blocking PVDF membranes with protein in 5% skim milk for 60 min, primary antibodies were applied to them at 4°C for 8 h. The chemiluminescent detection of protein blots was performed after adding the horseradish peroxidase (HRP)-conjugated secondary antibody. The full scans of entire and original gels displayed in this study were provided in Supplementary file 1.
Clone formation assay
In six-well culture plates, colony formation experiments were carried out. The indicated cells were planted in each well at a density of 5×102 cells. Incubation was performed at 37°C and 5% CO2, with the medium being replaced every 3–4 days. After around 2 weeks, cell colonies were quantified and analyzed. For each cell line, three replicates were conducted in the experiment.
Flow cytometry assay
The indicated cells were then treated with 5-FU at 20 μM. Using Annexin V-FITC apoptosis detection kits, the apoptosis rate of cells was detected following the manufacturer’s instructions. Three replicates were conducted. The Annexin + PI + (Q1) was counted as dead cells. The Annexin + PI – (Q4) was counted as apoptosis cells. The Annexin – PI – (Q3) was counted as live cells. The Annexin – PI + (Q2) was counted as mechanical injured cells.
Animal model
Six-week-old male BALB/c nude mice (Beijing Vital River Laboratory Animal Co. Ltd, China) were obtained and bred under an SPF (specific pathogen-free) condition. LOXL2 or scramble stably downregulated SW48 cells were subcutaneously injected into the mice (6 mice/group). 7 days post injection, 5-FU treatment was performed every 3 days by gavage. The volume of tumors was continuously measured using a Vernier caliper. 25 days later, the mice were euthanatized to measure the tumor weight. The animal experimental protocols were following institutional guidelines approved by the Animal Care and Use Committee of Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine.
Statistical analysis
The means and standard deviations (SDs) are presented for three independent replicates using GraphPad Prism 9. The t-test and χ2 test were used for comparisons of differences in clinical features between groups. Two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to compare three or more groups, and two groups were compared with Student’s t-test. Spearman correlation analysis was applied for analyzing the correlation. p < 0.05 deemed statistically significant.
Results
Elevated LOXL2 expression positively correlated to the progression in colon cancer patients
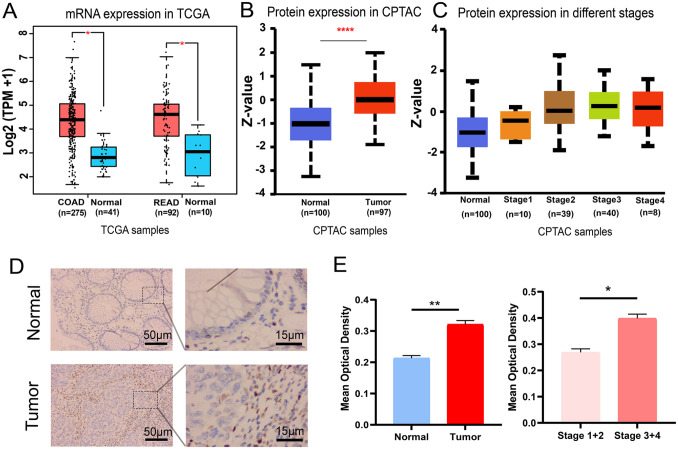
According to the expression profiles of the TCGA-COAD and TCGA-READ cohorts, a significant upregulation of LOXL2 mRNA expression was detected in tumor samples compared with normal tissues (Figure 1(A)). The elevated LOXL2 expression was also found in the protein level based on the CPTAC samples (Figure 1(B)). Moreover, the protein expression level was gradually increased with the progression of stages in CPTAC samples (Figure 1(C)). The expression of LOXL2 was further validated in clinical tissues with immunohistochemistry. Based on the MOD value, patients were separated into LOXL2-high and LOXL2-low groups, and the correlation between LOXL2 expression and clinical features was shown (Table 1). In tumor tissues, LOXL2 expression was increased compared with normal tissues, and representative images were displayed (Figure 1(D)). Compared to the normal group, the tumor group had a significantly higher MOD value (Figure 1(E)). Within the tumor group, the MOD value of stages III and IV was substantially higher than that of stages I and II (Figure 1(E)). These data indicated that elevated LOXL2 expression was positively related to the progression in CRC patients.
Figure 1.
Elevated LOXL2 expression was positively related to the progression of CRC. (A) The mRNA expression in the TCGA-COAD and TCGA-READ cohorts. One-way ANOVA test. *p < 0.05. (B) The protein expression in the CPTAC samples. Z-values represented the standard deviations from the median across samples. Student’s t-test. ****p < 0.0001. (C) The protein expression level of LOXL2 was found gradually increased with the progress of stages in CPTAC samples. (D) Representative immunohistochemical staining images in the normal and tumor tissues. Length scale bars are 50 µm at 100× magnification and 15 µm at 400× magnification. (E) The MOD values of LOXL2 in different subgroups (Normal, Tumor, Stages I and II, and Stages III and IV). Student’s t-test. **p < 0.01, *p < 0.05. Error bars, SD.
Table 1.
Relationship between LOXL2 expression and clinical features.
| Features | LOXL2-Low (N = 41) | LOXL2-High (N = 41) | p-value |
|---|---|---|---|
| Age | |||
| ⩾60 | 27 (65.9%) | 32 (78.0%) | 0.325 |
| <60 | 14 (34.1%) | 9 (22.0%) | |
| Gender | |||
| Male | 23 (56.1%) | 20 (48.8%) | 0.658 |
| Female | 18 (43.9%) | 21 (51.2%) | |
| Stage | |||
| I | 8 (19.5%) | 2 (4.9%) | < 0.001 |
| II | 27 (65.9%) | 12 (29.3%) | |
| III | 5 (12.2%) | 17 (41.5%) | |
| IV | 1 (2.4%) | 10 (24.4%) | |
| T | |||
| T1–T2 | 8 (19.5%) | 2 (4.9%) | 0.0915 |
| T3–T4 | 33 (80.5%) | 39 (95.1%) | |
| N | |||
| N0 | 35 (85.4%) | 15 (36.6%) | <0.001 |
| N1 | 4 (9.8%) | 11 (26.8%) | |
| N2 | 2 (4.9%) | 15 (36.6%) | |
| M | |||
| M0 | 40 (97.6%) | 31 (75.6%) | 0.00954 |
| M1 | 1 (2.4%) | 10 (24.4%) | |
LOXL2: lysyl oxidase-like protein 2. Bold values indicate statistical significance.
LOXL2 promoted the resistance of CRC cells to 5-FU
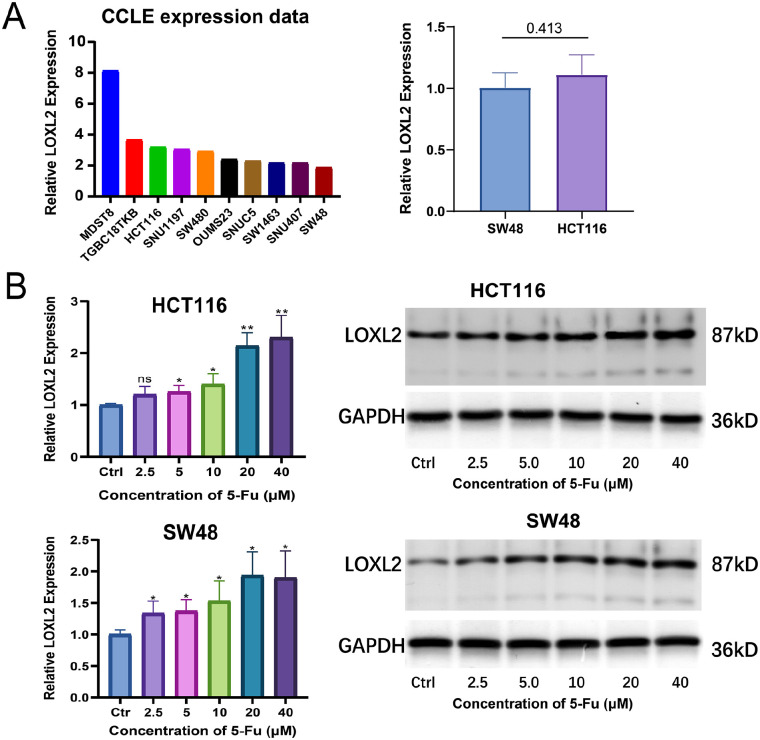
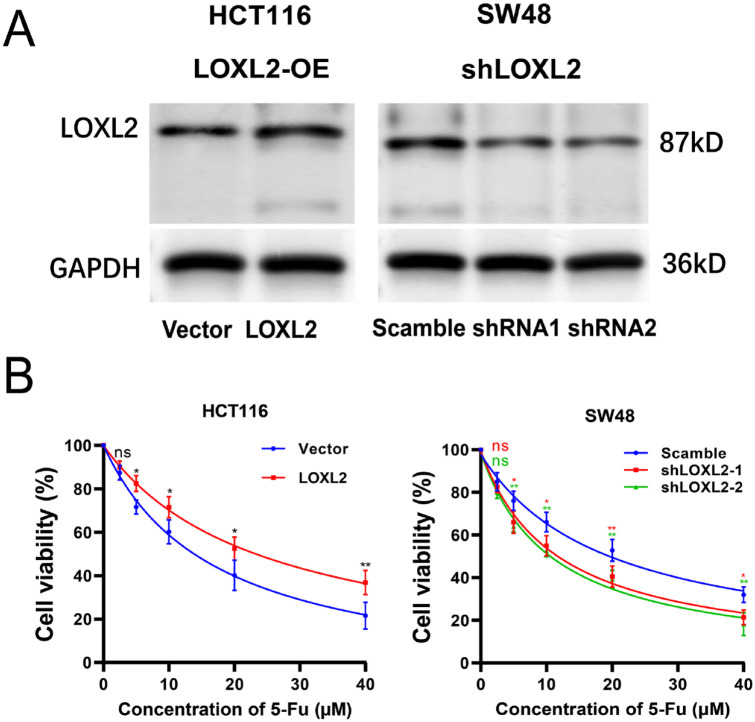
The mRNA expression levels of LOXL2 in multiple CRC cell lines were explored using the CCLE database. The top ten CRC cell lines with the highest expression were displayed (Figure 2(A)). Among them, the expression levels of LOXL2 in HCT116 and SW48 cells were determined with qRT-PCR, and there was no significant difference (Figure 2(A)). These two cell lines were selected for further experiments. First, HCT116 and SW48 cells were treated with various concentrations of 5-FU (2.5, 5, 10, 20, and 40 μM) for 48 h. Both the qRT-PCR and WB results showed that the expression levels of LOXL2 increased with the rising of 5-FU concentrations, especially at 20 and 40 μM (Figure 2(B)). Then, cell viability was detected with CCK8 assay in HCT116/Vector, HCT116/LOXL2, SW48/Scamble, and SW48/shLOXL2 cells treated with 5-FU at 48 h. The overexpression and knockdown efficiencies were validated with WB (Figure 3(A)). IC50 values of 5-FU were measured with curvilinear regression equations based on the cell viability at different concentrations. In details, the IC50 values for HCT116/Vector, HCT116/LOXL2, SW48/Scamble, SW48/shLOXL2-1, and SW48/shLOXL2-2 cells were 16.85, 24.29, 19.22, 11.47, and 10.81 μM, respectively (Figure 3(B)). These data demonstrated that the overexpression of LOXL2 promoted the resistance of CRC cells to 5-FU, and the knockdown of LOXL2 increased the sensitivity of CRC cells to 5-FU.
Figure 2.
LOXL2 expression was positively correlated with 5-FU concentration in CRC cells. (A) Relative LOXL2 expression of top 10 CRC cell lines in CCLE database (Left); Relative LOXL2 expression in SW48 and HCT116 cell lines tested by qRT-PCR (Right). (B) Relative LOXL2 mRNA and protein expression in HCT116 and SW48 cells treated with gradient concentrations of 5-FU (2.5, 5, 10, 20, 40 μM) determined at 48 h by qRT-PCR and WB. Two-way ANOVA and Tukey’s multiple comparison test was used for statistical analysis. Error bars, SD.
**p < 0.01, *p < 0.05.
Figure 3.
LOXL2 promoted the cell viability of CRC cells treated with 5-FU. The scramble and shLOXL2 vector were transfected into SW48 cells, while HCT116 cells were transfected with LOXL2 expression and empty vector. (A) The LOXL2 overexpression in HCT116 cell line and knockdown efficiencies in SW48 cell line were validated by Western blotting. (B) The cell abilities were measured by CCK8 assays. The IC50 values of 5-FU for HCT116/Vector, HCT116/LOXL2, SW48/Scamble, SW48/shLOXL2-1, and SW48/shLOXL2-2 cells were 16.85, 24.29, 19.22, 11.47, and 10.81 μM, respectively.
ns: not significant.
**p < 0.01, *p < 0.05.
LOXL2 enhanced the proliferation and inhibited 5-FU-induced apoptosis in CRC cells
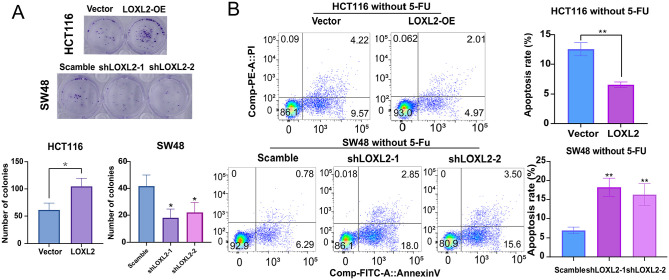
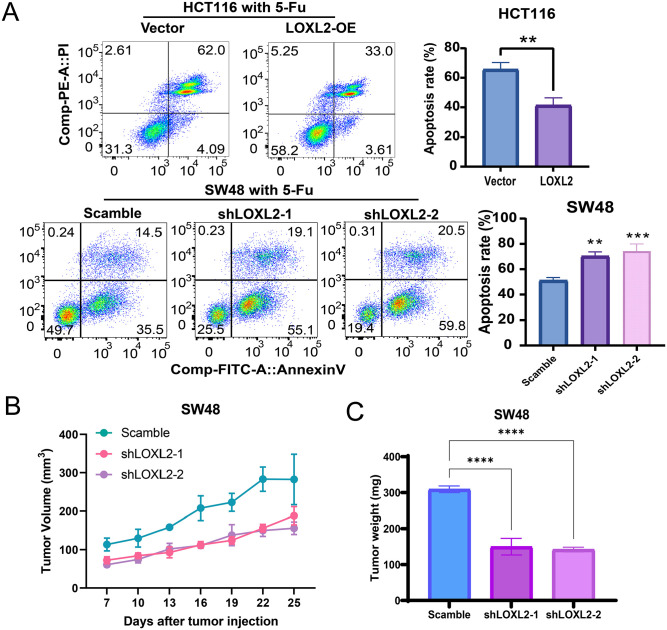
The growth-promoting effect of LOXL2 on CRC cells was further verified using clone formation assays. The overexpression of LOXL2 significantly increased the number of cell colonies, and the knockdown of LOXL2 significantly decreased the number of cell colonies (Figure 4(A)). Then, the apoptosis rates of CRC cells treated without or with 5-FU at a concentration of 10 μM at 48 h were detected with the Annexin V-FITC apoptosis detection kits. The apoptosis rate was significantly decreased when LOXL2 was overexpressed and was significantly increased when LOXL2 was knocked down. Furthermore, LOXL2 overexpression could typically inhibit the CRC cells’ apoptosis without or with 5-FU treatment, and vice versa (Figures 4(B) to 5(A)). Besides, the mice xenografts model described, upon silencing LOXL2 in SW48 cells, that the SW48 exhibited a more torpidly growth with 5-FU treatment, with a lower tumor weight (Figure 5(B) and (C)). These data demonstrated that LOXL2 enhanced proliferation and inhibited 5-FU-induced apoptosis in CRC cells.
Figure 4.
LOXL2 enhanced the proliferation and inhibited the apoptosis in CRC cells without 5-FU treatment. (A) Clone formation assays in HTC116 and SW48 cell lines. Overexpressed LOXL2 significantly increased the number of HCT116 cell colonies, while the knockdown of LOXL2 significantly decreased the number of SW48 cell colonies. (B) The apoptosis rates of CRC cells treated without 5-FU were detected with the flow cytometry assays. Student’s t-test or two-way ANOVA and Tukey’s multiple comparison test were used for significance determination. Error bars, SD.
***p < 0.001, **p < 0.01,*p < 0.05.
Figure 5.
LOXL2 enhanced the proliferation and inhibited 5-FU-induced apoptosis in CRC cells. (A) The apoptosis rates of CRC cells treated with 5-FU at a concentration of 10 μM for 48 h were detected with the flow cytometry assays. The apoptosis rate was significantly decreased when LOXL2 was overexpressed and was significantly increased when LOXL2 was knocked down. LOXL2 or scramble stably downregulated SW48 cells were subcutaneously injected into the mice, and 5-FU treatment was conducted every 3 days. The tumor growth volume (B) and weight (C) were measured. Student’s t-test or two-way ANOVA and Tukey’s multiple comparison test were used for significance determination. Error bars, SD.
***p < 0.001, **p < 0.01, *p < 0.05.
Elevated LOXL2 up-regulated BCL2 through the Hedgehog signaling pathway
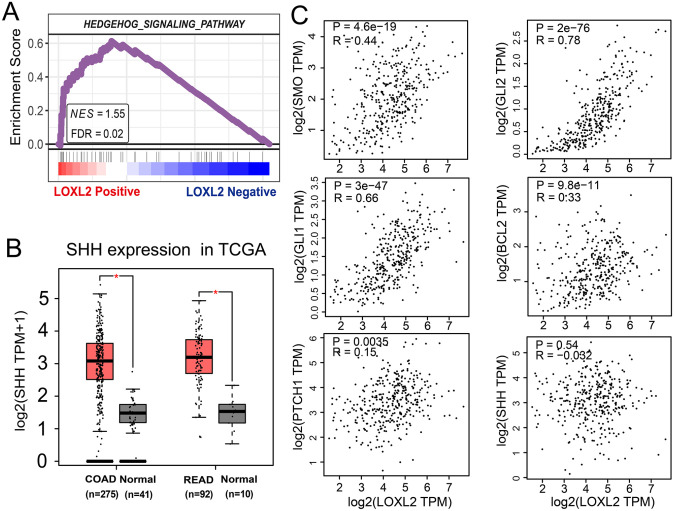
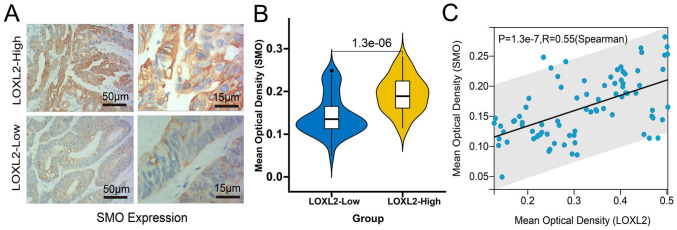
Next, we performed gene set enrichment analysis (GSEA) in TCGA-COAD and TCGA-READ samples from the TCGA database to identify the probable mechanism of LOXL2 in CRC. The results revealed a substantial positive correlation between LOXL2 expression and the Hedgehog signaling pathway. The false discovery rate (FDR) was 0.02, and the normalized enrichment score (NES) was 1.54 (Figure 6(A)). Moreover, the mRNA expression of Sonic Hedgehog (SHH), encoding the sonic hedgehog protein, was also significantly up-regulated in TCGA samples (Figure 6(B)). Through the mRNA expression correlation analyses with the GEPIA2 web tool, we found no significant correlation between LOXL2 and SHH (Figure 6(C)). However, the expression of LOXL2 was found to be positively correlated with other critical molecules of the Hedgehog pathway, such as SMO (R = 0.44, p < 0.001), GLI1 (R = 0.66, p < 0.001), GLI2 (R = 0.78, p < 0.001) (Figure 6(C)). Weak correlation was found with PTCH1 (R = 0.15, p < 0.01) and SHH (R = −0.032, p = 0.54) (Figure 6(C)). Subsequently, the expression of SMO in clinical samples with high and low expression of LOXL2 was determined with the immunohistochemical method, and the MOD was calculated (Figure 7(A)). Wilcoxon test showed that the expression of SMO in the samples with high expression of LOXL2 was also significantly increased (Figure 7(B)), and Spearman correlation analysis also showed that there was a significant positive correlation between LOXL2 and SMO in protein expression level, with a correlation coefficient of 0.55 (Figure 7(C)).
Figure 6.
Correlations between LOXL2 expression and Hedgehog signaling pathway. (A) The GSEA result in the TCGA-COAD and TCGA-READ samples with Pearson method. The Hedgehog signaling pathway was significantly enriched in the LOXL2-positive side. (B) The mRNA expression of SHH was significantly up-regulated in the TCGA-COAD and TCGA-READ samples compared with normal samples. (C) Spearman correlation analyses between the expression of LOXL2 with SMO (R = 0.44, p < 0.001), GLI1 (R = 0.66, p < 0.001), GLI2 (R = 0.78, p < 0.001), PTCH1 (R = 0.15, p < 0.01), BCL2 (R = 0.33, p < 0.001) and SHH (R = −0.032, p = 0.54). One-way ANOVA.
*p < 0.05.
Figure 7.
Validation of the correlation between the LOXL2 and SMO expression with immunohistochemistry. (A) Representative immunohistochemical staining images of SMO in the LOXL2-High and LOXL2-Low expressed tissues. Length scale bars are 50 µm at 100× magnification and 15 µm at 400× magnification. (B) The differential MOD values of SMO in the LOXL2-High and LOXL2-Low expressed groups compared with the Wilcoxon test. (C) The Spearman correlation between the MOD values of SMO and LOXL2 in the clinical samples (R = 0.55, p < 0.001).
It is known that SMO and PTCH1 are the two membrane receptors of the Hedgehog pathway. 23 Normally, PTCH1 protein inhibits the activity of SMO, which will be released when the Hedgehog binds the patched protein, promoting the nuclear import of the transcriptional factor GLI. 24 Moreover, the anti-apoptotic BCL2 has been proved to be transcriptionally regulated by the sonic Hedgehog signaling pathway through GLI1. 17 It was suggested that LOXL2 could reverse the apoptosis reduced by 5-FU through the Hedgehog/BCL2 signaling pathway.
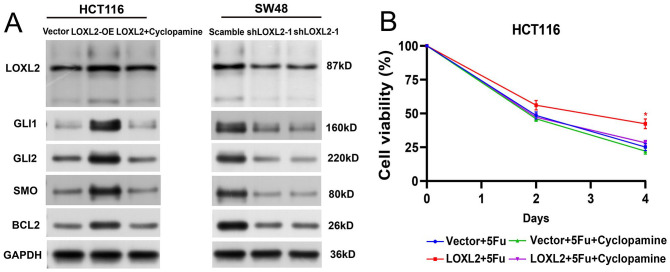
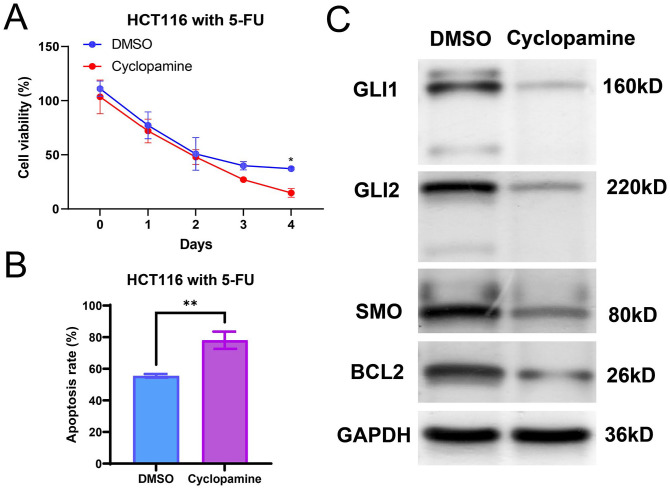
Cyclopamine, a direct antagonist of SMO, has been used to be one of the specific inhibitors of Hedgehog signaling. 25 Subsequently, we detected the protein levels of LOXL2, SMO, GLI1, GLI2, and BCL2 in the LOXL2-overexpressing HCT116 cells, LOXL2-overexpressing HCT116 cells treated with cyclopamine, and LOXL2-knockdown SW48 cells by WB. The results showed that the protein levels of SMO, GLI1, GLI2, and BCL2 increased after the overexpression of LOXL2 while decreasing after the knockdown of LOXL2 (Figure 8(A)). Especially, the up-regulation of BCL2 mediated by the overexpression of LOXL2 was reversed by the treatment of cyclopamine (40 nM, 48 h) (Figure 8(A)). Then, CCK8 assays indicated that the cell viability of LOXL2-overexpressing HCT116 cells was significantly inhibited by the cyclopamine treatment (Figure 8(B)). Moreover, the specific antagonists for Hedgehog/BCL2 signaling pathway were utilized for fathoming the effects of this pathway on 5-FU resistance of HCT116. As the results demonstrated, in comparison with the control, the cell viability was lower and the apoptosis rate was higher, with significance. It indicated that the inhibitory effects of 5-FU on HCT116 cells were enhanced with cyclopamine treatment (Figure 9(A) and (B)). The reduced expression levels of SMO, GLI1, GLI2, and BCL2 further indicated the blocking of the Hedgehog pathway (Figure 9(C)). These data demonstrated that elevated LOXL2 reduced the 5-FU sensitivity by up-regulating the activity of the Hedgehog/BCL2 signaling pathway.
Figure 8.
Elevated LOXL2 upregulated BCL2 through the Hedgehog signaling pathway. (A) Protein levels of LOXL2, SMO, GLI1, GLI2, and BCL2 in the LOXL2-overexpressing HCT116 cells, LOXL2-overexpressing HCT116 cells treated with cyclopamine, and LOXL2-knockdown SW48 cells were determined by Western blotting. SMO, GLI1, GLI2, and BCL2 were positively correlated to LOXL2. Especially, the up-regulation of BCL2 mediated by LOXL2 was reversed by the treatment of cyclopamine (40 nM). (B) Cell viabilities were detected by the CCK8 assays in HCT116 cells. The enhancing effect on cell proliferation by LOXL2 was significantly reversed by cyclopamine (40 nM). Two-way ANOVA with Tukey’s multiple comparisons test.
*p < 0.05.
Figure 9.
Cyclopamine promoted CRC cells 5-FU sensitivity. Hedgehog/BCL2 signaling pathway-specific antagonists cyclopamine (40 nM) was used on HCT116 cells treated with 5-Fu (10 μM). (A) Cell viabilities were detected by the CCK8 assays in HCT116 cells at day 0, day 1, day 2, day 3, and day 4. (B) The apoptosis rates of CRC cells were detected by the flow cytometry assays. (C) Protein levels of SMO, GLI1, GLI2, and BCL2 in HCT116 were determined by Western blots.
Discussion
CRC poses a significant threat to human health and accounts for a large proportion of cancer-associated deaths. 26 The development of laparoscopic surgery, resection, radiotherapy, and chemotherapy has made little improvement in increasing cure rates. 27 As an important chemotherapeutic molecule, 5-FU is often applied for CRC management under conditions of locoregional or distant invasion. However, this treatment could fail due to innate or acquired drug resistance in patients. 28 This article has tried to elucidate that LOXL2 may reduce 5-FU sensitivity in CRC via the Hedgehog/BCL2 signaling pathway.
Past research has confirmed the association between the LOXL2 expression pattern and CRC metastasis. 29 Our results showed that LOXL2 was considerably increased in CRC samples and drug-resistant cell lines, which continued to increase with progression. Consistent results have been documented in a previous study. It reported that LOXL2 was highly expressed in the extracellular matrix and CRC cells, which was associated with tumor-node-metastasis stage and distant metastasis. 30 Together, we could affirm that LOXL2 was highly expressed in the interstitial micro-environment of CRC cells.
After understanding the involvement of LOXL2 in CRC, we would like to explore its association with 5-FU resistance. The LOXL2 expression was altered in CRC cells and then treated with 5-FU. There was evidence showing the association between LOXL2 expression and sensitization toward gemcitabine in pancreatic cancer. 31 In our study, LOXL2 expression was enhanced in CRC cells resistant to 5-FU. Upregulation of LOXL2 has been shown to play a contributory role in the 5-FU resistance of CRC cells. 12 For verification purposes, we upregulated and downregulated LOXL2 in CRC cells, and increased 5-FU resistance was observed after LOXL2 upregulation.
Apoptosis triggered by chemotherapy drugs has been important in chemotherapy. 32 To understand the roles of LOXL2 in CRC cell proliferation and apoptosis, we observed the growth of CRC cell colonies after LOXL2 overexpression or knockdown. Evidently, the ability of CRC cell colony formation was enhanced after LOXL2 overexpression and diminished after LOXL2 knockdown. CRC cell apoptosis induced by 5-FU, as opposed to proliferation, was decreased after upregulating LOXL2 and enhanced after downregulating LOXL2. A previous study reported that LOXL2 deficiency impaired CRC cell proliferation and provokes apoptosis. It can be concluded that LOXL2 was able to accelerate CRC cell proliferation and decelerate apoptosis evoked by 5-FU.
The molecular mechanism of LOXL2 has been further explored. The Hedgehog signaling has a widespread reputation in CRC tumorigenesis. 33 The abnormal Hedgehog activation could promote cancer stem cells with therapy resistance in CRC cells. 34 Our results elicited that LOXL2 expression was positively correlated with the Hedgehog pathway, evidenced by its positive correlation with SMO, GLI1, and GLI2, all of which are components of the Hedgehog pathway. 35 SMO is the main cell membrane receptor of the Hedgehog pathway, which can activate the transcription of downstream GLI proteins and their downstream target genes in the nucleus. 36 BCL2 is regarded as a hub gene to determine apoptosis in CRC. 37 Following the overexpression of LOXL2, levels of SMO, GLI1, GLI2, and BCL2 were augmented, while the opposite trend was noticed after LOXL2 knockdown, indicating a regulatory action of LOXL2 on BCL2 and the Hedgehog pathway.
The signaling pathway has also been further verified with an inhibitor cyclopamine specific to Hedgehog pathways. Intriguingly, the upregulation of SMO, GLI1, GLI2, and BCL2 in CRC cells mediated by LOXL2 overexpression was abolished after Cyclopamine treatment. In addition, our results elicited that LOXL2-overexpressing CRC cells manifested abated viability after cyclopamine treatment. CCCTC binding factor facilitated CRC cell proliferation and resistance to 5-FU through the P53-Hedgehog axis. 38 The miR-206 regulated BCL2 expression to mediate the 5-FU resistance of CRC cells. 39 Similarly, LOXL2 mediated 5-FU sensitivity in CRC cells through the Hedgehog/BCL2 axis.
To summarize, upregulation of LOXL2 reduced 5-FU sensitivity in CRC by activating the Hedgehog signaling pathway and elevating BCL2 expression. There are also some limitations in the mechanism exploration of this study. One limitation is that SMO is only inhibited with Cyclopamine in the protein level without being knocked down in the mRNA level. The off-target effects are not explored in-depth. The second limitation is the enzymatic or non-enzymatic functions of LOXL2 are not considered. The third limitation rests with the interaction between LOXL2 and SMO and the unanswered question of whether LOXL2 interacts with SMO directly or indirectly. In future studies, we will continue to explore the in-depth molecular mechanism to find answers.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221139203 for LOXL2 reduces 5-FU sensitivity through the Hedgehog/BCL2 signaling pathway in colorectal cancer by Zhize Qiu, Shiqi Qiu, Wenli Mao, Wu Lin, Qiqi Peng and Hao Chang in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: ZQ and SQ conceived and designed the project. ZQ, WM, and WL acquired and interpreted the data. ZQ wrote the paper. HC contributed to the bioinformatic analyses.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was performed in compliance with the Declaration of Helsinki. Zhuhai Hospital’s Ethics Committee approved the experiment. All patients signed informed consent forms.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Project of Zhuhai Science and Technology Innovation Bureau (ZH22036201210109PWC).
ORCID iDs: Zhize Qiu  https://orcid.org/0000-0001-7108-3593
https://orcid.org/0000-0001-7108-3593
Hao Chang  https://orcid.org/0000-0001-6038-5617
https://orcid.org/0000-0001-6038-5617
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A.Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–64 [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP.Colorectal cancer. Lancet 2014;383:1490–502 [DOI] [PubMed] [Google Scholar]
- 3.Azwar S, Seow HF, Abdullah M, Faisal Jabar M, Mohtarrudin N.Recent updates on mechanisms of resistance to 5-fluorouracil and reversal strategies in colon cancer treatment. Biology 2021;10:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V.5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther 2020;206:107447. [DOI] [PubMed] [Google Scholar]
- 5.Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N.5-Fluorouracil resistance mechanisms in colorectal cancer: from classical pathways to promising processes. Cancer Sci 2020;111:3142–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallet SD, Ricard-Blum S.Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem 2019;63:349–64 [DOI] [PubMed] [Google Scholar]
- 7.Ye M, Song Y, Pan S, Chu M, Wang ZW, Zhu X.Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol Ther 2020;215:107633. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Zhu Y.The function and mechanisms of action of LOXL2 in cancer (review). Int J Mol Med 2015;36:1200–4 [DOI] [PubMed] [Google Scholar]
- 9.Cuevas EP, Eraso P, Mazón MJ, Santos V, Moreno-Bueno G, Cano A, Portillo F.LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway. Sci Rep 2017;7:44988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F.A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. Embo J 2005;24:3446–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres S, Garcia-Palmero I, Herrera M, Bartolomé RA, Peña C, Fernandez-Aceñero MJ, Padilla G, Peláez-García A, Lopez-Lucendo M, Rodriguez-Merlo R, García de, Herreros A, Bonilla F, Casal JI.LOXL2 is highly expressed in cancer-associated fibroblasts and associates to poor colon cancer survival. Clin Cancer Res 2015;21:4892–902 [DOI] [PubMed] [Google Scholar]
- 12.Zheng GL, Liu YL, Yan ZX, Xie XY, Xiang Z, Yin L, Wang QQ, Chong DC, Xue GL, Xu LL, Zhou K, Wang Q.Elevated LOXL2 expression by LINC01347/miR-328-5p axis contributes to 5-FU chemotherapy resistance of colorectal cancer. Am J Cancer Res 2021;11:1572–85 [PMC free article] [PubMed] [Google Scholar]
- 13.Neophytou CM, Trougakos IP, Erin N, Papageorgis P.Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers 2021;13:4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu A.The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol Ther 2022;230: 107943. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Liu Q, Yan A, Chang H, Ding Y, Tao J, Qiao C.Metformin revert insulin-induced oxaliplatin resistance by activating mitochondrial apoptosis pathway in human colon cancer HCT116 cells. Cancer Med 2020;9:3875–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen NM, Cho J.Hedgehog pathway inhibitors as targeted cancer therapy and strategies to overcome drug resistance. Int J Mol Sci 2022; 23:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigelow RL, Chari NS, Unden AB, Spurgers KB, Lee S, Roop DR, Toftgard R, McDonnell TJ.Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem 2004;279:1197–205 [DOI] [PubMed] [Google Scholar]
- 18.Geyer N, Gerling M.Hedgehog signaling in colorectal cancer: all in the stroma? Int J Mol Sci 2021;22:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Z, Kang B, Li C, Chen T, Zhang Z.GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 2019;47:W556–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S.UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017;19:649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER, 3rd, Barretina J, Gelfand ET, Bielski CM, Li H, Hu K, Andreev-Drakhlin AY, Kim J, Hess JM, Haas BJ, Aguet F, Weir BA, Rothberg MV, Paolella BR, Lawrence MS, Akbani R, Lu Y, Tiv HL, Gokhale PC, de Weck A, Mansour AA, Oh C, Shih J, Hadi K, Rosen Y, Bistline J, Venkatesan K, Reddy A, Sonkin D, Liu M, Lehar J, Korn JM, Porter DA, Jones MD, Golji J, Caponigro G, Taylor JE, Dunning CM, Creech AL, Warren AC, McFarland JM, Zamanighomi M, Kauffmann A, Stransky N, Imielinski M, Maruvka YE, Cherniack AD, Tsherniak A, Vazquez F, Jaffe JD, Lane AA, Weinstock DM, Johannessen CM, Morrissey MP, Stegmeier F, Schlegel R, Hahn WC, Getz G, Mills GB, Boehm JS, Golub TR, Garraway LA, Sellers WR.Next-generation characterization of the cancer cell line encyclopedia. Nature 2019;569:503–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP.Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigafoos AN, Paradise BD, Fernandez-Zapico ME.Hedgehog/GLI signaling pathway: transduction, regulation, and implications for disease. Cancers 2021;13:3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande I, Liang J, Hedeen D, Roberts KJ, Zhang Y, Ha B, Latorraca NR, Faust B, Dror RO, Beachy PA, Myers BR, Manglik A.Smoothened stimulation by membrane sterols drives Hedgehog pathway activity. Nature 2019;571:284–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee ST, Welch KD, Panter KE, Gardner DR, Garrossian M, Chang CW.Cyclopamine: from cyclops lambs to cancer treatment. J Agric Food Chem 2014;62:7355–62 [DOI] [PubMed] [Google Scholar]
- 26.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB.Colorectal cancer. Lancet 2019;394:1467–80 [DOI] [PubMed] [Google Scholar]
- 27.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T.Colorectal cancer. Nat Rev Dis Primers 2015;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond WA, Swaika A, Mody K.Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 2016;8:57–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park PG, Jo SJ, Kim MJ, Kim HJ, Lee JH, Park CK, Kim H, Lee KY, Kim H, Park JH, Dong SM, Lee JM.Role of LOXL2 in the epithelial-mesenchymal transition and colorectal cancer metastasis. Oncotarget 2017;8:80325–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui X, Wang G, Shen W, Huang Z, He H, Cui L.Lysyl oxidase-like 2 is highly expressed in colorectal cancer cells and promotes the development of colorectal cancer. Oncol Rep 2018;40:932–42 [DOI] [PubMed] [Google Scholar]
- 31.Rückert F, Joensson P, Saeger HD, Grützmann R, Pilarsky C.Functional analysis of LOXL2 in pancreatic carcinoma. Int J Colorectal Dis 2010;25:303–11 [DOI] [PubMed] [Google Scholar]
- 32.Xiong L, Wu F, Wu Q, Xu L, Cheung OK, Kang W, Mok MT, Szeto LLM, Lun CY, Lung RW, Zhang J, Yu KH, Lee SD, Huang G, Wang CM, Liu J, Yu Z, Yu DY, Chou JL, Huang WH, Feng B, Cheung YS, Lai PB, Tan P, Wong N, Chan MW, Huang TH, Yip KY, Cheng AS, To KF.Aberrant enhancer hypomethylation contributes to hepatic carcinogenesis through global transcriptional reprogramming. Nat Commun 2019;10:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Zhu X, Liu W, Ruan T, Tao K.Hedgehog signaling pathway in colorectal cancer: function, mechanism, and therapy. Onco Targets Ther 2017;10:3249–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das PK, Islam F, Lam AK.The roles of cancer stem cells and therapy resistance in colorectal carcinoma. Cells 2020;9:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu A.Proteostasis in the Hedgehog signaling pathway. Semin Cell Dev Biol 2019;93:153–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar SK, Roy I, Anchoori RK, Fazli S, Maitra A, Beachy PA, Khan SR.Targeted inhibition of hedgehog signaling by cyclopamine prodrugs for advanced prostate cancer. Bioorg Med Chem 2008;16:2764–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng T, Lan Z, Zhao X, Niu L, Chen C, Zhang W.Comprehensive bioinformatics analysis of functional molecules in colorectal cancer. J Gastrointest Oncol 2022;13:231–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai Q, Li Q, He C, Fang Y, Lin S, Cai J, Ding J, Zhong Q, Zhang Y, Wu C, Wang X, He J, Liu Y, Yan Q, Li A, Liu S.CTCF promotes colorectal cancer cell proliferation and chemotherapy resistance to 5-FU via the P53-Hedgehog axis. Aging 2020;12:16270–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng X, Fu R.miR-206 regulates 5-FU resistance by targeting Bcl-2 in colon cancer cells. Onco Targets Ther 2018;11:1757–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221139203 for LOXL2 reduces 5-FU sensitivity through the Hedgehog/BCL2 signaling pathway in colorectal cancer by Zhize Qiu, Shiqi Qiu, Wenli Mao, Wu Lin, Qiqi Peng and Hao Chang in Experimental Biology and Medicine