Abstract
Many cancer types metastasize to bone. This propensity may be a product of genetic traits of the primary tumor in some cancers. Upon arrival, cancer cells establish interactions with a variety of bone resident cells during the process of colonization. These interactions, to a large degree, dictate cancer cell fates at multiple steps of the metastatic cascade, from single cells to overt metastases. The bone microenvironment may even influence cancer cells to subsequently spread to multiple other organs. Therefore, it is imperative to spatiotemporally delineate the evolving cancer-bone crosstalk during bone colonization. In this review, we provide a summary of the bone microenvironment and its impact on bone metastasis. Based on the microscopic anatomy, we tentatively define a roadmap of cancer cells’ journey through bone relative to various microenvironment components, including the potential of bone to function as a launch pad for secondary metastasis. Finally, we examine common and distinct features of bone metastasis from different cancer types. Our goal is to stimulate future studies leading to the development of a broader scope of potent therapies.
ToC blurb
This Review discusses the bone microenvironment and its impact on bone metastasis, defining a roadmap of the cancer cell journey through bone relative to various microenvironment components and in different cancer types as well as providing insight into new therapeutic targets.
Introduction
Bone and bone marrow together represent a highly complex environment. This complexity results from the intricate spatial organization of many different resident cell types and their agile temporal dynamics. The major functions of bone include mechanical support and hematopoiesis. The former function is carried out by the mineral part of bone, which is built and maintained primarily by osteoblasts [G], osteocytes [G] , osteoclasts [G] , their precursors and mesenchymal stem cells (MSCs). The latter function involves a hierarchy of cells, including hematopoietic stem cells (HSCs), a variety of intermediate progenitor cells, as well as matured blood cells. MSCs and their decedent cells cooperate with the hematopoietic cells at different levels of the hierarchy and play important roles in regulating hematopoiesis. In addition, bone and bone marrow are highly vascularized. Arteries enter long bones from the periosteum, branch into smaller arterioles, and form capillaries (Type H capillaries [G]) at the metaphyseal and epiphyseal regions (Figure 1). The blood then drains into sinusoidal network (Type L capillaries [G]) that extend in the reverse direction, converge into central vein, and eventually exit the medullary cavity1. Furthermore, different blood vessels are accompanied by different mural or perivascular cells as well. Arteries, arterioles, Type-H, and Type-L capillaries are covered by perivascular cells that are αSMA+NG2+, PDGFRβ+nestin-GFPhighNG2+, PDGFRβ+NG2+, and LEPR+nestin-GFPlowPDGFRα+, respectively2-6. Therefore, the vasculature in the bone is heterogeneous. Nerves, including sensory and sympathetic neurons and their supportive cells7, usually accompany blood vessels. Together, this miscellany of cells constitutes the bone microenvironment (BME) and function in a delicate balance to maintain bone mass and integrity (Box 1).
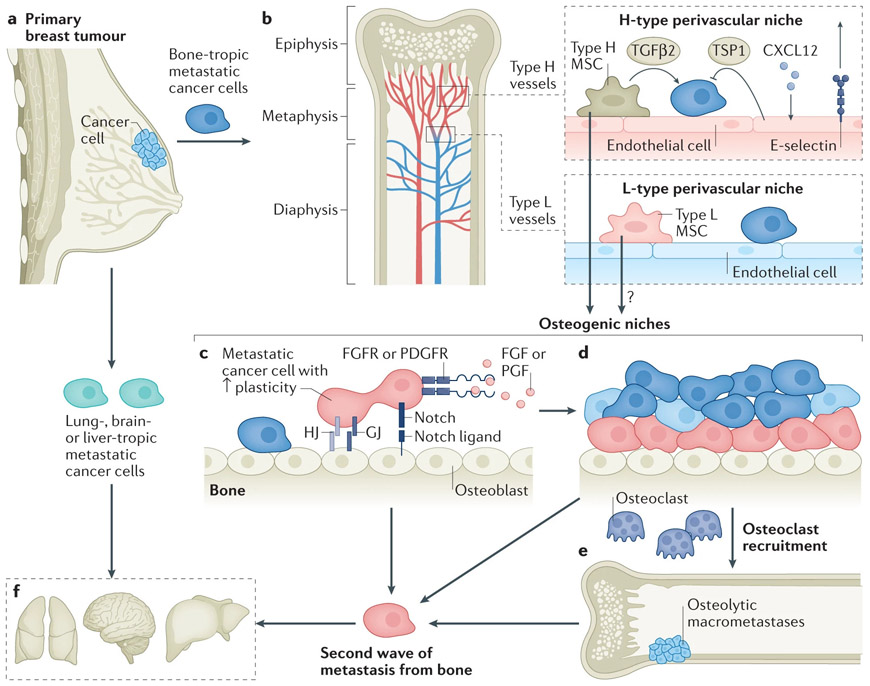
Figure 1. The journey of DTCs toward bone metastasis and beyond.
a. Metastatic organotropism may be encoded by genetic traits and arise in primary tumors by various mechanisms.
b. Blood vessels may provide the first foothold for disseminated tumor cells (DTCs). The vasculature in the bone marrow is highly heterogeneous. Most notably, the capillaries can be classified into the H-type, which connects to arterioles, and L-type, which connects to veins. Type H capillaries are localized to metaphyseal regions as well as in parallel to the endosteal surface. In contrast, Type L capillaries are mostly within diaphysis and are sinusoidal. Endothelial cells in Type H and Type L capillaries express high and low level of CD31 and endomucin, respectively. The perivascular niches harbor dormant DTCs. H-type and L-type vessels may represent different niches with both endothelial and perivascular cells differing from one another. TGFβ2 produced by perivascular mesenchymal cells and TSP1 produced by endothelial cells may mediate cellular quiescence of cancer cells. The counteraction between CXCL12 and E-selectin may also determine the fate and positioning of cancer cells relative to the niche. The perivascular mesenchymal cells possess mesenchymal stem cell (MSC) activities and may contribute to osteogenesis.
c. The osteogenic niche promotes progression of DTC toward micrometastases through multiple mechanisms, including direct interaction by heterotypic adherens junctions (hAJs), gap junctions (GJs), and Notch signaling. The osteogenic cells may also secrete TSC, which activates integrin signaling. Furthermore, paracrine signaling of FGF2 and PDGF-DD produced by the bone microenvironment enhances the phenotypic plasticity of cancer cells.
d. As micrometastases grow, cancer cells that remain adjacent to osteogenic cells may maintain their plasticity whereas those that are pushed away may revert to a more differentiated status.
e. Recruitment and activation of osteoclasts start the vicious cycle and drive the progression toward osteolytic macrometastases. This is the phase that causes symptoms and leads to diagnosis in the clinic.
f. The interaction with osteogenic cells leads to increased cancer cell stemness and phenotypic plasticity, which may fuel further dissemination to multiple other organs.
The second wave of metastasis from bone may be less organotropic, which is distinctive from the initial wave of metastasis.
Box 1: The multi-dimensional view of bone microenvironment.
Spatial organization of bone
In long bones, osteoclasts, osteoblasts and their precursors are predominantly localized at the surface of cortical bones (termed endosteum) and trabecular bones, which constitute the “endosteal” or “osteogenic” niche1. Differentiated osteocytes are embedded into the bone matrix. Mesenchymal stem cells (MSCs), the multipotent stem cells of osteoblasts, chondrocytes and adipocytes, are usually found adjacent to blood vessels, or the “perivascular” niche. Hematopoietic stem cells (HSCs), on the other hand, were found to be in both osteogenic and perivascular niche224-226.
The blood capillaries can be divided into two types: H and L. Type H capillaries are localized to metaphyseal regions as well as in parallel to the endosteal surface. In contrast, Type L capillaries are mostly within diaphysis and are sinusoidal227,228.
Furthermore, arteries, arterioles, Type-H, and Type-L capillaries are surrounded by perivascular cells that express different markers3,229,230Besides MSCs, there is a distinct class of perivascular cells in the bone marrow, namely CXCL12-abundant-reticular cells (CARs)231-233. Single cell RNA-seq defined subsets of CARs with transcriptomic profile characteristic of osteogenic cells or adipocytes. They preferentially localize to arteriolar and sinusoidal vessels, respectively, and can shape the local microenvironment through cytokine secretion234.
Although most studies implicate the perivascular niche as the major location of HSCs, there are debates as to whether the adjacent vasculature is arteriolar or sinusoidal4,225,235. The endosteal niche was also suggested to host engrafted HSCs40. Perivascular and endothelial niches may be physically close to one another and even share some niche components due to the coupling between angiogenesis and osteogenisis228. Furthermore, megakaryocytes may also provide a unique HSC niche and maintain HSC quiescience236-239.
Temporal dynamics of bone
In addition to the daily production of blood cells by the bone marrow, it is estimated that 5-25% of all skeleton is replenished every year in healthy adults240 with osteoclasts absorbing old bone and osteoblasts depositing new bone241. Pathological conditions can significantly impact bone turnover. For instance, diabetes and obesity both increase resorption of old bones and decrease formation of new bones242-244. In particular, bone fracture induces a healing process involving development of hematoma, acute inflammation, resolution of inflammation, formation of soft callus in association with neoangiogenesis, growth of woven bones (newly formed bone), and finally remodeling of woven bones to healed bones245,246.
Taken together, bone is exquisitely organized and agilely dynamic. Therefore, the BME needs to be investigated in a well-defined spatiotemporal context.
Bone and bone marrow are frequently affected by metastasis from cancers in multiple organs, including breast, prostate, colon, lung, bladder, kidney, and head/neck. The proclivity of these cancer types to colonize bone remains poorly understood and may be related to the fact that the BME is enriched with factors and niches that nurture stem cells.
Fully developed metastatic disease has devastating consequences for the function of bone and accelerates cancer progression. Current standard-of-care therapies target the ability of cancer cells to resorb bone, which presumably does not occur until late stage of bone colonization in most cancer types. While such therapies undeniably improve quality of life, patient survival is not significantly elongated. Additional therapeutic strategies may be revealed with a deeper understanding of the process of early bone colonization, including the initial interactions between disseminated tumor cells (DTCs) and the various microenvironment niches, and the subsequent progression toward bone-deconstructing overt bone metastases.
Here we present a review on the topic of bone metastatic diseases, focusing on tumor cell interactions with the microenvironment during the journey from primary tumor to bone and, as more recently unveiled, from bone to additional target organs. We will summarize our knowledge of bone metastasis as a sequence of connected steps using the example of breast cancer and other representative cancer types to highlight the spectrum of tumor driven interactions in the BME that uniquely describe the metastatic journey. Specifically, we will compare the metastatic journey of disseminated cells from breast cancer, which is the most studied tumor type with the highest incidence of bone metastasis among the cancers we discuss, with other selected solid tumors which differ mechanistically according to their phenotype (prostate-osteoblastic), treatment resistance (renal-osteolytic) and their immunologic properties (multiple myeloma-osteolytic). Finally, we will review newly emerging therapeutic targets that may mediate different steps of bone colonization.
The metastatic journey from breast to bone
To obtain a relatively integrated view, we will use mostly breast cancer studies as examples for the discussion of the metastatic journey to bone, since there are more experimental models available compared with other cancers (Table 1, Box 2) and, as a result, a larger number of published studies exist for analysis. However, insights from other cancers can sometimes facilitate deeper understanding and boarder discussion, and therefore, will be mentioned with cancer type specified. Briefly, we will summarize the process through which cancer cells disseminate from primary tumors, establish the initial foothold that facilitates dormancy, begin proliferation under the influence of the (altering) BME, recruit osteoclasts to trigger a vicious osteolytic cycle, and finally further metastasize to multiple other organs.
Table 1:
Experimental models of bone metastasis
| Transplantation routes |
Cancer types | Pros | Cons | |
|---|---|---|---|---|
| Direct injection into circularion (experimental metastasis models) | Left ventrical85,213 | Breast, prostate, renal, colorectal, lung, head and neck, multiple myeloma, … |
|
|
| Tibia or femur bones214 | Breast, prostate, renal, colorectal, lung, head and neck, multiple myeloma, … |
|
|
|
| Illiac artery42,215 | Breast and prostate |
|
|
|
| Caudal artery216 | Breast, prostate, renal, lung, osteosarcoma… |
|
|
|
| Othtopic injection (spontaneous metastasis) | Mammary fat pad217 | Breast |
|
|
| Nipple218 | Breast | |||
| Prostate219 | Prostate | |||
| Renal capsule220 | Renal | |||
| Rectal221 | Colorectal | |||
| Lung222,223 | Lung | |||
| Perimaxillary gingival submucosa | Head and neck |
Box 2. Limitations of current models will need to be overcome with innovation of new models.
Immunocompetent vs. immunocompromised models.
Findings in the current literature are predominantly based on transplanation of human cell lines to immunocompromised hosts, which severely limits our ability to investigate the roles of immune cells in bone colonization. Only a couple of available murine cell lines spontaneously metastaize to bones, including AT378 and 4T1.2247. Considering the unique immune milieu provided by the bone marrow, it is imperative to develop more syngeneic models to allow systematic characterization of the mutual impact of cancer cells and the BME. This need is especially urgent for certain tumor types or subtypes exhibiting stronger bone tropisms, e.g., prostate cancer and ER+ breast cancer.
Patient-derived xenograft (PDX) vs. cell lines
Cell lines maintained in culture are subjected to artificial selective pressures and may genetically drift to lose pathologically relevant heterogeneity. PDXs can overcome this caveat to some extent248. A challenge for establishing PDX models of bone metastasis, aside from the rareness of metastatic tissues, is the need for orthotopic transplantation, which in this case is to bone rather than breast. This is presumably important for the maintenance of the critical cancer-microenvironment interaction249. A robust and efficient pipeline will need to be developed toward this end in future research.
In vivo vs. ex vivo models
The BME can be partially recapitulated ex vivo. In the simplest case, osteogenic cells can be co-cultured with cancer cells in 3D suspension media42. Interestingly, this admixture forms heterotypic organoids with the two cell types either well intermixed or organized into a shell-core structure. Cell-cell interactions can be dissected using the 3D organoids. Bone fragments can also be used ex vivo to host cancer cells192. When seeded to appropriate scaffolds and stimulated by specific cytokines, osteocytes can differentiate into osteoblasts and become minimized to mimic the BME48,250,251, which can provide cancer cells with a representative environment. The ideal ex vivo models will need to be scalable and inclusive of major bone and bone marrow components, including the mesenchymal lineage, hematopoietic lineage, and endothelial cells. Compared to in vivo models, the ex vivo platforms are more amenable to molecular manipulation and high throughput screening. Importantly, the ex vivo setting will also allow utilization of human cells so that cancer-niche interactions can be studied in a human-human setting.
Hematopoietic stem cells (HSCs):
Cells that are multipotent and responsible for production of all blood cells.
Mesenchymal stem cells (MSCs):
Cells that are multipotent and responsible for production of mesenchymal cells, including osteoblasts, chondrocytes, adipocytes and fibroblasts.
Perivascular niche:
The microenvironmental location adjacent to a blood vessel. The components include endothelial cells, pericytes, and HSCs. The pericytes exhibit MSC activities.
Endosteal niche:
The microenvironmental location at the endosteal surface. It is enriched with osteoblasts, osteoprogenitors as well as osteoclasts. Transplanted hematopoietic stem cells often adhere to this niche.
Osteoblastic niche:
The microenvironmental location enriched with osteoblasts. It overlaps with the endosteal niche but more restrictively refers to locations adjacent to osteoblasts.
Osteogenic niche:
The microenvironmental locations including endosteum and trabecular bones, where osteogenesis occurs. It is enriched with osteoblasts and precursor cells. It overlaps with the endosteal niche, but also includes trabecular bones while lacks the osteoclast component by definition.
The pre-metastatic niche:
Potential destination of metastasis in distant organs before the actual arrival of metastatic cells. It is different from normal tissue because of interactions with bone marrow-derived cells stimulated by primary tumors.
Metastasis organotropism:
The observations that metastasis does not occur randomly to all organs but rather preferentially affects a specific set of distant organs.
Seed-and-soil hypothesis:
Stephen Paget first postulated that metastatic seeds spread throughout the organism but only colonize in congenial soil. This hypothesis has been used to explain metastatic organotropism and provides a conceptual framework to study cancer-environment (analogous to seed-soil) interactions.
Epithelial-to-mesenchymal transition (EMT):
A process through which epithelial cells lose cell-cell adhesions and other epithelial traits but acquire mesenchymal characteristics, including migration and invasion. Recent studies demonstrate that EMT is a continuum and there exists a hybrid status with both epithelial and mesenchymal features. The hybrid EMT phenotype has been linked to cancer stemness, or the ability to regenerate a tumor.
Phenotypic plasticity:
The potential of a cell to alter its phenotypic characteristics in response to environmental stimuli. The ability to switch between epithelial and mesenchymal phenotypes is considered one example of phenotypic plasticity.
Osteoblasts:
Cells that are responsible for synthesis and mineralization of new bones during development and bone remodeling. They are derived from mesenchymal linage, usually localize at the surface of bone matrix and can differentiate into osteocytes.
Osteoclasts:
Cells that are responsible for resorption of bones. They are derived from myeloid cell lineage. Matured osteoclasts are multi-nuclear and function in close coordination with osteoblasts.
Osteocytes:
Cells that are derived from osteoblasts and become embedded into the bone matrix.
H-type capillaries:
Vascular networks in the bone marrow that continue from arterioles and precede sinusoid vein vessels. They are surrounded by osteoprogenitor cells and couple osteogenesis and angiogenesis.
L-type capillaries:
Sinusoid veins that continue from the H-type capillaries and converge on central vein in the medullar cavity of bone marrow.
Osteogenesis: The process of osteoblast and osteocyte differentiation and formation of new bones.
Osteosclerosis:
Bone abnormality characterized by excessive hardening of bone and an increase of bone density.
Darwinian selection:
An evolution process during which individuals with greatest fitness among a population survive the selective pressure exerted by the environment. In cancer biology, it was adopted to understand how cancer cells with the most enabling genetic traits progress and expand over other cancer cells under the selective pressure from the microenvironment.
The endothelial tip cells:
Cells as part of the endothelium that sprout branches of blood vessels.
Periostin:
An extracellular matrix component encoded by the POSTN gene. It is a ligand of integrins and plays important roles in the niche supporting normal and cancer stem cells.
Cabozantinib:
is a small molecule inhibitor of the tyrosine kinases c-Met, VEGFR2, AXL and RET. It is approved to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma.
Embolization:
Blockade of blood vessels by an agglomerate of cancer cells or other substance.
Pre-metastatic alterations in bone
Breast tumors may systemically impact distant organs even before metastasis occurs8. The development of a “pre-metastatic niche” was first characterized in lungs and implicated changes in the bone marrow. Specifically, bone marrow-derived VEGFR1+ cells can be mobilized by primary tumors and recruited to the lungs before the arrival of metastatic cancer cells9. These cells can prepare the lung tissues and make them more amenable to metastatic seeding. Subsequent studies provided further details of this process and expanded our knowledge of the mechanisms of the pre-metastatic niche development (e.g., ref10-12). In some cases this niche was shown to suppress, rather than promote, metastatic seeding13,14. Overall, these studies also confirmed that the bone marrow acts as a remote responder to primary tumors and as a source of cells recruited to other organs for pre-metastatic niche formation. However, these studies did not address the questions of how the BME is altered in this process and whether such alterations affect potential metastatic seeding to bone itself.
Independent from the above studies, it has long been observed that breast tumors skewed hematopoiesis toward the myeloid lineage with cells of abnormal functionality 15-17. We and other groups have shown that some breast tumors can induce systemic accumulation of immature myeloid cells that are immunosuppressive, known as myeloid-derived suppressor cells (MDSCs)18,19. However, the level of MDSC accumulation varies across different tumors and may be dictated by tumor-intrinsic characteristics such as the epithelial-mesenchymal transition (EMT) status and levels of mTOR signaling in cancer cells20,21. Although the systemic roles of MDSCs in tumor progression have been intensively investigated, their local impact on bone metastasis remain poorly defined.
The rise of bone-tropic metastatic seeds in primary tumors
Before cancer cells embark on the metastatic journey, their fate and destination may already be partly determined. The nonrandom distribution of metastases to specific organs is referred to as metastatic organotropism, and is a long-standing clinical observation22. The organs frequently affected by breast cancer metastasis include bone, lung, brain and liver. Different breast cancer subtypes exhibit largely different organ preferences: while luminal-like tumors (mostly estrogen receptor positive [ER+]) tend to metastasize first to bone, basal-like tumors (mostly ER−, progesterone receptor negative [PR-] and Her2-, also known as triple negative breast cancer [TNBC]) aggressively disseminate initially to visceral organs, including the lungs23,24. Moreover, visceral metastases usually occur within 5 years after surgical removal of primary tumors. In contrast, ER+ bone metastases are often diagnosed after a much longer latency, and the risk of late-onset bone metastases persists for years to decades25,26. This inter-subtype discrepancy remains largely unexplained.
Although at a lower frequency compared to ER+ breast cancer, bone metastases still occur in TNBC. Our previous work (XZ) based on experimental metastasis models (Table 1, Box 2) suggests that SRC activity in TNBC cells may be linked to proclivity for bone metastasis mainly through potentiating CXCR4 and/or IGFR-AKT signaling cascades in cancer cells. The bone-specific role of SRC results from abundant expression of cognate ligands of CXCR4 and IGF1R, which are CXCL12 and IGF1 respectively, secreted by stromal cells in the BME 26. Interestingly, cancer cells with relatively higher SRC activity can be enriched in primary tumors by Darwinian selection [G] (the major conceptual framework of our understanding of the metastatic cascade27). when there is a similar enrichment of CXCL12 and IGF128. Therefore, the mimicry of bone cytokine milieu in the primary tumor may pre-select metastatic seeds that might be ‘primed’ to survive and grow in the BME.
Cancer cells may also demonstrate “osteomimicry”, which refers to the evolution of cancer cells to exhibit bone resident cell phenotypes 29,30. Osteomimicry mostly occurs in cancer cells that have already metastasized to bone, and therefore represents a later step in the journey. Of note, in orthotopic or subcutaneous tumors in mice, ectopic bone tissues were occasionally observed31, which suggests a osteomimicry-like process in non-bone tissues seemingly independent of the presence of bone metastasis. How such a process in primary tumors relates to later bone metastasis remains unknown. It is reasonable to hypothesize that cancer cells undergoing osteomimicry and thus assuming bone cell phenotypes may confer selective advantages on DTCs and drive bone-tropic metastasis.
Of note, genes and pathways mediating bone metastasis are not expected to confer selective advantages in primary tumors as the interactions with bone have not yet occurred. The microenvironmental pressure is not yet available at this point to drive bone-tropic genetic selection or reactive adaptation. This is especially true for late-onset bone recurrences during which metastases must undergo prolonged parallel evolution in bone, thereby allowing them to become distinct from primary tumors. However, resemblance of the microenvironment between primary and metastatic tumors may drive convergent evolution and result in overlapping phenotypic profiles. Therefore, both the occurrences of seed pre-selection and/or osteomimicry are plausible hypotheses to explain the paradox that bone tropism is predictable in primary tumors (Fig.1a).
The perivascular niche and metastasis dormancy
The first niche that DTCs encounter upon arrival in BME may play important roles in determining the subsequent metastatic process. A study in prostate cancer showed that inoculation of cancer cells to peripheral blood induced egress of HSCs from the bone marrow32, which led to the hypothesis that DTCs and HSCs both share and compete for the same niches. This hypothesis gained support from recent studies in experimental metastasis models (Table 1) of breast cancer. Ghajar et al., demonstrated that DTCs (introduced by intra-cardiac injection, see Table 1) stay close to blood vessels after extravasation. Moreover, thrombospondin 1 (TSP1) produced by endothelial cells induced dormancy in DTCs 33. Using the same approach, Price et al. corroborated this conclusion and elucidated that endothelial cell-derived E-selectin and CXCL12 induced the migration of DTCs toward the endosteal surface and the retention of DTCs at the perivascular niche, respectively34. More recently, it was suggested that the perivascular niche renders cancer cells resistant to chemotherapies through integrin signaling35. Furthermore, perivascular NG2+ cells were also shown to reinforce dormancy through secretion of TGFβ236.Taken together, it has become increasingly clear that the perivascular niche is the first foothold of DTCs in the bone marrow and plays an important role in determining cancer cell fates (Fig. 1b).
Much remains to be discovered about cancer cell dormancy and the perivascular niche. Additional pathways in cancer cells may induce dormancy, including those involving LIFR and MSK1 through regulating STAT3 activities and cell differentiation status, respectively37,38. Blood vessels and perivascular cells are highly heterogeneous in the bone marrow as previously discussed. It will be important to determine what specific type of blood vessels (i.e., arteriolar, H-type capillaries, or L-type capillaries) preferentially constitute the niche of dormancy (Fig. 1b). This information may reconcile the seemingly conflicting finding that vascular E-selectin stimulates mesenchymal-to-epithelial transition and promotes proliferation of DTCs through WNT signaling39. Thus, the understanding of cancer-niche interactions will likely benefit from a deeper and more precise characterization of the normal bone marrow microenvironment.
The osteogenic niche and metastasis outgrowth
In addition to the perivascular niche, other microenvironment niches may also regulate the fate of DTCs. The endosteal surface of cortical bones and the surface of trabecular bones harbor osteoblasts and represent sources of new bone. This region is termed the “endosteal” niche and also hosts HSCs and other hematopoietic progenitor cells40,41. We [XZ] and others observed that bone micrometastases (BMMs) are usually in close contact with cells that have osteogenic potential including MSCs, osteoprogenitors, pre-osteoblasts and osteoblasts, but not osteoclasts42-44. Thus, we used the term the “osteogenic niche” in this Review to collectively refer to these cell types. Using the intra-iliac injection-based experimental metastasis model (Table 1), we [XZ] showed that the cancer-osteogenic niche interaction was mediated by heterotypic adherens and gap junctions and stimulated multiple pathways inside of BMMs, including mTOR and calcium signaling, which can drive proliferation of cancer cells42,43,45(Fig. 1c,d). Also, osteoblast-produced cytokines (e.g., FGF2 and PDGF-DD) can induce epigenomic reprogramming via activation of EZH2, which in turn confers stemness on BMMs in the context of ER+ breast cancer xenograft models. Here, epigenetic changes were in part reflected by a transient and reversible loss of ER expression and the emergence of a hybrid-EMT phenotype particularly in cancer cells directly interacting with the osteogenic cells46 (Fig. 1c,d). Multiple other crosstalk mechanisms between cancer cells and osteogenic cells were discovered, including tumor derived JAGGED1-induced Notch signaling in osteoblasts which activated production of TGFβ and led to activation of osteoclasts (see later sections for more discussions)47. Interestingly, activation of the Notch pathway in cancer cells confered resistance of experimental bone metastasis to chemotherapies in multiple xenograft models (Fig. 1c) 43. In addition, in an MSC-derived ex vivo model (Table1, Box 2), MSCs produced tenascin (TNC) upon interaction with cancer cells48. TNC is a well-described stem cell niche component49 that signals through integrins to promote tumor progression 48,50. Taken together, it has become increasingly clear that the osteogenic niche may foster metastasis outgrowth, which represents one mechanism for activation of proliferation, or the termination of dormancy.(Fig. 1d).
Many aspects of the osteogenic niche need to be better understood. Conditioned medium of breast cancer-educated osteoblasts was shown to suppress tumor growth and osteoclastogenesis in vitro51, suggesting that the secretome of osteoblasts in culture produce the opposing effect compared to observations in vivo discussed above. Moreover, in multiple myeloma bone metastasis, the osteogenic niche was shown to induce dormancy rather than terminate it52. Again, these seemingly conflicting observations may be explained by differences in experimental models and/or the diversity of bone niches and the exquisite spatiotemporal arrangement of the BME. In this regard, accurately mapping cancer-niche interactions to a single cell resolution will significantly benefit bone metastasis research.
The vicious cycle and osteolytic bone metastasis
The hallmark of overt bone metastasis in breast cancer is the recruitment and activation of osteoclasts through paracrine relay between cancer cells and osteoblasts. Specifically, cancer cells can produce PTHrP, which induces osteoblasts to secrete RANKL53,54. The RANKL- RANK pathway is a master regulator of osteoclastogenesis55. Resorption of bone matrix by osteoclasts leads to the release of TGFβ and IGFs, which reciprocally act on cancer cells to stimulate further progression56-58. Altogether, these processes form an osteolytic vicious cycle (Fig. 1e). Many recently discovered pathways converge to regulate this cycle and promote bone metastasis, including VCAM159 generated by tumors that recruits osteoclasts, integrin signaling activated in cancer cells60, RON signaling activated by MSP in both cancer cells61 and osteoclasts62, Notch signaling activated mutually between cancer cells47 and osteoblasts43, and IL-6 released by osteoblasts or senescent stromal cells that activate osteoclasts47,63. The osteolysis caused by metastasis leads to skeletal related events (SRE), including bone pain, spinal cord compression, hypercalcemia, and pathological fractures. Therapies targeting osteoclasts significantly improve patient quality of life, thereby confirming the vicious cycle as a paradigm of late-stage bone metastasis64 (Fig. 1e,f). The molecular details of this paradigm have been increasingly elucidated in the past few decades and have been summarized by excellent recent reviews65,66.
Further dissemination from bone to other organs
Establishment of bone metastasis may not be the final step of the journey. In recent studies collectively surveying over four thousand breast cancer patients, the first metastasis diagnosed was found in a single organ rather than multiple organs in 74% of patients23,24,67. However, while breast cancers of patients with different subtypes exhibit distinct metastatic distributions 23,24, autopsies of breast cancer patients revealed a high percentage of metastases in multiple organs68-70. In fact, only 6% of patients had single organ metastases68, which led to the previous conclusion that “breast cancer was nonselective in its metastatic targets”69. These seemingly contradictory observations may be reconciled by the function of the BME in invigorating a second wave of metastasis with reduced organotropism (Fig. 1f).
The notion that cancer cells in bone can further disseminate has been indirectly suggested by a few observations. First, the presence of DTCs in the bone marrow of patients with breast cancer was associated with recurrences that are not restricted to bone71,72. Second, roughly two-thirds of patients with bone-only metastases later developed other metastases73,74. Third, in postmenopausal patients, adjuvant treatment with bisphosphonates, a bone-targeting agent, was associated with a reduction of all distant metastases and improved overall survival75, especially in DTC+ patients76,77. Although each of these observations could have alternative explanations (e.g., bisphosphonates might have direct inhibitory effects on metastatic cells in other organs), they collectively suggest that DTC and bone metastasis are tightly associated with metastases in other organs, and therefore, might be the source of further dissemination.
We recently demonstrated that the interaction with osteogenic cells can invigorate cancer cells for further dissemination78 Breast cancer-derived circulating tumor cells (CTCs) in bone metastasis-carrying animals exhibited a stronger stem cell-like phenotype than those in mice with orthotopic breast tumors or lung metastases. An evolving barcode system79,80 was used to delineate the phylogenetic relationship among spontaneous metastatic lesions in various organs, showing that at least a proportion of metastases in visceral organs were closely related to bone metastases78. Mechanistically, the BME induced transient epigenomic reprogramming driven by EZH2and increased phenotypic plasticity of BMMs compared to primary tumors and macrometastases in breast cancer models 46. Remarkably, inducible knockdown of EZH2 in cancer cells did not alter growth of initial bone metastasis but abolished further metastasis from bone78. Together, this suggests that bone can serve as a robust ‘launch pad’ for secondary metastasis, as opposed to a terminal destination, in the metastatic cascade (Fig. 1g,h).
Of note, further metastasis from bone is a different process than tumor self-seeding or cross seeding. Self-seeding refers to the observation that CTCs may return to seed their tumours of origin, whereas during cross-seeding, CTCs infiltrate other pre-existing tumors in the same host81. In contrast, bone metastases provide an environment enabling cancer cells to seed other organs and establish secondary metastases de novo.
The specific epigenomic reprogramming process behind secondary metastasis from bone appears fundamentally different from the Darwinian selection known to drive tumor progression. The latter usually operates on stable genetic traits and result in irreversible changes in tumor clonal structures82. In contrast, the reprogramming induced by BME appears to be transient and reversible as shown using experimental bone metastasis models of ER+ breast cancer46. Numerous previous studies, including many referenced earlier in this Review, suggested that Darwinian selection is the basis of organotropic metastasis26,28,83-85. This concept may be accurate for the first wave of metastasis directly from primary tumors. However, in recent studies we demonstrated a fundamentally different metastasis process from bone46,78. Specifically, mice carrying established bone lesions of breast and prostate cancers subsequently developed further metastases in multiple organs. Interaction with the BME enabled multi-organ metastasis of cells that were initially nonmetastatic and genetically homogeneous (immediately expanded from a single cell), thereby ruling out Darwinian selection as the major driving mechanism. An evolving barcode system facilitates the dissection of metastatic evolution and supports that many metastases in non-bone organs may result from further spread of spontaneous bone metastases. Significantly, targeting EZH2, an epigenomic modifying enzyme, in cancer cells abolished the secondary metastasis from bone. Together, these findings suggest that the adaptive epigenomic alterations induced in metastatic cancer cells in the bone marrow may enable the second wave of metastasis with reduced organotropism as compared to the first wave.Taken together, coordination of genetic and epigenetic mechanisms may provide a more complete view of the metastatic cascade from localized primary tumors to terminal-stage multi-organ metastases and may also reconcile two seemingly contradicting observations in breast cancer care: strong organotropism of first-site metastasis23,24 vs. multi-organ distribution of metastases toward the terminal stage of diseases68-70.
Missing links in the journey
Our knowledge of the bone metastasis journey is far from complete. A few key questions need to be addressed to strengthen our understanding of the spatiotemporal evolution of breast cancer cells in bone.
First, the relationship between different microenvironment niches needs to be better defined. In particular, the perivascular and osteogenic niches both harbor cancer cells, raising the question of how these niches are related to one another. There is considerable evidence suggesting that they enforce different cellular fates of cancer cells (Fig. 2a,b). In particular, the perivascular and osteogenic niches seem to be associated with cellular quiescence and proliferation in breast cancer models, respectively33,34,42,45. However, there is a lack of consensus as to general roles of these niches in dormancy in different cancer types. For instance, when murine and human multiple myeloma cells were intravaneously transplanted into mice, osteoblasts in the osteogenic niche were observed to turn on the dormancy program, whereas osteoclast activity could wake the dormant cells52,86. Thus, the exact roles of different niches may be cancer type-specific. A further question is whether these different types of niches may be inter-convertible. For instance, the perivascular MSCs may undergo osteogenic differentiation87,88, thereby creating an adjacent osteogenic niche and altering DTC fate42,45 (Fig. 2c). In the case of breast cancer, the generation of a new osteogenic niche from perivascular MSCs may terminate DTC dormancy and initiate metastatic colonization 42,45. Finally, DTC fate may be altered through DTC relocation from one type of niche to another. In breast cancer mouse models, perivascular MSCs co-localized with dormant DTCs36. These MSCs may be activated by osteogenic signals released from sites of bone turnover or injury and migrate to the site through chemotaxis36. Indeed, DTCs can form unique protrusions that tether to the migrating MSCs89. This co-migration mechanism may allow DTCs to relocate from the perivascular niche to the osteogenic niche (Fig. 2d). Taken together, the dynamics of various types of niches may profoundly impact the course of bone colonization. The application of high-resolution, spatiotemporal mapping of cancer-niche interactions will help distinguish the abovementioned possibilities and reconcile many seemingly contradictory observations.
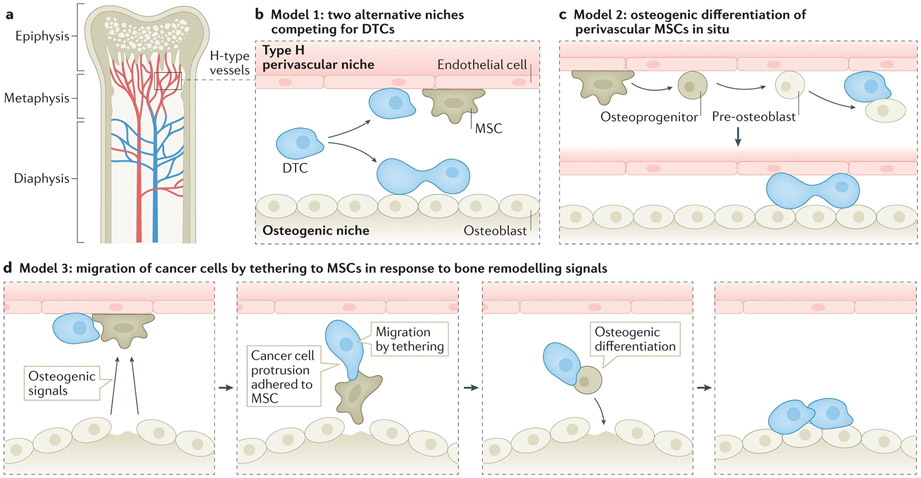
Figure 2. The possible relationship between different microenvironment niches during early-stage bone metastasis.
a. The vascular network in the bone marrow is in close proximity to trabecular bones and endosteum, where osteogenic cells localize. The perivascular niche and osteogenic niche may have a few possible relationships during bone metastasis.
b. Model 1: The two niches may compete for DTCs. DTCs localize to the perivascular niche and osteogenic niche, after which they may enter dormancy or begin proliferation, respectively.
c. Model 2: The perivascular mesenchymal cells of Type H vessels possess MSC activities and may differentiate into osteogenic cells. Therefore, in situ differentiation may create a new osteogenic niche adjacent to perivascular niche, and may terminate dormancy and trigger proliferation of cancer cells.
d. Model 3: Dormant DTCs and quiescent MSCs co-localize in the perivascular niche. Bone homeostasis or pathological bone injuries release osteogenic signals to mobilize MSCs. Cancer cells may form specialized protrusions to attach to MSCs that are undergoing chemotaxis toward the source of osteogenic signals. Upon arrival, the MSCs differentiate into osteoblasts. The associated cancer cells remain in the newly formed osteogenic niche and begin proliferation.
Second, the transition from asymptomatic to osteolytic bone metastasis needs to be characterized. In breast cancer, bone metastasis often occurs late, as long as years to even decades after primary tumor removal25. The prolonged asymptomatic phase is poorly understood. This phase likely occurs prior to the vicious cycle, which would otherwise lead to severe symptoms. We know very little about the initiation of the vicious cycle. It has been suggested that cancer cells can produce the soluble form of VCAM1, thereby recruiting osteoclast progenitors59. Interestingly, VCAM1 is a target gene of HIF1α in endothelial cells 90. Thus, the accumulation of tumor mass in asymptomatic metastasis may exacerbate hypoxia and activate HIF1α-VCAM1 signaling90. Whether this signaling axis in cancer cells and/or endothelial cells contributes to the initiation of the vicious cycle will need to be tested in future investigations. In general, onset of the vicious cycle may represent termination of dormancy. Therefore, any physiological or pathological cues that induce osteoclastogenesis could potentially awaken dormant cancer cells. These cues and their underlying molecular mechanisms need to be identified and therapeutically targeted.
Finally, the observation of further dissemination of bone metastases raises many questions. Among these, the timing of dissemination may be the most urgent to address. Hypothetically, dissemination could occur early when source bone metastases are still microscopic. As a result, metastases diagnosed in other organs may be seeded from bone rather than the primary tumor even if there are no overt bone metastases. Related to this point, our data suggest that the size of metastases does not necessarily correlate with their position in the phylogenetic hierarchy as indicated by evolving barcodes78. This is consistent with the notion that small bone lesions may already begin to spur metastatic seeds to other organs. One provocative little understood association is the relationship between dormancy and the second wave of metastasis. Like HSCs, cancer cells with stem cell properties are often more quiescent but persistent91. Though requiring further validation, it is conceivable that interaction with the BME may confer stemness on DTCs 46,78,92, which may be accompanied by dormancy. Thus, the potential for further metastasis may be harbored in cancer cells that appear indolent. Further studies will be needed to reveal the trigger for further dissemination and to determine how this event is related to the initiation of local bone colonization. It also remains unclear whether the ability to empower further metastasis is unique to bone. Recent genomic analyses revealed frequent metastasis-to-metastasis seeding93,94. Future studies and analyses with deeper sequencing and larger sample size may help elucidate preferred sources of metastatic seeds.
Bone metastasis of different cancer types
In what follows, we summarize the bone metastatic cascade for prostate cancer, kidney cancer, and multiple myeloma. These cancers were selected in order to highlight significant mechanistic and/or phenotypic differences. The role of bone specific adaption is suggested by cancer type-specific differences in phenotypes of bone metastases (osteolytic, osteoblastic, or mixed) and, as more recently observed, differences in resistance mechanisms to bone targeted therapies.
The propensity for creating sclerotic metastases Multiple myeloma, as a marrow based malignancy, introduces unique immunologic factors, while sharing features with kidney cancer in creating almost exclusively osteolytic bone metastases.
Prostate cancer
Prostate cancer metastasizes predominantly to bone usually after development of castration resistance95. Bone metastases of prostate cancer have many commonalities with those of breast cancer, including clinical treatments95 and key roles of certain molecular pathways (e.g., TGFβ and IGF1)96-98 (Fig. 3a). This is largely because the vicious cycle between osteoclasts and prostate cancer cells, similar to breast cancer cells, is a major driver of bone colonization56. Moreover, we recently showed that disseminated prostate cancer cells, like breast cancer cells, adhere to osteogenic cells and form heterotypic gap junctions when inoculated into bone in mice. Interaction with the BME similarly invigorated further metastasis to multiple organs78. Despite these shared properties, bone metastases of prostate cancer are predominantly osteosclerotic [G] with excessive bone formation outperforming the excessive bone resorption99,100. Although our knowledge is still scarce, a few aspects of prostate cancer biology have been uncovered to explain this uniqueness.
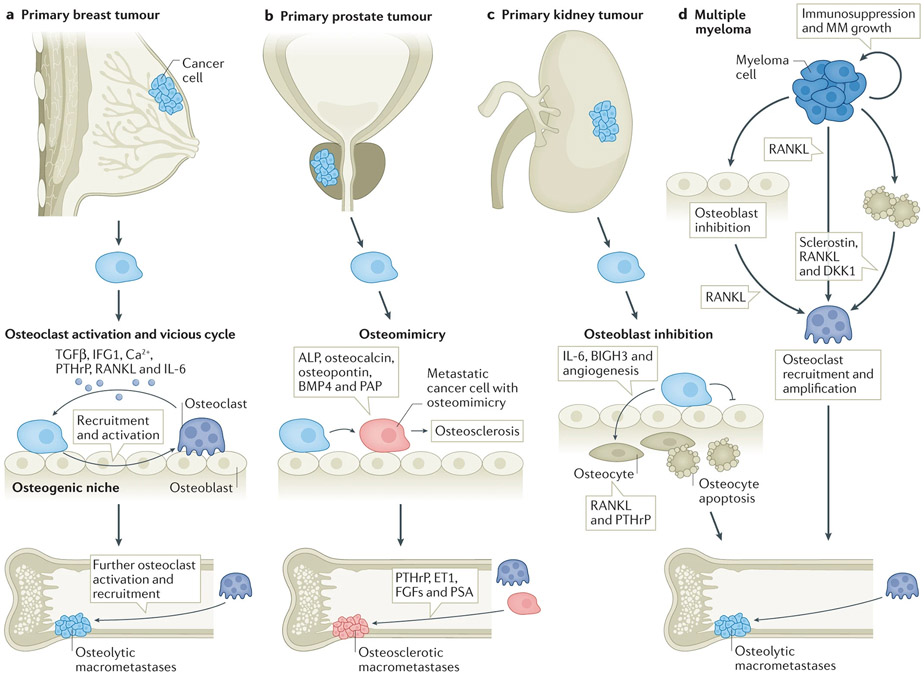
Figure 3. The relationship between primary tumor and the vicious cycle of late-stage bone metastasis in different cancer types.
a. For breast cancer, DTCs awaken from dormancy to create osteolytic macrometatases by both paracrine and heterotypic hAJ and gap junction interactions in the osteogenic niche, which directly and indirectly stimulate osteoclast recruitment and activation. Osteoclast activity, in turn, releases TGFβ, IGF1, Ca++, and other growth factors from bone that further stimulate tumor proliferation. This is the classic ‘vicious cycle’.
b. For prostate cancer, osteomimicry of DTCs in the osteogenic niche harnesses both the anabolic and lytic components of normal bone homeostasis, leading to osteolysis (PSA) and/or osteosclerosis (PAP). Tumor cells induces osteosclerosis via secretion of osteogenic factors such as ALP, osteocalcin, osteopontin, and BMP4. Osteolysis is induced via secretion of PTHrP, ET1, and IGF1. This global alteration toward bone-like phenotypes may be driven by RUNX2. The underlying genomics of osteomimicry, and why it is not as predominant in other tumor types is not known.
c. For kidney cancer, the road to bone destruction is more indirect than breast or prostate cancer, and resembles that for multiple myeloma. DTCs create a vicious cycle via paracrine inhibition of osteoblast function, and osteocyte apoptosis. Consequently, the adverse impact on the anabolic component of the osteogenic niche creates an environment that increases the RANKL/OPG ratio, promoting osteoclast recruitment and activity that creates predominantly lytic macrometastases. The details of interactions in the perivascular and osteogenic niche are likely tightly linked, as neovascular induction is a prominent component of kidney cancer bone metastasis.
d. Multiple myeloma (MM) is remains almost exclusively bone organatropic. Interactions in the osteogenic niche are driven by crosstalk between multiple myeloma cells and osteocytes, osteoblasts, and osteoclasts. Osteolysis is induced via secretion of RANKL by MM cells, and amplified by RANKL from apoptotic osteocytes, and inhibited osteoblasts. Immunosuppression enabling MM proliferation and progression is provoked by immune dysregulation, influencing T-cell immunity, natural killer cell function, and the antigen presenting capacity of dendritic cells; and via MDSC (myeloid derived suppressor cells) amplification by osteoclasts.
After prostate cancer cells reach the BME, they increasingly exhibit properties of osteoblasts, including the secretion of osteoblast-characteristic molecules such as alkaline phosphatase, , osteocalcin, osteopontin, and bone morphogenic proteins (BMPs) 101,102. The acquisition of these properties is referred to as “osteomimicry”, and is often stimulated by osteoblasts. Osteomimicry of prostate cancer can be driven by cancer cell expression of RUNX2 , a transcription factor regulating osteoblast differentiation103. RUNX2 expression is negatively regulated by the PTEN-FOXO1 signaling axis104,105. In addition, prostate cancer cells in the BME express cytokines, including WNTs106, PTHrP107, ET-1108, and FGFs109, which are known to favor osteoblast proliferation and differentiation (Fig. 3b). In particular, the WNT pathway plays important roles in regulating osteoblast differentiation and function110. Prostate cancer cells can secrete WNT ligands to induce osteogenesis [G] 106 and may also activate intrinsic WNT signaling to promote invasion111. Thus, prostate cancer cells ‘blend’ into the osteogenic environment, and seem to evolve to reinforce and benefit from this environment. It was also recently shown in the C4-2B cell-based intrafemoral injection (Table 1, Box 2) that prostate cancer cells can stimulate osteogenesis in vivo by secreting BMP4 which induces endothelial cells to become osteoblasts112. Reciprocally, a recent study has shown that calvariae of new born mice (enriched with osteoblasts) can secrete into conditioned medium multiple dormancy-inducing factors that employ distinct signaling pathways in prostate cancer cells, including the p38MAPK signaling pathway113. What remains to be elucidated, however, is how the osteogenic transcriptional program is activated inside of prostate cancer cells in the bone milieu, whether driven by genetic selection or epigenetic adaptation. Similarly notable is why the osteomimicry phenomenon is so pronounced in prostate cancer and whether this phenomenon may be underlying the strong bone tropism of prostate cancer metastasis. Further research will be required to address these questions.
Compared with other cancer types, prostate cancer cells uniquely express a number of genes such as those encoding prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP). PSA has been widely used as a biomarker to monitor tumor burden in patients114. Interestingly, PSA is a serine protease. Its activities lead to activation of PTHrP115, TGFβ116 and IGF1117, all of which play important roles in driving the osteolytic vicious cycle. On the other hand, PAP stimulates osteoblast differentiation, increases collagen synthesis and enhances expression of ALP 118,119. The coordinated action of these prostate-specific enzymes may further enhance abnormal osteogenesis and lead to osteomimicry (Fig. 3b).
Kidney cancer
RCCBMs have unique phenotypic aspects that distinguish them from breast and prostate cancer. The bone metastasis from kidney cancer are almost exclusively osteolytic120-123. Our knowledge of the premetastatic niche that leads to this phenotype is more limited than with breast and prostate cancer and is the focus of current work by our group and others124-127. Moreover, little is understood about dormancy and the relative influences of the perivascular versus osteogenic niches on metastatic progression. The interactions between kidney cancer and bone resident cells during the end-stage of the vicious cycle promote bone destruction that is more resistant to treatment with bone targeting agents, like bisphosphonates and denosumab120,128,129. Whether this resistance results from Darwinian selection and/or epigenomic reprogramming is unknown. Defining interactions between kidney cancer and the microenvironment will be important for identifying targeted therapeutic strategies that are more effective.
Similar to other cancers, kidney cancers express high levels of cadherin 11 and CXCR4, which confer a predisposition for homing to the osteogenic niche130-132. However, it is unclear whether disseminated kidney cancer cells form heterotypic adherens junctions and/or heterotypic gap junctions with osteogenic cells, as is seen in breast cancer45. Interestingly, the pathologic mechanism emerging for kidney cancer (Fig. 3c) appears to share more commonalities with multiple myeloma, where osteocyte apoptosis and osteoblast inhibition have been observed in patients with lytic bone lesions 133-135. The kidney cancer ‘vicious cycle’ in bone appears to be driven by interactions between kidney cancer cells and osteoblasts as well as osteocytes, rather than osteoclasts124 (Fig. 3c). In the course of investigating these unique aspects of kidney cancer bone metastasis progression, our group (RS) showed that cabozantinib [G] , which has osteoanabolic activity, reversed osteoblast inhibition and reduces SREs in a preclinical model136. This mechanism is consistent with clinical observations of improved survival in patients with kidney cancer and bone metastases treated with cabozantinib137-139. There are likely to be new treatment strategies discovered as the kidney cancer vicious cycle in the osteogenic niche is further defined.
Recent findings point to the osteogenic niche as an essential driver of treatment-resistant bone destruction in RCC. Both preclinical studies and patient samples have demonstrated increased osteocyte apoptosis (unpublished data RS) near lytic bone lesions promoted by tumor secretion of BIGH3, a TGFβ induced protein124. Until recently, BIGH3 was mostly known for causing apoptosis in human retinal pericytes140 and for being downregulated in melorheostosis141, a rare bone disease characterized by linear hyperostosis (excessive osteogenesis). BIGH3 is not structurally related to the TGFβ family, and is known to bind to integrins and other extracellular matrix proteins in the BME that mediate cell adhesion and migration142. BIGH3 is upregulated in kidney and colorectal cancer cells143. In addition, BIGH3 inhibits differentiation of mature osteoblasts in vitro and in vivo144. In normal bone biology, an osteolytic response is counterbalanced by bone formation110,145. Pathologic osteolysis is thus promoted by impaired bone formation. In MM, inhibition of bone formation by DKK1 (Dickkopf-related protein 1) secreted by myeloma cells in the BME was found to contribute to induced osteolysis146. Studies by our group have highlighted the pro-osteolytic properties of BIGH3 in RCCBM. BIGH3 and IL-6 (similar to breast cancer) secreted from tumor cells both: 1. inhibit osteoblast differentiation, thereby reducing anabolic activity and the healing of osteolytic lesions; and 2. induce osteocyte apoptosis, creating a premetastatic niche that is pro-osteolytic and potentially associated with increased disease burden131,136. The osteolytic environment is further promoted by secretion of RANKL and PTHrP by both invading tumor cells and apoptotic osteocytes131 (Fig. 3c).
In the perivascular niche, kidney cancer cells are likely spurred toward other organs. Metastatic kidney cancer cells express high levels of CXCR4, a chemokine receptor, , indicative of an affinity for the perivascular niche and bone marrow space 147-149. And although events are not well characterized, the response is a defining characteristic of metastic disease. Kidney cancer metastases in most organs are hypervascular, thereby inducing an angiogenic response during progression150,151. For bone, this angiogenesis appears to be essential for tumor growth. Indeed, treatments that target neovascularization, such as embolization [G] , are frequently used in conjuction with surgical intervention123,152. In patients, hypervascularity has been attributed to a common mutation in the gene encoding the von Hippel–Lindau (VHL) E3 ubiquitin ligase which normally interacts with and targets HIF-1α for proteasomal degradation153. Mutation in the VHL tumor suppressor gene leads to the stabilization of HIF-1α and initiates gene transcription of its target genes, leading to up-regulation of several angiogenic factors and growth factors154,155.Angiogenic factors that are highly expressed by kidney cancer bone metastases include HIF-1α, VEGF, and angiopoietin-1122,131,156-158. The impact of VHL-HIF signaling is not restricted to the initial tumorigenesis, but gets expanded to drive further metastasis via epigenetic mechanisms159. Further research is needed to determine the role of the neovascular response in awakening dormant kidney cancer cells and/or promoting their propagation to other distant organs.
Multiple myeloma
Multiple myeloma (MM) causes almost exclusively osteolytic lesions that frequently lead to SREs such as pathological fractures, spinal cord compression, and the need for radiotherapeutic or surgical intervention135. The hallmarks of MM bone disease include both homotypic and heterotypic interactions in the BME that promote bone destruction by dysregulating the normal homeostatic balance160,161. Exclusive bone organotropism distinguishes MM, with both regional and vascular spread to other bones, but rarely to other organs. Further elucidation of the bone specificity of MM will help in understanding why MM differs in secondary organotropism from other tumors, such as breast cancer, that produce a second wave of metastasis to other organs from bone.
Similar to kidney cancer cells (Fig. 3d), myeloma cells are able to suppress osteoblast function and induce osteocyte apoptosis133,135. During the progression of MM, osteocytes directly interact with multiple myeloma cells that adhere to cells in the osteogenic niche via the VLA-4/VCAM-1 integrin system135,160. Such interactions stimulate osteocytes to produce sclerostin, Dickkopf-1 (DKK-1), and RANKL160. This stimulates the recruitment of osteoclast precursors and decreases Wnt signaling, thereby leading to the inhibition of osteoblast differentiation134. Sclerostin is a protein secreted by osteocytes that impedes activation of the canonical Wnt pathway, inhibits osteoblast differentiation and mineralization, and induces osteoblast apoptosis162. Direct interactions with myeloma cells also induce osteocyte apoptosis, leading to the creation of a pre-metastatic niche for myeloma cells134,162. This has been demonstrated in MM patients who have reduced numbers of viable osteocytes and suppressed osteoblast activity133,135. In addition, other factors such as BIGH3 and its transactivator, KLF10 (Kruppel-like transcriptional factor induced by TGFβ) which are overexpressed in the BME in RCC143, are less well studied in the BME with MM. Most evidence indicates that KLF10 is a tumor suppressor in MM, and is down-regulated in both MM cell lines and patient samples163. Overexpression of KLF10 causes myeloma apoptosis via the β-cateniin pathway163. Further work is needed to identify whether KLF10 and BIGH3 are significant factors in osteolysis induced by MM.
A multilayered pathophysiologic mechanism creates the characteristic destructive bone process. Notch signaling is induced by myeloma cells, which express Notch family and Jagged135 ligands in their membranes. The resulting pathway activation increases RANKL production by myeloma cells164. In addition, osteocyte and osteoblast production of RANKL amplify osteoclast activity and synergize with osteoblast inhibition . MM cells also induce release of pro-osteoclastogenic factors, including IL-6135, IL-11162, Activan A165, and MIP-1α166. Overall, crosstalk between the bone resident cells and myeloma cells, driven by bidirectional Notch signaling, promotes bone deconstruction and MM proliferation.
Because MM is not a solid tumor and arises in the bone marrow as a malignancy from cells of immune origin, it has unique immunologic properties that distinguish its progression. Immunosuppression in the BME contributes to MM growth161,167. In the presence of MM, the BME is altered from the interplay between MM cells, mature osteoblasts, and osteoclasts52,86. Interactions with osteoclasts potentiate immune dysregulation, influencing T-cell immunity, natural killer cell function, and the antigen presenting capacity of dendritic cells168. Osteoclasts promote the expansion of T helper 17 (Th17) lymphocytes and myeloid derived suppressor cells (MDSC), thereby inhibiting cytotoxic T and NK cells that target MM cells161. Taken together, there is a loss of tumor specific lymphocytes (CD4+ T helper cells and CD8+ cytotoxic T cells), and a rise in immune suppressor cells in the MM cell-containing BME. As a consequence, osteolytic lesions that arise in MM are treated with strategies that differ from kidney cancer and other solid tumors, in that treatment options include immunotherapy that enhances the host anti-myeloma immunity169,170 . In the last 5-10 years, treatment options introduced include immunomodulatory agents, proteasome inhibitors, duratumumab (an anti-CD38 antibody), newer generations of monoclonal antibodies, and CAR (chimeric antigen receptor)-T cells. The result has been substantial, with a near doubling of 5-year relative survival rage in the last 20 years (increased from 32% in 1996 to 55% in 2016)171,172.
New therapeutic targets
Bone targeting therapies, such as bisphosphonates, were introduced in the late 1990s for the purpose of improving clinical outcomes for patients with metastastic bone disease. Their effectiveness is related to their ability to inhibit bone resorption. Accordingly, the results of bisphosphonate therapy of patients with bone metastasis from breast, lung and prostate cancers, and with multiple myeloma, have been positive, including reducing the risk of fracture and bone pain 75,173-176, as well as prolonging progression free survival and reducing mortality 173,177-179. There is mixed evidence regarding whether bisphosphonates extend overall survival 75,128,157,175,178,180. In contrast, patients with renal cell carcinoma (RCC) bone metastasis (RCCBM) have been relatively resistant to bisphosphonate treatment128,157,180. In recent years RCCBM has become the most common solid tumor bone metastasis requiring surgical intervention for treatment and/or palliation despite patients with RCC having a lower incidence of bone metastasis compared with breast, prostate, and lung cancer patients181,182. Because of the inconsistent treatment response of RCCBM to bone targeted therapy such as bisphosphonates and denosumab 121,183, additional interventions for the purposes of palliation (rather than cure) are often the only remaining option. Most often, these treatments include radiation for bone pain (in nearly 80% of patients), and surgical intervention to treat or prevent an impending fracture (28%) 183,184 .
Anti-resorption treatments can stabilize bone metastases by mitigating the vicious cycle185. Two major classes of drugs, namely bisphosphonates and denosumab, are used to target osteoclasts by inducing apoptosis186,187 and preventing activation188, respectively. Despite their effectiveness of strengthening the bone and improving quality of life, they do not significantly prolong overall survival of patients with bone metastases185, and about two-thirds of patients with breast cancer and bone metastases later develop metastases in other organs73,74. Thus, additional therapies are urgently needed. In this section, we will discuss novel potential therapeutic targets emerging from recent research.
Targeting dormant DTCs, asymptomatic BMMs and their niches
It has become increasingly clear that the vicious cycle is not initiated immediately upon DTC arrival in the bone marrow. The asymptomatic stage of bone colonization may persist for years or even decades in different cancer types25,189. This stage may be divided into two phases characterized by the presence of quiescent DTCs and proliferative BMMs, respectively. As previously discussed, the cellular and molecular status of these early-stage lesions is determined by their interactions with different microenvironment niches.
There are two challenges in targeting DTCs and BMMs. First, clinical information from corresponding primary tumors may not be applicable to these populations. DTCs and BMMs only represent a small fraction of cells residing in primary tumors. Moreover, cancer-niche interactions provide therapeutic resistance and vulnerability not seen in primary tumors. For instance, gap junctions between cancer cells and osteogenic cells provide a channel for calcium flux into the former and result in activation of calcium signaling and unexpected therapeutic sensitivity to arsenic trioxide45. ER+ breast cancer cells may transiently lose their ER expression and acquire endocrine resistance under the influence of the osteogenic cells46. The integrin-mediated signaling between the perivascular niche and DTCs causes resistance to chemotherapies35. These findings implicate new targets to disrupt cancer-niche interactions, and treating these targets may eliminate DTCs and BMMs either directly or through sensitization to traditional therapies. The second challenge in targeting DTCs and BMMs is their “invisibility” to current diagnostic technologies, which makes it difficult to define clinical endpoints in adjuvant settings other than overt recurrence190. Since recurrences often occur years later and in only 20-40% of patients25, clinical trials would need to recruit many patients with long follow-up times to capture enough events. To overcome this challenge, biomarkers that distinguish patients with high recurrence risk are urgently needed.
Recent studies have started to reveal potential therapeutic targets to abolish the tumorigenic capacity of DTCs and BMMs. For example, E-selectin based on its role in inducing phenotype plasticity in cancer cells as discussed above might serve as a potential target 39. Blockade of E-selectin activity might force DTCs to remain dormant. Notably, a small molecular inhibitor of E-selectin is being investigated for treatment of leukemia191. (Fig. 4a). As previously mentioned, osteoblasts and their progenitor cells provide several targetable mechanisms to drive metastasis progression, including the activation of mTOR by heterotypic adherens junctions, calcium signaling by gap junctions, Notch signaling by Jagged-1, and EZH2 by FGFR and PDGFR signaling. Indeed, pharmacologically inhibition of mTOR, adherens junctions, calcium signaling and gap junctions impeded bone colonization in experimental metastasis models42,43,45-47,78,192 (Fig. 3b).
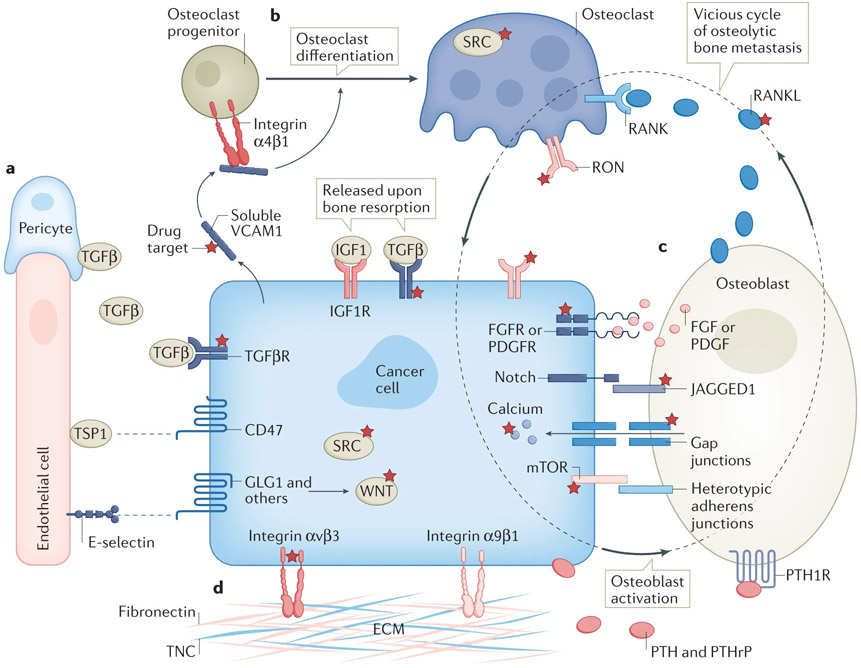
Figure 4. Emerging therapeutic targets in bone metastasis.
a) Molecular crosstalk between cancer cells and perivascular niche cells including endothelial cells and pericytes.
b) Therapeutic targets in differentiation of osteoclast progenitor cells into matured osteoclasts.
c) Direct cell-cell interactions and paracrine between cancer cells and osteogenic cells.
d) The integrin pathways mediating interaction between cancer cells with extracellular matrix during bone metastasis.
The dotted circle indicates the paradigm of vicious cycle that includes secretion of PTH and PTHrP by cancer cells that activates osteoblasts, the secretion of RANKL by osteoblasts that drives osteoclast differentiation, and the release of TGFβ and IGF1 from bone matrix upon bone resorption that reciprocally promotes cancer cell progression. Molecules with targeted therapies available are highlighted with reticle signs.
Another therapeutic strategy is to reinforce dormancy without necessarily killing DTCs and/or BMMs. Toward this end, agonists of TSP1 signaling are compelling potential therapies. Although TSP1 agonist mimetic peptides have been available, and may be exploited in metastatic models193(Fig. 4a). As discussed earlier, the conversion or alteration from perivascular to osteogenic niche may terminate dormancy and initiate proliferation (Fig. 2). Osteogenesis may be a major driving force of the initiation of proliferation upon dormancy termination, either as part of normal bone turnover or bone repair/remodeling. Therefore, it is conceivable that perturbation of osteogenesis may affect outgrowth of DTCs and/or BMMs. Indeed, this notion is consistent with the observation that bisphosphonates (which inhibit bone turnover194) reduce breast cancer metastases specifically in postmenopausal women75, in whom bone turnover is increased 195. The termination of dormancy may also be mediated by a number of signals, including soluble VCAM1 produced by cancer cells59 (Fig. 4b) and periostin [G] produced by endothelial tip cells [G] 33. These molecules represent potential targets to keep DTCs dormant. Specifically, VCAM1 signals through α4 integrin, which can be blocked by monoclonal antibodies or small molecular inhibitors196. For periostin, a monoclonal antibody targeting the cancer-specific isoform has recently been applied to xenograft models of breast cancer and exhibited efficacies in combatting chemoresistance197. Although promising, the above agents have not been tested in models of metastasis dormancy. Moreover, the metastasis preventative strategy entails persistent long-term treatment, which may be challenging in the clinical setting.
Finally, dormant DTCs may be eradicated after being mobilized through perturbation of important niche components. Since DTCs and HSCs share common niches, strategies to mobilize HSCs can also stimulate DTCs to leave their niches. Administration of G-CSF, which can mobilize HSCs, increased leukemic stem cells (LSCs) in the circulation of acute myeloid leukemia 198 and patients with breast cancer199. Similarly, both HSCs and DTCs express CXCR4, which mediate their homing and retention to the niche. The cognate ligand of CXCR4, CXCL12, is highly expressed by niche cells. Consistent with this roles in HSCs, , perturbation of the CXCR12-CXCR4 axis by a CXCR4 inhibitor (AMD3100) and in prostate cancer and lymphoblastic leukemia models resulted in release of cancer cells into circulation32,200. In combination with other therapies, this mobilization can lead to effective elimination of dormant and therapy-resistant cancer cells201.
Targeting the vicious cycle
Several pathways were recently discovered to participate in the vicious cycle mainly through driving osteoclast differentiation and functions. The RON kinase is expressed by both bone metastatic cells of breast cancer and osteoclasts. A specific RON inhibitor is currently being tested in clinical trials62 (NCT03292536) (Fig. 4c). Inside of osteoclasts, the SRC kinase promotes the transduction of signals toward differentiation202 and production of bone-degrading enzymes203. As a result, inhibition of SRCmay achieve dual inhibitory effects on both cancer cells and osteoclasts, thereby representing a possible strategy to impede the vicious cycle. This hypothesis has been validated in pre-clinical metastasis models of breast cancer (both experimental metastases based on intra-cardiac injection and spontaneous metastases from orthotopic tumors, see Table 1 and Box 2) via genetic knockdown of SRC178 or application of a SRC inhibitor, dasatinib26. Notch signaling mediates crosstalk between cancer cells and osteoblasts and renders cancer cells resistant to chemotherapies as part of the vicious cycle47,58. A therapeutic antibody against Jagged-1, the pertinent Notch ligand, represents a promising agent to block bone metastasis43. Likewise, integrin β3 is induced in cancer cells by the BME, mediates chemo-resistance and may be targeted by nanotherapy60, possibly through interaction with fibronectin (Fig. 4d). Senescent osteoblasts produce IL-6 to promote osteoclastogenesis and bone metastasis progression of breast cancer cells63. The stromal components other than osteoclasts and osteoblasts may also play important roles in facilitating the vicious cycle. Inhibition of the p38MAPK/MK2 pathway in stromal cells by small molecule inhibitors could also limit bone metastasis progression of breast cancer in mice 204. TGFβ plays pivotal roles in multiple stages of colonization including early dormancy (Fig. 4a) and later vicious cycle, although the directions of effects may not be consistent. Nevertheless, TGFβ signaling has been targeted in myeloma pre-clinical models with the neutralizing antibody 1D11 which exhibits efficacy in combination with other therapies 205.
A common challenge for all above therapies is potential toxicity due to the pleotropic roles of the targeted pathways. The acidic environment and the positively charged bone matrix may be leveraged to overcome this challenge. For instance, conjugation with negatively charged bisphosphonates can significantly enrich antibodies and chemical inhibitors in the BME, thereby reducing drug effects on other organs206-208.
Immunotherapies
The bone marrow has unique immunological properties as it is where all immune cells are produced. For example, immature immune cells, especially myeloid cells, may negatively impact adaptive immunity. Moreover, the high level of TGFβ in the bone marrow may also blunt immune responses. Therefore, it is reasonable to hypothesize that the BME is immunosuppressive. In support of this notion, retrospective analysis suggests that patientsof triple negative breast cancers with bone metastases respond poorly to combined atezolizumab, an immune checkpoint inhibitor, and nab-paclitaxel, despite overall significant responses in all patients209. Similarly, ipilimumab exhibited limited effects on castration-resistant prostate cancer210, which may be due to skewing of T helper subsets toward the Th17 subtype under the influence of TGFβ211. Future studies will be needed to further characterize the immune microenvironment in the bone marrow and to better understand how this microenvironment may affect responses to immunotherapies in bone metastasis.
Conclusions and perspectives
The colonization of DTCs in bone is driven by intimate interactions between cancer cells and the spatiotemporally dynamic environment. DTC fate regulation may be achieved through intricate organization of microenvironmental cells of various lineages and subtle differences between cells of the same lineage. Both normal homeostasis and pathological repair and/or remodeling induce reorganization and reprogramming of bone cells, which may in turn impact bone metastasis progression. Thus, future investigation of bone metastasis will benefit from approaches that can precisely map the BME at a single-cell resolution. Complementarily, real-time intra-vital imaging will be needed to provide information about temporal dynamics of cancer and bone cells. Taken together, multi-dimensional models of bone colonization need to be established in order to fill the missing links of a cancer cell’s journey toward, within, and beyond BME .
In addition to exerting pressure for genetic selection, the BME also seems to trigger adaptation of cancer cells through epigenomic reprogramming. Interestingly, the reprogramming appears to cause a de-differentiation process and render metastatic cells more stem-like. As a result, the BME may fuel further metastasis to other organs, as demonstrated in pre-clinical models46,78. This hypothesis will need to be tested in more models and detailed mechanisms will need to be elucidated. However, it is compelling to notice that the reprogramming may be in part driven by EZH2 46,78. EZH2 inhibitors are currently under clinical investigations212. Future studies will be needed to further characterize this process, in terms of the onset, kinetics, reversibility, and exact molecular drivers. Clinically, it will be interesting to examine if EZH2 treatment can prevent patients with bone-only metastases from developing other metastases. If invisible bone metastases already begin to seed other organs, the treatment may even apply to all patients to stop the potential spread from bone. It also remains to be determined if specific microenvironment niches in other metastatic sites can cause similar effects. The compound actions of clonal selection and epigenetic reprogramming may lead to more complicated metastatic evolution, which has been suggested by recent genomic sequencing studies.
Despite many commonalities, overt bone metastases of different cancer types exhibit important distinctions. For instance, strong osteomimicry and pivotal roles of osteocytes in the vicious cycle distinguish bone metastases of prostate cancer and kidney cancer, respectively. It is important to understand how various cancer types evolve to utilize different pathways and microenvironment factors to facilitate bone colonization. This knowledge may explain distinct therapeutic responses such as the resistance of kidney cancer to bisphosphonates and denosumab. Although it is difficult to compare cancer cells of different origins, their impact on various cell types in the microenvironment may be readily compared to delineate cancer type-specific interaction mechanisms.
ACKNOWLEDGEMENTS
We would like to thank Hilda Chan and Laura Michie for editing the manuscript. X.H.-F.Z. is supported by US Department of Defense DAMD W81XWH-16-1-0073, DAMD W81XWH-20-1-0375, NCI R01CA183878, NCI R01CA251950, NCI R01CA221946, NCI R01CA227904, NCI U01CA252553, Breast Cancer Research Foundation, and McNair Foundation. RLS is supported by US DOD KCRP W81XWH-20-1-0895, and UT MD Anderson Cancer Center Knowledge Gap Award, and Institutional Research Grant.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Clarke B Normal bone anatomy and physiology. Clinical journal of the American Society of Nephrology : CJASN, doi: 10.2215/CJN.04151206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Saunders TL, Enikolopov G & Morrison SJ Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462, doi: 10.1038/nature10783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou BO, Yue R, Murphy MM, Peyer JG & Morrison SJ Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168, doi: 10.1016/j.stem.2014.06.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunisaki Y et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643, doi: 10.1038/nature12612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusumbe AP et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384, doi: 10.1038/nature17638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez-Ferrer S et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834, doi: 10.1038/nature09262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazill JM, Beeve AT, Craft CS, Ivanusic JJ & Scheller EL Nerves in Bone: Evolving Concepts in Pain and Anabolism. J Bone Miner Res 34, 1393–1406, doi: 10.1002/jbmr.3822 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAllister SS & Weinberg RA The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16, 717–727, doi: 10.1038/ncb3015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan RN et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827, doi: 10.1038/nature04186 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Z et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun 9, 5395, doi: 10.1038/s41467-018-07810-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y et al. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 30, 243–256, doi: 10.1016/j.ccell.2016.06.021 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Tyagi A et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun 12, 474, doi: 10.1038/s41467-020-20733-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catena R et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov 3, 578–589, doi: 10.1158/2159-8290.CD-12-0476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granot Z et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314, doi: 10.1016/j.ccr.2011.08.012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer MA et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun 9, 1250, doi: 10.1038/s41467-018-03600-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng P et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205, 2235–2249, doi: 10.1084/jem.20080132 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha P et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 181, 4666–4675, doi: 10.4049/jimmunol.181.7.4666 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming V et al. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol 9, 398, doi: 10.3389/fimmu.2018.00398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR & Carson WE 3rd. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat 140, 13–21, doi: 10.1007/s10549-013-2618-7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welte T et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat Cell Biol 18, 632–644, doi: 10.1038/ncb3355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim IS et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol 21, 1113–1126, doi: 10.1038/s41556-019-0373-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paget S THE DISTRIBUTION OF SECONDARY GROWTHS IN CANCER OF THE BREAST. The Lancet 133, 571–573, doi: 10.1016/S0140-6736(00)49915-0 (1889). [DOI] [PubMed] [Google Scholar]
- 23.Kennecke H et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 28, 3271–3277, doi: 10.1200/JCO.2009.25.9820 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Smid M et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res 68, 3108–3114, doi: 10.1158/0008-5472.CAN-07-5644 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Zhang XH, Giuliano M, Trivedi MV, Schiff R & Osborne CK Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 19, 6389–6397, doi: 10.1158/1078-0432.CCR-13-0838 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang XH et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78, doi: 10.1016/j.ccr.2009.05.017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanharanta S & Massague J Origins of metastatic traits. Cancer Cell 24, 410–421, doi: 10.1016/j.ccr.2013.09.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XH et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073, doi: 10.1016/j.cell.2013.07.036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awolaran O, Brooks SA & Lavender V Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast 30, 156–171, doi: 10.1016/j.breast.2016.09.017 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Bellahcene A & Castronovo V Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol 146, 95–100 (1995). [PMC free article] [PubMed] [Google Scholar]
- 31.Tan CC et al. Breast cancer cells obtain an osteomimetic feature via epithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction. Oncotarget 7, 79688–79705, doi: 10.18632/oncotarget.12939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiozawa Y et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. Journal of Clinical Investigation 121, 1298–1312, doi: 10.1172/JCI43414DS1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghajar CM et al. The perivascular niche regulates breast tumour dormancy. Nature Cell Biology 15, 807–817, doi: 10.1038/ncb2767 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price TT et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med 8, 340ra373, doi: 10.1126/scitranslmed.aad4059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson P et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat Cell Biol 21, 238–250, doi: 10.1038/s41556-018-0267-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobre AR et al. Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGF-β2. Nature Cancer 2, 327–339, doi: 10.1038/s43018-021-00179-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RW et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol 18, 1078–1089, doi: 10.1038/ncb3408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gawrzak S et al. MSK1 regulates luminal cell differentiation and metastatic dormancy in ER(+) breast cancer. Nat Cell Biol 20, 211–221, doi: 10.1038/s41556-017-0021-z (2018). [DOI] [PubMed] [Google Scholar]
- 39.Esposito M et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat Cell Biol 21, 627–639, doi: 10.1038/s41556-019-0309-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams GB et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603, doi: 10.1038/nature04247 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Wei Q & Frenette PS Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 48, 632–648, doi: 10.1016/j.immuni.2018.03.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210, doi: 10.1016/j.ccell.2014.11.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H et al. Therapeutic Antibody Targeting Tumor- and Osteoblastic Niche-Derived Jagged1 Sensitizes Bone Metastasis to Chemotherapy. Cancer Cell 32, 731–747 e736, doi: 10.1016/j.ccell.2017.11.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D et al. NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche. J Bone Oncol 13, 91–96, doi: 10.1016/j.jbo.2018.09.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H et al. The Osteogenic Niche Is a Calcium Reservoir of Bone Micrometastases and Confers Unexpected Therapeutic Vulnerability. Cancer Cell 34, 823–839 e827, doi: 10.1016/j.ccell.2018.10.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bado IL et al. The bone microenvironment increases phenotypic plasticity of ER+ breast cancer cells. Developmental Cell 56, 1100–1117.e1109, doi: 10.1016/j.devcel.2021.03.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sethi N, Dai X, Winter CG & Kang Y Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205, doi: 10.1016/j.ccr.2010.12.022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.San Martin R et al. Tenascin-C and Integrin alpha9 Mediate Interactions of Prostate Cancer with the Bone Microenvironment. Cancer Res 77, 5977–5988, doi: 10.1158/0008-5472.CAN-17-0064 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oskarsson T et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17, 867–874, doi: 10.1038/nm.2379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowy CM & Oskarsson T Tenascin C in metastasis: A view from the invasive front. Cell Adh Migr 9, 112–124, doi: 10.1080/19336918.2015.1008331 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shupp AB, Kolb AD, Mukhopadhyay D & Bussard KM Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers (Basel) 10, doi: 10.3390/cancers10060182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawson MA et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun 6, 8983, doi: 10.1038/ncomms9983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guise TA et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98, 1544–1549, doi: 10.1172/JCI118947 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guise TA et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 12, 6213s–6216s, doi: 10.1158/1078-0432.CCR-06-1007 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Yasuda H et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95, 3597–3602, doi: 10.1073/pnas.95.7.3597 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kingsley LA, Fournier PG, Chirgwin JM & Guise TA Molecular biology of bone metastasis. Mol Cancer Ther 6, 2609–2617, doi: 10.1158/1535-7163.MCT-07-0234 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Waning DL & Guise TA Molecular mechanisms of bone metastasis and associated muscle weakness. Clin Cancer Res 20, 3071–3077, doi: 10.1158/1078-0432.CCR-13-1590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ell B & Kang Y SnapShot: Bone Metastasis. Cell 151, 690–690 e691, doi: 10.1016/j.cell.2012.10.005 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Lu X et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714, doi: 10.1016/j.ccr.2011.11.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross MH et al. Bone-Induced Expression of Integrin beta3 Enables Targeted Nanotherapy of Breast Cancer Metastases. Cancer Res 77, 6299–6312, doi: 10.1158/0008-5472.CAN-17-1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welm AL et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A 104, 7570–7575, doi: 10.1073/pnas.0702095104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrade K et al. RON kinase: A target for treatment of cancer-induced bone destruction and osteoporosis. Sci Transl Med 9, doi: 10.1126/scitranslmed.aai9338 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo X et al. Stromal-Initiated Changes in the Bone Promote Metastatic Niche Development. Cell Rep 14, 82–92, doi: 10.1016/j.celrep.2015.12.016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coleman RE Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27, 165–176, doi: 10.1053/ctrv.2000.0210 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Esposito M, Guise T & Kang Y The Biology of Bone Metastasis. Cold Spring Harb Perspect Med 8, doi: 10.1101/cshperspect.a031252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coleman RE et al. Bone metastases. Nat Rev Dis Primers 6, 83, doi: 10.1038/s41572-020-00216-3 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Soni A et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 143, 471–478, doi: 10.1309/AJCPYO5FSV3UPEXS (2015). [DOI] [PubMed] [Google Scholar]
- 68.Cummings MC et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol 232, 23–31, doi: 10.1002/path.4288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Disibio G & French SW Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med 132, 931–939, doi: 10.1043/1543-2165(2008)132[931:MPOCRF]2.0.CO;2 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Lee YT Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol 23, 175–180, doi: 10.1002/jso.2930230311 (1983). [DOI] [PubMed] [Google Scholar]
- 71.Banys-Paluchowski M, Fehm T, Janni W, Solomayer EF & Hartkopf A Circulating and Disseminated Tumor Cells in Breast Carcinoma: Report from the Consensus Conference on Tumor Cell Dissemination during the 39th Annual Meeting of the German Society of Senology, Berlin, 27 June 2019. Geburtshilfe Frauenheilkd 79, 1320–1327, doi: 10.1055/a-1031-1120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tjensvoll K et al. Detection of disseminated tumor cells in bone marrow predict late recurrences in operable breast cancer patients. BMC Cancer 19, 1131, doi: 10.1186/s12885-019-6268-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coleman RE & Rubens RD The clinical course of bone metastases from breast cancer. Br J Cancer 55, 61–66, doi: 10.1038/bjc.1987.13 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman RE, Smith P & Rubens RD Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 77, 336–340, doi: 10.1038/bjc.1998.52 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Early Breast Cancer Trialists' Collaborative, G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386, 1353–1361, doi: 10.1016/S0140-6736(15)60908-4 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Solomayer EF et al. Influence of zoledronic acid on disseminated tumor cells in primary breast cancer patients. Ann Oncol 23, 2271–2277, doi: 10.1093/annonc/mdr612 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Hartkopf AD et al. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients - results from a large single-centre analysis. Eur J Cancer 50, 2550–2559, doi: 10.1016/j.ejca.2014.06.025 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Zhang W et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell, doi: 10.1016/j.cell.2021.03.011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalhor R, Mali P & Church GM Rapidly evolving homing CRISPR barcodes. Nat Methods 14, 195–200, doi: 10.1038/nmeth.4108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalhor R et al. Developmental barcoding of whole mouse via homing CRISPR. Science 361, doi: 10.1126/science.aat9804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim MY et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326, doi: 10.1016/j.cell.2009.11.025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nik-Zainal S et al. The life history of 21 breast cancers. Cell 149, 994–1007, doi: 10.1016/j.cell.2012.04.023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minn AJ et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524, doi: 10.1038/nature03799 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bos PD et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009, doi: 10.1038/nature08021 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang Y et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549, doi: 10.1016/s1535-6108(03)00132-6 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Chen Z, Orlowski RZ, Wang M, Kwak L & McCarty N Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood 123, 2204–2208, doi: 10.1182/blood-2013-07-517136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crisan M et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313, doi: 10.1016/j.stem.2008.07.003 (2008). [DOI] [PubMed] [Google Scholar]
- 88.da Silva Meirelles L, Caplan AI & Nardi NB In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299, doi: 10.1634/stemcells.2007-1122 (2008). [DOI] [PubMed] [Google Scholar]
- 89.Muscarella AM Unique cellular protrusions mediate breast cancer cell migration by tethering to osteogenic cells. npj Breast Cancer, doi: 10.1038/s41523-020-00183-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willam C, Schindler R, Frei U & Eckardt KU Increases in oxygen tension stimulate expression of ICAM-1 and VCAM-1 on human endothelial cells. Am J Physiol 276, H2044–2052, doi: 10.1152/ajpheart.1999.276.6.H2044 (1999). [DOI] [PubMed] [Google Scholar]
- 91.Shen S, Vagner S & Robert C Persistent Cancer Cells: The Deadly Survivors. Cell 183, 860–874, doi: 10.1016/j.cell.2020.10.027 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Eyre R et al. Microenvironmental IL1beta promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat Commun 10, 5016, doi: 10.1038/s41467-019-12807-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown D et al. Phylogenetic analysis of metastatic progression in breast cancer using somatic mutations and copy number aberrations. Nat Commun 8, 14944, doi: 10.1038/ncomms14944 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ullah I et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J Clin Invest 128, 1355–1370, doi: 10.1172/JCI96149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sturge J, Caley MP & Waxman J Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol 8, 357–368, doi: 10.1038/nrclinonc.2011.67 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Wan X et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 50, 695–703, doi: 10.1016/j.bone.2011.11.022 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fournier PG et al. The TGF-beta Signaling Regulator PMEPA1 Suppresses Prostate Cancer Metastases to Bone. Cancer Cell 27, 809–821, doi: 10.1016/j.ccell.2015.04.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen C et al. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate 75, 883–895, doi: 10.1002/pros.22971 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roudier MP et al. Histological, immunophenotypic and histomorphometric characterization of prostate cancer bone metastases. Cancer Treat Res 118, 311–339, doi: 10.1007/978-1-4419-9129-4_13 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Furesi G, Rauner M & Hofbauer LC Emerging Players in Prostate Cancer-Bone Niche Communication. Trends Cancer 7, 112–121, doi: 10.1016/j.trecan.2020.09.006 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Knerr K, Ackermann K, Neidhart T & Pyerin W Bone metastasis: Osteoblasts affect growth and adhesion regulons in prostate tumor cells and provoke osteomimicry. Int J Cancer 111, 152–159, doi: 10.1002/ijc.20223 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Hagberg Thulin M, Jennbacken K, Damber JE & Welen K Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies. Clin Exp Metastasis 31, 269–283, doi: 10.1007/s10585-013-9626-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akech J et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29, 811–821, doi: 10.1038/onc.2009.389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y et al. PTEN Loss Promotes Intratumoral Androgen Synthesis and Tumor Microenvironment Remodeling via Aberrant Activation of RUNX2 in Castration-Resistant Prostate Cancer. Clin Cancer Res 24, 834–846, doi: 10.1158/1078-0432.CCR-17-2006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H et al. FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res 71, 3257–3267, doi: 10.1158/0008-5472.CAN-10-2603 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dai J et al. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res 68, 5785–5794, doi: 10.1158/0008-5472.CAN-07-6541 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liao J et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer 123, 2267–2278, doi: 10.1002/ijc.23602 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson JB et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med 1, 944–949, doi: 10.1038/nm0995-944 (1995). [DOI] [PubMed] [Google Scholar]
- 109.Lee YC et al. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol Cell Proteomics 14, 471–483, doi: 10.1074/mcp.M114.039909 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Long F Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13, 27–38, doi: 10.1038/nrm3254 (2011). [DOI] [PubMed] [Google Scholar]
- 111.Nandana S et al. Bone Metastasis of Prostate Cancer Can Be Therapeutically Targeted at the TBX2-WNT Signaling Axis. Cancer Res 77, 1331–1344, doi: 10.1158/0008-5472.CAN-16-0497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin SC et al. Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer. Dev Cell 41, 467–480 e463, doi: 10.1016/j.devcel.2017.05.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu-Lee LY et al. Bone secreted factors induce cellular quiescence in prostate cancer cells. Sci Rep 9, 18635, doi: 10.1038/s41598-019-54566-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ercole CJ et al. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J Urol 138, 1181–1184, doi: 10.1016/s0022-5347(17)43543-9 (1987). [DOI] [PubMed] [Google Scholar]
- 115.Cramer SD, Chen Z & Peehl DM Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J Urol 156, 526–531, doi: 10.1097/00005392-199608000-00076 (1996). [DOI] [PubMed] [Google Scholar]
- 116.Dallas SL et al. Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J Cell Physiol 202, 361–370, doi: 10.1002/jcp.20147 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Cohen P, Peehl DM, Graves HC & Rosenfeld RG Biological effects of prostate specific antigen as an insulin-like growth factor binding protein-3 protease. J Endocrinol 142, 407–415, doi: 10.1677/joe.0.1420407 (1994). [DOI] [PubMed] [Google Scholar]
- 118.Kirschenbaum A, Liu XH, Yao S, Leiter A & Levine AC Prostatic acid phosphatase is expressed in human prostate cancer bone metastases and promotes osteoblast differentiation. Ann N Y Acad Sci 1237, 64–70, doi: 10.1111/j.1749-6632.2011.06198.x (2011). [DOI] [PubMed] [Google Scholar]
- 119.Ishibe M, Rosier RN & Puzas JE Human prostatic acid phosphatase directly stimulates collagen synthesis and alkaline phosphatase content of isolated bone cells. J Clin Endocrinol Metab 73, 785–792, doi: 10.1210/jcem-73-4-785 (1991). [DOI] [PubMed] [Google Scholar]
- 120.Santoni M et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? Journal of experimental & clinical cancer research : CR 34, 10, doi: 10.1186/s13046-015-0122-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wood SL & Brown JE Skeletal metastasis in renal cell carcinoma: current and future management options. Cancer Treat Rev 38, 284–291, doi: 10.1016/j.ctrv.2011.06.011 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Chen SC & Kuo PL Bone Metastasis from Renal Cell Carcinoma. Int J Mol Sci 17, doi: 10.3390/ijms17060987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin PP et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. The Journal of bone and joint surgery. American volume 89, 1794–1801, doi: 10.2106/JBJS.F.00603 (2007). [DOI] [PubMed] [Google Scholar]
- 124.Pan T et al. BIGH3 Promotes Osteolytic Lesions in Renal Cell Carcinoma Bone Metastasis by Inhibiting Osteoblast Differentiation. Neoplasia 20, 32–43, doi: 10.1016/j.neo.2017.11.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sasaki H et al. Beta IGH3, a TGF-beta inducible gene, is overexpressed in lung cancer. Japanese journal of clinical oncology 32, 85–89 (2002). [DOI] [PubMed] [Google Scholar]
- 126.Yamanaka M et al. BIGH3 is overexpressed in clear cell renal cell carcinoma. Oncology reports 19, 865–874 (2008). [PubMed] [Google Scholar]
- 127.Zhang W, Bado I, Wang H, Lo HC & Zhang XH Bone Metastasis: Find Your Niche and Fit in. Trends Cancer 5, 95–110, doi: 10.1016/j.trecan.2018.12.004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keizman D et al. Bisphosphonates combined with sunitinib may improve the response rate, progression free survival and overall survival of patients with bone metastases from renal cell carcinoma. Eur J Cancer 48, 1031–1037, doi: 10.1016/j.ejca.2012.02.050 (2012). [DOI] [PubMed] [Google Scholar]
- 129.Vrdoljak E et al. Bisphosphonates in patients with renal cell carcinoma and bone metastases: a sunitinib global expanded-access trial subanalysis. Future Oncol 11, 2831–2840, doi: 10.2217/fon.15.140 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Haber T et al. Bone Metastasis in Renal Cell Carcinoma is Preprogrammed in the Primary Tumor and Caused by AKT and Integrin alpha5 Signaling. J Urol 194, 539–546, doi: 10.1016/j.juro.2015.01.079 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Pan T et al. Three-dimensional (3D) culture of bone-derived human 786-O renal cell carcinoma retains relevant clinical characteristics of bone metastases. Cancer Lett 365, 89–95, doi: 10.1016/j.canlet.2015.05.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Satcher RL et al. Cadherin-11 in renal cell carcinoma bone metastasis. PLoS One 9, e89880, doi: 10.1371/journal.pone.0089880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giuliani N et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia 26, 1391–1401, doi: 10.1038/leu.2011.381 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Riquelme MA, Cardenas ER & Jiang JX Osteocytes and Bone Metastasis. Front Endocrinol (Lausanne) 11, 567844, doi: 10.3389/fendo.2020.567844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Terpos E, Ntanasis-Stathopoulos I & Dimopoulos MA Myeloma bone disease: from biology findings to treatment approaches. Blood 133, 1534–1539, doi: 10.1182/blood-2018-11-852459 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Pan T et al. Cabozantinib Reverses Renal Cell Carcinoma-Mediated Osteoblast Inhibition in Three-Dimensional Co-culture In Vitro and Reduces Bone Osteolysis In Vivo. Molecular Cancer Therapeutics, molcanther.0174.2019, doi: 10.1158/1535-7163.mct-19-0174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Choueiri TK et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17, 917–927, doi: 10.1016/S1470-2045(16)30107-3 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Choueiri TK et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 35, 591–597, doi: 10.1200/JCO.2016.70.7398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Escudier B et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J Clin Oncol 36, 765–772, doi: 10.1200/JCO.2017.74.7352 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Betts-Obregon BS et al. TGFbeta induces BIGH3 expression and human retinal pericyte apoptosis: a novel pathway of diabetic retinopathy. Eye (Lond) 30, 1639–1647, doi: 10.1038/eye.2016.179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim JE et al. A TGF-beta-inducible cell adhesion molecule, betaig-h3, is downregulated in melorheostosis and involved in osteogenesis. J Cell Biochem 77, 169–178, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 142.Klamer SE et al. BIGH3 modulates adhesion and migration of hematopoietic stem and progenitor cells. Cell Adh Migr 7, 434–449, doi: 10.4161/cam.26596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ivanov SV et al. Two novel VHL targets, TGFBI (BIGH3) and its transactivator KLF10, are up-regulated in renal clear cell carcinoma and other tumors. Biochem Biophys Res Commun 370, 536–540, doi: 10.1016/j.bbrc.2008.03.066 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thapa N, Kang KB & Kim IS Beta ig-h3 mediates osteoblast adhesion and inhibits differentiation. Bone 36, 232–242, doi: 10.1016/j.bone.2004.08.007 (2005). [DOI] [PubMed] [Google Scholar]
- 145.Raggatt LJ & Partridge NC Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285, 25103–25108, doi: 10.1074/jbc.R109.041087 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tian E et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349, 2483–2494, doi: 10.1056/NEJMoa030847 (2003). [DOI] [PubMed] [Google Scholar]
- 147.D'Alterio C et al. Concomitant CXCR4 and CXCR7 expression predicts poor prognosis in renal cancer. Curr Cancer Drug Targets 10, 772–781, doi: 10.2174/156800910793605839 (2010). [DOI] [PubMed] [Google Scholar]
- 148.Pan J et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer 5, 56, doi: 10.1186/1476-4598-5-56 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wehler TC et al. Strong expression of chemokine receptor CXCR4 by renal cell carcinoma correlates with advanced disease. J Oncol 2008, 626340, doi: 10.1155/2008/626340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Motzer RJ, Bander NH & Nanus DM Renal-cell carcinoma. N Engl J Med 335, 865–875, doi: 10.1056/NEJM199609193351207 (1996). [DOI] [PubMed] [Google Scholar]
- 151.Chambers A, Kundranda M, Rao S, Mahmoud F & Niu J Anti-angiogenesis Revisited: Combination with Immunotherapy in Solid Tumors. Curr Oncol Rep 23, 100, doi: 10.1007/s11912-021-01099-7 (2021). [DOI] [PubMed] [Google Scholar]
- 152.Geraets SEW, Bos PK & van der Stok J Preoperative embolization in surgical treatment of long bone metastasis: a systematic literature review. EFORT Open Rev 5, 17–25, doi: 10.1302/2058-5241.5.190013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chan DA, Sutphin PD, Yen SE & Giaccia AJ Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol 25, 6415–6426, doi: 10.1128/MCB.25.15.6415-6426.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Blancher C, Moore JW, Robertson N & Harris AL Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3'-kinase/Akt signaling pathway. Cancer Res 61, 7349–7355 (2001). [PubMed] [Google Scholar]
- 155.Wiesener MS et al. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer Res 61, 5215–5222 (2001). [PubMed] [Google Scholar]
- 156.Ivanyi P et al. Does the onset of bone metastasis in sunitinib-treated renal cell carcinoma patients impact the overall survival? World journal of urology, doi: 10.1007/s00345-015-1707-0 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Kalra S et al. Outcomes of Patients With Metastatic Renal Cell Carcinoma and Bone Metastases in the Targeted Therapy Era. Clinical genitourinary cancer 15, 363–370, doi: 10.1016/j.clgc.2017.01.010 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Osanto S & van der Hulle T Cabozantinib in the treatment of advanced renal cell carcinoma in adults following prior vascular endothelial growth factor targeted therapy: clinical trial evidence and experience. Ther Adv Urol 10, 109–123, doi: 10.1177/1756287217748867 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vanharanta S et al. Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat Med 19, 50–56, doi: 10.1038/nm.3029 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Atkinson EG & Delgado-Calle J The Emerging Role of Osteocytes in Cancer in Bone. JBMR Plus 3, e10186, doi: 10.1002/jbm4.10186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.An G et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood 128, 1590–1603, doi: 10.1182/blood-2016-03-707547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maroni P & Bendinelli P Bone, a Secondary Growth Site of Breast and Prostate Carcinomas: Role of Osteocytes. Cancers (Basel) 12, doi: 10.3390/cancers12071812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou M et al. KLF10 inhibits cell growth by regulating PTTG1 in multiple myeloma under the regulation of microRNA-106b-5p. Int J Biol Sci 16, 2063–2071, doi: 10.7150/ijbs.45999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Delgado-Calle J et al. Bidirectional Notch Signaling and Osteocyte-Derived Factors in the Bone Marrow Microenvironment Promote Tumor Cell Proliferation and Bone Destruction in Multiple Myeloma. Cancer Res 76, 1089–1100, doi: 10.1158/0008-5472.CAN-15-1703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Terpos E et al. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol 23, 2681–2686, doi: 10.1093/annonc/mds068 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Roussou M et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 23, 2177–2181, doi: 10.1038/leu.2009.130 (2009). [DOI] [PubMed] [Google Scholar]
- 167.Costa F et al. Expression of CD38 in myeloma bone niche: A rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget 8, 56598–56611, doi: 10.18632/oncotarget.17896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chung C Role of Immunotherapy in Targeting the Bone Marrow Microenvironment in Multiple Myeloma: An Evolving Therapeutic Strategy. Pharmacotherapy 37, 129–143, doi: 10.1002/phar.1871 (2017). [DOI] [PubMed] [Google Scholar]
- 169.Favaloro J et al. Myeloma skews regulatory T and pro-inflammatory T helper 17 cell balance in favor of a suppressive state. Leuk Lymphoma 55, 1090–1098, doi: 10.3109/10428194.2013.825905 (2014). [DOI] [PubMed] [Google Scholar]
- 170.Pratt G, Goodyear O & Moss P Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 138, 563–579, doi: 10.1111/j.1365-2141.2007.06705.x (2007). [DOI] [PubMed] [Google Scholar]
- 171.Gagelmann N et al. Development of CAR-T cell therapies for multiple myeloma. Leukemia 34, 2317–2332, doi: 10.1038/s41375-020-0930-x (2020). [DOI] [PubMed] [Google Scholar]
- 172.Mikhael J et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol 37, 1228–1263, doi: 10.1200/JCO.18.02096 (2019). [DOI] [PubMed] [Google Scholar]
- 173.D'Oronzo S, Coleman R, Brown J & Silvestris F Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J Bone Oncol 15, 004–004, doi: 10.1016/j.jbo.2018.10.004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Henry DH et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29, 1125–1132, doi: 10.1200/JCO.2010.31.3304 (2011). [DOI] [PubMed] [Google Scholar]
- 175.Lipton A et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur J Cancer 53, 75–83, doi: 10.1016/j.ejca.2015.09.011 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Scagliotti GV et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 7, 1823–1829, doi: 10.1097/JTO.0b013e31826aec2b (2012). [DOI] [PubMed] [Google Scholar]
- 177.Body JJ et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 111, 306–312, doi: 10.1016/j.pain.2004.07.011 (2004). [DOI] [PubMed] [Google Scholar]
- 178.De Marinis F et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 4, 1280–1288, doi: 10.1097/JTO.0b013e3181b68e5a (2009). [DOI] [PubMed] [Google Scholar]
- 179.Dearnaley DP, Mason MD, Parmar MK, Sanders K & Sydes MR Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. The Lancet. Oncology 10, 872–876, doi: 10.1016/S1470-2045(09)70201-3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McKay RR et al. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol 66, 502–509, doi: 10.1016/j.eururo.2014.02.040 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cronin KA, Ries LA & Edwards BK The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 120 Suppl 23, 3755–3757, doi: 10.1002/cncr.29049 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Noone AM et al. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 26, 632–641, doi: 10.1158/1055-9965.EPI-16-0520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Woodward E et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 48, 160–166, doi: 10.1016/j.bone.2010.09.008 (2011). [DOI] [PubMed] [Google Scholar]
- 184.Kinnane N Burden of bone disease. Eur J Oncol Nurs 11 Suppl 2, S28–31, doi: 10.1016/j.ejon.2007.07.002 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Mackiewicz-Wysocka M, Pankowska M & Wysocki PJ Progress in the treatment of bone metastases in cancer patients. Expert Opin Investig Drugs 21, 785–795, doi: 10.1517/13543784.2012.679928 (2012). [DOI] [PubMed] [Google Scholar]
- 186.Sato M et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88, 2095–2105, doi: 10.1172/JCI115539 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hughes DE et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10, 1478–1487, doi: 10.1002/jbmr.5650101008 (1995). [DOI] [PubMed] [Google Scholar]
- 188.Lacey DL et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov 11, 401–419, doi: 10.1038/nrd3705 (2012). [DOI] [PubMed] [Google Scholar]
- 189.Ghajar CM Metastasis prevention by targeting the dormant niche. Nat Rev Cancer 15, 238–247, doi: 10.1038/nrc3910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Risson E, Nobre AR, Maguer-Satta V & Aguirre-Ghiso JA The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer 1, 672–680, doi: 10.1038/s43018-020-0088-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barbier V et al. Endothelial E-selectin inhibition improves acute myeloid leukaemia therapy by disrupting vascular niche-mediated chemoresistance. Nat Commun 11, 2042, doi: 10.1038/s41467-020-15817-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang H et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat Commun 8, 15045, doi: 10.1038/ncomms15045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Denefle T et al. Thrombospondin-1 Mimetic Agonist Peptides Induce Selective Death in Tumor Cells: Design, Synthesis, and Structure-Activity Relationship Studies. J Med Chem 59, 8412–8421, doi: 10.1021/acs.jmedchem.6b00781 (2016). [DOI] [PubMed] [Google Scholar]
- 194.Allen MR & Burr DB Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know. Bone 49, 56–65, doi: 10.1016/j.bone.2010.10.159 (2011). [DOI] [PubMed] [Google Scholar]
- 195.Garnero P, Sornay-Rendu E, Chapuy MC & Delmas PD Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11, 337–349, doi: 10.1002/jbmr.5650110307 (1996). [DOI] [PubMed] [Google Scholar]
- 196.Chen Q & Massague J Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin Cancer Res 18, 5520–5525, doi: 10.1158/1078-0432.CCR-11-2904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nakazawa Y et al. Periostin blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci Rep 8, 4013, doi: 10.1038/s41598-018-22340-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Estey E et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol 12, 671–678, doi: 10.1200/JCO.1994.12.4.671 (1994). [DOI] [PubMed] [Google Scholar]
- 199.Fischer JC et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci U S A 110, 16580–16585, doi: 10.1073/pnas.1313594110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Boyerinas B et al. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121, 4821–4831, doi: 10.1182/blood-2012-12-475483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Becker PS et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br J Haematol 155, 182–189, doi: 10.1111/j.1365-2141.2011.08831.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Soriano P, Montgomery C, Geske R & Bradley A Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702, doi: 10.1016/0092-8674(91)90499-o (1991). [DOI] [PubMed] [Google Scholar]
- 203.Furuyama N & Fujisawa Y Regulation of collagenolytic protease secretion through c-Src in osteoclasts. Biochem Biophys Res Commun 272, 116–124, doi: 10.1006/bbrc.2000.2698 (2000). [DOI] [PubMed] [Google Scholar]
- 204.Murali B et al. Inhibition of the Stromal p38MAPK/MK2 Pathway Limits Breast Cancer Metastases and Chemotherapy-Induced Bone Loss. Cancer Res 78, 5618–5630, doi: 10.1158/0008-5472.CAN-18-0234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nyman JS et al. Combined treatment with a transforming growth factor beta inhibitor (1D11) and bortezomib improves bone architecture in a mouse model of myeloma-induced bone disease. Bone 91, 81–91, doi: 10.1016/j.bone.2016.07.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cole LE, Vargo-Gogola T & Roeder RK Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv Drug Deliv Rev 99, 12–27, doi: 10.1016/j.addr.2015.10.005 (2016). [DOI] [PubMed] [Google Scholar]
- 207.Farrell KB, Karpeisky A, Thamm DH & Zinnen S Bisphosphonate conjugation for bone specific drug targeting. Bone Rep 9, 47–60, doi: 10.1016/j.bonr.2018.06.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tian Z et al. Harnessing the power of antibodies to fight bone metastasis. Sci Adv 7, doi: 10.1126/sciadv.abf2051 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schmid P et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. New England Journal of Medicine 379, 2108–2121, doi: 10.1056/NEJMoa1809615 (2018). [DOI] [PubMed] [Google Scholar]
- 210.Beer TM et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 35, 40–47, doi: 10.1200/JCO.2016.69.1584 (2017). [DOI] [PubMed] [Google Scholar]
- 211.Jiao S et al. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 179, 1177–1190 e1113, doi: 10.1016/j.cell.2019.10.029 (2019). [DOI] [PubMed] [Google Scholar]
- 212.Italiano A et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19, 649–659, doi: 10.1016/S1470-2045(18)30145-1 (2018). [DOI] [PubMed] [Google Scholar]
- 213.Yoneda T, Sasaki A & Mundy GR Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat 32, 73–84, doi: 10.1007/BF00666208 (1994). [DOI] [PubMed] [Google Scholar]
- 214.Corey E et al. Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. Prostate 52, 20–33, doi: 10.1002/pros.10091 (2002). [DOI] [PubMed] [Google Scholar]
- 215.Yu C et al. Intra-iliac Artery Injection for Efficient and Selective Modeling of Microscopic Bone Metastasis. J Vis Exp, doi: 10.3791/53982 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kuchimaru T et al. A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries. Nat Commun 9, 2981, doi: 10.1038/s41467-018-05366-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Medina D The mammary gland: a unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia 1, 5–19, doi: 10.1007/BF02096299 (1996). [DOI] [PubMed] [Google Scholar]
- 218.Sflomos G et al. A Preclinical Model for ERalpha-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell 29, 407–422, doi: 10.1016/j.ccell.2016.02.002 (2016). [DOI] [PubMed] [Google Scholar]
- 219.Sato N et al. A metastatic and androgen-sensitive human prostate cancer model using intraprostatic inoculation of LNCaP cells in SCID mice. Cancer Res 57, 1584–1589 (1997). [PubMed] [Google Scholar]
- 220.Valta MP et al. Development of a realistic in vivo bone metastasis model of human renal cell carcinoma. Clin Exp Metastasis 31, 573–584, doi: 10.1007/s10585-014-9651-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kashtan H et al. Intra-rectal injection of tumour cells: a novel animal model of rectal cancer. Surg Oncol 1, 251–256, doi: 10.1016/0960-7404(92)90072-s (1992). [DOI] [PubMed] [Google Scholar]
- 222.Sakamoto S et al. New metastatic model of human small-cell lung cancer by orthotopic transplantation in mice. Cancer Sci 106, 367–374, doi: 10.1111/cas.12624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Martin CK et al. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Res 70, 8607–8616, doi: 10.1158/0008-5472.CAN-10-0850 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pinho S & Frenette PS Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 20, 303–320, doi: 10.1038/s41580-019-0103-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Crane GM, Jeffery E & Morrison SJ Adult haematopoietic stem cell niches. Nat Rev Immunol 17, 573–590, doi: 10.1038/nri.2017.53 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Mendez-Ferrer S et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer 20, 285–298, doi: 10.1038/s41568-020-0245-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sivaraj KK & Adams RH Blood vessel formation and function in bone. Development 143, 2706–2715, doi: 10.1242/dev.136861 (2016). [DOI] [PubMed] [Google Scholar]
- 228.Kusumbe AP, Ramasamy SK & Adams RH Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328, doi: 10.1038/nature13145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park D et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272, doi: 10.1016/j.stem.2012.02.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Logan M et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80, doi: 10.1002/gene.10092 (2002). [DOI] [PubMed] [Google Scholar]
- 231.Greenbaum A et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230, doi: 10.1038/nature11926 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pinho S et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 210, 1351–1367, doi: 10.1084/jem.20122252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sugiyama T, Kohara H, Noda M & Nagasawa T Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988, doi: 10.1016/j.immuni.2006.10.016 (2006). [DOI] [PubMed] [Google Scholar]
- 234.Baccin C et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol 22, 38–48, doi: 10.1038/s41556-019-0439-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Acar M et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130, doi: 10.1038/nature15250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bruns I et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20, 1315–1320, doi: 10.1038/nm.3707 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Heazlewood SY et al. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res 11, 782–792, doi: 10.1016/j.scr.2013.05.007 (2013). [DOI] [PubMed] [Google Scholar]
- 238.Olson TS et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249, doi: 10.1182/blood-2012-10-463414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhao M et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20, 1321–1326, doi: 10.1038/nm.3706 (2014). [DOI] [PubMed] [Google Scholar]
- 240.Quarta G, Cattaneo C & D'Elia M Determining 14C Content in Different Human Tissues: Implications for Application of 14C Bomb-Spike Dating in Forensic Medicine. Radiocarbon 55, 1845–1849, doi: 10.2458/azu_js_rc.55.16205 (2013). [DOI] [Google Scholar]
- 241.Rodan GA Bone homeostasis. Proceedings of the National Academy of Sciences of the United States of America, doi: 10.1073/pnas.95.23.13361 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ribot C et al. Obesity and postmenopausal bone loss: the influence of obesity on vertebral density and bone turnover in postmenopausal women. Bone 8, 327–331, doi: 10.1016/8756-3282(87)90062-7 (1987). [DOI] [PubMed] [Google Scholar]
- 243.Compston JE et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 124, 1043–1050, doi: 10.1016/j.amjmed.2011.06.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Krakauer JC et al. Bone loss and bone turnover in diabetes. Diabetes 44, 775–782, doi: 10.2337/diab.44.7.775 (1995). [DOI] [PubMed] [Google Scholar]
- 245.Einhorn TA & Gerstenfeld LC Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 11, 45–54, doi: 10.1038/nrrheum.2014.164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schell H et al. The haematoma and its role in bone healing. J Exp Orthop 4, 5, doi: 10.1186/s40634-017-0079-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bidwell BN et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med 18, 1224–1231, doi: 10.1038/nm.2830 (2012). [DOI] [PubMed] [Google Scholar]
- 248.Dobrolecki LE et al. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev 35, 547–573, doi: 10.1007/s10555-016-9653-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hoffman RM Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 15, 451–452, doi: 10.1038/nrc3972 (2015). [DOI] [PubMed] [Google Scholar]
- 250.Wang D et al. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 14, 893–903, doi: 10.1359/jbmr.1999.14.6.893 (1999). [DOI] [PubMed] [Google Scholar]
- 251.Qiao H & Tang T Engineering 3D approaches to model the dynamic microenvironments of cancer bone metastasis. Bone Res 6, 3, doi: 10.1038/s41413-018-0008-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]