Abstract
Background and aims
Observational studies have linked elevated blood pressure (BP) to impaired cognitive function. However, the functional and structural changes in the brain that mediate the relationship between BP elevation and cognitive impairment remain unknown. Using observational and genetic data from large consortia, this study aimed to identify brain structures potentially associated with BP values and cognitive function.
Methods and results
Data on BP were integrated with 3935 brain magnetic resonance imaging-derived phenotypes (IDPs) and cognitive function defined by fluid intelligence score. Observational analyses were performed in the UK Biobank and a prospective validation cohort. Mendelian randomisation (MR) analyses used genetic data derived from the UK Biobank, International Consortium for Blood Pressure, and COGENT consortium. Mendelian randomisation analysis identified a potentially adverse causal effect of higher systolic BP on cognitive function [−0.044 standard deviation (SD); 95% confidence interval (CI) −0.066, −0.021] with the MR estimate strengthening (−0.087 SD; 95% CI −0.132, −0.042), when further adjusted for diastolic BP. Mendelian randomisation analysis found 242, 168, and 68 IDPs showing significant (false discovery rate P < 0.05) association with systolic BP, diastolic BP, and pulse pressure, respectively. Most of these IDPs were inversely associated with cognitive function in observational analysis in the UK Biobank and showed concordant effects in the validation cohort. Mendelian randomisation analysis identified relationships between cognitive function and the nine of the systolic BP-associated IDPs, including the anterior thalamic radiation, anterior corona radiata, or external capsule.
Conclusion
Complementary MR and observational analyses identify brain structures associated with BP, which may be responsible for the adverse effects of hypertension on cognitive performance.
Keywords: Hypertension, Cognitive function, Blood pressure, Mendelian randomisation, Brain, Genome-wide association study
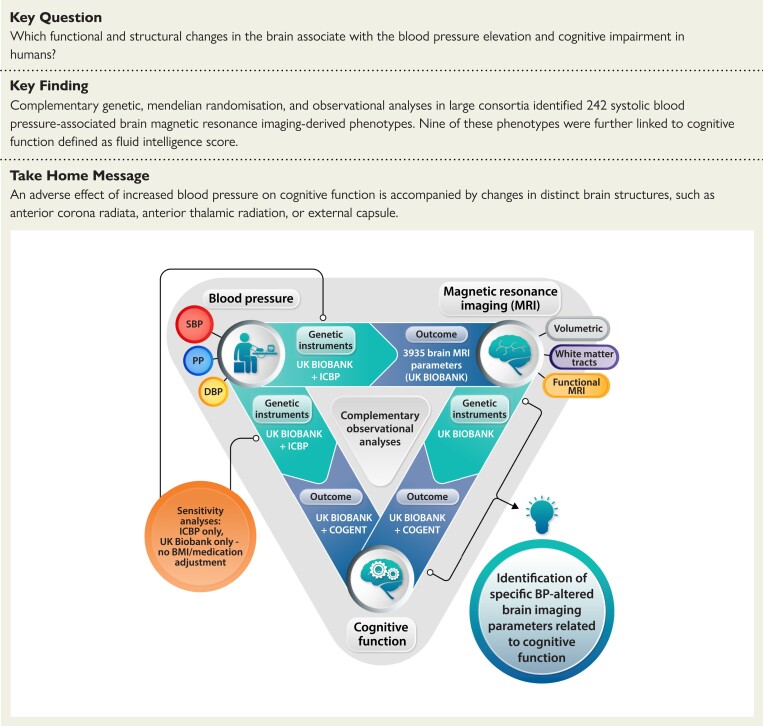
Structured Graphical Abstract
Structured Graphical Abstract.
BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; ICPB, International Consortium for Blood Pressure; PP, pulse pressure; SBP, systolic blood pressure.
See the editorial comment for this article ‘Hypertension, brain imaging phenotypes, and cognitive impairment: lessons from Mendelian randomization’, by E.L. Schiffrin and J.C. Engert, https://doi.org/10.1093/eurheartj/ehad187.
Translational perspective.
Three complementary approaches were used to identify brain structures associated with increased blood pressure (BP) that may potentially mediate BP-induced cognitive impairment. While clinical trials targeting specific brain structures may be challenging, focused imaging of these structures in future preclinical and clinical studies may, in due course, support a more personalised approach to hypertensive patients in relation to cognitive impairment. It may be possible that identification of imaging-derived phenotypes associated with hypertension and cognitive impairment can guide the choice of imaging surrogate biomarkers in future clinical studies of antihypertensive therapies. Finally, focused molecular characterisation, including RNA sequencing and tissue proteomics, of these brain structures may unravel altered pathways in hypertension and associated cognitive impairment.
Introduction
Elevated blood pressure (BP) is a major modifiable risk factor for mortality worldwide and leads to cerebrovascular diseases and dementia.1 Observational and prospective studies, as well as randomised clinical trials of antihypertensive therapies, have demonstrated that hypertension may be causally associated with impaired cognitive performance.2–7 At the same time, increased BP causes long-term changes in the brain.8–10 While the associations between BP and cognition may be mediated by alterations within cerebral structures and traits,8,10–15 the causal nature and exact mechanisms of these associations have been challenging to ascertain. Therefore, it is of primary clinical importance to identify specific brain areas mediating BP-dependent cognitive changes. Emerging genetic tools may enable this, as cognitive function is heritable; shares a common genetic variation with various brain imaging-derived phenotypes (IDPs), including parameters describing cortical and subcortical regions; and may be affected by early life factors.16–20 While randomised trials remain the state-of-the-art approach to infer causality between various clinical traits,21,22 causal inference approaches employing genetic instrumental variables through Mendelian randomisation (MR) sit at the interface of experimental and observational studies.21 At the same time, the increasing number of well-powered genome-wide association studies (GWAS) facilitates the identification of genetic proxies for complex phenotypes, such as BP and cognitive function, that can be used for MR analysis as both exposures and outcomes. More importantly, these may provide unique tools to indicate specific structures and functional phenotypes of the brain that can mediate this relationship.17,23,24 Identifying such IDPs may define novel radiomic predictors of cognitive impairment in hypertensive subjects. It will also specify candidate brain regions for subsequent molecular and cellular therapeutic targeting.
Accordingly, we designed a triangulation approach to identify brain structures potentially responsible for the effects of BP on cognitive function. Firstly, we comprehensively mapped brain structures potentially influenced by BP in midlife using MR analysis. Secondly, we elucidated the effects of BP on cognitive function in well-powered datasets. Finally, using MR analysis, we identified structures in the brain that accompany the detrimental effects of elevated BP on cognitive function in humans. The pattern of brain damage linked to BP by genetic causal inference methods was then validated in an independent, well-phenotyped, prospectively recruited cohort.
Methods
Univariable and multivariable Mendelian randomisation analyses testing the effect of BP indices on cognitive function and brain imaging-derived phenotypes
Uncorrelated (r2 < 0.2), instrumental variables associated with BP traits were selected from 885 single nucleotide polymorphisms (SNPs, Supplementary data online, Table S1) replicated in one-stage or two-stage analyses in GWAS on BP indices by Evangelou and colleagues,23 similar to previous studies.25,26 Estimates of effects of the above SNPs on BP were extracted from the meta-analysis of the UK Biobank and International Consortium for Blood Pressure (ICBP) [n = 757 601, systolic BP (SBP), diastolic BP (DBP), and pulse pressure (PP) analyses adjusted for body mass index (BMI) and antihypertensive medication use in both the UK Biobank and ICBP].23 Genome-wide significant (P < 5 × 10−8) SNPs were extracted, and palindromic SNPs with minor allele frequency (MAF) > 0.4 or SNPs with MAF <0.05 were excluded (see Supplementary data online, Figure S1A).
Summary statistics for each of the 3935 brain IDPs were derived from the GWAS of Smith and colleagues, including ∼33 000 UK Biobank participants,24 and used as an outcome in MR analyses. Brain IDPs were named according to the original nomenclature.24 In order to avoid use of reverse-causal instruments, all SNPs with a significantly larger variation explained in the outcome compared with exposure trait (nominal P < 0.05 in Steiger’s test27) were excluded from this analysis. Since brain IDPs strongly correlate with each other,9 we used a false discovery rate (FDR) P < 0.05 threshold for inverse variance–weighted (IVW) method using instrumental variables from the ICBP + UK Biobank meta-analysis to identify IDPs significantly affected by BP. These IDPs had to additionally demonstrate a nominally significant (P < 0.05) association with BP when (i) using the weighted median, MR–Egger and robust IVW sensitivity methods, which allowed relaxing certain assumptions, such as lack of genetic pleiotropy,21 of the MR framework, and (ii) instrumental variables from separate ICBP and UK Biobank cohorts were used in IVW MR analysis.
Genome-wide association study performed by Lee and colleagues, encompassing 257 841subjects from the UK Biobank and COGENT consortia,17 was used as a source of genetic estimates of the effect of selected SNPs on cognitive function. Mendelian randomisation analyses of the impact of BP indices on cognitive function included SNPs with a more significant effect on the exposure compared with outcome (nominal P < 0.05 in Steiger’s test27). Univariable and multivariable (i.e. testing effects of SBP, while adjusting for DBP and vice versa) two-sample MR analyses were performed using IVW method as well as sensitivity methods including weighted median, MR–Egger, and robust IVW methods.
Additional sensitivity analyses used the genetic estimates extracted from individual cohorts, i.e. ICBP (n = 299 024; SBP, DBP, and PP adjusted for BMI and antihypertensive medication use)28 and UK Biobank [n = 340 159; only SBP and DBP from the Neale lab analysis (http://www.nealelab.is/uk-biobank/) were used without adjusting for BMI; BP values were not adjusted for antihypertensive medication use]. These analyses are essential to acknowledge the possible impact of collider effects potentially caused by BMI and antihypertensive medication adjustments, both lacking in the UK Biobank–only BP GWAS, as well as moderate subject overlap (lacking in the ICBP-only MR analysis) in the primary MR analysis involving the ICBP + UK Biobank dataset. Since sensitivity analyses were less powered due to three to five times lower number of instrumental variables (see Supplementary data online, Figure S1A), we used a nominal significance level (P < 0.05 for IVW method) to retain IDPs affected by BP genetically defined by ICBP- and UK Biobank–only instruments.
(See Supplementary data online, Methods, for additional details on MR and rationale for control MR analyses concerning APOE locus, LDL-C level, and cognitive function.)
Mendelian randomisation analyses testing the effect of selected brain imaging-derived phenotypes on cognitive function
To test the effect of selected IDPs on cognitive function, we used uncorrelated (r2 < 0.2) SNPs associated with individual brain IDPs (significance threshold P < 3.16 × 10−8 set as previously described in a discovery UK Biobank sample of ∼22 000 subjects24) and replicated at the nominal level of significance P < 0.05 in an independent sample of ∼11 000 UK Biobank participants.24 The meta-analysis29 of both the above samples yielded genetic estimates used for subsequent MR analyses. In case SNPs were missing in the cognitive function GWAS, the best proxy (r2 > 0.8) was selected. All SNPs, selected as instrumental variables, were associated with selected brain IDPs at P < 3.16 × 10−8 (F-statistic > 10) and displayed a significantly larger effect on exposure compared with outcome (nominal P < 0.05 in Steiger’s test27).
UK Biobank study
The UK Biobank recruited 500 000 participants (aged 40–69 years) from 22 assessment centres across the UK between 2006 and 2010.30 Baseline biological measurements were recorded, and touchscreen questionnaires were administered, as described elsewhere.30 The UK Biobank received ethical approval from the North-West Multi-Center Research Ethics Committee (11/NW/03 820). All participants gave written informed consent before enrolment in the study, which was conducted by the principles of the Declaration of Helsinki. Blood pressure measurement was performed twice for each participant present at baseline (2006–10) and imaging (2014–19) visits using a digital sphygmomanometer Omron 705 IT or a sphygmomanometer with an inflatable cuff in conjunction with a stethoscope (see https://biobank.ctsu.ox.ac.uk/showcase/showcase/docs/Bloodpressure.pdf for details). Brain imaging was performed at the imaging visit using Siemens Skyra 3 T scanner according to a protocol (https://www.fmrib.ox.ac.uk/ukbiobank/protocol/ and https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/brain_mri.pdf) described previously in details.31,32 Cognitive function was defined as a fluid intelligence score (UK Biobank field ID: 20016), i.e. an unweighted sum of the number of correct answers given to the 13 fluid intelligence questions, and assessed at baseline, imaging, and first repeat imaging visits. Of note, this variable demonstrates a significant, negative genetic correlation with SBP and DBP (r = −0.14, P = 3.42 × 10−10/r = −0.16, P = 2.68 × 10−14, respectively) in the UK Biobank (Neale lab calculations available at https://ukbb-rg.hail.is/rg_browser/), which may suggest shared genetic background of these traits. Diagnosis of atrial fibrillation prior to attending the baseline visit was assessed using the UK Biobank field ID 131350.
Baseline data from a prospective cohort and case–control study
Hypertensive and normotensive subjects were prospectively recruited between November 2014 and December 2020 in the Heart and Brain research programme at IRCCS Neuromed (NCT03986957) and as part of a case–control study (NCT02310217). Baseline visit data from both studies were analysed. (See Supplementary data online, Methods, for details concerning recruitment, BP measurement, cognitive function assessment, and brain magnetic resonance imaging in this cohort).
Testing the observational association between BP indices, cognitive performance, and brain imaging-derived phenotypes
Since GWAS on BP performed by the UK Biobank and ICBP included subjects of European ancestry,23 based on self-reported ethnicity, we selected white, British participants of the UK Biobank study to assess the association between BP, cognitive function, and brain IDPs. An average of two automated, sitting BP readings, with no adjustment for BP-lowering medication use, was calculated at the baseline and imaging visits and used in the final analyses, while subjects with only one reading available were excluded. We did not exclude any UK Biobank subjects based on their disease history. Association between BP traits and cognitive function was tested at the baseline visit in the UK Biobank (2006–10, n = 138 602) using univariate general linear model (GLM). Associations between selected brain IDPs and BP indices or cognitive function were tested using data from the imaging visit (2014–19) and the multivariate GLM model. All GLM models included sex, age, and BMI set as covariates. The longitudinal analysis tested the effect of IDPs, assessed at the imaging visit, on the difference between the fluid intelligence score assessed at first repeat imaging (2019+) and imaging visits divided by the number of days between those visits. The effects of smoking status and alcohol intake frequency were investigated in separate models. Brain volumetric IDPs were scaled for the T1-based head size scaling factor. All brain IDPs were de-confounded by four technical imaging confounders related to scanner position. In order to obtain effect estimates in standard deviation units and approximate normal distribution, dependent and independent variables were transformed to normal scores. Similar analyses were performed in the observational prospective cohort study. Observational mediation analysis was performed using the R mediation package (ver. 4.5)33 with BMI, sex, and age used as covariates.
Software
Observational analyses were performed using SPSS (ver. 27.0). Mendelian randomisation analyses were performed in R (ver 4.0.3) utilising MendelianRandomization34,35 and TwoSampleMR packages.36 Figures were generated using ClustVis,37 R (ver 4.0.3), FSL,38 FreeSurfer,39 and GraphPad Prism (ver. 7.05).
Results
Effects of blood pressure on 3935 brain imaging-derived phenotypes
Mendelian randomisation utilises random segregation of alleles at gametogenesis and uses genetic polymorphisms as instrumental variables to estimate the effect of exposure on the outcome. Since BP highly correlates with brain IDPs in observational analyses,9 we investigated relationships between BP and brain IDPs by performing MR analysis, testing the effects of SBP, DBP, and PP on each of the 3935 brain IDPs (see Supplementary data online, Tables S2–S4). A total of 242, 168, and 68 brain IDPs, including primarily diffusion-weighted IDPs, volumetric, as well as resting-state functional IDPs, was significantly associated with SBP, DBP, and PP, respectively (see Supplementary data online, Tables S2–S4). Importantly, all the above associations remained significant at nominal P < 0.05 for any of the leave-one-out IVW MR analyses performed. SBP is significantly associated with the highest number of brain IDPs, compared with other BP indices, with a positive effect on the volume of specific brain structures and the mean diffusivity in diffusion-weighted IDPs and a negative effect on fractional anisotropy–related IDPs (see Supplementary data online, Table S2). Sensitivity analyses, performed separately in either ICBP or UK Biobank population, demonstrated a high correlation between MR estimates derived from various exposure BP GWAS (see Supplementary data online, Tables S2–S4, and Supplementary data online, Figure S2A–F, for comparison of IVW MR estimates calculated using genetic exposures derived separately from the UK Biobank and ICBP datasets). Moreover, IVW causal estimates obtained for 242, 168, and 68 brain IDPs associated with SBP, DBP, and PP, respectively, were slightly inflated for ICBP cohort–only analysis compared with meta-analysis of ICBP and UK Biobank (on average by a factor of 1.47, 1.26, and 1.53, respectively) or UK Biobank–only analysis [1.35 (SBP) and 1.24 (DBP)]. Causal estimates were less inflated in the UK Biobank–only analysis compared with the meta-analysis of the ICBP and UK Biobank [1.09 (SBP) and 1.02 (DBP)].
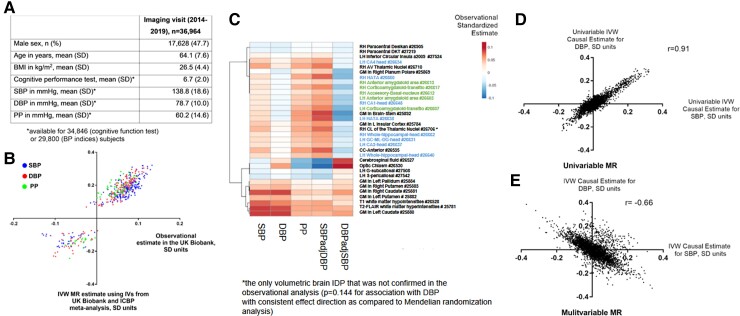
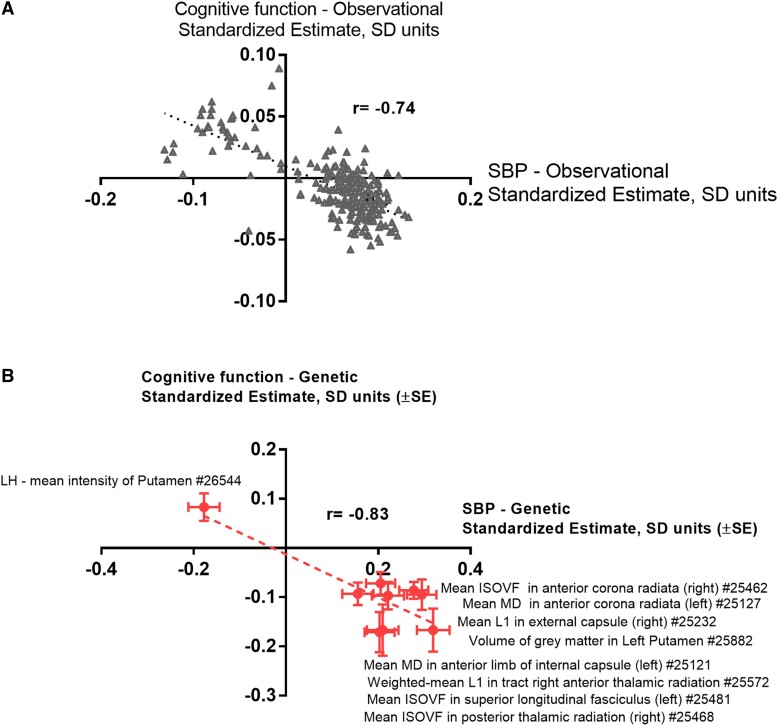
Most of the associations identified using genetic tools were consistent with the results from the observational analysis at the imaging visit in the UK Biobank (Figures 1A and B), including 33 volumetric IDPs associated with one of the BP parameters analysed (Figure 1C), as 95%/91%/82% of genetically SBP/DBP/PP–linked brain IDPs associated with SBP/DBP/PP in the observational analysis below Bonferroni-defined significance threshold (P < 0.05/3935, Supplementary data online, Tables S2–S4, S6-S8). Further adjustment for smoking status and alcohol intake frequency did not substantially influence observational analyses. As expected, estimates from the univariable MR analyses testing IDPs in relation to SBP or DBP were positively correlated (Figure 1D).
Figure 1.
Observational and genetic effects of BP on brain IDPs. Characteristics table of UK Biobank subjects with BP and/or cognitive function test available at the imaging visit (A). Comparison of Mendelian randomisation (using IVW method) and observational estimates concerning 242, 168, and 68 brain IDPs significantly affected by genetically instrumented SBP, DBP, and PP, respectively (B). Heatmap (C) presents observational effects of BP indices on all 33 brain volumetric IDPs that were significantly affected by either BP index (IDPs depicted in blue are related to the hippocampus, while IDPs depicted in green are related to the amygdala nuclei; GM, grey matter; LH/RH, left/right hemisphere; UK Biobank field IDs are depicted on the right). Scatter plots present genetic causal estimates corresponding to the effect of SBP and DBP on all 3935 brain IDPs considered in univariable (D) and multivariable (E) Mendelian randomisation analyses. IVW, inverse variance–weighted; SD, standard deviation; SBP/DBP, systolic/diastolic blood pressure; PP, pulse pressure; MR, Mendelian randomisation; IDP, imaging-derived phenotype.
Multivariable MR identified much lower number of IDPs compared with univariable MR and demonstrated that SBP and DBP associated with 18 and 33 IDPs, respectively (FDR P < 0.05 for multivariable IVW and nominal P < 0.05 for multivariable weighted median and multivariable MR–Egger using instrumental variables from the ICBP + UK Biobank meta-analysis). These effects were independent of BMI/antihypertensive medication use adjustment and moderate subject overlap in exposure and outcome GWAS as they were also confirmed (nominal P < 0.05 for multivariable IVW method) in the separate ICBP or UK Biobank analysis (see Supplementary data online, Tables S5 and S9). Surprisingly, the estimates derived from the multivariable MR analysis demonstrated their negative correlation, which suggests opposite, direct effects of SBP and DBP on some of the IDPs tested (Figure 1E); however, only 13 IDPs were significantly associated with both SBP and DBP with opposite direction of causal estimate (see Supplementary data online, Table S5). The above change of correlation pattern was observed for analyses restricted to the UK Biobank or ICBP only (see Supplementary data online, Figure S3A–D). Overall, 336 IDPs associated with either of the BP parameters, analysed in univariable or multivariable MR, were identified (see Supplementary data online, Figure S1B). These were mostly consistent with the findings from observational analysis (Figure 1B and Supplementary data online, Tables S6–S9).
Differential effects of blood pressure parameters on cognitive performance
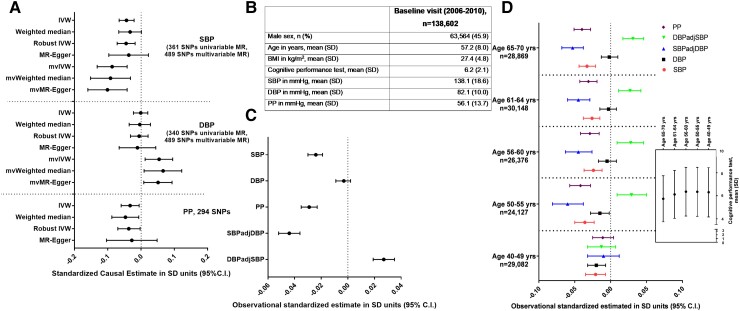
In the next stage of analysis, we aimed to place the effects of BP on IDPs in the context of the effects of individual BP parameters on cognitive function. Interestingly, MR analysis, using SNPs derived from the UK Biobank and ICBP meta-analysis as instrumental variables for BP, identified a negative effect of SBP (−0.044 standard deviation (SD); 95% confidence interval (CI) −0.066, −0.021) and PP, but not of DBP, on cognitive function using the IVW method with consistent effect estimates across sensitivity methods, i.e. weighted median, robust IVW, and MR–Egger (Figure 2A). Importantly, leave-one-out IVW analysis found that this association was not artificially inflated by any of the individual SNPs. Additional analyses performed separately in the ICBP or UK Biobank cohort, aimed to control for potential bias caused by a subject overlap in exposure and outcome GWAS and by BMI/antihypertensive medication use adjustment, confirmed the magnitude and the direction of causal estimates (see Supplementary data online, Figure S4A and B).
Figure 2.
Observational and genetic association of BP with cognitive function. Univariable and multivariable Mendelian randomisation analysis testing the total and direct effects of BP indices on cognitive function, respectively (A). Standardised causal estimates were calculated assuming SD of 18.58, 10.08, and 13.62 mmHg for systolic BP (SBP), diastolic BP (DBP), and pulse pressure (PP), respectively. Instrumental variables for Mendelian randomisation analysis were derived from the meta-analysis of the UK Biobank and ICBP consortium.23 At the same time, a genome-wide association study by Lee and colleagues was used as a source of genetic summary statistics concerning cognitive function.17 Observational association between various BP indices and cognitive function was tested at baseline visit in the UK Biobank (B) in the overall cohort (C) and according to quintiles of age (D). IVW, inverse variance–weighted; SD, standard deviation; SBP/DBP, systolic/diastolic blood pressure; PP, pulse pressure; MR, Mendelian randomisation; mv, multivariable.
Interestingly, a multivariable MR analysis showed that the direct effect of SBP on cognitive function, independent of DBP, was more pronounced than the effect obtained from the univariable MR analysis (−0.087 SD; 95% CI −0.132, −0.042, Figure 2A). At the same time, the independent impact of higher DBP on cognitive function was protective, after adjusting for SBP (Figure 2A). Again, the same effect in multivariable MR analysis, compared with univariable MR analysis, was observed when the analysis was performed separately in ICBP or UK Biobank cohort (see Supplementary data online, Figure S4A and B). The above findings were supported by observational analysis, which revealed a significant association of SBP and PP, but not DBP with cognitive function in the UK Biobank (Figure 2B and C). Moreover, when the damaging effect of SBP was taken into account in a single GLM model, higher DBP was associated with better cognitive performance (Figure 2C), and these associations remained robust to adjustment for smoking status, alcohol intake frequency, and the diagnosis of atrial fibrillation. We observed significant effects of SBP, particularly in people over 50 years of age, while in younger subjects, SBP and DBP appeared to have adverse effects on cognitive function (Figure 2D).
Additional sensitivity analyses further validated genetically predicted cognitive function,17 used in the current MR analyses. The genetically instrumented level of LDL cholesterol, used as a negative control exposure, showed no significant effect on cognitive function, irrespective of inclusion of SNPs from the APOE locus (see Supplementary data online, Figure S5). On the other hand, the rs429358 variant, constituting major genetic risk factor for Alzheimer’s disease40 and determining APOE ε4 allele, was associated with poorer cognitive function (B = −0.017 SD, SE = 0.004, P = 3 × 10−5). This analysis, used as a positive control, suggests that cognitive function, as defined in the current study, does capture at least part of the spectrum of cognitive domains that can be affected later in life by dementia.
Identification of brain imaging-derived phenotypes that associate with cognitive function and are linked to blood pressure
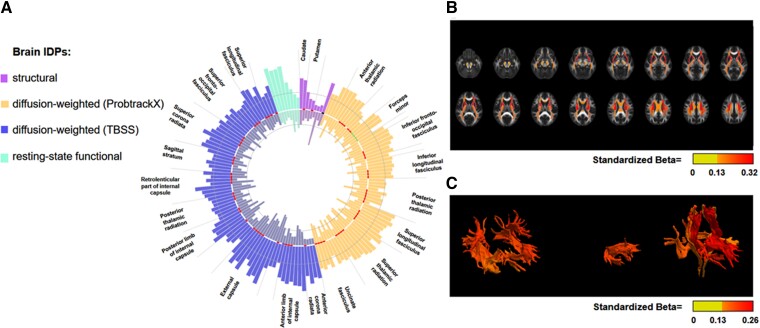
We next investigated the relationship between IDPs and cognitive function, focusing on IDPs that were identified as genetically and observationally linked to BP. An observational analysis confirmed that the majority of IDPs related to BP was associated with cognitive function, at FDR-corrected P < 0.05, and additional adjustment for smoking status and alcohol intake frequency did not substantially influence these results. These IDPs cover predominantly diffusion-weighted parameters with variable strength of association with SBP (Figure 3A–C). Importantly, the direction of estimates was predominantly opposite to the association with a particular BP index (see Figures 3A and 4A for SBP and Supplementary data online, Tables S6, S8, and S9) and remained significant (FDR P < 0.05, Supplementary data online, Table S6) for 138 brain IDPs following additional adjustment for SBP. To provide additional evidence that these IDPs may accompany adverse effects of BP on cognitive function, we estimated the effects of these IDPs on cognitive function by performing MR analyses. To narrow down analysis to the most reliably instrumented candidates, we identified 119, 29, and 7 IDPs, altered by SBP, PP, or SBP/DBP in multivariable MR analysis, with at least 3 uncorrelated instrumental variables available, respectively. This analysis has identified a total of 10 unique IDPs, including 9 related to SBP, that was associated with cognitive function (FDR-corrected P < 0.05 using IVW method; see Supplementary data online, Tables S6, S8 and S9, for all MR results concerning IDPs with at least 1 IV available). These IDPs included parameters related to putamen as well as white matter tracts such as the anterior corona radiata, anterior thalamic radiation, or anterior limb of the internal capsule (Figure 4B). Inspection of leave-one-out IVW plots of the nine SBP-associated IDPs, potentially related to cognitive function, revealed that rs13107325 SNP from the solute carrier family 39 member 8 (SLC39A8) locus had a predominant impact on IVW estimates concerning two putamen IDPs. In comparison, the strength of association of two brain IDPs concerning superior longitudinal fasciculus and anterior limb of internal capsule got attenuated to P = 0.06 after exclusion of single SNP (see Supplementary data online, Figure S6). Notably, except for two IDPs related to the putamen and one related to the anterior thalamic radiation, six remaining IDPs demonstrated association with SBP below the stricter Bonferroni-defined significance threshold using the weighted median MR approach.
Figure 3.
Observational association of brain IDPs with SBP and cognitive function. The circular bar blot presents absolute standardised estimates of the association of 155 imaging-derived phenotypes (IDPs) with SBP (outer bars) and cognitive function (inner bars). Presented IDPs demonstrated significant genetic and observational association with SBP and significant observational association with cognitive function (nominal P < 0.05; 150 IDPs with FDR-adjusted P < 0.05) in the opposite direction compared with the association with SBP. Shaded circles demonstrate a standardised observational estimate of 0.05 (SBP) or 0.025 (cognitive function). Tracts and structures from either hemisphere and appearing more than three times (diffusion-weighted IDPs) or one time (structural IDPs) among 155 IDPs are depicted. (B) Heatmap shows the association’s strength between the cerebral regions identified by diffusion-weighted magnetic resonance imaging with TBSS analyses and the link with SBP. (C) Heatmap shows the association’s strength between the cerebral regions identified by diffusion-weighted magnetic resonance imaging with PROBTRACK analyses and the connection with SBP.
Figure 4.
Relation between observational and genetic estimates linking brain IDPs to SBP or to cognitive function. Observational, standardised coefficients concerning 242 brain IDPs, genetically affected by SBP (FDR P < 0.05 for the IVW method and nominal P < 0.05 for the weighted median, MR–Egger, and robust IVW methods using instrumental variables from the ICBP + UK Biobank meta-analysis and nominal P < 0.05 for IVW method using instrumental variables from the separate ICBP or UK Biobank analysis), corresponding to their association with cognitive function or SBP at the imaging visit in the UK Biobank are depicted on a scatter plot (A). After the exclusion of 38 potential outlier points (Betacognitive function > 0 and BetaSBP < 0), the correlation coefficient was r = −0.37, yet retaining statistical significance (P = 5.6 × 10−8). Mendelian randomisation estimates linking nine IDPs, affecting cognitive function in MR analysis at FDR P < 0.05, to SBP or cognitive function are presented on panel (B) (MD, mean diffusivity; ISOVF, isotropic or free water volume fraction; LH, left hemisphere; UK Biobank field IDs are depicted on the right).
Associations concerning individual IDPs could be confirmed in an observational analysis either directly (IDPs concerning putamen or anterior limb of the internal capsule) or indirectly using different IDPs related to the same brain structure (see Supplementary data online, Tables S6, S8, and S9). Notably, a significant negative correlation exists between genetic estimates of cognitive function, SBP, and the nine IDPs identified (Figure 4B). Additionally, using UK Biobank data, we analysed the effects of 9 brain IDPs (quantified at imaging visit) on the longitudinal change of fluid intelligence score in 2054 subjects between imaging and first repeat imaging visits. We found nominally significant adverse effects of IDPs related to the anterior thalamic radiation (right hemisphere) and anterior corona radiata (left hemisphere) on the change of fluid intelligence score (see Supplementary data online, Figure S7A and B). This further supports their role as potential mediators of detrimental effects of SBP on cognitive function, making these IDPs a potential target for pharmacological therapy of cognitive impairment in hypertension.
Validation in an observational prospective cohort and case–control study
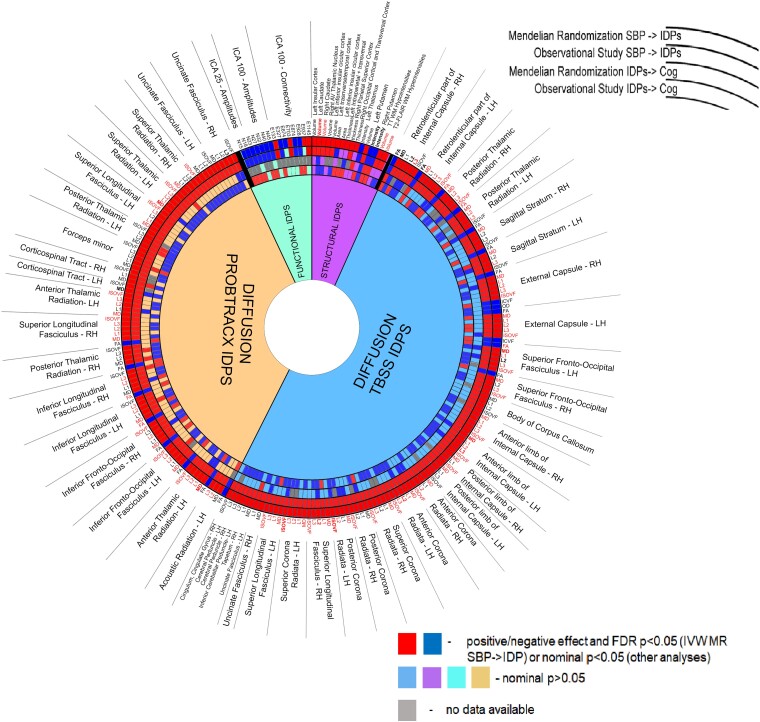
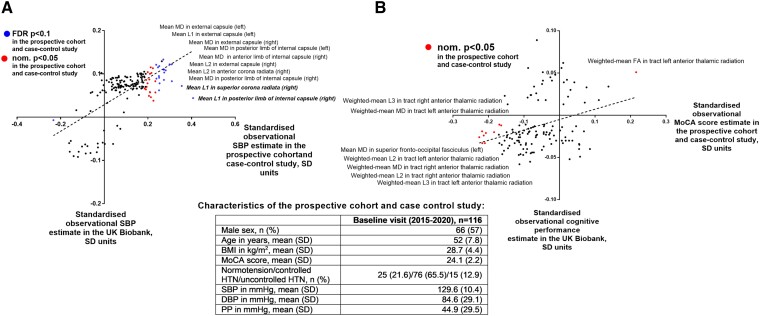
As discussed above, genetic and observational analyses using the UK Biobank and ICBP cohorts identified 242 brain IDPs genetically linked to SBP which were concomitantly associated with cognitive function (Figure 5). To replicate and translate our findings into clinically relevant setting, we tested selected IDPs for association with SBP or Montreal Cognitive Assessment score–defined cognitive function in a cohort of 116 prospectively recruited, well-phenotyped hypertensive and normotensive subjects (see Supplementary data online, Table S10). Since SBP associates with cognitive function and the largest number of IDPs, we focused subsequent validation on SBP-related IDPs. The standardised betas of the association between (i) SBP and IDPs and (ii) IDPs and cognitive function were compared between the UK Biobank cohort and baseline data from the prospective cohort and case–control study with (i) a strong (r = 0.67, P < 0.0001; Figure 6A) and (ii) a moderate (r = 0.33, P < 0.0001, Figure 6B) correlation, respectively, which demonstrates a translational potential of our UK Biobank–based findings into clinically relevant subjects, in the context of SBP-induced brain changes quantified with magnetic resonance imaging. The latter moderate correlation may be explained by a difference in cognitive function, estimated as fluid intelligence in the UK Biobank cohort or Montreal Cognitive Assessment score in the prospective cohort and case–control study. Despite this, the top principal component, calculated using loadings of 30 IDPs (see Supplementary data online, Table S11), related to structures genetically influencing cognitive function in the UK Biobank and available in the observational prospective cohort and case–control study, significantly correlated with Montreal Cognitive Assessment score in the two latter studies (r = −0.192, P = 0.039) and fluid intelligence score in the UK Biobank (r = −0.069, P < 0.001), where it mediated 7.4% (95% CI 2.9%–15.0%) of the total effect of SBP on fluid intelligence score. This additionally supports a translational perspective of our findings in the context of BP-induced changes of cognitive function that are mediated by specific IDPs. The association of 21 brain IDPs with SBP was directly replicated at FDR P < 0.1, while the anterior thalamic radiation tract, present among 9 brain IDPs genetically associated with cognitive function in the UK Biobank, demonstrated the most robust association with Montreal Cognitive Assessment score in the observational prospective cohort and case–control study (Figure 6A and B and Supplementary data online, Table S10).
Figure 5.
Summary of associations between brain IDPs, SBP, and cognitive function identified using observational and genetic approaches. Circular plot presents an association summary for 242 brain imaging-derived phenotypes (IDPs) significantly (FDR P < 0.05 for the IVW method and nominal P < 0.05 for the weighted median, MR–Egger, and robust IVW methods using instrumental variables from the ICBP + UK Biobank meta-analysis and nominal P < 0.05 for IVW method using instrumental variables from the separate ICBP or UK Biobank analysis) affected by SBP. Red/dark blue squares correspond to a significant (at least nominal P < 0.05), positive/negative association with SBP using MR or observational analysis (two outer circles) and cognitive function using MR (at least 1 instrumental variable available) or observational analysis in the UK Biobank (two inner rings). Grey squares correspond to brain IDPs with no instrumental variables available for MR analysis linking them to cognitive function. Brain IDPs labelled in red are associated with SBP below the Bonferroni-defined significance threshold (P < 0.05/3935) using a weighted median MR approach. IDP, imaging-derived phenotype; RH/LH, right/left hemisphere; WM, white matter; MD, mean diffusivity; ISOVF, isotropic or free water volume fraction; FA, fractional anisotropy; ICVF, intra-cellular volume fraction.
Figure 6.
Comparison of results obtained in the prospective cohort and case–control study and the UK Biobank. One hundred seventy brain IDPs, out of 242 genetically affected by SBP in the MR analyses of the UK Biobank and ICBP cohorts, were extracted and tested for association with SBP and Montreal Cognitive Assessment score (only those 127 IDPs with FDR P < 0.05 for association with cognitive function in the UK Biobank) in 116 subjects from the prospective cohort and case–control study. Standardised, observational estimates were compared with the ones obtained in the UK Biobank. IDPs associated at nominal P < 0.05/FDR P < 0.1 with SBP (A) or Montreal Cognitive Assessment score (B) in the prospective cohort and case–control study are depicted in red/blue. Top (based on P values) brain IDPs associated with SBP or Montreal Cognitive Assessment score in the prospective cohort and case–control study are named (MD, mean diffusivity; HTN, hypertension; FA, fractional anisotropy; MoCA, Montreal Cognitive Assessment; SD, standard deviation; SBP/DBP, systolic/diastolic blood pressure; PP, pulse pressure; UK Biobank field IDs are depicted on the right).
Discussion
Using a triangulation approach based on combined observational and genetic causal inference approaches, we found an effect of BP on cognitive function and identified, for the first time, a set of specific brain structures that potentially respond to differences in SBP among 3935 brain IDPs (see the Structured Graphical Abstract). Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which SBP acts on cognitive function. We then leveraged the data obtained from a prospectively recruited cohort and case–control study to confirm the identified pattern of damage linked by genetic instruments to SBP.
While previous attempts to identify brain IDPs linked to BP were observational,10,13,14,41 we demonstrate a relationship between BP and a specific set of IDPs using genetic causal inference methods. These include the anterior thalamic radiation, superior longitudinal fasciculus, and the forceps minor tracts10 as well as total volume of white matter hyperintensities previously suggested both observationally13,14 and in MR.42 However, contrary to observational analysis, we found no evidence of the genetic association between total volume of white matter hyperintensities and cognitive function. While white matter hyperintensities have been previously linked to dementia43–45 or Alzheimer’s disease but not to general cognitive function,19 our results may suggest that the effect of BP on cognitive performance may be mediated by specific white matter tracts, making the absolute total brain quantification of white matter hyperintensities a less sensitive indicator for this association in hypertension. For example, the external capsule, anterior corona radiata, and anterior thalamic radiation are strongly linked to SBP and associate with cognitive function, as evidenced by the MR analysis. Noteworthy, the two latter tracts are additionally associated with longitudinal change in cognitive function in 2054 UK Biobank subjects. While the level of cognitive function in a particular timepoint may be a derivative of various factors, the exact, overtime route of the effect of brain IDPs, including those identified in the current study, on cognitive function should be validated in the future sufficiently powered longitudinal cohort studies. Furthermore, putamen has been identified as one of the key brain structures linked to both SBP and cognitive function in observational and genetic analyses. This is important, as putamen is a part of the basal ganglia essential in stimulus response, learning, or planning strategies.46
Importantly, our validation cohort study illustrated a similar pattern of SBP effects on key brain IDPs in a relatively small sample of hypertensive and normotensive subjects in whom no conventional radiological damage was evident in clinical routine magnetic resonance imaging, and we were able to directly translate the pattern of main features of cerebral injury associated with cognitive function from genetic association to the hypertensive and normotensive subjects. This is important as it suggests that these may serve as prodromic indicators of ongoing cerebral damage due to elevated BP. Further validation of this important finding will require focused large-scale magnetic resonance imaging studies in hypertensive subjects.
Another interesting conclusion arising from the current study is that structural changes in the brain occur mainly in response to the difference between SBP and DBP. Most volumetric IDPs were associated with PP and mutually adjusted SBP and DBP in the multivariable MR analysis. At the same time, we identified several resting-state functional IDPs potentially responding to SBP or DBP rather than to PP. Observationally, BP-associated resting-state functional IDPs were associated with cognitive function. However, this observation was not supported by the MR approach. This may be caused by low number of good genetic instruments available for these IDPs24 and by the intrinsic link between cerebral blood flow and blood oxygen level–dependent (BOLD) signal, with the latter that may be more linked to the cerebral haemodynamic alterations than inherited factors.
Finally, our study supports the existence of a modest, potentially causal, detrimental effect of higher SBP and PP, but not DBP, on cognitive performance in midlife, which may serve as additional evidence regarding the role of hypertension in dementias.47,48 This is important in the context of the prevalence of hypertension. Since various forms of dementia may create a continuum spectrum rather than a strict dichotomy between Alzheimer’s disease and vascular dementia,49,50 fluid intelligence score, investigated in the current study, may be best suited to evaluate the complexity of this pathological condition.51 Previous MR studies linking BP to the cognitive function provided conflicting results, likely due to different sets of SNPs used as instrumental variables for BP indices.12,52,53 Our study included a much larger set of BP-specific instrumental variables derived from the UK Biobank and ICBP meta-analysis23 and used more powered GWAS on the cognitive function.17 The null effect of DBP on cognitive function observed in the univariable analysis is intriguing, as higher DBP became protective after adjusting for SBP. Taken together with the opposite effect of SBP and DBP on many IDPs identified in multivariable MR analysis, this may reflect the impact of arterial stiffening on increased pulsatility in the brain microvasculature with increased penetration of forward and backward travelling pressure waves54 as previously associated with white matter hyperintensities.55
Several limitations should be considered when interpreting the results. Most of the MR analyses performed were characterised by minor overlap of subjects in exposure and outcome GWAS. While analyses linking BP indices to brain IDPs and IDPs to cognitive function were characterised by a relatively small (<10%) subject overlap, this was not the case for a major analysis linking BP (instrumental variables derived from the meta-analysis of the UK Biobank and ICBP23) to cognitive performance (instrumental variables and genetic estimates derived from GWAS performed in the UK Biobank and COGENT17). Nevertheless, effect estimates linking BP to cognitive function were consistent across MR methods and different exposure GWAS used in the current study. Our study was primarily performed using middle-aged subjects from the UK Biobank; thus, extrapolating the results to older subjects, particularly at risk for dementia, is challenging. Since it was demonstrated that early-onset, rather than late-onset, hypertension predisposes to cognitive impairment in the later life,56 we may speculate that lifetime exposure to elevated BP, as assumed by an MR approach, plays a major role in the impairment of brain function, even in older people. Another limitation that should be noted is the modest number of subjects in IDP GWAS (∼33 000) and thus a relatively small number, or even lack, of genetic instruments available for specific IDPs.24 While multiple levels of evidence support the association of certain IDPs with BP or cognitive function, false positive associations may have arisen due to the multiple tests performed. In contrast to observational studies, MR-based causal inference should not be affected by confounding factors and reverse causality, although less likely owing to the careful selection of genetic instruments, cannot be entirely excluded. All the above emphasise the need for further epidemiological characterisation of BP–IDPs–cognitive function axis, and when possible, randomised trials remain state-of-the-art to address causal pathways between modifiable traits and human diseases.21
In summary, this is the first study comprehensively, genetically, and observationally to link BP parameters, IDPs, and cognitive function. While cognitive function and volumetric IDPs seem to be related predominantly to SBP and PP, SBP associates with several IDPs that further display genetic effects on cognitive function and may serve as novel diagnostic and therapeutic targets in hypertension associated cognitive impairment.
Supplementary Material
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 69257.
Contributor Information
Mateusz Siedlinski, Department of Internal Medicine, Jagiellonian University Medical College, ul. Skarbowa 1, 31-121 Krakow, Poland; Centre for Cardiovascular Sciences, Queen’s Medical Research Institute, University of Edinburgh, 47 Little France Crescent, Edinburgh EH16 4TJ, UK; Center for Medical Genomics OMICRON, Jagiellonian University Medical College, ul. Kopernika 7c, 31-034 Kraków, Poland.
Lorenzo Carnevale, Department of Angiocardioneurology and Translational Medicine, I.R.C.C.S. INM Neuromed, Via Atinense, 18, 86077 Pozzilli, Italy.
Xiaoguang Xu, Division of Cardiovascular Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, 46 Grafton Street, Manchester M13 9NT, UK.
Daniela Carnevale, Department of Angiocardioneurology and Translational Medicine, I.R.C.C.S. INM Neuromed, Via Atinense, 18, 86077 Pozzilli, Italy; Department of Molecular Medicine, Sapienza University of Rome, Viale Regina Elena, 291 - 00161 Roma, Italy.
Evangelos Evangelou, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, South Kensington Campus, London SW7 2AZ, UK; Department of Hygiene and Epidemiology, University of Ioannina Medical School, University Campus, University of Ioannina, P.O. Box: 1186, 451 10, Ioannina, Greece; Department of Biomedical Research, Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, University Campus GR -451 15, Ioannina, Greece.
Mark J Caulfield, William Harvey Research Institute, NIHR Biomedical Research Centre at Barts, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Pasquale Maffia, School of Infection & Immunity, College of Medical, Veterinary and Life Sciences, University of Glasgow, University Avenue, Glasgow G12 8QQ, UK; Department of Pharmacy, School of Medicine and Surgery, University of Naples Federico II, Via Domenico Montesano 49, 80131 Napoli, Italy.
Joanna Wardlaw, Centre for Clinical Brain Sciences, UK Dementia Research Institute, University of Edinburgh, 49 Little France Crescent, Edinburgh EH16 4SB, UK.
Nilesh J Samani, Department of Cardiovascular Sciences, University of Leicester, University Road, Leicester LE1 7RH, UK; NIHR Leicester Biomedical Research Centre, Glenfield Hospital, Groby Road, Leicester LE3 9QP, UK.
Maciej Tomaszewski, Division of Cardiovascular Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, 46 Grafton Street, Manchester M13 9NT, UK; Division of Medicine, Manchester Academic Health Science Centre, Manchester University NHS Foundation Trust, Oxford Road, Manchester M13 9WL, UK.
Giuseppe Lembo, Department of Angiocardioneurology and Translational Medicine, I.R.C.C.S. INM Neuromed, Via Atinense, 18, 86077 Pozzilli, Italy; Department of Molecular Medicine, Sapienza University of Rome, Viale Regina Elena, 291 - 00161 Roma, Italy.
Michael V Holmes, Bristol Medical School, Population Health Sciences, University of Bristol, Queens Road, Bristol BS8 1QU, UK; Medical Research Council, Integrative Epidemiology Unit, University of Bristol, Queens Road, Bristol BS8 1QU, UK.
Tomasz J Guzik, Department of Internal Medicine, Jagiellonian University Medical College, ul. Skarbowa 1, 31-121 Krakow, Poland; Centre for Cardiovascular Sciences, Queen’s Medical Research Institute, University of Edinburgh, 47 Little France Crescent, Edinburgh EH16 4TJ, UK; Center for Medical Genomics OMICRON, Jagiellonian University Medical College, ul. Kopernika 7c, 31-034 Kraków, Poland.
Author contributions
Mateusz Siedlinski, PhD (Conceptualization: Lead; Data curation: Equal; Formal analysis: Lead; Methodology: Lead; Visualization: Lead; Writing—original draft: Lead), Lorenzo Carnevale, PhD (Formal analysis: Supporting; Investigation: Supporting; Visualization: Supporting; Writing—review & editing: Supporting), Xiaoguang Xu, PhD (Formal analysis: Supporting; Methodology: Supporting; Resources: Supporting), Daniela Carnevale (Investigation: Supporting; Resources: Supporting; Writing—review & editing: Supporting), Evangelos Evangelou, PhD (Formal analysis: Supporting; Resources: Supporting; Writing—review & editing: Supporting), Mark J. Caulfield, MD, PhD (Resources: Supporting; Writing—review & editing: Supporting), Pasquale Maffia, PhD (Investigation: Supporting; Writing—review & editing: Supporting), Joanna Wardlaw, MD, PhD (Investigation: Supporting; Writing—review & editing: Supporting), Nilesh J Samani, MD, PhD (Investigation: Supporting; Writing—review & editing: Supporting), Maciej Tomaszewski (Investigation: Supporting; Methodology: Supporting; Resources: Supporting; Writing—review & editing: Supporting), Giuseppe Lembo, MD, PhD (Conceptualization: Supporting; Investigation: Supporting; Resources: Supporting; Writing—review & editing: Supporting), Michael V. Holmes, MD, PhD (Conceptualization: Supporting; Methodology: Supporting; Writing—review & editing: Supporting), and Tomasz J Guzik, MD PhD MSc(oxon) (Conceptualization: Lead; Data curation: Supporting; Formal analysis: Supporting; Funding acquisition: Lead; Investigation: Equal; Methodology: Supporting; Project administration: Lead; Resources: Lead; Supervision: Lead; Writing—review & editing: Lead)
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
The UK Biobank data are available on application to the UK Biobank for the data access (http://www.ukbiobank.ac.uk/). GWAS summary statistics were obtained from: https://www.thessgac.org/data (cognitive function; COGENT and UK Biobank meta-analysis), https://open.win.ox.ac.uk/ukbiobank/big40/(GWAS on 3935 brain imaging-derived phenotypes in ∼33 000 UK Biobank participants), http://www.nealelab.is/uk-biobank/(automated SBP and DBP reading GWAS in the UK Biobank), and NHGRI–EBI Catalog of human GWAS (meta-analysis of ICBP and UK Biobank). The ICBP GWAS summary statistics can be assessed through the ICBP consortium.
Funding
This study was funded by the European Research Council (ERC and InflammaTENSION; ERC-CoG-726318; to T.J.G.), British Heart Foundation (FS/14/49/30838 and FS/4yPhD/F/20/34127A to T.J.G.) and as part of the British Heart Foundation Centre for Research Excellence at the University of Edinburgh (RE/18/5/34216), CVD ERA-CVD (PLAQUEFIGHT; 01KL1808 and ImmuneHyperCog to G.L. Ministry of Health Italy; and T.J.G.; NCBiR, Poland), and Italian Ministry of Health ‘Ricerca Corrente’ and ‘RF-2018-12366235’ to G.L. Dr Holmes works in a unit that receives funding from the UK Medical Research Council and is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/23/33512) and the National Institute for Health Research Oxford Biomedical Research Centre. M.T. and X.X. are supported by the British Heart Foundation (PG/19/16/34270) and Kidney Research UK (RP_017_20180302 and RP_013_20190305). J.W. is supported by the UK Dementia Research Institute funded by MRC, Alzheimer’s Society, and Alzheimer’s Research UK. P.M. is supported by the British Heart Foundation grants (PG/19/84/34771, FS/19/56/34893A, PG/21/10541, PG/21/10634).
References
- 1. Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923–1994. 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SPRINT MIND Investigators for the SPRINT Research Group; Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, et al. . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Hayden KM, May NS, Haring B, Liu Z, Henderson VW, et al. . Association between blood pressure levels and cognitive impairment in older women: a prospective analysis of the Women’s Health Initiative Memory Study. Lancet Healthy Longev 2022;3:e42–e53. 10.1016/S2666-7568(21)00283-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007;64:1734–1740. 10.1001/archneur.64.12.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. 10.1016/S1474-4422(05)70141-1 [DOI] [PubMed] [Google Scholar]
- 6. Sun D, Thomas EA, Launer LJ, Sidney S, Yaffe K, Fornage M. Association of blood pressure with cognitive function at midlife: a Mendelian randomization study. BMC Med Genomics 2020;13:121. 10.1186/s12920-020-00769-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters R, Xu Y, Fitzgerald O, Aung HL, Beckett N, Bulpitt C, et al. . Blood pressure lowering and prevention of dementia: an individual patient data meta-analysis. Eur Heart J 2022;43:4980–4990. 10.1093/eurheartj/ehac584 [DOI] [PubMed] [Google Scholar]
- 8. Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res 2019;124:1025–1044. 10.1161/CIRCRESAHA.118.313260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, et al. . Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 2018;562:210–216. 10.1038/s41586-018-0571-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carnevale L, D'Angelosante V, Landolfi A, Grillea G, Selvetella G, Storto M, et al. . Brain MRI fiber-tracking reveals white matter alterations in hypertensive patients without damage at conventional neuroimaging. Cardiovasc Res 2018;114:1536–1546. 10.1093/cvr/cvy104 [DOI] [PubMed] [Google Scholar]
- 11. Lyall DM, Celis-Morales CA, Anderson J, Gill JM, Mackay DF, McIntosh AM, et al. . Associations between single and multiple cardiometabolic diseases and cognitive abilities in 474 129 UK Biobank participants. Eur Heart J 2017;38:577–583. 10.1093/eurheartj/ehw528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferguson AC, Tank R, Lyall LM, Ward J, Welsh P, Celis-Morales C, et al. . Association of SBP and BMI with cognitive and structural brain phenotypes in UK Biobank. J Hypertens 2020;38:2482–2489. 10.1097/HJH.0000000000002579 [DOI] [PubMed] [Google Scholar]
- 13. Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR, et al. . Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J 2019;40:2290–2300. 10.1093/eurheartj/ehz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wartolowska KA, Webb AJS. Midlife blood pressure is associated with the severity of white matter hyperintensities: analysis of the UK Biobank cohort study. Eur Heart J 2020;42:750–757. 10.1093/eurheartj/ehaa756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. . Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012;11:1039–1047. 10.1016/S1474-4422(12)70241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao B, Luo T, Li T, Li Y, Zhang J, Shan Y, et al. . Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat Genet 2019;51:1637–1644. 10.1038/s41588-019-0516-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. . Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 2018;50:1112–1121. 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao B, Zhang J, Ibrahim JG, Luo T, Santelli RC, Li Y, et al. . Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits (n = 17,706). Mol Psychiatry 2019;26:3943–3955. 10.1038/s41380-019-0569-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sargurupremraj M, Suzuki H, Jian X, Sarnowski C, Evans TE, Bis JC, et al. . Cerebral small vessel disease genomics and its implications across the lifespan. Nat Commun 2020;11:6285. 10.1038/s41467-020-19111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Backhouse EV, Shenkin SD, McIntosh AM, Bastin ME, Whalley HC, Valdez Hernandez M, et al. . Early life predictors of late life cerebral small vessel disease in four prospective cohort studies. Brain 2021;144:3769–3778. 10.1093/brain/awab331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richmond RC, Davey Smith G. Mendelian randomization: concepts and scope. Cold Spring Harb Perspect Med 2022;12:a040501. 10.1101/cshperspect.a040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. . Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 2018;50:1412–1425. 10.1038/s41588-018-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SM, Douaud G, Chen W, Hanayik T, Alfaro-Almagro F, Sharp K, et al. . An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat Neurosci 2021;24:737–745. 10.1038/s41593-021-00826-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siedlinski M, Jozefczuk E, Xu X, Teumer A, Evangelou E, Schnabel RB, et al. . White blood cells and blood pressure: a Mendelian randomization study. Circulation 2020;141:1307–1317. 10.1161/CIRCULATIONAHA.119.045102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eales JM, Jiang X, Xu X, Saluja S, Akbarov A, Cano-Gamez E, et al. . Uncovering genetic mechanisms of hypertension through multi-omic analysis of the kidney. Nat Genet 2021;53:630–637. 10.1038/s41588-021-00835-w [DOI] [PubMed] [Google Scholar]
- 27. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 2017;13:e1007081. 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Consortium for Blood Pressure Genome-Wide Association Studies; Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. 10.1038/nature10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–2191. 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. . UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alfaro-Almagro F, Jenkinson M, Bangerter NK, Andersson JLR, Griffanti L, Douaud G, et al. . Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 2018;166:400–424. 10.1016/j.neuroimage.2017.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, et al. . Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 2016;19:1523–1536. 10.1038/nn.4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: r package for causal mediation analysis. J Stat Softw 2014;59:1–38. 10.18637/jss.v059.i0526917999 [DOI] [Google Scholar]
- 34. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017;46:1734–1739. 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Broadbent JR, Foley CN, Grant AJ, Mason AM, Staley JR, Burgess S. MendelianRandomization v0.5.0: updates to an R package for performing Mendelian randomization analyses using summarized data. Wellcome Open Res 2020;5:252. 10.12688/wellcomeopenres.16374.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. . The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res 2015;43:W566–W570. 10.1093/nar/gkv468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 39. Fischl B. FreeSurfer. Neuroimage 2012;62:774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Holland D, et al. . A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat Genet 2021;53:1276–1282. 10.1038/s41588-021-00921-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carnevale L, Maffei A, Landolfi A, Grillea G, Carnevale D, Lembo G. Brain functional magnetic resonance imaging highlights altered connections and functional networks in patients with hypertension. Hypertension 2020;76:1480–1490. 10.1161/HYPERTENSIONAHA.120.15296 [DOI] [PubMed] [Google Scholar]
- 42. Taylor-Bateman V, Gill D, Georgakis M, Malik R, Munroe P, Traylor M, et al. . Cardiovascular risk factors and MRI markers of cerebral small vessel disease: a Mendelian randomization study. Neurology 2021;98:e343–e351. 10.1212/WNL.0000000000013120 [DOI] [PubMed] [Google Scholar]
- 43. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019;18:684–696. 10.1016/S1474-4422(19)30079-1 [DOI] [PubMed] [Google Scholar]
- 44. Georgakis MK, Duering M, Wardlaw JM, Dichgans M. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology 2019;92:e1298–e1308. 10.1212/WNL.0000000000007142 [DOI] [PubMed] [Google Scholar]
- 45. Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2019;76:81–94. 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol 2008;86:141–155. 10.1016/j.pneurobio.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 47. Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. . Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016;68:e67–e94. 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med 2014;12:206. 10.1186/s12916-014-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santisteban MM, Iadecola C. Hypertension, dietary salt and cognitive impairment. J Cereb Blood Flow Metab 2018;38:2112–2128. 10.1177/0271678X18803374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS One 2020;15:e0231627. 10.1371/journal.pone.0231627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hagenaars SP, Gale CR, Deary IJ, Harris SE. Cognitive ability and physical health: a Mendelian randomization study. Sci Rep 2017;7:2651. 10.1038/s41598-017-02837-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Warren HR, Evangelou E, Cabrera CP, Gao H, Ren M, Mifsud B, et al. . Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet 2017;49:403–415. 10.1038/ng.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chirinos JA. Large artery stiffness, microvascular function, and cardiovascular risk. Circ Cardiovasc Imaging 2016;9:e005903. 10.1161/CIRCIMAGING.116.005903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aribisala BS, Morris Z, Eadie E, Thomas A, Gow A, Valdes Hernandez MC, et al. . Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension 2014;63:1011–1018. 10.1161/HYPERTENSIONAHA.113.02735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suvila K, Lima JAC, Yano Y, Tan ZS, Cheng S, Niiranen TJ. Early-but not late-onset hypertension is related to midlife cognitive function. Hypertension 2021;77:972–979. 10.1161/HYPERTENSIONAHA.120.16556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK Biobank data are available on application to the UK Biobank for the data access (http://www.ukbiobank.ac.uk/). GWAS summary statistics were obtained from: https://www.thessgac.org/data (cognitive function; COGENT and UK Biobank meta-analysis), https://open.win.ox.ac.uk/ukbiobank/big40/(GWAS on 3935 brain imaging-derived phenotypes in ∼33 000 UK Biobank participants), http://www.nealelab.is/uk-biobank/(automated SBP and DBP reading GWAS in the UK Biobank), and NHGRI–EBI Catalog of human GWAS (meta-analysis of ICBP and UK Biobank). The ICBP GWAS summary statistics can be assessed through the ICBP consortium.