Abstract
Both experimental and clinical findings linking vitamin D to cardiovascular (CV) risk have prompted consideration of its supplementation to improve overall health. Yet several meta-analyses do not provide support for the clinical effectiveness of this strategy. Meanwhile, the understanding of the roles of vitamin D in the pathophysiology of CV diseases has evolved. Specifically, recent work has revealed some non-classical pleiotropic effects of vitamin D, increasing the complexity of vitamin D signalling. Within particular microenvironments (e.g. dysfunctional adipose tissue and atherosclerotic plaque), vitamin D can act locally at cellular level through intracrine/autocrine/paracrine feedforward and feedback circuits. Within atherosclerotic tissues, ‘local’ vitamin D levels may influence relevant systemic consequences independently of its circulating pool. Moreover, vitamin D links closely to other signalling pathways of CV relevance including those driving cellular senescence, ageing, and age-related diseases—among them CV conditions. This review updates knowledge on vitamin D biology aiming to clarify the widening gap between experimental and clinical evidence. It highlights the potential reverse causation confounding correlation between vitamin D status and CV health, and the need to consider novel pathophysiological concepts in the design of future clinical trials that explore the effects of vitamin D on atherosclerosis and risk of CV events.
Keywords: Vitamin D, Vitamin D receptor, Atherosclerosis, Inflammation, Adipose tissue, Senescence
Graphical Abstract
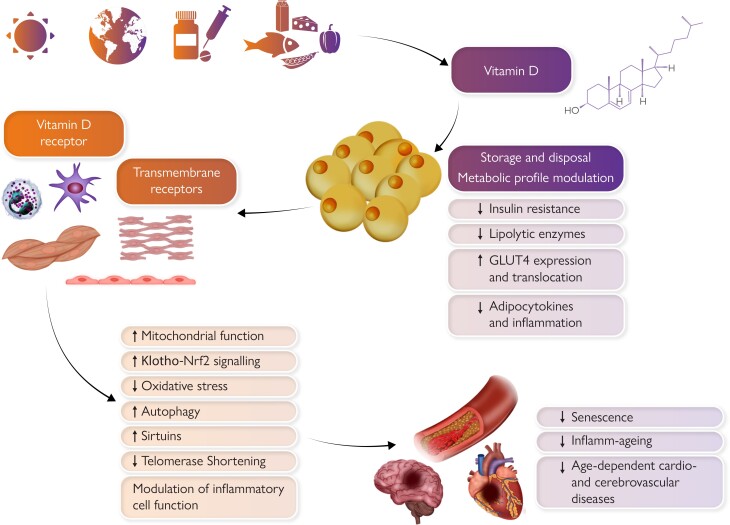
Graphical Abstract.
Pleiotropic effects of vitamin D on the cardiovascular system. Sunlight exposure, exogenous dietary, and pharmacological supply primarily determine vitamin D levels. Vitamin D is then stored within the adipose tissue which influences its systemic availability. Pleiotropic vitamin D effects on the cardiovascular system are mediated by intracellular vitamin D receptor or other transmembrane receptors and include beneficial modulation of senescence, inflamm-ageing, and adiposopathy. Clinical translation of such findings still requires more complete preclinical evidence, especially on newly defined mechanisms such as epigenetic senescence, and also better standardization among clinical studies (e.g. definition of deficiency, distinctions between fortified food and pharmacologic supplementation).
Introduction
The role of vitamin D in cardiovascular (CV) health remains an elusive aspect of contemporary cardiology. A plethora of publications over the last decade have prompted a critical reappraisal of the evidence regarding the health benefits of vitamin D supplementation. Epidemiological observations have consistently linked low circulating levels of vitamin D to CV risk, but randomized clinical trials and meta-analyses have not shown a benefit of supplementation.1 Such a dichotomy raises many questions about potential confounders (e.g. limited outdoor physical activity, exogenous vitamin D supplementation, and calcium supplementation regimens).2 Furthermore, enlarging the gamut of CV risk factors beyond the traditional, e.g. including inflammation, might shed light on the role of this vitamin and help to understand its therapeutic potential. Obesity has long been considered a target for vitamin D supplementation,3,4 and qualitative fat abnormalities (i.e. adiposopathy) with different distributions of obesity have gained increasing recognition.5,6 This evolution has prompted a focus on the tissue microenvironment, a concept that may extend from visceral adipose tissue to ectopic fat depots, to the atherosclerotic plaque, the myocardium, and the brain.7 Beyond the recognition of its ‘non-classical’ effects, we now know that circulating concentrations of vitamins may not reflect their activity in tissues. Also, locally produced and stored vitamin D may have systemic functional consequences. This state-of-the-art review summarizes the evolving scientific knowledge regarding local and systemic effects of vitamin D in primary and secondary CV prevention. Furthermore, we propose that categorizing patients according to vitamin D levels might aid risk stratification and allocation of supplementation. Lastly, we provide a critical assessment of CV conditions proposed to derive benefit from vitamin D supplementation.
Vitamin D axis: systemic and local contribution to cardiovascular risk
Vitamin D biology and interaction with other cardiovascular relevant pathways
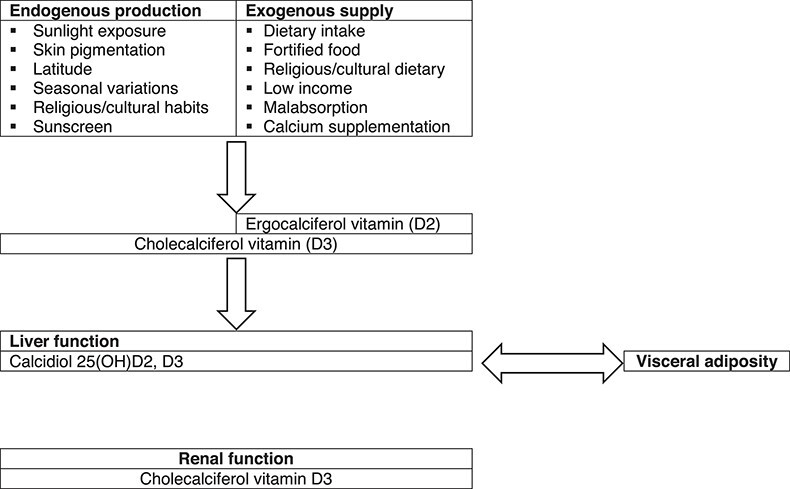
The substantial controversy surrounding vitamin D results from its complex biology (Figure 1). Sunlight exposure accounts for ∼80% of vitamin D synthesis (i.e. cholecalciferol). Exogenous dietary supply (i.e. cholecalciferol or ergocalciferol) has rather limited relevance under physiological conditions. The degree of endogenous production and exogenous intake depends on several factors. Indeed, the conversion from 7-dehydrocholesterol—the endogenous cholecalciferol precursor—to the active 1,25(OH)2D (i.e. calcitriol) requires multiple steps. The rate-limiting enzyme of this pathway, CYP27B1, is tightly regulated by feedback control mechanisms involving parathyroid hormone, calcitonin, the vitamin D catabolic enzyme CYP24A1, and activated vitamin D itself. Ageing and sequestration within adipose tissue add further layers to the complex vitamin D balance (Table 1). In particular, such sequestration critically determines vitamin D availability which relates to adipose tissue function/dysfunction rather than to its quantity.8 Ergocalciferol and cholecalciferol then require a two-step hydroxylation to become biologically active and reach target organs. Within the bloodstream, vitamin D mainly circulates as 25-hydroxyvitamin D combined with its carrier protein, vitamin D binding protein (VDBP). Under physiological conditions, circulating serum 25-hydroxyvitamin D level ranges between 20 and 50 ng/mL.9
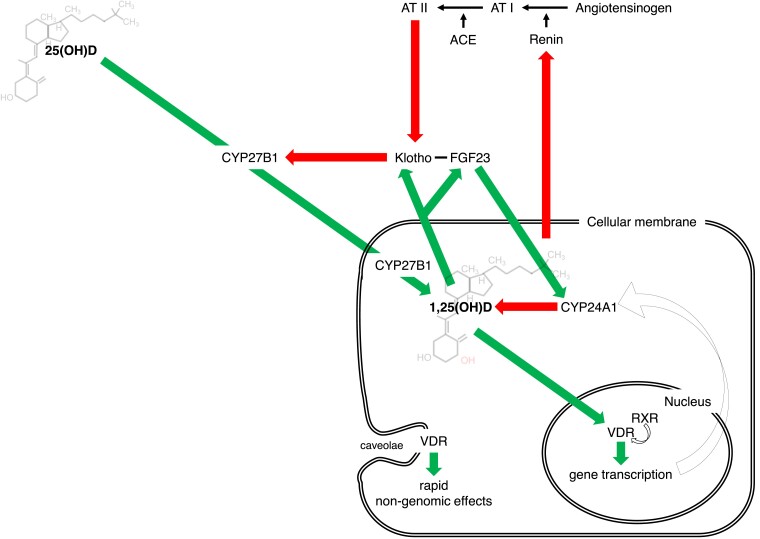
Figure 1.
Vitamin D axis is a complex system regulated at several levels and connected with many signals of cardiovascular relevance. Beyond the renal control (under parathyroid hormone), local vitamin D axes involve intracrine/autocrine/paracrine feedforward and feedback circuits. Furthermore, vitamin D system intertwines closely with other pathways of cardiovascular relevance: renin–angiotensin–aldosterone system and Klotho-FGF23.
Table 1.
Determinants of circulating vitamin D levels
|
No longer considered a mere vitamin but rather a lipophilic ‘steroid-like’ hormone, vitamin D exerts its biological response through the binding with the vitamin D receptor (VDR). The heterodimeric form of VDR—composed of the active vitamin D (1,25(OH)2D) and the retinoid × receptor (RXR α, β, or γ)—acts as transcription factor targeting specific vitamin D response elements (VDRE) located in the promoter region of target genes. VDR was initially detected in tissues involved in calcium/phosphate balance including bone, kidney, intestine, and parathyroid glands. The evidence of additional vitamin D effects prompted the recognition of VDR expression in cellular components of different organs such as the skin, immune, and CV systems. Within the CV system, VDR is expressed by endothelial cells (ECs), vascular smooth muscle cells (VSMCs), immune cells, and cardiomyocytes.10 Genomic actions of activated VDR then depend on allosteric modulation, VDRE location, and epigenetic modifications in different cell types.11 After the completion of the human genome, RNA sequencing and then microarrays have become the assays of choice for describing the vitamin D-transcriptome. The VDR affects the transcription of up to 5% of the whole human genome: some 20 000 target genes. However, primary target genes should be discriminated from delayed-reacting ‘secondary’ targets for which vitamin D encodes transcription factors, co-factors, and/or chromatin modifiers.12 Thus, the number of VDR target genes in a single cell type might be restricted to a few hundreds. Common target genes include those involved in calcium/phosphate homoeostasis (e.g. osteocalcin) and inflammation (e.g. cathelicidin antimicrobial peptide). Rapid non-genomic VDR effects depend on membrane receptors such as PDIA3 (protein disulphide isomerase family A member 3). PDIA3 is an endoplasmic reticulum chaperone located in cell membrane, cytoplasm, mitochondria, and nucleus that requires the interaction with caveolin 1 to trigger the rapid response to vitamin D. PDIA3 but not VDR is essential for the activation of protein kinase C-phospholipase A2 cascade and MAPK and WNT/NOTCH pathways. Disrupting the PDIA3 gene results in bone abnormalities and loss of protection against UV-induced DNA damage.13
Far from operating in isolation, vitamin D intertwines closely with other pathways pivotal in the CV system. Several experimental studies confirmed the inhibitory role of vitamin D on the renin–angiotensin–aldosterone system (RAAS) with effects on blood pressure and cardiac hypertrophy in mice.14,15 In turn, RAAS activation suppresses the renal expression of Klotho, essential for effective FGF23 signalling. These interactions comprise a complex feedback loop in which high FGF23 levels suppress vitamin D signal—by targeting both CYP27B1 and CYP24A1—eventually leading to RAAS activation16 (Figure 1).
Role of vitamin D in cellular senescence and ageing
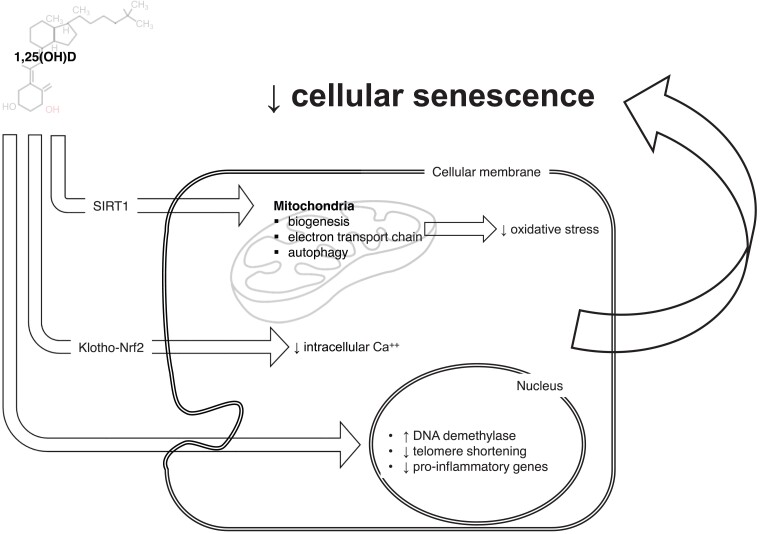
New intriguing hypotheses link vitamin D deficiency—in conjunction with autophagy and Klotho-Nrf2 signalling—with cellular senescence, ageing, and age-related diseases17 (Figure 2). Cellular senescence develops as a cumulative effect of cell stressors eventually leading to a permanent cell growth arrest and the development of the senescence-associated secretory pattern (SASP). This programme contributes importantly to ‘inflamm-ageing’ the chronic low-grade inflammatory status that develops with ageing and participates in the pathophysiology of most CV diseases.18,19 Vitamin D can preserve mitochondrial function and limit oxidative stress by facilitating mitochondrial biogenesis, the electron transport chain, and autophagy.20,21 Specifically, vitamin D activates pro-survival autophagy as a protective mechanism to inhibit oxidative stress and apoptosis with critical effects on bone and CV health. Through autophagy, vitamin D mediates osteoclastogenesis and thus the risk of osteoporosis.21 Within the vessel wall, vitamin D restores oxidized low-density lipoprotein-impaired autophagy22 and prevents endothelial dysfunction.23 Furthermore, vitamin D-mediated autophagy also exerts beneficial effects on failing/ageing heart.24,25 With ageing, autophagy and vitamin D share the same trajectory, being characterized by inactivation and deficiency, respectively. Vitamin D deficiency may also accelerate ageing and ageing-related disease. Vitamin D may influence the ageing determinants NAD+-dependent protein acetylases sirtuins, calcium signalling homoeostasis, mitochondrial function, and oxidative stress regulation.17 By augmenting Klotho-Nrf2 signalling, vitamin D can limit oxidative stress by preventing the rise in intracellular Ca++. At the nuclear level, vitamin D reduces the rate of telomere shortening26 and controls the epigenetic landscape of its promoters by stimulating key DNA demethylases that alter epigenetic aspects of ageing.27 This effect likely involves the hypermethylation of Klotho’s promoter region causing a reduced level of this mediator and the progression of age-related phenomena.28 In addition to those mechanisms, vitamin D directly modulates inflammatory cell activation again blunting inflamm-ageing.29
Figure 2.
Vitamin D system might participate in senescence-ageing processes. Through a wide range of cellular and nuclear mechanisms, vitamin D can modulate many aspects of cellular senescence: mitochondrial survival and activity, intracellular calcium concentrations, and direct/epigenetic control on gene transcription. Those mechanisms may further explain the tight pathophysiological link between vitamin D axis and age-related diseases, including cardiovascular diseases.
The wide anti-inflammatory effects of vitamin D arise locally through intracrine, autocrine, and paracrine feedforward and feedback circuits.30 The stimulation of toll-like receptors (TLRs) amplifies CYP27B1 and VDR expression in macrophages in an autocrine manner, whereas interferon-γ produced by the Th1 lymphocyte subset determines the same effect through a paracrine pathway. In turn, when macrophages release 1,25(OH)2D, it drives VDR-dependent inhibitory feedback on lymphocyte activation.31 Such findings underscore the importance of 25(OH)D in macrophages, as it exerts direct effects on both innate and adaptive immunity. The co-existent expression of CYP27B1 and VDR characterizes many cell types and indicates the intracrine metabolism of vitamin D as a potential candidate to modulate their effects in different diseases.32 However, this plethora of experimental evidence about senolytic effect of vitamin D requires clinical translation in randomized trials.33
Vitamin D insufficiency/deficiency: definition and dispute
Defining reference ranges for vitamin D has long presented challenges. The preferential use of blood 25(OH)D levels reflects its relative abundance (1000-fold higher than 1,25(OH)2D) and stability (half-life: 15 days vs. 15 h for 1,25(OH)2D). 25(OH)D circulates both in its free form and bound to carrier proteins, mainly VDBP. Specifically, VDBP affinity is greater for the inactivated [24,25(OH)2D] and the depot [25(OH)D] forms of vitamin D rather than the active 1,25(OH)2D. VDBP deletion in mice does not lead to skeletal abnormalities or calcium imbalance so that a direct pathophysiological role for VDBP is unlikely.32 On the other hand, the measurement of the free, unbound form of 25(OH)D has been proved challenging, because of its low levels and the need for radioactive tracers. Yet, enzyme-linked immunosorbent assays for detecting free 25(OH)D are currently under development. Beyond analytical variability, 25(OH)D concentrations show seasonal variations due to their mechanisms of synthesis with higher levels during sunny seasons. Given such complexity, proposed vitamin D range calculators take into account different confounders, although their use still needs further validation.34
As a result, the thresholds defining vitamin D deficiency significantly differ across health organizations ranging from serum levels of 30–10 ng/mL. However, there is general agreement with those proposed by the former US Institute of Medicine now known as the Academy of Medicine9 (Table 2). The US population has a substantial prevalence of vitamin D insufficiency (41.4%) or deficiency (28.9%).38 These estimates increase substantially with the application of more stringent thresholds.39,40 Eastern European countries report lower levels of vitamin D than northern ones.37 Vitamin D status is also suboptimal in Western Europe, and especially in the UK. Finally, non-Western immigrants in European countries showed a very poor vitamin D status, with a mean 25(OH)D levels lower than locally born and people living in their countries of origin.37
Table 2.
Proposed thresholds for vitamin D deficiency definition
| 25(OH)D | Institute of Medicine | Endocrine Society | EFSA, 201635 | SACN, 201636 | ECTS, 201937 | US prevalencea | European prevalenceb |
|---|---|---|---|---|---|---|---|
| Normal values | ≥20 ng/mL | ≥30 ng/mL | ≥20 ng/mL | NA | ≥ 20 ng/mL | ||
| Insufficiency | 12–20 ng/mL | 20–29 ng/mL | – | – | 20–10 ng/mL | 41.4% |
|
| Deficiency | <12 ng/mL | <20 ng/mL | <20 ng/mL | <10 ng/mL | <10 ng/mL | 28.9% |
EFSA, European Food Safety Authority; SACN, Scientific Advisory Committee on Nutrition; ECTS, European Calcified Tissue Society.
Prevalence refers to the thresholds of the Institute of Medicine.
Prevalence of vitamin D deficiency refers to the threshold of the Endocrine Society.
Vitamin D and cardio-metabolic risk
Vitamin D activity on adipose tissue microenvironment
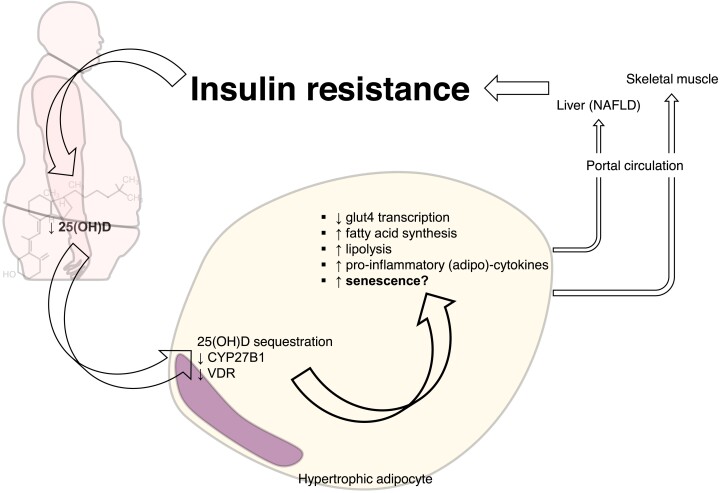
Visceral adipose tissue stores about three-quarters of the total cholecalciferol and one-third of 25(OH)D. But vitamin D depots within the adipose tissue depend little on circulating levels. Indeed, adipocytes express all key enzymes required for vitamin D hydroxylation. Within the adipose tissue, vitamin D signals in paracrine and autocrine manners. In its active form, 1,25(OH)2D improves glut4 expression and translocation, suppresses fatty acid synthesis from glucose, augments lipolytic enzymes, and inhibits adipokines and inflammatory signalling41 (Figure 3). Nevertheless, VDR knockout mice are lean and resist diet-induced obesity. Even adipose-specific VDR deletion did not resolve this apparent paradox.42 Less is known about the role of vitamin D in adipocyte hypertrophy, the hallmark of adiposopathy and associated dysfunction. Hypertrophic adipocytes show necrotic-like abnormalities as the vascular tree becomes deficient and hypoxia ensues. Lipolysis and insulin resistance characterize this microenvironment with effects on other tissues (e.g. liver and skeletal muscle) through free fatty acids and pro-inflammatory mediators released in the portal vein.43 More recent studies have further characterized the hypertrophic adipocyte as a senescent cell showing the typical pro-inflammatory SASP44 (Figure 3). As mentioned above, the vitamin D axis can influence several pathways involved in adipocyte senescence: mitochondrial respiration,45 autophagy,46 intracellular Ca++ concentration,47 and epigenetic clocks (i.e. methylation/acetylation of both DNA and histone and the expression of micro-RNAs).48,49 Vitamin D generally exerts anti-inflammatory effects on adipocytes. 1,25(OH)2D indeed prevents and even reverses monocyte chemotaxis.50 Nevertheless, dysfunctional hypertrophic adipocytes have blunting of the entire vitamin D axis and an associated feedback transcriptional response characterized by augmentation of CYP27A1 and VDR, and suppression of CYP27B1.51,52 Microarray approaches are deciphering the target cytokines and the role of miRNAs as components of adipocyte SASP.53
Figure 3.
Dysfunctional hypertrophic adipocytes show blunted vitamin D signalling. Vitamin D receptor and the hydroxylase CYP27B1 are blunted in dysfunctional hypertrophic adipocyte. Alongside the sequestration of 25(OH)D within visceral adipose tissue, suppression of vitamin D signalling sustains metabolic disturbances not only in adipose tissue but also systemically.
Vitamin D deficiency and adiposopathy: clinical evidence
Visceral adiposity links consistently with vitamin D deficiency/insufficiency, as originally reported by the Framingham Heart Study.54 Furthermore, recent studies also showed an association with the unhealthy obesity pattern.55 A tight interplay exists between vitamin D axis and visceral adiposity. Not only do concentrations of 25(OH)D critically determine the VDR gene expression in adipocytes, but sun exposure and skin reflectance also associate with visceral adiposity.56,57 25(OH)D levels increase linearly as a function of visceral adiposity reduction during lifestyle modification programmes, with dynamic local changes in vitamin D metabolism.58–60 This effect is even more evident with massive weight loss achieved through a very low-calorie ketogenic diet61 or bariatric surgery.62 The expression of CYP27B1 indeed rises after weight loss and decreases during overweight along with vitamin D sequestration.63 As a result, obese subjects have a muted response to vitamin D supplementation.64 Further supporting a causative role in adiposopathy, vitamin D deficiency is associated with ectopic fat deposition. Few studies have so far focused on the association between vitamin D and epicardial adipose tissue (EAT), reporting an association with both thickness and dysfunction while resisting regression after replacement therapy.65,66 Similarly, preliminary data indicate an association between vitamin D deficiency, fatty degeneration of skeletal muscle,67 and the osteosarcopenic obese pattern.68 Although limited to association studies such evidence deserves further investigation. Recent evidence suggests a role for EAT in inflammation by hosting tertiary lymphoid organs (TLOs). In consideration of the role of TLOs as the regulator of B cell response with relevant effect on atherogenesis and recovery after myocardial infarction69–71 and in keeping with the immunomodulatory function of vitamin D, whether CV effects of such mediators may depend in part on local immune responses within ectopic fat is an intriguing question for future investigations.
Vitamin D deficiency and cardio-metabolic profile: clinical evidence
Extensive literature supports the association between vitamin D deficiency and non-alcoholic fatty liver disease (NAFLD). In this context, vitamin D may blunt the activation of hepatic stellate cells and have a broad immunomodulatory role for resident immune cells.72 Of importance, vitamin D deficiency might promote NAFLD progression independently of other risk factors (e.g. hypertriglyceridaemia and insulin resistance). In patients, analyses of polymorphisms in genes from the vitamin D axis further strengthened this association, whereas recent studies are deepening the effects on liver senescence mechanisms.73–75 Low serum 25(OH)D levels indeed correlate with the severity of steatosis and necro-inflammatory injury. This histopathological pattern is consistent with the suppression of TLRs by vitamin D and the increase of VDR expression in non-parenchymal liver cells including hepatic stellate cells, Kupffer cells, natural killer T cells, and sinusoidal ECs. Furthermore, vitamin D may protect against a high-fat diet by inducing autophagy. Yet, the causality of vitamin D deficiency in NAFLD is hard to prove. Indeed, the lack of vitamin D can be a consequence of its lower synthesis by the dysfunctional liver. This may explain why clinical trials of vitamin D supplementation generally reported a metabolic improvement without a significant effect on liver injury.76–81 Similarly, the notion that insulin resistance itself may account for vitamin D deficiency—rather than being its consequence—is gaining attention. Insulin resistance can suppress the expression of CYP2R1 in the liver and CYP27B1 in the kidney.82 Although vitamin D deficiency and insulin resistance can both be treated, beneficial effects derive only from increased insulin sensitivity. Whether improving insulin sensitivity may increase vitamin D levels and/or the effectiveness of supplementation strategies in CV prevention remains unresolved. Epidemiological data indicate that chronic deficit of vitamin D parallels with the clinical manifestations of insulin resistance. Vitamin D deficiency very frequently accompanies diabetes and associates with a higher risk of developing diabetes.83 The most recent meta-analysis and Mendelian randomization studies have evaluated more deeply the role of vitamin D status in the development and progression of diabetes. A significant, yet non-causal, association links the risk of developing diabetes with vitamin D deficiency in ∼300 000 participants.84 Yet, the same meta-analysis indicates adiposopathy and unhealthy lifestyles as risk factors for diabetes, confounding conclusions regarding the causality of vitamin D levels. Furthermore, clinical studies and meta-analyses agree on the close link of vitamin D deficiency with hypertension and dyslipidaemia as reviewed elsewhere.85,86 The pooled relative risk of incident hypertension in more than 300 000 participants has been estimated at 0.88 (0.81–0.97) per 10 ng/mL increment in 25(OH)D levels,87 whereas in 25 000 children and adolescents vitamin D associated with a favourable lipid profile.88 Yet, the effects of vitamin D supplementation on blood pressure and serum lipids remain unproven.89,90
The ‘intraplaque’ vitamin D system
Vitamin D activity in the atherosclerotic plaque microenvironment
Intimal inflammation drives many aspects of atherogenesis.91 The atherosclerotic plaque microenvironment represents the prototypical milieu in which the vitamin D axis locally exerts its ‘non-classical’ effects via paracrine and autocrine processes.92 The first recognition of VDR expression within the plaque dates to 2012.93 Our research group first localized VDR in human samples and observed how its expression was proportional to the infiltrate of pro-inflammatory M1 macrophages and the likelihood of future adverse CV events.94 As exposure to 1,25(OH)2D limited the expression of TLR-4 on M1 macrophages in vitro, we hypothesized that VDR expression in pro-inflammatory macrophages constituted a negative feedback loop. Intraplaque macrophages participate critically in the local intraplaque vitamin D system: they express all the enzymes for its synthesis as well as VDR thereby potentially regulating the axis in an intracrine, autocrine, and paracrine manner. The genome/epigenome of human monocytes shows regulation by vitamin D at multiple levels.95 The result is an extensive control on macrophage function, not limited to phenotype polarization and anti-inflammatory activity.30,96
Vitamin D prevents the formation of foam cells by intraplaque macrophages by inhibiting endoplasmic reticulum stress, the expression of scavenger receptors,97 and autophagy, triggered via the PTPN6/SHP-1 pathway.22 As with monocytes, the vitamin D axis may exert autocrine feedback on neutrophils as these granulocytes themselves express CYP27B1 and VDR. This point still lacks experimental demonstration.
Dendritic cells (DCs) provide another source of 1,25(OH)2D. Transplantation of apoptosis-resistant DCs into LDLR−/− recipient mice indeed slows atherosclerotic progression.98 This finding likely arises from an autocrine loop that results in DC regression to a less mature stage characterized by impaired chemotaxis and suppression of pro-inflammatory cytokines and costimulatory molecules (i.e. CD40, CD80, and CD86).99 T lymphocytes may have a further role, especially the regulatory (Treg) subtype, as the VDR direct controls their signature transcription factor FOXP3 under.100 Beyond the immune system, the pleiotropic activity of vitamin D involves different cell types of relevance in atherosclerosis. 1,25(OH)2D influences VSMCs directly via VDR. 1,25(OH)2D stimulates the expression of vascular endothelial growth factor by VSMC and regulates their proliferation, migration, calcification, and tissue factor expression. Furthermore, vitamin D signalling could prevent senescence in VSMCs, likely through the inhibition of local angiotensin-II signalling.101
Vitamin D and atherosclerotic burden: clinical evidence
Experiments with LDLR- and/or VDR-deficient mice demonstrated beneficial effects of vitamin D signalling on atherosclerosis progression. Likewise, clinical studies generally agree on the relationship between 25(OH)D and subclinical atherosclerosis. A recent pooled analysis of 21 studies matching ∼2000 vitamin D deficiency/insufficiency patients with controls reported a significant difference in carotid intima-media thickness and prevalence of carotid plaques.102 Two subsequent meta-analyses confirmed these findings and support the beneficial effects of vitamin D supplementation on this biomarker.103,104 The association between vitamin D levels and coronary atherosclerotic burden or composition remains less clear. A single systematic review dating 2014 did not find consistent evidence for the association with coronary artery calcification (CAC) and highlighted the relevant differences across studies.105 The heterogeneity in the definition of vitamin D deficiency/insufficiency and CAC criteria precluded a quantitative meta-analysis. Later studies did not solve this issue even when considering other biomarkers of plaque vulnerability106–113 (Table 3). Polymorphisms in the VDR gene were suggested as determinants of such a variability.114–116 A recent Mendelian randomization analysis supported a causal role for vitamin D deficiency as well as the beneficial effects of its supplementation on CV health. Of interest, this analysis found a non-linear L-shaped relationship, a feature formerly overlooked in randomized clinical trials and Mendelian randomization studies.117
Table 3.
Summary of the main studies addressing the association between vitamin D deficiency and coronary artery calcium score and biomarkers of atherosclerotic plaque vulnerability
| Authors | Year | Study design (patient number) | Outcome(s) | Results |
|---|---|---|---|---|
| Martín-Reyes et al.106 | 2016 | Cross-sectional (270) | Categorized CAC |
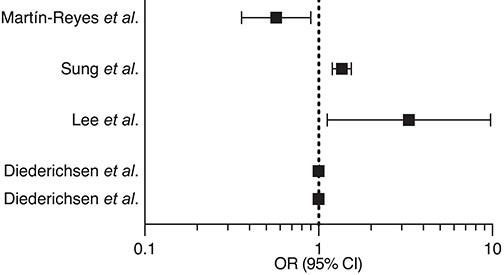
|
| Sung et al.107 | 2016 | Cross-sectional (180,918) | CAC | |
| Lee et al.108 | 2016 | Cross-sectional (195) | ||
| Diederichsen et al.109 | 2017 | Prospective (1227) | Incident CAC CAC progression |
|
| Sajjadieh et al.111 | 2020 | Cross-sectional (67) | CAC | There was no correlation between CAC and vitamin D levels (r = −0.03) |
| Anis et al.112 | 2020 | RCT (48w FU) (44) | CAC | CAC progression did not differ between treatment arms (calcitriol vs. paricalcitol; P = 0.76) |
| Rodrigues et al.113 | 2021 | Cross-sectional (140) | CAC | Excess visceral fat (OR 4.0 [1.4–11.7]) but not vitamin D is associated with subclinical atherosclerosis |
CAC, coronary artery calcification; OR, odds ratio; CI, confidence Interval; FU, follow-up.
From vulnerable plaque to vulnerable patients: the role of vitamin D on blood and myocardium/brain vulnerability
Vitamin D deficiency and thrombogenicity
The evolution of an atherosclerotic plaque to produce a clinical thrombotic event requires a ‘perfect storm’ in which a pro-thrombotic milieu in the fluid phase of blood meets the ‘solid state’ of a culprit plaque91,118,119 (Figure 3). Endothelial functions contribute to the crosstalk between the atheroma and circulation and influence critically the vulnerability of the plaque and that of the patient. ECs express VDR constitutively, whereas in stress conditions (e.g. disturbed flow, pro-oxidant environment, hyperglycaemia) CYP27B1 may induce this receptor. Impaired vitamin D signalling in ECs enhances the interaction of leucocytes with the endothelium (i.e. rolling, adhesion, and migration).120 Furthermore, VDR silencing/vitamin D supplementation revealed the multifaceted actions of the vitamin D axis in ECs that involve: apoptosis, oxidative stress and nitric oxide generation, lipid metabolism, and interaction with platelets.121,122 The effect on platelets depends in part on the reduction of adhesion molecules on the EC surface and EC-derived microparticles and with integrin activation on the platelet surface. These broad effects of vitamin D on endothelial function can influence arterial stiffness (assessed by brachial artery flow-mediated dilation or by pulse wave velocity), as is common in patients with vitamin D deficiency. Conversely, the effects of vitamin D supplementation on markers of vascular function remain controversial, often showing only slight or insignificant alterations.123,124
Along with the aforementioned factors (i.e. chronic low-grade inflammatory status, chemokines/adhesion molecules, and EC dysfunction), the pro-thrombotic milieu depends on tissue factor expression, coagulation factors, and platelet activation. As these features do not yet clearly associate with vitamin D biology, this topic has become an intriguing research problem.125 The anti-thrombogenic effect of vitamin D was first documented in venous thromboembolism, whereas studies on VDR-deleted mice found increased platelet aggregation and imbalance between antithrombin/thrombomodulin and tissue factor expression.126–128 Accordingly, preliminary clinical studies reported an inverse association between vitamin D deficiency and mean platelet volume, a biomarker of platelet reactivity.129,130 A recent randomized clinical trial testing a weekly dose of 60 000 IU of 25(OH)D in diabetic patients reported an anti-inflammatory inhibitory effect on platelet activation and immune cell aggregate in the intervention arm.131
Vitamin D and its effects on myocardium and brain vulnerability and remodelling
Reperfusion strategies now comprise the cornerstone of the management of many acute ischaemic complications of atherosclerosis (i.e. ST-segment elevation myocardial infarction and many ischaemic strokes). Yet, the benefit of reperfusion comes at the cost of massive inflammatory and oxidative responses, potentially exacerbating damage to the hypoxic tissues, as well as bleeding risks following thrombolytic therapy or anti-platelet treatment in those undergoing percutaneous intervention. Optimal healing requires a timely orchestration of multiple processes.132,133 Cytokines and growth factors dynamically modulate resolution and repair, as well as local angiogenic responses. Also, the no-reflow phenomenon can impair the microcirculation in ischaemically injured tissues. Vitamin D deficiency may associate indirectly with impaired microvascular function as highlighted by the association of low coronary flow reserve.134,135
Moreover, given its immunomodulatory properties and the widespread expression of VDR in myocardial tissue, vitamin D may influence post-infarction cardiac remodelling. VDR deletion causes myocardial hypertrophy independently of RAAS activation and calcium abnormalities, while vitamin D supplementation may reverse cardiac steatosis and interstitial fibrosis.15,136,137 Although few experimental studies specifically focused on post-ischaemic injury, some suggest a beneficial effect of vitamin D on left ventricular remodelling.137–139 Animal experiments have supported population-based studies140,141 and the EVITA trial seemed to exclude a link between vitamin D and RAAS.142,143 The VINDICATE trial suggested a beneficial effect of vitamin D supplementation on the left ventricular structure and function144 but meta-analyses generally failed to show significant benefits145–149 (Table 4). Mechanistic studies on vitamin D in myocardial remodelling are lacking. It is, therefore, difficult to establish whether any clinical association reflects causality.152
Table 4.
Summary of randomized clinical trials investigating the effects of vitamin D supplementation on myocardial infarction
| Authors | Year | Intervention (patient number) | Outcome(s) | Results |
|---|---|---|---|---|
| Zittermann et al. EVITA trial 150 | 2017 | Cholecalciferol 4000 IU/die i.m for 3 years (200 HF patients and control group) |
|
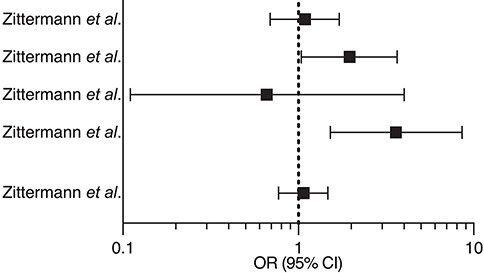
|
| Zittermann et al. EVITA trial 143 | 2020 | Cholecalciferol 4000 IU/die i.m for 3 years (200 HF patients and control group) |
|
|
| Zittermann et al. EVITA trial 142 | 2018 | Cholecalciferol 4000 IU/die i.m for 3 years (83 HF patients and control group) |
|
Vitamin D did not affect RAAS system: renin (r = 0.157) or aldosteronee (r = 0.048) |
| Zittermann et al. EVITA trial 151 | 2019 | Cholecalciferol 4000 IU/die i.m for 3 years (200 HF patients and control group) |
|
Vitamin D did not improve myocardial function |
| Witte et al. VINDICATE study 144 | 2016 | Cholecalciferol 4000 IU/die i.m. (n = 80 HF patients and control group) |
|
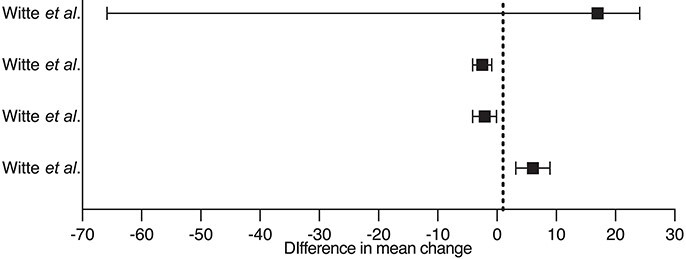
|
CI, confidence interval; OR, odds ratio; HF, heart failure; MCS, mechanical circulatory support; RAAS, renin–angiotensin–aldosterone system; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; 6MWT, 6-min walking test.
In distinction with the myocardium, the blood–brain barrier (BBB) tightly regulates the accessibility of immune cells to the parenchyma. Protection of BBB integrity153,154 and antioxidant activity155,156 are key effects of vitamin D in the early phases of ischaemic brain injury. Vitamin D might also contribute to late neuroprotection by preventing neuronal apoptosis157 and promoting angiogenesis.158 Experimental evidence agrees with clinical studies in which vitamin D levels correlate with the radiological estimation of ischaemic volume159–161 and with functional outcome.162 Yet, few studies have so far investigated the effects of vitamin D supplementation on stroke outcomes. Such studies have yielded inconsistent results, although they differed greatly in design and outcomes assessed163–168 (Table 5).
Table 5.
Summary of randomized clinical trials investigating the effects of vitamin D supplementation on stroke outcome
| Authors | Year | Intervention (patient number) | Outcome(s) | Results |
|---|---|---|---|---|
| Narasimhan et al. 163 | 2017 | Cholecalciferol 6 lac IU i.m. (n = 30 per group) |
|
Stroke outcome significantly improved in vitamin D deficient—but not insufficient—patients once supplemented (mean 6.39 ± 4.56 vs. 2.50 ± 2.20; P < 0.001) |
| Sari et al. 164 | 2018 | Cholecalciferol 300 000 IU i.m. (n = 36 per group) |
|
3.09 ± 0.99 vs. 3.34 ± 5.28; P = 0.794
2.34 ± 1.31 vs. 1.60 ± 1.16; P = 0.018 59.18 ± 22.15 vs. 41.87 ± 20.42; P = 0.002 38.72 ± 14.14 vs. 24.75 ± 14.76; P < 0.001 |
| Momosaki et al. 165 | 2019 | Cholecalciferol 2 000 IU/die i.m. (n = 49/48) |
|
19 ± 15 vs. 20 ± 13; P = 0.480
27%–20% vs. 25%–18%; P = 0.770;0.740 19%–20% vs. 23%–20%; P = 0.770;0.600 |
| Rist et al. 166 from the VITAL study | 2021 | Cholecalciferol 2 000 IU/die i.m. in primary prevention (n = 104/93 per group) |
Nagi scale
Rosow-Breslau scale Katz AD scale |
OR 1.01 [95% CI 0.52–1.97]
OR 0.92 [95% CI 0.59–1.67] OR 1.03 [95% CI 0.31–3.42] |
| Torrisi et al. 167 | 2021 | Cholecalciferol 2 000 IU/die i.m. (n = 15/14) per group) |
MADRS
FIM GSE (all at 12 weeks) |
Both groups improved mood and functional recovery independently of vitamin D supplementation |
| Wang et al. 168 | 2021 | Cholecalciferol 600 IU/die i.m. (n = 72/51 per group) |
mRS
FSS (both at 3 months) |
FSS and mRS significanlty lowered in the supplementation group (P < 0.01 for both) |
CI, confidence interval; OR, odds ratio; SSS, Scandinavian Stroke Scale; BRS, Brunnstrom recovery staging; FAS, functional ambulation scale; MBI, modified Barthel index; BBS, Berg balance scale; MADRS, Montgomery Aasberg depression rating scale; FIM, functional independent measures; GSE, general self-efficacy; mRS, modified Rankin score; FSS, fatigue severity scale.
Global cardiovascular risk related to vitamin D deficiency and supplementation: clinical evidence
Considerable observational evidence links vitamin D deficiency with poor CV prognosis. In the ARIC study, the risk of incident heart failure during 21 years of follow-up was two-fold higher in the lowest quintile (≤17 ng/mL) of 25(OH)D.169 A large body of observational studies has further characterized the CV risk associated with vitamin D deficiency. The data consistently associate vitamin D deficiency with increased incidence and prevalence of subclinical and clinical atherosclerotic disease, myocardial infarction, ischaemic stroke, and peripheral artery disease.2 However, the poor prognosis associated with vitamin D deficiency is not CV-specific, as NHANES III reported a 26% higher overall mortality in those with lower levels of vitamin D.170 Indeed, a critical reappraisal of observational studies raises issues of reverse causation. Vitamin sufficiency may reflect good health in subjects engaged in physical activity and healthy diet. Whereas the Cooper Center Longitudinal Study recently confirmed a benefit of physical activity of vitamin D sufficiency,171 the negative effect on %fat would further contribute to the rise of circulating 25(OH)D in the EpiFloripa Aging Cohort Study.172 Similarly, unhealthy dietary patterns associate not only directly with vitamin D deficiency but also promote other aspects of dysmetabolism further reducing 25(OH)D levels.173
We thus possess a plethora of observational and experimental studies relating to the health effects of vitamin D. Yet, from a practical clinical perspective, what matters most is whether screening for vitamin D status, and supplementation in those found to be vitamin D deficient, can improve patient outcomes. In line with this ‘D-lemma’—as repeatedly quoted by Holick—substantial evidence now shows that vitamin D supplementation does not confer CV benefit.
The origins of tailored randomized clinical trials date back to 2008, when the first conflicting data emerged from interventional studies.174–176 Vitamin D Assessment (ViDA) study and VITamin D and OmegA-3 TriaL (VITAL) were registered almost simultaneously. They both reassuringly reported similar results despite different vitamin D dosing schedules, monthly for ViDA and daily for VITAL. Despite a significant rise in serum 25(OH)D concentrations, the interventions had no effect on the cumulative incidence of CV events (11.8% vs. 11.5% and 6.5% vs. 6.4% for ViDA and VITAL, respectively). Accumulating evidence that vitamin D supplementation benefits primarily deficient people initially rose questions regarding the ViDA and VITAL trials, due to the broad range of serum 25(OH)D levels in the enrolled cohorts, although it now seems established that their findings do not depend on the baseline 25(OH)D levels or CV history.177 Furthermore, evidence from Mendelian randomization studies converges in supporting vitamin D’s inefficacy in reducing CV risk.178,179 An extensive meta-analysis of 21 randomized clinical trials enrolling 83 291 participants reported no benefit of vitamin D supplementation on major adverse CV events, myocardial infarction, stroke, CV, or overall mortality.1 Two other contemporary meta-analyses have provided similar results.180,181 Similarly, recent randomized clinical trials failed to prove a benefit of vitamin D supplementation on diabetes risk (D2d study)182 and associated renal impairment (ancillary study to the VITAL),183 hypertension,184 or CV mortality (D-Health and Finnish vitamin D trials)185,186 (Table 6). On the contrary, most recent trials showed beneficial effects of vitamin D supplementation on body composition and cardio-metabolic/inflammatory biomarkers.187–189 Such results were not unexpected as vitamin D supplementation recently showed favourable effects on atherosclerotic CV disease score without reducing major adverse CV event incidence.190
Table 6.
Summary of randomized clinical trials investigating the effects of vitamin D supplementation on CV risk
| Authors | Year | Intervention (patient number) | Outcome(s) | Results |
|---|---|---|---|---|
| Pittas et al. D2d study 182 | 2019 | Cholecalciferol 4000 IU/die i.m (1211 patients with pre-diabetes) |
|
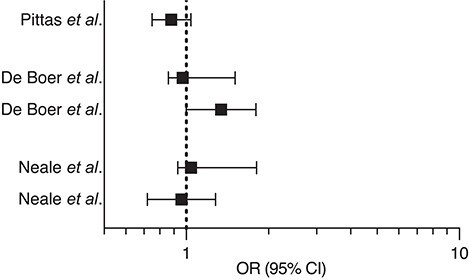
|
| de Boer et al. VITAL trial 183 | 2019 | Cholecalciferol 2000 IU/die i.m (333 patients with diabetes) |
|
|
| Neale et al. D-Health trial 185 | 2022 | Cholecalciferol 60 000 IU/month i.m (10662 older people and control group) |
|
|
| Theiler-Schwetz et al. Styrian Vitamin D Hypertension Trial 184 | 2022 | Cholecalciferol 2800 IU/die i.m (100 hypertensive patients and control group) |
|
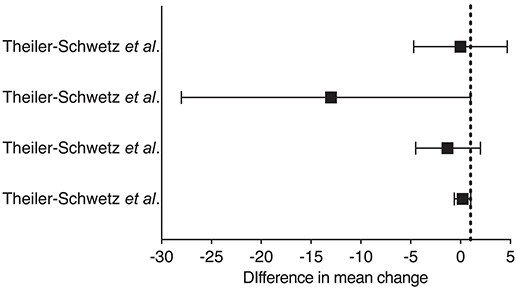
|
CI, confidence interval; OR, odds ratio; HR, hazard ratio; eGFR, estimated glomerular filtration rate; ACR, albumin–creatinine ratio; BP, blood pressure; PWV, pulse wave velocity.
Shortcomings and outlook
Vitamin D deficiency affects populations worldwide. It appears prevalent in Western countries which show a strong relationship of vitamin D deficiency with CV disease risk and prevalence. Yet, such associations do not establish a causal relationship. During the past decade, experimental evidence on the vitamin D axis and CV health generated important advances, but a wide gap still exists between the research realm and practical clinical measures. Many mechanisms may link vitamin D deficiency with cardio-metabolic risk, atherosclerosis, and its complications (Graphical Abstract). Experimental studies require more rigour. For example, VDR deletion does not address the rapid actions of vitamin D that do not require altered gene transcription. Molecular and epigenetic senescence are newly appreciated mechanisms likely regulated by the vitamin D axis worthy of further investigation. Very recent studies indeed support a potential senolytic activity of vitamin D supplementation, mainly targeting epigenetic ageing and telomerase activity.27,191 The epigenetic clocks might then furnish the intermediate link between vitamin D and CV outcome. Such a change of view—interpreting vitamin D as senolytic—may then help to design future experimental clinical studies.
Ultimately, the field needs to address the inconsistencies in clinical translation. Determinants of vitamin D synthesis, intake, and body distribution require standardization in the design of future clinical trials. Distinctions between fortified food and pharmacologic supplementation is another critical point, compounded by the widespread use of calcium supplements.2 The available data do not justify the screening for vitamin D deficiency in asymptomatic adults.172,192 Vitamin D supplementation has no proven CV benefit with the exception of end-stage renal disease, and even the current thresholds for vitamin D deficiency are still bone-driven. Framing ‘non-classical’—often local—effects of the vitamin D axis with traditional categories will not allow to clarify the extent to which vitamin D status influences cardio-metabolic risk or serves as an independent biomarker of good health. The putative link between vitamin D and cardio-metabolic risk is likely complex and future studies should take reverse causation into account rigorously.
Acknowledgements
None declared.
Contributor Information
Federico Carbone, First Clinic of Internal Medicine, Department of Internal Medicine, University of Genoa, 6 viale Benedetto XV, 16132 Genoa, Italy; IRCCS Ospedale Policlinico San Martino, Genoa—Italian Cardiovascular Network, Genoa, Italy.
Luca Liberale, First Clinic of Internal Medicine, Department of Internal Medicine, University of Genoa, 6 viale Benedetto XV, 16132 Genoa, Italy; IRCCS Ospedale Policlinico San Martino, Genoa—Italian Cardiovascular Network, Genoa, Italy.
Peter Libby, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Fabrizio Montecucco, First Clinic of Internal Medicine, Department of Internal Medicine, University of Genoa, 6 viale Benedetto XV, 16132 Genoa, Italy; IRCCS Ospedale Policlinico San Martino, Genoa—Italian Cardiovascular Network, Genoa, Italy.
Data availability
No new data were generated or analysed in support of this research.
Funding
L.L. receives funding support from the Swiss Heart Foundation. P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892, 1R01HL163099-01, and 1R01HL163099-01), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund. This work was supported by grants from Italian Ministry of Health—5 × 1000 funds 2020 to F.M.
References
- 1. Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol 2019;4:765–776. 10.1001/jamacardio.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michos ED, Cainzos-Achirica M, Heravi AS, Appel LJ. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC focus seminar. J Am Coll Cardiol 2021;77:437–449. 10.1016/j.jacc.2020.09.617 [DOI] [PubMed] [Google Scholar]
- 3. Duan L, Han L, Liu Q, Zhao Y, Wang L, Wang Y. Effects of vitamin D supplementation on general and central obesity: results from 20 randomized controlled trials involving apparently healthy populations. Ann Nutr Metab 2020;76:153–164. 10.1159/000507418 [DOI] [PubMed] [Google Scholar]
- 4. Dewansingh P, Reckman GAR, Mijlius CF, Krijnen WP, van der Schans CP, Jager-Wittenaar H, et al. Protein, calcium, vitamin D intake and 25(OH)D status in normal weight, overweight, and obese older adults: a systematic review and meta-analysis. Front Nutr 2021;8:718658. 10.3389/fnut.2021.718658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med 2018;48:6–17. 10.1016/j.ejim.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 6. Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020;126:1477–1500. 10.1161/CIRCRESAHA.120.316101 [DOI] [PubMed] [Google Scholar]
- 7. Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 2018;137:1391–1406. 10.1161/CIRCULATIONAHA.117.029617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chattranukulchai Shantavasinkul P, Nimitphong H. Vitamin D and visceral obesity in humans: what should clinicians know? Nutrients 2022;14:3075. 10.3390/nu14153075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr 2011;14:938–939. 10.1017/S1368980011000565 [DOI] [PubMed] [Google Scholar]
- 10. The Human Protein Atlas . The Human Protein Atlas, VDR. https://www.proteinatlas.org/ENSG00000111424-VDR (26 September 2022, date last accessed).
- 11. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77–98. 10.1007/s00223-012-9619-0 [DOI] [PubMed] [Google Scholar]
- 12. Carlberg C. Vitamin D and its target genes. Nutrients 2022;14:1354. 10.3390/nu14071354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donati S, Palmini G, Aurilia C, Falsetti I, Miglietta F, Iantomasi T, et al. Rapid nontranscriptional effects of calcifediol and calcitriol. Nutrients 2022;14:1291. 10.3390/nu14061291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol 2007;103:521–524. 10.1016/j.jsbmb.2006.12.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011;124:1838–1847. 10.1161/CIRCULATIONAHA.111.032680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurpas A, Supel K, Idzikowska K, Zielinska M. FGF23: a review of its role in mineral metabolism and renal and cardiovascular disease. Dis Markers 2021;2021:8821292. 10.1155/2021/8821292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berridge MJ. Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J Physiol 2017;595:6825–6836. 10.1113/JP274887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020;41:2974–2982. 10.1093/eurheartj/ehz961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liberale L, Badimon L, Montecucco F, Luscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol 2022;79:837–847. 10.1016/j.jacc.2021.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Latham CM, Brightwell CR, Keeble AR, Munson BD, Thomas NT, Zagzoog AM, et al. Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front Physiol 2021;12:660498. 10.3389/fphys.2021.660498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhutia SK. Vitamin D in autophagy signaling for health and diseases: insights on potential mechanisms and future perspectives. J Nutr Biochem 2022;99:108841. 10.1016/j.jnutbio.2021.108841 [DOI] [PubMed] [Google Scholar]
- 22. Kumar S, Nanduri R, Bhagyaraj E, Kalra R, Ahuja N, Chacko AP, et al. Vitamin D3-VDR-PTPN6 axis mediated autophagy contributes to the inhibition of macrophage foam cell formation. Autophagy 2021;17:2273–2289. 10.1080/15548627.2020.1822088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uberti F, Lattuada D, Morsanuto V, Nava U, Bolis G, Vacca G, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab 2014;99:1367–1374. 10.1210/jc.2013-2103 [DOI] [PubMed] [Google Scholar]
- 24. Lee TL, Lee MH, Chen YC, Lee YC, Lai TC, Lin HY, et al. Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Front Pharmacol 2020;11:604700. 10.3389/fphar.2020.604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendoza A, Karch J. Keeping the beat against time: mitochondrial fitness in the aging heart. Front Aging 2022;3:951417. 10.3389/fragi.2022.951417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zarei M, Zarezadeh M, Hamedi Kalajahi F, Javanbakht MH. The relationship between vitamin D and telomere/telomerase: a comprehensive review. J Frailty Aging 2021;10:2–9. 10.14283/jfa.2020.33 [DOI] [PubMed] [Google Scholar]
- 27. Vetter VM, Sommerer Y, Kalies CH, Spira D, Bertram L, Demuth I. Vitamin D supplementation is associated with slower epigenetic aging. Geroscience 2022;44:1847–1859. 10.1007/s11357-022-00581-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai Y, Chen D, Xu T. DNA methylation aberrant in atherosclerosis. Front Pharmacol 2022;13:815977. 10.3389/fphar.2022.815977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carpenter AB, Smailer S. Measurement of rheumatoid factor isotypes in the clinical laboratory. Immunol Invest 1989;18:765–773. 10.3109/08820138909030597 [DOI] [PubMed] [Google Scholar]
- 30. Ao T, Kikuta J, Ishii M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021;11:1624. 10.3390/biom11111624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 2009;182:4289–4295. 10.4049/jimmunol.0803736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chun RF, Shieh A, Gottlieb C, Yacoubian V, Wang J, Hewison M, et al. Vitamin D binding protein and the biological activity of vitamin D. Front Endocrinol (Lausanne) 2019;10:718. 10.3389/fendo.2019.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raffaele M, Vinciguerra M. The costs and benefits of senotherapeutics for human health. Lancet Healthy Longev 2022;3:e67–e77. 10.1016/S2666-7568(21)00300-7 [DOI] [PubMed] [Google Scholar]
- 34. Ferrari D, Lombardi G, Banfi G. Concerning the vitamin D reference range: pre-analytical and analytical variability of vitamin D measurement. Biochem Med (Zagreb) 2017;27:030501. 10.11613/BM.2017.030501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. EFSA Authority . Dietary reference values for vitamin D, 2016. https://www.efsa.europa.eu/it/efsajournal/pub/4547 (18 May 2022, date last accessed).
- 36. (SACN) SACoN . The Scientific Advisory Committee on Nutrition (SACN) recommendations on vitamin D, 2016. https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (14 March 2023, date last accessed).
- 37. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol 2019;180:P23–P54. 10.1530/EJE-18-0736 [DOI] [PubMed] [Google Scholar]
- 38. Liu X, Baylin A, Levy PD. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br J Nutr 2018;119:928–936. 10.1017/S0007114518000491 [DOI] [PubMed] [Google Scholar]
- 39. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911–1930. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 40. Shea MK, Houston DK, Tooze JA, Davis CC, Johnson MA, Hausman DB, et al. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the health, aging and body composition study. J Am Geriatr Soc 2011;59:1165–1174. 10.1111/j.1532-5415.2011.03476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nimitphong H, Guo W, Holick MF, Fried SK, Lee MJ. Vitamin D inhibits adipokine production and inflammatory signaling through the vitamin D receptor in human adipocytes. Obesity 2021;29:562–568. 10.1002/oby.23109 [DOI] [PubMed] [Google Scholar]
- 42. Bennour I, Haroun N, Sicard F, Mounien L, Landrier JF. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes Rev 2022;23:e13453. 10.1111/obr.13453 [DOI] [PubMed] [Google Scholar]
- 43. Szymczak-Pajor I, Miazek K, Selmi A, Balcerczyk A, Sliwinska A. The action of vitamin D in adipose tissue: is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders? Int J Mol Sci 2022;23:956. 10.3390/ijms23020956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvonen MT, Salehzadeh F, et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat Med 2021;27:1941–1953. 10.1038/s41591-021-01501-8 [DOI] [PubMed] [Google Scholar]
- 45. Ricciardi CJ, Bae J, Esposito D, Komarnytsky S, Hu P, Chen J, et al. 1,25-Dihydroxyvitamin D3/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. Eur J Nutr 2015;54:1001–1012. 10.1007/s00394-014-0778-9 [DOI] [PubMed] [Google Scholar]
- 46. Cruciani S, Garroni G, Pala R, Cossu ML, Ginesu GC, Ventura C, et al. Metformin and vitamin D modulate inflammation and autophagy during adipose-derived stem cell differentiation. Int J Mol Sci 2021;22:6686. 10.3390/ijms22136686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sergeev IN. Vitamin D-cellular Ca(2+) link to obesity and diabetes. J Steroid Biochem Mol Biol 2016;164:326–330. 10.1016/j.jsbmb.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 48. Santaniello S, Cruciani S, Basoli V, Balzano F, Bellu E, Garroni G, et al. Melatonin and vitamin D orchestrate adipose derived stem cell fate by modulating epigenetic regulatory genes. Int J Med Sci 2018;15:1631–1639. 10.7150/ijms.27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jonas MI, Kurylowicz A, Bartoszewicz Z, Lisik W, Jonas M, Kozniewski K, et al. Vitamin D receptor gene expression in adipose tissue of obese individuals is regulated by miRNA and correlates with the pro-inflammatory cytokine level. Int J Mol Sci 2019;20:5272. 10.3390/ijms20215272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol 2012;280:36–43. 10.1016/j.cellimm.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 51. Clemente-Postigo M, Munoz-Garach A, Serrano M, Garrido-Sanchez L, Bernal-Lopez MR, Fernandez-Garcia D, et al. Serum 25-hydroxyvitamin D and adipose tissue vitamin D receptor gene expression: relationship with obesity and type 2 diabetes. J Clin Endocrinol Metab 2015;100:E591–E595. 10.1210/jc.2014-3016 [DOI] [PubMed] [Google Scholar]
- 52. Bonnet L, Karkeni E, Couturier C, Astier J, Defoort C, Svilar L, et al. Four days high fat diet modulates vitamin D metabolite levels and enzymes in mice. J Endocrinol 2021;248:87–93. 10.1530/JOE-20-0198 [DOI] [PubMed] [Google Scholar]
- 53. Landrier JF, Derghal A, Mounien L. MicroRNAs in obesity and related metabolic disorders. Cells 2019;8:859. 10.3390/cells8080859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–248. 10.2337/db09-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cordeiro A, Campos B, Pereira SE, Saboya CJ, Ramalho A. Inadequacy of vitamin D nutritional status in individuals with metabolically unhealthy obesity phenotype: the relevance of insulin resistance. Diabetes Metab Syndr Obes 2020;13:4131–4139. 10.2147/DMSO.S256132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yuzbashian E, Asghari G, Hedayati M, Zarkesh M, Mirmiran P, Khalaj A. Determinants of vitamin D receptor gene expression in visceral and subcutaneous adipose tissue in non-obese, obese, and morbidly obese subjects. J Steroid Biochem Mol Biol 2019;187:82–87. 10.1016/j.jsbmb.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 57. Klinedinst BS, Meier NF, Larsen B, Wang Y, Yu S, Mochel JP, et al. Walking in the light: how history of physical activity, sunlight, and vitamin D account for body fat—a UK Biobank Study. Obesity 2020;28:1428–1437. 10.1002/oby.22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wamberg L, Christiansen T, Paulsen SK, Fisker S, Rask P, Rejnmark L, et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue—the effect of obesity and diet-induced weight loss. Int J Obes (Lond) 2013;37:651–657. 10.1038/ijo.2012.112 [DOI] [PubMed] [Google Scholar]
- 59. Gangloff A, Bergeron J, Pelletier-Beaumont E, Nazare JA, Smith J, Borel AL, et al. Effect of adipose tissue volume loss on circulating 25-hydroxyvitamin D levels: results from a 1-year lifestyle intervention in viscerally obese men. Int J Obes (Lond) 2015;39:1638–1643. 10.1038/ijo.2015.118 [DOI] [PubMed] [Google Scholar]
- 60. Gangloff A, Bergeron J, Lemieux I, Tremblay A, Poirier P, Almeras N, et al. Relationships between circulating 25(OH) vitamin D, leptin levels and visceral adipose tissue volume: results from a 1-year lifestyle intervention program in men with visceral obesity. Int J Obes (Lond) 2020;44:280–288. 10.1038/s41366-019-0347-7 [DOI] [PubMed] [Google Scholar]
- 61. Buscemi S, Buscemi C, Corleo D, De Pergola G, Caldarella R, Meli F, et al. Obesity and circulating levels of vitamin D before and after weight loss induced by a very low-calorie ketogenic diet. Nutrients 2021;13:1829. 10.3390/nu13061829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A, et al. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl 2019;9:20–31. 10.1038/s41367-019-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Elkhwanky MS, Kummu O, Piltonen TT, Laru J, Morin-Papunen L, Mutikainen M, et al. Obesity represses CYP2R1, the vitamin D 25-hydroxylase, in the liver and extrahepatic tissues. JBMR Plus 2020;4:e10397. 10.1002/jbm4.10397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Oliveira LF, de Azevedo LG, da Mota Santana J, de Sales LPC, Pereira-Santos M. Obesity and overweight decreases the effect of vitamin D supplementation in adults: systematic review and meta-analysis of randomized controlled trials. Rev Endocr Metab Disord 2020;21:67–76. 10.1007/s11154-019-09527-7 [DOI] [PubMed] [Google Scholar]
- 65. Dozio E, Briganti S, Vianello E, Dogliotti G, Barassi A, Malavazos AE, et al. Epicardial adipose tissue inflammation is related to vitamin D deficiency in patients affected by coronary artery disease. Nutr Metab Cardiovasc Dis 2015;25:267–273. 10.1016/j.numecd.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 66. Gurses KM, Tokgozoglu L, Yalcin MU, Kocyigit D, Evranos B, Yorgun H, et al. Epicardial fat thickness is increased in vitamin D deficient premenopausal women and does not decrease after short-term replacement. J Atheroscler Thromb 2015;22:582–589. 10.5551/jat.28381 [DOI] [PubMed] [Google Scholar]
- 67. Scott D, Joham A, Teede H, Gibson-Helm M, Harrison C, Cassar S, et al. Associations of vitamin D with inter- and intra-muscular adipose tissue and insulin resistance in women with and without polycystic ovary syndrome. Nutrients 2016;8:774. 10.3390/nu8120774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Di Filippo L, De Lorenzo R, Giustina A, Rovere-Querini P, Conte C. Vitamin D in osteosarcopenic obesity. Nutrients 2022;14:1816. 10.3390/nu14091816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chang HX, Zhao XJ, Zhu QL, Hou Q, Li Y. Removal of epicardial adipose tissue after myocardial infarction improves cardiac function. Herz 2018;43:258–264. 10.1007/s00059-017-4555-4 [DOI] [PubMed] [Google Scholar]
- 70. Horckmans M, Bianchini M, Santovito D, Megens RTA, Springael JY, Negri I, et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation 2018;137:948–960. 10.1161/CIRCULATIONAHA.117.028833 [DOI] [PubMed] [Google Scholar]
- 71. Hendricks S, Dykun I, Balcer B, Totzeck M, Rassaf T, Mahabadi AA. Epicardial adipose tissue is a robust measure of increased risk of myocardial infarction—a meta-analysis on over 6600 patients and rationale for the EPIC-ACS study. Medicine (Baltimore) 2021;100:e28060. 10.1097/MD.0000000000028060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Karatayli E, Stokes CS, Lammert F. Vitamin D in preclinical models of fatty liver disease. Anticancer Res 2020;40:527–534. 10.21873/anticanres.13981 [DOI] [PubMed] [Google Scholar]
- 73. Holmes D. NAFLD: vitamin D-induced autophagy prevents steatosis. Nat Rev Endocrinol 2017;13:190. [DOI] [PubMed] [Google Scholar]
- 74. Al-Ghamdi HA, Al Fayez FF, Bima AI, Khawaji TM, Elsamanoudy AZ. Study of cellular senescence and vitamin D deficiency in nonalcoholic fatty liver disease and the potential protective effect of vitamin D supplementation. J Clin Exp Hepatol 2021;11:219–226. 10.1016/j.jceh.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang Z, Moon R, Thorne JL, Moore JB. NAFLD And vitamin D: evidence for intersection of microRNA-regulated pathways. Nutr Res Rev 2021;28:1–20. 10.1017/S095442242100038X [DOI] [PubMed] [Google Scholar]
- 76. Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, et al. The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr 2017;11:S975–S982. 10.1016/j.dsx.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 77. Mansour-Ghanaei F, Pourmasoumi M, Hadi A, Ramezani-Jolfaie N, Joukar F. The efficacy of vitamin D supplementation against nonalcoholic fatty liver disease: a meta-analysis. J Diet Suppl 2020;17:467–485. 10.1080/19390211.2019.1624671 [DOI] [PubMed] [Google Scholar]
- 78. Guo XF, Wang C, Yang T, Li S, Li KL, Li D. Vitamin D and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Food Funct 2020;11:7389–7399. 10.1039/D0FO01095B [DOI] [PubMed] [Google Scholar]
- 79. Wei Y, Wang S, Meng Y, Yu Q, Wang Q, Xu H, et al. Effects of vitamin D supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Int J Endocrinol Metab 2020;18:e97205. 10.5812/ijem.97205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rezaei S, Tabrizi R, Nowrouzi-Sohrabi P, Jalali M, Shabani-Borujeni M, Modaresi S, et al. The effects of vitamin D supplementation on anthropometric and biochemical indices in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Pharmacol 2021;12:732496. 10.3389/fphar.2021.732496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sindhughosa DA, Wibawa IDN, Mariadi IK, Somayana G. Additional treatment of vitamin D for improvement of insulin resistance in non-alcoholic fatty liver disease patients: a systematic review and meta-analysis. Sci Rep 2022;12:7716. 10.1038/s41598-022-11950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trimarco V, Manzi MV, Mancusi C, Strisciuglio T, Fucile I, Fiordelisi A, et al. Insulin resistance and vitamin D deficiency: a link beyond the appearances. Front Cardiovasc Med 2022;9:859793. 10.3389/fcvm.2022.859793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rafiq S, Jeppesen PB. Insulin resistance is inversely associated with the status of vitamin D in both diabetic and non-diabetic populations. Nutrients 2021;13:1742. 10.3390/nu13061742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One 2018;13:e0194127. 10.1371/journal.pone.0194127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carbone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol 2014;6:260–276. 10.4330/wjc.v6.i5.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Surdu AM, Pinzariu O, Ciobanu DM, Negru AG, Cainap SS, Lazea C, et al. Vitamin D and its role in the lipid metabolism and the development of atherosclerosis. Biomedicines 2021;9:172. 10.3390/biomedicines9020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol 2013;28:205–221. 10.1007/s10654-013-9790-2 [DOI] [PubMed] [Google Scholar]
- 88. Kelishadi R, Farajzadegan Z, Bahreynian M. Association between vitamin D status and lipid profile in children and adolescents: a systematic review and meta-analysis. Int J Food Sci Nutr 2014;65:404–410. 10.3109/09637486.2014.886186 [DOI] [PubMed] [Google Scholar]
- 89. Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res 2011;50:303–312. 10.1016/j.plipres.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 90. He S, Hao X. The effect of vitamin D3 on blood pressure in people with vitamin D deficiency: a system review and meta-analysis. Medicine (Baltimore) 2019;98:e15284. 10.1097/MD.0000000000015284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Montecucco F, Carbone F, Schindler TH. Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatments. Eur Heart J 2016;37:1268–1283. 10.1093/eurheartj/ehv592 [DOI] [PubMed] [Google Scholar]
- 92. Carbone F, Montecucco F. The role of the intraplaque vitamin d system in atherogenesis. Scientifica (Cairo) 2013;2013:620504. 10.1155/2013/620504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schnatz PF, Nudy M, O’Sullivan DM, Jiang X, Cline JM, Kaplan JR, et al. The quantification of vitamin D receptors in coronary arteries and their association with atherosclerosis. Maturitas 2012;73:143–147. 10.1016/j.maturitas.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Carbone F, Satta N, Burger F, Roth A, Lenglet S, Pagano S, et al. Vitamin D receptor is expressed within human carotid plaques and correlates with pro-inflammatory M1 macrophages. Vascul Pharmacol 2016;85:57–65. 10.1016/j.vph.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 95. Carlberg C. Vitamin D signaling in the context of innate immunity: focus on human monocytes. Front Immunol 2019;10:2211. 10.3389/fimmu.2019.02211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang X, Zhao Y, Zhu X, Guo Y, Yang Y, Jiang Y, et al. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J Cell Physiol 2019;234:6917–6926. 10.1002/jcp.27450 [DOI] [PubMed] [Google Scholar]
- 97. Oh J, Riek AE, Darwech I, Funai K, Shao J, Chin K, et al. Deletion of macrophage vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep 2015;10:1872–1886. 10.1016/j.celrep.2015.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, et al. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 2009;119:2367–2375. 10.1161/CIRCULATIONAHA.108.807537 [DOI] [PubMed] [Google Scholar]
- 99. Bartels LE, Hvas CL, Agnholt J, Dahlerup JF, Agger R. Human dendritic cell antigen presentation and chemotaxis are inhibited by intrinsic 25-hydroxy vitamin D activation. Int Immunopharmacol 2010;10:922–928. 10.1016/j.intimp.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 100. Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol 2010;30:2495–2503. 10.1161/ATVBAHA.110.215459 [DOI] [PubMed] [Google Scholar]
- 101. Valcheva P, Cardus A, Panizo S, Parisi E, Bozic M, Lopez Novoa JM, et al. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 2014;235:247–255. 10.1016/j.atherosclerosis.2014.05.911 [DOI] [PubMed] [Google Scholar]
- 102. Lupoli R, Vaccaro A, Ambrosino P, Poggio P, Amato M, Di Minno MN. Impact of vitamin D deficiency on subclinical carotid atherosclerosis: a pooled analysis of cohort studies. J Clin Endocrinol Metab 2017;102:2146–2153. 10.1210/jc.2017-00342 [DOI] [PubMed] [Google Scholar]
- 103. Stienstra R, van Poorten JF. Spinal anaesthesia with bupivacaine. Br J Anaesth 1989;62:112–113. 10.1093/bja/62.1.112-b [DOI] [PubMed] [Google Scholar]
- 104. Saidifard N, Tangestani H, Djafarian K, Shab-Bidar S. Serum vitamin D level and carotid intima-media thickness: a systematic review and meta-analysis of observational studies and randomized control trials. Horm Metab Res 2020;52:305–315. 10.1055/a-1153-0657 [DOI] [PubMed] [Google Scholar]
- 105. Malik R, Aneni EC, Roberson L, Ogunmoroti O, Ali SS, Shaharyar S, et al. Measuring coronary artery calcification: is serum vitamin D relevant? Atherosclerosis 2014;237:734–738. 10.1016/j.atherosclerosis.2014.10.087 [DOI] [PubMed] [Google Scholar]
- 106. Martin-Reyes R, Franco-Pelaez JA, Lorenzo O, Gonzalez-Casaus ML, Pello AM, Acena A, et al. Plasma levels of monocyte chemoattractant protein-1, n-terminal fragment of brain natriuretic peptide and calcidiol are independently associated with the complexity of coronary artery disease. PLoS One 2016;11:e0152816. 10.1371/journal.pone.0152816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sung KC, Chang Y, Ryu S, Chung HK. High levels of serum vitamin D are associated with a decreased risk of metabolic diseases in both men and women, but an increased risk for coronary artery calcification in Korean men. Cardiovasc Diabetol 2016;15:112. 10.1186/s12933-016-0432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee S, Ahuja V, Masaki K, Evans RW, Barinas-Mitchell EJ, Ueshima H, et al. A significant positive association of vitamin D deficiency with coronary artery calcification among middle-aged men: for the ERA JUMP Study. J Am Coll Nutr 2016;35:614–620. 10.1080/07315724.2015.1118651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Diederichsen SZ, Gronhoj MH, Mickley H, Gerke O, Steffensen FH, Lambrechtsen J, et al. CT-detected growth of coronary artery calcification in asymptomatic middle-aged subjects and association with 15 biomarkers. JACC Cardiovasc Imaging 2017;10:858–866. 10.1016/j.jcmg.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 110. Moradi M, Foroutanfar A. Evaluation of vitamin D levels in relation to coronary CT angiographic findings in an Iranian population. Vasc Health Risk Manag 2017;13:361–367. 10.2147/VHRM.S142721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sajjadieh H, Sajjadieh A, Koopaei ZK, Oveisgharan S. Correlation between vitamin D level and coronary artery calcification. J Res Med Sci 2020;25:51. 10.4103/jrms.JRMS_1080_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Anis KH, Pober D, Rosas SE. Vitamin D analogues and coronary calcification in CKD stages 3 and 4: a randomized controlled trial of calcitriol versus paricalcitol. Kidney Med 2020;2:450–458. 10.1016/j.xkme.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rodrigues IG, Pinho CPS, Sobral Filho D, Leao APD, Oliveira MCM, Barbosa GP, et al. The impact of visceral fat and levels of vitamin D on coronary artery calcification. Rev Assoc Med Bras (1992) 2021;67:88–93. 10.1590/1806-9282.67.01.20200388 [DOI] [PubMed] [Google Scholar]
- 114. Lu S, Guo S, Hu F, Guo Y, Yan L, Ma W, et al. The associations between the polymorphisms of vitamin D receptor and coronary artery disease: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3467. 10.1097/MD.0000000000003467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ Cardiovasc Genet 2016;9:349–356. 10.1161/CIRCGENETICS.116.001396 [DOI] [PubMed] [Google Scholar]
- 116. Tabaei S, Motallebnezhad M, Tabaee SS. Vitamin D receptor (VDR) gene polymorphisms and risk of coronary artery disease (CAD): systematic review and meta-analysis. Biochem Genet 2021;59:813–836. 10.1007/s10528-021-10038-x [DOI] [PubMed] [Google Scholar]
- 117. Zhou A, Selvanayagam JB, Hypponen E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur Heart J 2022;43:1731–1739. 10.1093/eurheartj/ehab809 [DOI] [PubMed] [Google Scholar]
- 118. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005;111:3481–3488. 10.1161/CIRCULATIONAHA.105.537878 [DOI] [PubMed] [Google Scholar]
- 119. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 2017;136:1155–1166. 10.1161/CIRCULATIONAHA.117.029870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bozic M, Alvarez A, de Pablo C, Sanchez-Nino MD, Ortiz A, Dolcet X, et al. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: implications for atherosclerosis development. PLoS One 2015;10:e0136863. 10.1371/journal.pone.0136863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stach K, Kalsch AI, Nguyen XD, Elmas E, Kralev S, Lang S, et al. 1alpha,25-dihydroxyvitamin D3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells. Cardiology 2011;118:107–115. 10.1159/000327547 [DOI] [PubMed] [Google Scholar]
- 122. Ding Y, Liao W, Yi Z, Xiang W, He X. Cardioprotective role of vitamin D receptor in circulating endothelial cells of ApoE-deficient mice. Int J Clin Exp Med 2015;8:5065–5074. [PMC free article] [PubMed] [Google Scholar]
- 123. Beveridge LA, Khan F, Struthers AD, Armitage J, Barchetta I, Bressendorff I, et al. Effect of vitamin D supplementation on markers of vascular function: a systematic review and individual participant meta-analysis. J Am Heart Assoc 2018;7:e008273. 10.1161/JAHA.117.008273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen NC, Hsu CY, Mao PC, Dreyer G, Wu FZ, Chen CL. The effects of correction of vitamin D deficiency on arterial stiffness: a systematic review and updated meta-analysis of randomized controlled trials. J Steroid Biochem Mol Biol 2020;198:105561. 10.1016/j.jsbmb.2019.105561 [DOI] [PubMed] [Google Scholar]
- 125. Mohammad S, Mishra A, Ashraf MZ. Emerging role of vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules 2019;9:649. 10.3390/biom9110649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, Sudo T, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem 2004;279:35798–35802. 10.1074/jbc.M404865200 [DOI] [PubMed] [Google Scholar]
- 127. Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation 2000;102:2867–2872. 10.1161/01.CIR.102.23.2867 [DOI] [PubMed] [Google Scholar]
- 128. Martinez-Moreno JM, Herencia C, de Oca A M, Munoz-Castaneda JR, Rodriguez-Ortiz ME, Diaz-Tocados JM, et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J 2016;30:1367–1376. 10.1096/fj.15-272872 [DOI] [PubMed] [Google Scholar]
- 129. Korzonek-Szlacheta I, Hudzik B, Nowak J, Szkodzinski J, Nowak J, Gasior M, et al. Mean platelet volume is associated with serum 25-hydroxyvitamin D concentrations in patients with stable coronary artery disease. Heart Vessels 2018;33:1275–1281. 10.1007/s00380-018-1182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hajimoradi B, Hosseini B, Alirezaei T, Pourmotahari F. The association of 25-hydroxy vitamin D level with mean platelet volume in patients with acute coronary syndrome. Cardiovasc Hematol Disord Drug Targets 2022;22:52–57. 10.2174/1871529X22666220418111905 [DOI] [PubMed] [Google Scholar]
- 131. Johny E, Jala A, Nath B, Alam MJ, Kuladhipati I, Das R, et al. Vitamin D supplementation modulates platelet-mediated inflammation in subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Front Immunol 2022;13:869591. 10.3389/fimmu.2022.869591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Montecucco F, Liberale L, Bonaventura A, Vecchie A, Dallegri F, Carbone F. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep 2017;19:11. 10.1007/s11883-017-0646-1 [DOI] [PubMed] [Google Scholar]
- 133. Carbone F, Busto G, Padroni M, Bernardoni A, Colagrande S, Dallegri F, et al. Radiologic cerebral reperfusion at 24 h predicts good clinical outcome. Transl Stroke Res 2019;10:178–188. 10.1007/s12975-018-0637-8 [DOI] [PubMed] [Google Scholar]
- 134. Karohl C, Vaccarino V, Veledar E, Goldberg J, Tangpricha V, Bellasi A, et al. Vitamin D status and coronary flow reserve measured by positron emission tomography: a co-twin control study. J Clin Endocrinol Metab 2013;98:389–397. 10.1210/jc.2012-3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Capitanio S, Sambuceti G, Giusti M, Morbelli S, Murialdo G, Garibotto G, et al. 1,25-Dihydroxy Vitamin D and coronary microvascular function. Eur J Nucl Med Mol Imaging 2013;40:280–289. 10.1007/s00259-012-2271-0 [DOI] [PubMed] [Google Scholar]
- 136. Glenn DJ, Cardema MC, Gardner DG. Amplification of lipotoxic cardiomyopathy in the VDR gene knockout mouse. J Steroid Biochem Mol Biol 2016;164:292–298. 10.1016/j.jsbmb.2015.09.034 [DOI] [PubMed] [Google Scholar]
- 137. Philouze C, Martin JC, Riva C, Marziou A, Defoort C, Couturier C, et al. Vitamin D3 supplementation alleviates left ventricular dysfunction in a mouse model of diet-induced type 2 diabetes: potential involvement of cardiac lipotoxicity modulation. Cardiovasc Drugs Ther 2022;36:245–256. 10.1007/s10557-021-07143-9 [DOI] [PubMed] [Google Scholar]
- 138. Zhang L, Yan X, Zhang YL, Bai J, Hidru TH, Wang QS, et al. Vitamin D attenuates pressure overload-induced cardiac remodeling and dysfunction in mice. J Steroid Biochem Mol Biol 2018;178:293–302. 10.1016/j.jsbmb.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 139. Padoan L, Beltrami AP, Stenner E, Beleu A, Ruscio M, Sinagra G, et al. Left ventricular adverse remodeling after myocardial infarction and its association with vitamin D levels. Int J Cardiol 2019;277:159–165. 10.1016/j.ijcard.2018.08.052 [DOI] [PubMed] [Google Scholar]
- 140. Nolte K, Herrmann-Lingen C, Platschek L, Holzendorf V, Pilz S, Tomaschitz A, et al. Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction. ESC Heart Fail 2019;6:262–270. 10.1002/ehf2.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Radovanovic D, Stoickov V, Pechanova O, Scanlan AT, Jakovljevic V, Stojanovic E. The relationships between 25-hydroxyvitamin D and echocardiographic parameters in female basketball players. Clin J Sport Med 2022;32:e492–e498. 10.1097/JSM.0000000000001041 [DOI] [PubMed] [Google Scholar]
- 142. Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, et al. Effects of vitamin D supplementation on renin and aldosterone concentrations in patients with advanced heart failure: the EVITA trial. Int J Endocrinol 2018;2018:5015417. 10.1155/2018/5015417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zittermann A, Ernst JB, Prokop S, Fuchs U, Berthold HK, Gouni-Berthold I, et al. A 3 year post-intervention follow-up on mortality in advanced heart failure (EVITA vitamin D supplementation trial). ESC Heart Fail 2020;7:3754–3761. 10.1002/ehf2.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Witte KK, Byrom R, Gierula J, Paton MF, Jamil HA, Lowry JE, et al. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol 2016;67:2593–2603. 10.1016/j.jacc.2016.03.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rodriguez AJ, Mousa A, Ebeling PR, Scott D, de Courten B. Effects of vitamin D supplementation on inflammatory markers in heart failure: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 2018;8:1169. 10.1038/s41598-018-19708-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhao JD, Jia JJ, Dong PS, Zhao D, Yang XM, Li DL, et al. Effect of vitamin D on ventricular remodelling in heart failure: a meta-analysis of randomised controlled trials. BMJ Open 2018;8:e020545. 10.1136/bmjopen-2017-020545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chunbin W, Han W, Lin C. Efficacy of vitamin D on chronic heart failure among adults. Int J Vitam Nutr Res 2020;90:49–58. 10.1024/0300-9831/a000487 [DOI] [PubMed] [Google Scholar]
- 148. Wang T, Liu Z, Fu J, Min Z. Meta-analysis of vitamin D supplementation in the treatment of chronic heart failure. Scand Cardiovasc J 2019;53:110–116. 10.1080/14017431.2019.1612084 [DOI] [PubMed] [Google Scholar]
- 149. Naghedi A, Haghaninejad H, Varastehravan H, Naghedi A, Farshadi N. Effect of vitamin D supplements on left ventricular ejection fraction in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Rev Port Cardiol (Engl Ed) 2021;40:447–455. 10.1016/j.repc.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 150. Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J 2017;38:2279–2286. 10.1093/eurheartj/ehx235 [DOI] [PubMed] [Google Scholar]
- 151. Zittermann A, Ernst JB, Prokop S, Fuchs U, Gruszka A, Dreier J, et al. Vitamin D supplementation of 4000 IU daily and cardiac function in patients with advanced heart failure: the EVITA trial. Int J Cardiol 2019;280:117–123. 10.1016/j.ijcard.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 152. Niccoli G, Del Buono MG. Vitamin D and left ventricular adverse remodeling: does association imply causation? Int J Cardiol 2019;277:200–201. 10.1016/j.ijcard.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 153. Won S, Sayeed I, Peterson BL, Wali B, Kahn JS, Stein DG. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One 2015;10:e0122821. 10.1371/journal.pone.0122821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sayeed I, Turan N, Stein DG, Wali B. Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp Neurol 2019;312:63–71. 10.1016/j.expneurol.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 155. Velimirovic M, Jevtic Dozudic G, Selakovic V, Stojkovic T, Puskas N, Zaletel I, et al. Effects of vitamin D3 on the NADPH oxidase and matrix metalloproteinase 9 in an animal model of global cerebral ischemia. Oxid Med Cell Longev 2018;2018:3273654. 10.1155/2018/3273654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Vahidinia Z, Khassafi N, Tameh AA, Karimian M, Zare-Dehghanani Z, Moradi F, et al. Calcitriol ameliorates brain injury in the rat model of cerebral ischemia-reperfusion through Nrf2/HO-1 signalling axis: an in silico and in vivo study. J Stroke Cerebrovasc Dis 2022;31:106331. 10.1016/j.jstrokecerebrovasdis.2022.106331 [DOI] [PubMed] [Google Scholar]
- 157. Sadeghian N, Shadman J, Moradi A, Ghasem Golmohammadi M, Panahpour H. Calcitriol protects the blood-brain barrier integrity against ischemic stroke and reduces vasogenic brain edema via antioxidant and antiapoptotic actions in rats. Brain Res Bull 2019;150:281–289. 10.1016/j.brainresbull.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 158. Bao GQ, Yu JY. Vitamin D3 promotes cerebral angiogenesis after cerebral infarction in rats by activating Shh signaling pathway. Eur Rev Med Pharmacol Sci 2018;22:7069–7077. 10.26355/eurrev_201810_16179 [DOI] [PubMed] [Google Scholar]
- 159. Turetsky A, Goddeau RPJR, Henninger N. Low serum vitamin D is independently associated with larger lesion volumes after ischemic stroke. J Stroke Cerebrovasc Dis 2015;24:1555–1563. 10.1016/j.jstrokecerebrovasdis.2015.03.051 [DOI] [PubMed] [Google Scholar]
- 160. Nie Z, Ji XC, Wang J, Zhang HX. Serum levels of 25-hydroxyvitamin D predicts infarct volume and mortality in ischemic stroke patients. J Neuroimmunol 2017;313:41–45. 10.1016/j.jneuroim.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 161. Li YY, Wang YS, Chen Y, Hu YH, Cui W, Shi XY, et al. Association of serum 25(OH) D levels with infarct volumes and stroke severity in acute ischemic stroke. J Nutr Health Aging 2018;22:97–102. 10.1007/s12603-017-0926-z [DOI] [PubMed] [Google Scholar]
- 162. Yarlagadda K, Ma N, Dore S. Vitamin D and stroke: effects on incidence, severity, and outcome and the potential benefits of supplementation. Front Neurol 2020;11:384. 10.3389/fneur.2020.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Narasimhan S, Balasubramanian P. Role of vitamin D in the outcome of ischemic stroke—a randomized controlled trial. J Clin Diagn Res 2017;11:CC06–CC10. 10.7860/JCDR/2017/24299.9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sari A, Durmus B, Karaman CA, Ogut E, Aktas I. A randomized, double-blind study to assess if vitamin D treatment affects the outcomes of rehabilitation and balance in hemiplegic patients. J Phys Ther Sci 2018;30:874–878. 10.1589/jpts.30.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Momosaki R, Abo M, Urashima M. Vitamin D supplementation and post-stroke rehabilitation: a randomized, double-blind, placebo-controlled trial. Nutrients 2019;11:1295. 10.3390/nu11061295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rist PM, Buring JE, Cook NR, Manson JE, Rexrode KM. Effect of vitamin D and/or omega-3 fatty acid supplementation on stroke outcomes: a randomized trial. Eur J Neurol 2021;28:809–815. 10.1111/ene.14623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Torrisi M, Bonanno L, Formica C, Arcadi FA, Cardile D, Cimino V, et al. The role of rehabilitation and vitamin D supplementation on motor and psychological outcomes in poststroke patients. Medicine (Baltimore) 2021;100:e27747. 10.1097/MD.0000000000027747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wang L, Zhao XM, Wang FY, Wu JC, Wang Y. Effect of vitamin D supplementation on the prognosis of post-stroke fatigue: a retrospective cohort study. Front Neurol 2021;12:690969. 10.3389/fneur.2021.690969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, et al. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: the ARIC (Atherosclerosis Risk in Communities) study. JACC Heart Fail 2015;3:347–356. 10.1016/j.jchf.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–1637. 10.1001/archinte.168.15.1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Farrell SW, Meyer KJ, Leonard D, Shuval K, Barlow CE, Pavlovic A, et al. Physical activity, adiposity, and Serum vitamin D levels in healthy women: the Cooper Center Longitudinal Study. J Womens Health (Larchmt) 2022;31:957–964. 10.1089/jwh.2021.0402 [DOI] [PubMed] [Google Scholar]
- 172. Ceolin G, Confortin SC, da Silva AAM, Rech CR, d’Orsi E, Rieger DK, et al. Association between physical activity and vitamin D is partially mediated by adiposity in older adults: EpiFloripa Aging Cohort Study. Nutr Res 2022;103:11–20. 10.1016/j.nutres.2022.03.001 [DOI] [PubMed] [Google Scholar]
- 173. Abiri B, Valizadeh M, Nasreddine L, Hosseinpanah F. Dietary determinants of healthy/unhealthy metabolic phenotype in individuals with normal weight or overweight/obesity: a systematic review. Crit Rev Food Sci Nutr 2022:1–18. 10.1080/10408398.2021.2025036 [DOI] [PubMed] [Google Scholar]
- 174. Scragg RKR. Overview of results from the Vitamin D Assessment (ViDA) study. J Endocrinol Invest 2019;42:1391–1399. 10.1007/s40618-019-01056-z [DOI] [PubMed] [Google Scholar]
- 175. Manson JE, Bassuk SS, Buring JE, Group VR. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J Steroid Biochem Mol Biol 2020;198:105522. 10.1016/j.jsbmb.2019.105522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cummings SR, Rosen C. VITAL Findings—a decisive verdict on vitamin D supplementation. N Engl J Med 2022;387:368–370. 10.1056/NEJMe2205993 [DOI] [PubMed] [Google Scholar]
- 177. Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 2022;18:96–110. 10.1038/s41574-021-00593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Huang T, Afzal S, Yu C, Guo Y, Bian Z, Yang L, et al. Vitamin D and cause-specific vascular disease and mortality: a Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med 2019;17:160. 10.1186/s12916-019-1401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun 2020;11:1647. 10.1038/s41467-020-15421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chandler PD, Wang L, Zhang X, Sesso HD, Moorthy MV, Obi O, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 2015;73:577–593. 10.1093/nutrit/nuv012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ganmaa D, Enkhmaa D, Nasantogtokh E, Sukhbaatar S, Tumur-Ochir KE, Manson JE. Vitamin D, respiratory infections, and chronic disease: review of meta-analyses and randomized clinical trials. J Intern Med 2022;291:141–164. 10.1111/joim.13399 [DOI] [PubMed] [Google Scholar]
- 182. Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med 2019;381:520–530. 10.1056/NEJMoa1900906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. de Boer IH, Zelnick LR, Ruzinski J, Friedenberg G, Duszlak J, Bubes VY, et al. Effect of vitamin D and Omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA 2019;322:1899–1909. 10.1001/jama.2019.17380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Theiler-Schwetz V, Trummer C, Grubler MR, Keppel MH, Zittermann A, Tomaschitz A, et al. Effects of vitamin D supplementation on 24-hour blood pressure in patients with low 25-hydroxyvitamin D levels: a randomized controlled trial. Nutrients 2022;14:1360. 10.3390/nu14071360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Neale RE, Baxter C, Romero BD, McLeod DSA, English DR, Armstrong BK, et al. The D-health trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diab Endocrinol 2022;10:120–128. 10.1016/S2213-8587(21)00345-4 [DOI] [PubMed] [Google Scholar]
- 186. Virtanen JK, Nurmi T, Aro A, Bertone-Johnson ER, Hypponen E, Kroger H, et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: a randomized controlled trial. Am J Clin Nutr 2022;115:1300–1310. 10.1093/ajcn/nqab419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Cojic M, Kocic R, Klisic A, Kocic G. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: a 6-month follow up randomized controlled study. Front Endocrinol (Lausanne) 2021;12:610893. 10.3389/fendo.2021.610893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Indhavivadhana S, Boonyachan W, Rattanachaiyanont M, Wongwananuruk T, Techatraisak K, Sa-Nga-Areekul N. Effectiveness of vitamin D2 supplementation on high-sensitivity C-reactive protein and other metabolic indices in menopausal Thai women: a randomized-controlled trial. Gynecol Endocrinol 2022;38:83–89. 10.1080/09513590.2021.1988560 [DOI] [PubMed] [Google Scholar]
- 189. Thams L, Stounbjerg NG, Hvid LG, Molgaard C, Hansen M, Damsgaard CT. Effects of high dairy protein intake and vitamin D supplementation on body composition and cardiometabolic markers in 6–8-y-old children-the D-pro trial. Am J Clin Nutr 2022;115:1080–1091. 10.1093/ajcn/nqab424 [DOI] [PubMed] [Google Scholar]
- 190. Desouza C, Chatterjee R, Vickery EM, Nelson J, Johnson KC, Kashyap SR, et al. The effect of vitamin D supplementation on cardiovascular risk in patients with prediabetes: a secondary analysis of the D2d study. J Diabetes Complications 2022;36:108230. 10.1016/j.jdiacomp.2022.108230 [DOI] [PubMed] [Google Scholar]
- 191. Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H. Effects of vitamin D3 supplementation on epigenetic aging in overweight and obese African Americans with suboptimal vitamin D status: a randomized clinical trial. J Gerontol A Biol Sci Med Sci 2019;74:91–98. 10.1093/gerona/gly223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Force USPST, Krist AH, Davidson KW, Mangione CM, Cabana M, Caughey AB, et al. Screening for vitamin D deficiency in adults: US preventive services task force recommendation statement. JAMA 2021;325:1436–1442. 10.1001/jama.2021.3069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.