Abstract
Studies over the last decade have markedly broadened our understanding of store-operated Ca2+ channels (SOCs) and their roles in kidney diseases and podocyte dysfunction. Podocytes are terminally differentiated glomerular visceral epithelial cells which are tightly attached to the glomerular capillary basement membrane. Podocytes and their unique foot processes (pedicels) constitute the outer layer of the glomerular filtration membrane and the final barrier preventing filtration of albumin and other plasma proteins. Diabetic nephropathy and other renal diseases are associated with podocyte injury and proteinuria. Recent evidence demonstrates a pivotal role of store-operated Ca2+ entry (SOCE) in maintaining structural and functional integrity of podocytes. This article reviews the current knowledge of SOCE and its contributions to podocyte physiology. Recent studies of the contributions of SOC dysfunction to podocyte injury in both cell culture and animal models are discussed, including work in our laboratory. Several downstream signaling pathways mediating SOC function in podocytes also are examined. Understanding the pivotal roles of SOC in podocyte health and disease is essential, as SOCE-activated signaling pathways are potential treatment targets for podocyte injury-related kidney diseases.
Keywords: Ca2+ signaling, kidney disease, Orai1, podocyte, proteinuria, STIM1, store-operated Ca2+ channel
Impact statement
Podocyte integrity is critical for normal glomerular filtration, and podocyte injury is implicated in the pathogenesis of proteinuria in kidney diseases. During the past decade, store-operated Ca2+ entry (SOCE)-mediated Ca2+ signaling has been demonstrated as a central mechanism for maintaining podocyte integrity, and it is increasingly evident that abnormal store-operated Ca2+ channel (SOC) function contributes to podocyte damage. This review integrates recent progress on podocyte physiology and pathophysiology with new insights regarding SOCE-initiated signaling. These advances in our understanding of the mechanisms of physiological podocyte homeostasis and pathological podocyte injury are providing the foundation to develop drugs targeting podocyte SOC to treat kidney diseases.
Physiology and molecular biology of store-operated Ca2+ channels
Store-operated Ca2+ channels (SOC), which open when intracellular Ca2+ stores are depleted, play central roles in podocyte signaling. 1 Physiologically, G protein coupled receptors and receptor tyrosine kinases activate phospholipase C (PLC) β and γ isoforms, respectively, which in turn hydrolyze phosphatidylinositol 4,5-bisphosphate, yielding diacylglycerol and inositol 1,4,5-trisphosphate (IP3).2,3 IP3 interacts with its (sarco)endoplasmic reticular membrane receptors to elicit Ca2+ discharge from (sarco)endoplasmic reticulum.4,5 Depletion of these Ca2+ stores activates SOC in the plasma membrane, allowing extracellular Ca2+ to enter the cell.
Initially termed capacitative Ca2+ entry, store-operated Ca2+ entry (SOCE), via SOC was first proposed in 1976 by Putney, who studied the refilling of intracellular Ca2+ stores after their depletion by the PLC-linked agonists carbachol and phenylephrine. 6 Subsequent studies combining Ca2+ imaging and patch clamp techniques demonstrated that endoplasmic reticular Ca2+ depletion in leukemic T cells 4 and mast cells activated Ca2+ conductance. 5 More recent research defined the biophysical and pharmacological hallmarks of SOC. Multiple SOC subtypes with distinct biophysical properties, for example, Ca2+ selective and nonselective cationic SOC, have been identified,4,5,7–10 and SOC subtype expression is cell type- and tissue-specific.4,5,8,9,11 However, a property of all SOCs is the dependence of their activation on depletion of their internal Ca2+ stores, not on cytosolic Ca2+ concentration.9,12,13 This unique property distinguishes SOCs from nonselective Ca2+-activated cation channels.
In the decades following discovery of SOCs, intensive research effort encompassing physiology, pharmacology, cell biology and molecular biology has focused upon identifying the molecular components of SOCs and delineating their gating mechanisms. Around the turn of the 21st century, the transient receptor potential canonical (TRPC) channel family garnered the most attention,1,14 and numerous studies identified critical contributions of TRPC channel isoforms to SOC gating.15–21 However, important differences in the pharmacological and biophysical properties of SOCs versus TRPC channels questioned whether TRPC proteins are truly SOC components. Concurrently, the mechanisms activating SOCs were examined. Three major mechanisms were proposed involving (1) diffusible messengers, 22 (2) vesicle fusion/exocytosis, 23 and (3) physical interaction of IP3 receptors located on ER and Ca2+ channels on cell plasma membrane 24 including TRPC channels. Evidence for these mechanisms was equivocal until 2005 to 2006, when stromal interaction molecules (STIM) and Orai proteins were revealed by gene array and high throughput RNA interference screening.25–29 STIM1 contains a single transmembrane domain, is localized in the endoplasmic reticular (ER) membrane, and functions as a sensor monitoring luminal Ca2+ concentration in the ER. When ER Ca2+ is depleted, STIM1 aggregates and migrates toward ER-plasma membrane junctions, leading to physical interaction with Orai1, which is the pore-forming unit of SOCs located on the cell plasma membrane, at STIM1: Orai1 stoichiometries ranging from 1:1 to 2:1. This interaction activates Orai1, permitting Ca2+ influx from the extracellular fluid into the cytosol.30–32
In addition to STIM1 and Orai1, STIM2, a closely-related mammalian STIM1 homolog, and the mammalian Orai1 homologues Orai2 and Orai3 also may constitute and/or regulate SOC, but with distinct functional properties.33–38 The recent identification of Orai1 α and β splicing variants39,40 and the STIM1 splicing variant STIM1L,41–44 which generate SOCs with distinct signaling and regulatory properties, adds another layer of complexity to Orai/STIM-constituted SOCE. Several TRPC isoforms, which initially were proposed as SOC molecular components before Orai1 and STIM1 were discovered, may also function as SOC by interacting with STIM1 and/or Orai1.45–52 The composition of SOCs is still under investigation.
SOC in podocytes
Podocytes are pivotal determinants of the molecular selectivity of glomerular filtration. Located on the external face of the glomerular basement membrane (GBM), these terminally differentiated, polarized, highly specialized visceral epithelial cells constitute the glomerular filtration barrier’s outermost layer (Figure 1).53–55 Several primary processes extend from the podocyte cell body, from which further extend secondary and even tertiary foot processes, termed pedicels. Those structures encircle the basement membrane surrounding the glomerular capillaries. 54 Spanning the narrow gaps between adjacent foot processes are slit diaphragms composed of highly specialized adhesion molecules including nephrin, Neph1 and other junctional proteins. Slit diaphragms contain pores, 30 to 40 nm wide, which permit free filtration of water, electrolytes and small organic solutes from the glomerular capillaries to the urinary (Bowman’s) space, while restricting filtration of albumin and other plasma proteins. 56
Figure 1.
Podocyte structure and connections with glomerular basement membrane.
CD2AP: CD2-associated protein; ER: endoplasmic reticulum; GBM: glomerular basement membrane; Neph1: nephrin 1; SD: slit diaphragm; STIM1: stromal interaction molecule 1; TRPC6: transient receptor potential.
Under physiological conditions, the intact GBM and podocytes maintain essentially protein-free glomerular filtration. However, inflammatory, metabolic or mechanical stimuli associated with various diseases provoke distinctive and substantial changes in podocyte morphology termed foot process simplification and effacement. During this process, cytoskeletal re-arrangements widen and shorten the individual foot processes; in more severe cases, the secondary and tertiary processes are resorbed into the primary processes.54,57–59 Foot process effacement and simplification exposes the outer face of the GBM, increasing the protein permeability of the glomerular filtration barrier and producing pathological proteinuria.
As in vascular smooth muscle and glomerular mesangial cells, the structural integrity of podocytes depends on the maintenance of Ca2+ homeostasis inside the cell. In podocytes, multiple signaling mechanisms controlling intracellular Ca2+ concentrations converge on plasma membrane ion channels. Podocyte Ca2+ signaling has been studied most extensively in TRPC5 and especially TRPC6 channels.60–65 Recently, SOCE and the SOC components Orai1 and STIM1 have been reported in podocytes.66,67
In 2013, podocyte SOCE was first reported by Yang et al., 66 who studied signaling mechanisms activating podocyte apoptosis in response to high glucose concentrations. Using Ca2+ imaging, they showed that thapsigargin, a (sarco)endoplasmic reticular ATPase inhibitor which depletes ER Ca2+ stores, activated SOCE in cultured podocytes. Recent electrophysiology evidence from our group also supports existence of SOC in podocytes. By use of whole cell patch clamp, we recorded thapsigargin-stimulated inward currents in cultured human podocytes which were blocked by La3+, an inhibitor of SOCE. 67 Acting on its AT1 receptors, angiotensin II, an important regulator of podocyte physiology and pathology,63,64 activates the PLC-IP3 signaling pathway, which could open SOCs via IP3-induced ER Ca2+ release. Indeed, we demonstrated in human podocytes that angiotensin II evoked robust inward Ca2+ currents which were suppressed by the SOC blocker 3,5-bis(trifluoromethyl)pyrazole 2. 67
Expression of the channel or channel-gating proteins in cultured mouse and human podocytes further demonstrated the fundamental role of SOCE. In 2015, Xu et al. 68 reported that the saturated fatty acid palmitate induced STIM1 oligomerization, the initial step in SOC activation, in ER membranes of cultured mouse podocytes, possibly through PLC signaling. As expected, palmitate elicited SOCE, 68 although the functional consequences of palmitate-stimulated SOCE were not evaluated. 68
Although mounting biochemical, biological and functional evidence unambiguously supports existence of SOC in podocytes, this evidence was obtained almost exclusively in cultured cells, and in vivo data are lacking. Future studies should be directed toward confirming podocyte SOC in intact animals. Furthermore, to date all biochemical and molecular studies of SOC in podocytes have focused on Orai1 and STIM1, the prototypical SOC channel and gating proteins, respectively. However, homologs (Orai2, Orai3, and STIM2)33–38 and splicing variants (Orai1α, Orai1β, and STIM1L)39–44 of the two proteins have not been studied in podocytes. These homologs and splicing variants reportedly function as SOC or gate/regulate SOC in other cell types.33–44 Moreover, the location of SOC in podocytes should be identified, because the downstream pathways and functions of SOC in foot processes may differ from those in the cell body. For instance, TRPC6 activation in the podocyte cell body modulates gene expression, 69 but in foot process alters slit diaphragm permeability.70,71
It should be noted that various SOC subtypes may exist with cell type–specific distributions and distinct biophysical and pharmacological profiles. 1 Although formation of highly Ca2+-selective SOC by homo- or heteromultimeric Orai subunits is widely accepted, nonselective SOCs may also be formed by Orai-TRPC channel interactions.40,72 Indeed, Orai1-TRRPC1 interactions were indispensable for functional SOCE activation in human embryonic kidney (HEK293) cells 45 and osteoclasts. 48 Although podocytes possess Orai1 and TRPCs 5 and 6, whether these proteins act independently or cooperatively is unclear and awaits more molecular, biophysical, and pharmacological evidence.
Physiological impact of podocyte SOCs
Podocytes integrate with the glomerular endothelial cells to build and maintain the GBM. Collectively, podocytes, GBM, and the glomerular endothelium with its surface glycocalyx constitute the interface that effects glomerular filtration. The slit diaphragms bridging the adjacent podocyte foot processes impose steric hindrance selectively limiting filtration of protein-size molecules. As in many cell types, podocyte structure and function are largely regulated by intracellular Ca2+ signals and podocyte Ca2+ homeostasis is, to a large extent, attributed to plasma membrane Ca2+ channels. Emerging evidence suggests that SOC and its downstream signaling help maintain podocyte integrity and, thus, normal glomerular filtration.
Miao et al. 73 found that STIM1 and Orai1 overexpression lowered contents of the slit diaphragm proteins podocin and CD2-associated protein (CD2AP) while increasing content of the cytoskeletal protein α-actinin-4. All these proteins are essential for podocyte integrity and normal structure of the glomerular filtration barrier (Figure 1). As expected, overexpression of STIM1 and Orai1 increased podocyte permeability. 73 Although they did not assess the impact of podocin, CD2AP and α-actinin overexpression on SOC function, Miao et al. reported direct evidence that SOC signaling proteins are associated with podocyte structure and function. Recently, in cultured human podocytes we also found that SOCE regulates abundance of the slit diaphragm protein nephrin. 67
Foot process morphology and podocyte function highly depend on the actin cytoskeleton. Regardless of the initial insult, the ultimate pathway for podocyte damage is actin cytoskeleton rearrangement and dysfunction. 56 Recently, both Kim et al. 74 and our group 67 demonstrated in cultured podocytes that Orai1-mediated SOCE contributed to normal distribution and organization of cytoskeleton proteins. Enhancement of the Ca2+ signaling resulted in disorganization of cytoskeleton and actin remodeling, an indication of podocyte injury.67,74
Pathophysiological relevance of SOC in podocytes
Podocytes are terminally differentiated epithelial cells. Their limited regenerative capacity and their vulnerability to various diseases make podocyte injury particularly important in glomerular pathology. Loss of sufficient numbers of podocytes inevitably leads to glomerulosclerosis and eventual loss of the nephron. Podocyte structural and functional integrity depend on intracellular Ca2+ homeostasis; thus, disruption of intracellular Ca2+ signaling contributes to podocyte injury and glomerular disease. In addition to the well-described cause–effect relationship between TRPC6 overactivation in podocytes and focal segmental glomerulosclerosis/proteinuria,61–64,75 recent evidence indicates that abnormal SOC Ca2+ signaling also contributes to podocyte-associated glomerular disease. Miao et al. 73 demonstrated elevated abundances of mRNA encoding STIM1 and Orai1 in renal cortex of mice with adriamycin-induced nephropathy versus control mice. STIM1 and Orai1 overexpression decreased contents of slit diaphragm proteins podocin and CD2AP, leading to increased permeability of mouse podocytes. 73
The leading cause of chronic kidney disease in the United States, diabetic nephropathy (DN) is characterized by microalbuminuria in its early stages which intensifies into fulminant proteinuria as the disease progresses. Also in the early stages of DN, glomerular hyperfiltration imposes shear stress which damages podocytes. 76 Podocyte injury and loss disrupts the glomerular filtration barrier, allowing plasma proteins to pass from the glomerular capillaries into Bowman’s space. Excessive protein in the proximal tubular lumen overwhelms tubular capacity for endocytosis, allowing albumin and other plasma proteins to spill into the urine. The contributions of abnormal SOC signaling to podocyte pathology in DN are the focus of ongoing, intensive research. Jin et al. 77 found that both STIM1 and STIM2 contents increased in kidneys of rats and patients with diabetic kidney disease. STIM1 and Orai1 overexpression increased Ca2+ influx in cultured mouse podocytes, and increased Ca2+ entry provoked podocyte epithelial-to-mesenchymal transition (EMT) in humans and rats with DN.77,78 Furthermore, increased podocyte STIM1 content augmented Orai1-mediated Ca2+ entry in rats with diabetic kidney disease. 78 Moreover, deletion of STIM1 in cultured podocytes not only ameliorated high glucose-induced podocyte apoptosis and EMT, but also enhanced podocyte autophagy.77,78 Thus, disordered SOC contributes to podocyte injury in DN.
Podocytes are among several insulin signaling targets in kidney. 79 Insulin receptor signaling is pivotal for podocyte function, and perturbation of podocyte insulin signaling compromises the glomerular filtration barrier, causing proteinuria.80–82 Recently, Kim et al. 74 reported increased podocyte Orai1 plasma membrane trafficking through a Vesicle Associated Membrane Protein 2 (VAMP2)-dependent mechanism in response to insulin stimulation, resulting in enhanced SOCE. Insulin-activated SOCE in podocytes triggered actin remodeling and transepithelial albumin leakage. Intensification of SOCE-induced podocyte injury by insulin signaling was further validated in animals. Genetic orai1 overexpression in mice results in podocyte foot processes effacement, compromising the glomerular filtration barrier. In contrast, podocyte-specific Orai1 ablation blunts insulin-stimulated SOCE, synaptopodin depletion, and proteinuria. In diabetic mice, Kim et al. 74 showed that podocyte damage and proteinuria coincided with increased Orai1 expression at the hyperinsulinemic stage, and that suppression of Orai1 Ca2+ signaling ameliorated the detrimental effects of elevated insulin.
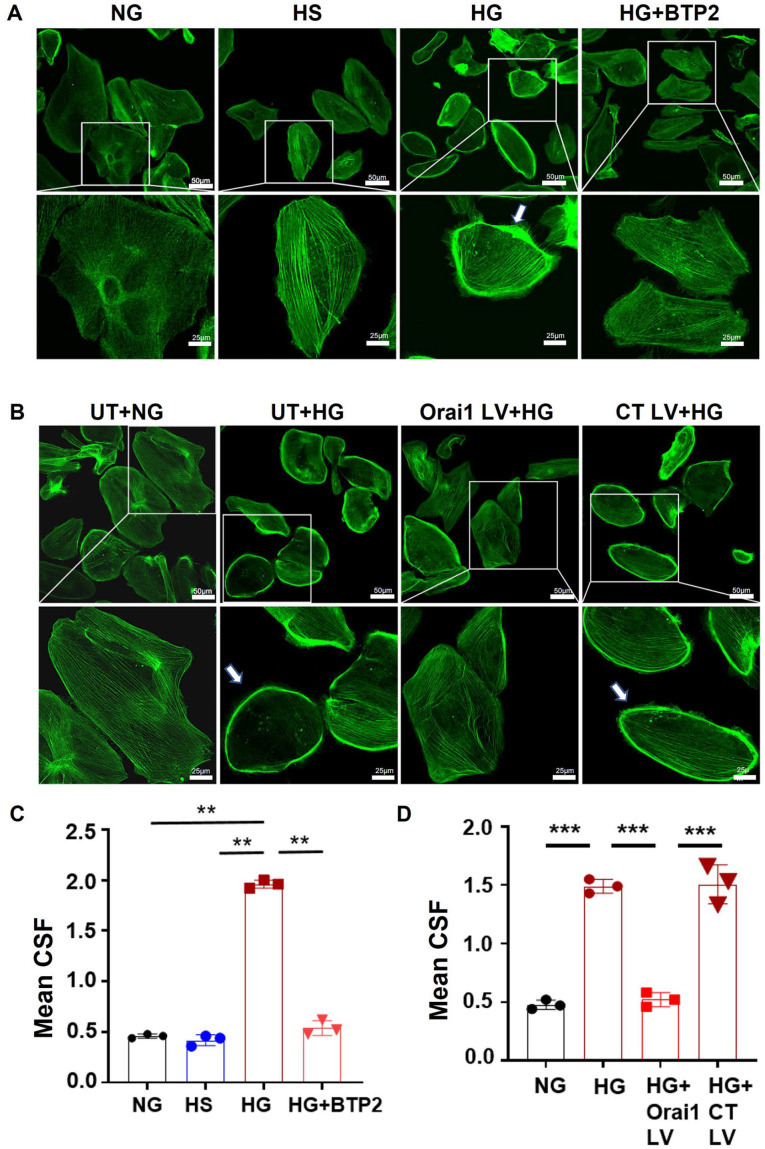
Recently, we demonstrated in cultured podocytes that exposure to elevated glucose concentration-dependently increased Orai1 protein content and SOCE. Furthermore, high glucose provoked overt F-actin remodeling and lowered nephrin content, indicating podocyte injury (Figure 2). Importantly, both pharmacological Orai1 inhibition by BTP2 or genetic orai1 ablation via CRISPR-Cas9 lentivirus prevent these indicators of podocyte injury (Figure 2). Since hyperglycemia is the main pathogenic factor promoting podocyte injury in DN, 83 these results strongly suggest enhanced SOCE in podocytes is a pivotal contributor to DN pathogenesis.
Figure 2.
Confocal microscopic images, showing contribution of SOCE to podocyte cytoskeleton organization. (A & B): Representative immunofluorescence staining of F-actin of podocytes with different treatments. (C & D): Statistical analysis of data from experiments presented in A and B, respectively (Adapted from Tao et al. 67 with permission of the American Society for Biochemistry and Molecular Biology).
SOC-initiated signaling in podocytes
Store-operated Ca2+ channels are essential to myriad cellular processes including exocytosis, enzyme regulation, gene transcription, proliferation, and apoptosis. 1 Not surprisingly, multiple downstream pathways mediate SOCE-induced physiological and pathological consequences. The diverse SOC signaling pathways could be cell-type specific and cell function dependent. In podocytes, studies on SOC signaling have focused on the intracellular pathways regulating cell phenotype transition, turnover and structural integrity.
Inflammation and immune mechanisms contribute to DN pathogenesis.84–87 Receptors for the Fc domains of immunoglobulin G antibodies (FcγRs) trigger phagocytosis, antibody-dependent cellular cytotoxicity, release of inflammatory mediators, and other effector functions. 88 Preventing FcγR activation alleviated renal hypertrophy, inflammation and fibrosis in diabetic mice, suggesting that targeting FcγR may prove renoprotective against DN. 89 Podocyte SOCE signaling is augmented in DN, and increased Ca2+ signaling induced podocyte EMT in diabetic kidney,77,78 a phenomenon associated with renal fibrosis. 90 Jin et al.77,78 demonstrated that FcγRII activation mediated SOCE-activated podocyte EMT, and inhibition of SOCE by STIM1 silencing blunted high glucose-induced FcγRII activity and podocyte injury. 77 Accordingly, targeting the SOCE-FcγRII signaling pathway might be a powerful strategy to treat inflammatory kidney diseases, including DN.
Activation of calcineurin, a serine/threonine phosphatase, requires increased intracellular Ca2+ concentrations. In cardiomyocytes and vascular endothelial cells, SOCE-activated calcineurin signaling mediated SOCE-induced hypertrophy91,92 and apoptosis. 93 Moreover, calcineurin-nuclear factor of activated T cells (NFAT) mediated downstream signaling in podocytes initiated by NMDA receptor- and TRPC6-mediated Ca2+ entry.70,71,94,95 Recently, Kim et al. 74 reported that calcineurin is also a downstream SOCE effector in podocytes. In cultured podocytes and in diabetic mice, calcineurin activation contributed to podocyte injury and proteinuria induced by insulin-activated SOCE. Thus, the insulin-SOCE-calcineurin signaling cascade in podocytes may be pivotal to the pathogenesis of renal disease.
Calpains, a family of Ca2+-activated cysteine proteases highly responsive to increased intracellular Ca2+, also mediate SOCE-initiated signaling in podocytes.96–98 Activation of TRPC6 in podocytes led to calpain activation.97,98 Interestingly, Farmer et al. 97 demonstrated that calpain is activated by its direct interaction with TRPC6, rather than TRPC6-mediated Ca2+ influx. Recently, we demonstrated calpain activation by SOCE-mediated, high glucose-induced injury in cultured human podocytes. 67 Since pharmacological SOC inhibition decreased and SOC activation increased calpain activity, 67 the calpain activation in our study could be ascribed to Ca2+ influx through SOCs.
Collectively, multiple pathways have been shown to mediate SOCE signaling in podocytes (Figure 3). These diverse signaling pathways are concordant with the multiple functions of SOC, all of which are critical for podocyte homeostasis.
Figure 3.

SOCE-initiated signaling pathways in podocytes. In diabetic nephropathy and many other kidney diseases, the activities of one or more of these pathways are upregulated and the increased signaling results in podocyte injury. Broken arrows: putative triggers of the signaling pathways.
EMT: epithelial–mesenchymal transition; ER: endoplasmic reticulum; SOCE: store-operated Ca2+ entry; STIM1: stromal interaction molecule 1.
Concluding remarks
This review has summarized the functions and downstream signaling of SOC in podocytes and its physiological and pathological impact. The evidence reviewed herein demonstrates unequivocally that SOCE is essential to podocyte integrity. As in other cell types, multiple mechanisms regulate SOC function in podocytes, and diverse intracellular messengers mediate SOC Ca2+ signaling. Intriguingly, the most substantial evidence relates to the contributions of SOC to cellular processes associated with disease states, for example, ultrastructural changes effecting podocyte simplification and effacement. Therefore, SOC signaling pathways are promising therapeutic targets for podocyte-associated renal diseases. However, several factors must be considered when pursuing such strategies. First, SOCs are ubiquitously expressed in the body, both in excitable and non-excitable cells. 1 Systemic application of SOC modulators in humans will have broad effects, including potential “off-target” consequences within and beyond the diseased organ or system. Second, SOC function is cell type specific. For instance, activation of SOC in glomerular mesangial cells inhibits extracellular matrix production, which is beneficial in diabetic kidney,99–101 but in proximal tubular cells, SOC activation exacerbates renal fibrosis. 102 Moreover, some evidence in the same cell types from different laboratories seem contradictory. For instance, Soni and Adebiyi 103 reported that SOC stimulated mesangial cell proliferation and synthesis of extracellular matrix proteins; in contrast, SOC inhibited these phenomena in our studies.99–101 Similarly, Zeng et al. 104 demonstrated that SOC inhibition exacerbates proteinuria in DN mice by impairing proximal tubular endocytosis of albumin, a finding divergent from the adverse effects of SOC on proximal tubular cells reported by Mai et al. 102 Finally, although many SOC inhibitors have been used over the past 30 years, none has proven to be purely SOC selective. 105 Therefore, podocyte-specific SOC signaling and its regulatory mechanisms must be delineated further to permit interrogation of strategies targeting SOC pathways for treatment of podocyte-related kidney diseases. Indeed, developing targeted delivery of selective agents to podocytes to modulate SOC holds particular promise for addressing SOC-mediated podocyte injury.
Footnotes
Authors’ Contributions: YT drafted, and RTM, KWM, and RM revised and edited the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by awards from the National Institute of Diabetes and Digestive and Kidney Disease (DK115424-01, to RM), the National Heart, Lung and Blood Institute (1R01HL153703-01A1 and 1K01HL139859a, to KWM), a Translational Project Award from American Heart Association (20TPA35500045, to RM), and a Predoctoral Fellowship from American Heart Association (903925, to YT).
ORCID iD: Yu Tao  https://orcid.org/0000-0003-1128-3176
https://orcid.org/0000-0003-1128-3176
References
- 1.Parekh AB, Putney JW., Jr.Store-operated calcium channels. Physiol Rev 2005;85:757–810 [DOI] [PubMed] [Google Scholar]
- 2.Parekh AB.Store-operatd CRAC channels: function in health and disease. Nat Rev 2010;9:399–410 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhari S, Mallet RT, Shotorbani PY, Tao Y, Ma R.Store-operated calcium entry: pivotal roles in renal physiology and pathophysiology. Exp Biol Med 2021;246:305–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis RS, Cahalan MD.Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul 1989;1:99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoth M, Penner R.Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992;355:353–6 [DOI] [PubMed] [Google Scholar]
- 6.Putney JW., Jr.Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther 1976; 198:375–84 [PubMed] [Google Scholar]
- 7.Hahn J, Jung W, Kim N, Uhm DY, Chung S.Characterization and regulation of rat microglial Ca2+ release-activated Ca2+ (CRAC) channel by protein kinases. GLIA 2000;31:118–24 [PubMed] [Google Scholar]
- 8.Albert AP, Large WA.A Ca2+ -permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol 2002;538:717–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM.The properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem 2001;276:7782–90 [DOI] [PubMed] [Google Scholar]
- 10.Su Z, Guo X, Barker DS, Shoemaker RL, Marchase RB, Blalock JE.A store-operated nonselective cation channel in human lymphocytes. Cell Mol Neurobiol 2005;25:625–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma R, Smith S, Child A, Carmines PK, Sansom SC.Store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol 2000;278:F954–61 [DOI] [PubMed] [Google Scholar]
- 12.Hoth M, Penner R.Calcium release-activated calcium current in rat mast cells. J Physiol 1993;465:359–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofer AM, Fasolato C, Pozzan T.Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+]. J Cell Biol 1998;140:325–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putney JW., Jr.The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol 2004;14:282–6 [DOI] [PubMed] [Google Scholar]
- 15.Liu XB, Singh BB, Ambudkar IS.TRPC1 is required for functional store-operated Ca2+ channels. J Biol Chem 2003;278:11337–43 [DOI] [PubMed] [Google Scholar]
- 16.Zagranichnaya TK, Wu X, Villereal ML.Endogenous TRPC1, TRPC3 and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem 2005;280:29559–69 [DOI] [PubMed] [Google Scholar]
- 17.Liu XB, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS.Molecular analysis of a store-operated and OAG sensitive non-selective cation channel: heteromeric assembly of TRPC1-TRPC3. J Biol Chem 2005;280:21600–6 [DOI] [PubMed] [Google Scholar]
- 18.Freichel M, Vennekens R, Olausson J, Hoffmann M, Müller C, Stolz S, Scheunemann J, Weibgerber P, Flockerzi V.Functional role of TRPC proteins in vivo: lessons from TRPC-deficient mouse models. Biochem Biophys Res Comm 2004;322:1352–8 [DOI] [PubMed] [Google Scholar]
- 19.Lievremont JP, Bird G, Putney JW.Canonical transient receptor potential TRPC7 can function both as receptor- and store-operated channel in human embryonic kidney HEK293 cells. Am J Physiol Cell Physiol 2004;287:C1709–16 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC.TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol 2004;287:C357–64 [DOI] [PubMed] [Google Scholar]
- 21.Ma R, Rundle D, Jacks J, Koch M, Downs T, Tsiokas L.Inhibitor of myogenic family: a novel suppressor of store-operated Ca2+ currents through an interaction with TRPC1. J Biol Chem 2003;278:52763–72 [DOI] [PubMed] [Google Scholar]
- 22.Randriamampita C, Tsien RY.Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature 1993;364:809–14 [DOI] [PubMed] [Google Scholar]
- 23.Fasolato C, Hoth M, Oenner R.A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem 1993;268: 20737–40 [PubMed] [Google Scholar]
- 24.Irvine RF.“Quantal” Ca2+ release and the control of Ca2+ entry by inositol phosphates: a possible mechanism. FEBS Lett 1990;263:5–9 [DOI] [PubMed] [Google Scholar]
- 25.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA.STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005;169:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Meyer T.STIM is a Ca2+ store sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 2005;15:1235–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A.A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006;441:179–85 [DOI] [PubMed] [Google Scholar]
- 28.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP.CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006;312:1220–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiluiza D, Krishna S, Schumacher VA, Schlondorff J.Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2). J Biol Chem 2013;288:18407–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL.STIM and Orai-dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem 2009;284:22501–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YDX, Gill DL.Calcium signaling by STIM and Orai: intimate coupling details revealed. Sci Signal 2010;3:pe42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nwokonko RM, Cai X, Loktionova NA, Wang Y, Zhou Y, Gill DL.The STIM-Orai pathway conformational coupling between STIM and Orai in the activation of store-operated Ca2+ entry. Adv Exp Med Biol 2017;993:83–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL.STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol 2006;16:1465–70 [DOI] [PubMed] [Google Scholar]
- 34.Dehaven WI, Smyth JT, Boyles RR, Putney JW.Calcium inhibition and calcium potentiation of Orail1, Orail2 and Orail3 calcium-release-activated calcium channels. J Biol Chem 2007;282:17548–56 [DOI] [PubMed] [Google Scholar]
- 35.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R.CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol 2007; 17:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motiani RK, Abdullaev IF, Trebak M.A novel native store-operated calcium channel encoded by Orai3. J Biol Chem 2010;285:19173–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J.Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS ONE 2011;6:e19285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez RA, Wan J, Song S, Smith KA, Gu Y, Tauseef M, Tang H, Makino A, Mehta D, Yuan JXJ. Upregulated expression of STIM2, TRPC6, and Orai2 contributes to the transition of pulmonary arterial smooth muscle cells from a contractile to proliferative phenotype. Am J Physiol Cell Physiol 2015;308:C581–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukushima M, Tomita T, Janoshazi A, Putney JW.Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J Cell Sci 2012;125:4354–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desi PN, Zhang X, Wu S, Janoshaz A, Bolimuntha S, Putney JW, Trebak M.Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci Signal 2015;8:ra74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darbellay D, Amaudeau S, Bader CR, Konig S, Bernheim L.STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol 2011;194:335–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA.STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol 2012;52:136–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horinouchi T, Higashi T, Higa T, Terada K, Mai Y, Aoyagi H, Hatate C, Nepal P, Horiguchi M, Harada T, Miwa S.Different binding property of STIM1 and its novel splice variant STIM1L to Orai1, TRPC3, and TRPC6 channels. Biochem Biophys Res Commun 2012;428:252–8 [DOI] [PubMed] [Google Scholar]
- 44.Sauc S, Bulla M, Nunes P, Orci L, Marchetti A, Antigny F, Bernheim L, Cosson P, Frieden M, Demaurex N.STIM1L traps and gates Orai1 channels without remodeling the cortical ER. J Cell Sci 2015;128:1568–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim MS, Zeng W, Yuan J, Shin DM, Worley P, Muallem S.Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem 2009;284:9733–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S.TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 2007;42:205–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S.STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 2007;9:636–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ong EC, Nesin V, Long CL, Bai CX, Guz JL, Ivanov IP, Abramowitz J, Birnbaumer L, Humphrey MB, Tsiokas L.A TRPC1 protein-dependent pathway regulates osteoclast formation and function. J Biol Chem 2013;288:22219–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S.Deletion of TRPC3 in mice reduced store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology 2009; 137:1509–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L.A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. PNAS 2009;106:3202–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L.Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/ICRAC channels. PNAS 2008;105:2895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L.Orai proteins interact with TRPC channels and confer responsiveness to store depletion. PNAS 2007;104:4682–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daehn IS, Duffield JS.The glomerular filtration barrier: a structural target for novel kidney therapies. Nat Rev Drug Discov 2021;20:770–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavenstädt H, Kriz W, Kretzler M.Cell biology of the glomerular podocyte. Physiol Rev 2003;83:253–307 [DOI] [PubMed] [Google Scholar]
- 55.Benzing T, Salant D.Insights into glomerular filtration and albuminuria. N Engl J Med 2021;384:1437–46 [DOI] [PubMed] [Google Scholar]
- 56.Blaine J, Dylewski J.Regulation of the actin cytoskeleton in podocytes. Cells 2020;9:1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ning L, Suleiman H, Miner JH.Synaptopodin deficiency exacerbates kidney disease in a mouse model of Alport syndrome. Am J Physiol Renal 2021;321:F12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q, Gulati A, Lemaire M, Nottoli T, Bale A, Tufro A.Rho-GTPase Activating Protein myosin MYO9A identified as a novel candidate gene for monogenic focal segmental glomerulosclerosis. Kidney Int 2021;99:1102–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sever S.Role of actin cytoskeleton in podocytes. Pediatr Nephrol 2021; 36:2607–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian D, Jacobo SMP, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstadt H, Hsu HH, Schlondorff J, Ramos A, Greka A.Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Scien Signal 2010;3:ra77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB.A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005;308:1801–4 [DOI] [PubMed] [Google Scholar]
- 62.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J.Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 2007;18:29–36 [DOI] [PubMed] [Google Scholar]
- 63.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, Staruschenko A.Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int 2014;86:506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson M, Roshanravan H, Khine J, Dryer SE.Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol 2014;229:434–42 [DOI] [PubMed] [Google Scholar]
- 65.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A.Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 2013;123:5298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H, Zhao B, Liao C, Zhang R, Meng K, Xu J, Jiao J.High glucose-induced apoptosis in cultured podocytes involves TRPC6-dependent calcium entry via the RhoA/ROCK pathway. Biochem Biophys Res Commun 2013;434:394–400 [DOI] [PubMed] [Google Scholar]
- 67.Tao Y, Chaudhari S, Yazdizadeh Shotorbani P, Ding Y, Chen Z, Kasetti R, Zode G, Ma R.Enhanced Orai1-mediated store-operated Ca2+ channel/calpain signaling contributes to high glucose-induced podocyte injury. J Biol Chem 2022;298:101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu S, Nam SM, Kim JH, Das R, Choi SK, Nguyen TT, Quan X, Choi SJ, Chung CH, Lee EY, Lee IK, Wiederkehr A, Wollheim CB, Cha SK, Park KS.Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis 2015;6:e1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nijenhuis T, Sloan AJ, Hoenderop JG, Kistler AD, Bakker M, Bindels RJM, de Boer RA, Moller CC, Hamming I, Navis G, Wetzels JFM, Berden JHM, Reiser J, Faul C, van der Vlag J.Angiotensin II contributes to podocyte injury by increasing TRPC6 expressing via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 2011;179:1719–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu H, Kistler A, Faridi MH, Meyer JO, Tryniszewska B, Mehta D, Yue L, Dryer S, Reiser J.Synaptopodin limits TRPC6 podocyte surface expression and attenuates proteinuria. J Am Soc Nephrol 2016;27:3308–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asano-Matsuda K, Ibrahim S, Takano T, Matsuda J.Role of Rho GTPase interacting proteins in subcellular compartments of podocytes. Int J Mol Sci 2021;22:3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, Kaufmann U, Jani PK, Lacruz RS, Flockerzi V.Orai2 modulates store-operated calcium entry and T cell-mediated immunity. Nat Commun 2017;8:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miao L, Wei D, Zhang Y, Liu J, Lu S, Zhang A, Huang S.Effects of stromal interaction molecule 1 or Orai1 overexpression on the associated proteins and permeability of podocytes. Nephrology 2016;21:959–67 [DOI] [PubMed] [Google Scholar]
- 74.Kim JH, Hwang KH, Dang BTN, Eom M, Kong ID, Gwack Y, Yu S, Gee HY, Birnbaumer L, Park KS, Cha SK.Insulin-activated store-operated Ca2+ entry via Orai1 induces podocyte actin remodeling and causes proteinuria. Nat Commun 2021;12:6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gigante M, Caridi G, Montemurno E, Soccio M, d’Apolito M, Cerullo G, Aucella F, Schirinzi A, Emma F, Massella L, Messina G, De Palo T, Ranieri E, Ghiggeri GM, Gesualdo L.TRPC6 mutation in children with steriod-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol 2011;6:1626–34 [DOI] [PubMed] [Google Scholar]
- 76.Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M.Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 2019;143:38–42 [DOI] [PubMed] [Google Scholar]
- 77.Jin J, Ye M, Hu K, Gong J, He Q.STIM promotes the epithelial-mesenchymal transition of podocytes through regulation of FcrRII activity in diabetic nephropathy. Histo Histopathol 2019;34:671–82 [DOI] [PubMed] [Google Scholar]
- 78.Jin J, Wu D, Zhao L, Zou W, Shen W, Tu Q, He Q.Effect of autophagy and stromal interaction molecule 1 on podocyte epithelial-mesenchymal transition in diabetic nephropathy. Int J Clin Exp Pathol 2018;11:2450–9 [PMC free article] [PubMed] [Google Scholar]
- 79.Rogacka D.Insulin resistance in glomerular podocytes: potential mechanisms of induction. Arch Biochem Biophys 2021;710:109005. [DOI] [PubMed] [Google Scholar]
- 80.Welsh GI, Hale L, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstadt H, Tavare JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJM. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 2010;12:329–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VE, Ranjan S, Wolter J, Kohli S, Shahzad K, Heidel F, Krueger M, Schwenger V, Moeller M, Kalinski T, Reiser J, Chavakis T, Isermann B.Defective podocyte insulin signaling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in daibetic nephropathy. Nat Commun 2015;6:6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fornoni A.Proteinuria, the podocyte, and insulin resistance. N Engl J Med 2010;363:2068–9 [DOI] [PubMed] [Google Scholar]
- 83.Liu WJ, Reiser J, Park TS, Liu Z, Ishibe S.New insights into diabetic kidney disease: the potential pathogenesis and therapeutic targets. J Diabetes Res 2017;2017:3945469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navarro-Gonzalez JF, Mora-Fernandez C.The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19:433–42 [DOI] [PubMed] [Google Scholar]
- 85.Gurley SB, Ghosh S, Johnson SA, Azushima K, Sakban RB, George SE, Maeda M, Meyer TW, Coffman TM.Inflammation and immunity pathways regulate genetic susceptibility to diabetic nephropathy. Diabetes 2018;67:2096–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hickey FB, Martin F.Diabetic kidney disease and immune modulation. Curr Opin Pharmacol 2013;13:602–12 [DOI] [PubMed] [Google Scholar]
- 87.Wada J, Makino H.Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 2016;12:13–26 [DOI] [PubMed] [Google Scholar]
- 88.Gessner JE, Heiken H, Tamm A, Schmidt RE.The IgG Fc receptor family. Ann Hematol 1998;76:231–48 [DOI] [PubMed] [Google Scholar]
- 89.Lopez-Parra V, Mallavia B, Lopez-Franco O, Ortiz-Munoz G, Oguiza A, Recio C, Blanco J, Nimmerjahn F, Egido J, Gomez-Guerrero C.Fc receptor deficiency attenuates diabetic nephropathy. J Am Soc Neprhol 2012;23:1518–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ying Q, Wu G.Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail 2017;39: 474–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossum DBV, Patterson RL, Ma HT, Gill DL. Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels: comparison of coupling and function. J Biol Chem 2000;275:28562–8 [DOI] [PubMed] [Google Scholar]
- 92.Pang Y, Hunton DL, Bounelis P, Marchase RB.Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes. Diabetes 2002;51:3461–7 [DOI] [PubMed] [Google Scholar]
- 93.Tamareille S, Mignen O, Capiod T, Rücker-Martin C, Feuvray D.High glucose-induced apoptosis through store-operated calcium entry and calcineurin in human umbilical vein endothelial cells. Cell Calcium 2006;39:47–55 [DOI] [PubMed] [Google Scholar]
- 94.Kim EY, Anderson M, Dryer SE.Sustained activation of N-methyl-D-aspartate receptors in podoctyes leads to oxidative stress, mobilization of transient receptor potential canonical 6 channels, nuclear factor of activated T cells activation, and apoptotic cell death. Mol Pharmacol 2012;82:728–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P.The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 2008;14:931–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Metwally E, Zhao G, Wang Q, Zhang YQ.Ttm50 facilitates calpain activation by anchoring it to calcium stores and increasing its sensitivity to calcium. Cell Res 2021;31:433–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farmer LK, Rollason R, Whitcomb DJ, Ni L, Goodliff A, Lay AC, Birnbaumer L, Heesom KJ, Xu SZ, Saleem MA, Welsh GI.TRPC6 binds to and activates calpain, independent of its channel activity, and regulates podocyte cytoskeleton, cell adhesion, and motility. J Am Soc Nephrol 2019;30:1910–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verheijden KAT, Sonneveld R, Bakker-van Bebber M, Wetzels JFM, van der Vlag J, Nijenhuis T. The calcium-dependent protease calpain-1 links TRPC6 activity to podocyte injury. J Am Soc Nephrol 2018;29:2099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu P, Wand Y, Davis ME, Zuckerman JE, Chaudhari S, Begg M, Ma R.Store-operated Ca2+ channel in mesangial cells inhibits matrix protein expression. J Am Soc Nephrol 2015;26:2691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu P, Ren Y, Ma Y, Wang Y, Jiang H, Chaudhari S, Davis ME, Zuckerman JE, Ma R.Negative regulation of Smad1 pathway and collagen IV expression by store-operated Ca2+ entry in glomerular mesangial cells. Am J Physiol Renal Physiol 2017;312:F1090–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chaudhari S, Li W, Wang Y, Jiang H, Ma Y, Davis ME, Zuckerman JE, Ma R.Store-operated calcium entry suppressed the TGF-b1/Smad3 signaling pathway in glomerular measngial cells. Am J Physiol Renal Physiol 2017;313:F729–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mai X, Shang J, Liang S, Yu B, Yuan J, Lin Y, Luo R, Zhang F, Liu Y, Lv X, Li C, Liang X, Wang W, Zhou J.Blockade of Orai1 store-operated calcium entry protects against renal fibrosis. J Am Soc Nephrol 2016;27:3063–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soni H, Adebiyi A.Urotensin II-induced store-operated Ca2+ entry contributes to glomerular mesangial cell proliferation and extracellular matrix protein production under high glucose conditions. Sci Rep 2017;7:18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng B, Chen GL, Garcia-Vaz E, Bhandari S, Daskoulidou N, Berglund LM, Jiang H, Hallett T, Zhou LP, Huang L, Xu ZH, Nair V, Nelson RG, Ju W, Kretzler M, Atkin SL, Gomez MF, Xu SZ.Orai channels are critical for receptor-mediated endocytosis of albumin. Nat Commun 2017;8:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jairaman A, Prakriya M.Molecular pharmacology of store-operated CRAC channels. Channels 2013;7:402–14 [DOI] [PMC free article] [PubMed] [Google Scholar]