Abstract
Introduction:
Evidence suggests that cancer treatment-related toxicity is under-reported by clinicians. We sought to compare patient- and clinician-reported acute toxicity among patients undergoing radiotherapy for primary breast cancer, and to determine factors associated with patient-clinician discordance.
Methods and Materials:
Patient responses from a weekly PRO-CTCAE-based assessment were matched to clinician assessments of acute toxicity during treatment. Weighted kappa (κ) statistics were used to evaluate agreement between patient and clinician assessments. Linear regression, logistic regression, and generalized estimating equation models were used to identify covariates associated with discordance.
Results:
Overall, 842 patient-clinician assessment pairs from 376 unique patients were analyzed. Total symptom burden score was higher for patients than clinicians (4.7 vs. 2.3, p<0.01). Dermatitis, pruritis, pain, and edema items were classified as having minimal agreement (κ of 0.25, 0.23, 0.20, and 0.25, respectively). Fatigue (κ 0.17) and psychosocial (0.03) patient-clinician pairs were found to have no agreement. The linear regression demonstrated that assessments by patients who identified as Black or African American were associated with a 0.13 point decrease in discordance (95% CI −0.25, −0.01) while time from the start of treatment was associated with increased discordance (95% CI 0.07, 0.12).
Conclusion:
For patients undergoing breast radiotherapy, discordance in patient- and clinician- symptom reporting is high and increases as treatment progresses. The mechanism of reduced discordance among Black or African American patients warrants further investigation. Prospective studies are needed to determine if interventions for lower severity symptoms, which are commonly overlooked by clinicians, can reduce symptom burden and improve patient quality of life during radiotherapy.
Introduction
The use of patient-reported outcomes (PROs), symptom assessments reported directly by the patient, has been shown to reduce symptom burden and improve overall survival for patients receiving cancer treatment (1,2). There is a growing body of literature on the use of PROs in both clinical practice (3) and as a measurable endpoint in clinical trials (4,5). However, the collection of PROs highlights a lack of desired reliability with regards to clinician reporting of patient symptoms, with only moderate agreement demonstrated between patient-reported and clinician-reported outcomes (6,7).
To date, most literature on the use of PROs and their comparison to clinician-reported outcomes is limited to patients who are receiving systemic oncologic treatment. Unlike oncologic treatments that are delivered systemically, radiotherapy is often delivered to a specific anatomic disease site, resulting in a symptom profile that includes more localized and visible effects such as radiation esophagitis and dermatitis, respectively. Thus, while prior literature has demonstrated discordance between patient and clinician reports of symptoms for systemic therapies, less is known about symptoms that may be more specific to radiotherapy (8). Additionally, little is known about patient characteristics and factors that may predict for patient-clinician agreement pertaining to symptom assessments.
A subset of items from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), a validated tool used to collect PROs among patients with cancer, was validated for specific anatomic disease sites to assess acute toxicities and side effects of treatment during radiotherapy (9). Subsequently, an assessment was developed and implemented for patients undergoing radiotherapy for primary breast cancer at a large, comprehensive cancer center (10). Herein, we compare patient reports of acute toxicities during breast radiotherapy to clinician assessments in order to analyze differences in patient and clinician reports, and to determine if patient characteristics or treatment factors are associated with differences in patient-clinician symptom assessments.
Methods
Setting and Participants
From June of 2019 to July of 2020, a subset of patients undergoing radiotherapy for primary breast cancer at a large, multi-center comprehensive cancer center completed a weekly assessment distributed via an online patient portal to evaluate patient-reported toxicity as part of an institutional pilot. Details of patient-reported outcomes and clinician experience using ePROs during and after radiotherapy have been previously reported (10). Patients were included if a clinician-reported assessment from an on-treatment visit was available within three days of a completed patient assessment. The institutional review board approved this study.
Patient and clinical variables
We collected self-reported patient demographic data at the time of treatment initiation, including age, race/ethnicity, education level, and employment status. Additionally, home distance from treatment center (miles) and socioeconomic index (scaled, 0–100) using the University of Wisconsin’s Neighborhood Atlas (11) were collected. We also assessed prior receipt of chemotherapy, location of treatment (main urban campus or a suburban regional clinical site), and the type of radiotherapy treatment patients received (partial breast radiation, whole breast radiation, or radiation with inclusion of the regional nodes).
Symptom reporting assessments and definitions of discordance
A 7–17-question response-adapted PRO-CTCAE-based survey addressed toxicities including skin changes or radiation dermatitis, pain in the radiated area, swelling, tenderness, and fatigue (10). Additionally, the survey included items on anxiety and worry from the Generalized Anxiety Disorder 2-item (GAD-2) screening tool (12). The surveys were assigned three days prior to the patients’ scheduled on-treatment visit with their radiation oncologists. The survey asked patients to report their symptoms over a 7-day recall period. Further details are described in a prior publication (10).
During radiotherapy treatment, patients were assessed weekly in clinic by their radiation oncologist and members of their care team (nurses, advanced practitioners). Treatment-related toxicity was reported by clinicians using the CTCAE v4.0 (13,14), the Visual Analog Scale for pain (15), and a binary question on patient psychosocial wellbeing. We collected all clinician-reported evaluations during the above study period. Clinician-reported assessments were paired to patient-completed surveys based on patient MRN and were considered a matched pair if they were dated within three days of each other.
Using each pair of matched patient-clinician assessments (pairings outlined in Supplemental Table 1), we generated average discordance scores by calculating the difference between each pair and dividing them by the number of pairs assessed (discordance score = ((patient skin toxicity score – clinician dermatitis score) + (patient fatigue score – clinician fatigue score) + (patient breast enlargement score – clinician breast edema score) + (patient itchy skin score – clinician pruritis score) + (patient pain score – clinician pain score) + (patient psychosocial score – clinician psychosocial score))/6). We also calculated total symptom burden scores by adding all six symptom items. For patient items in which there were multiple sub-items (i.e. the patient assessment of skin toxicity asked patients to rate the severity of skin breakdown and skin discoloration) the maximum score was used. For individual patient assessment items that contained different domains (i.e. fatigue severity and distress) composite scores were extrapolated from the domain scores according to the algorithm described by Basch et al (16). The average discordance score was used as a continuous outcome variable. To quantify high patient-clinician discordance, we binarized the variable to compare patients with discordance of at least one point versus those with discordance less than one point. This binarization included only comparisons where patients scored symptoms higher (more severe) than clinicians, which represented the vast majority of cases.
For scores that were assessed on different scales between patients and clinicians (i.e. patient-reported pain was evaluated on 0–4 scale while clinician-reported was evaluated on a 0–10 scale), we recalibrated scores so that they were evaluated on the same scale. For example, for psychosocial side effects, clinicians indicated yes or no if symptoms were present, whereas patients responded on a 0 to 3 scale. We rescaled patient responses such that scores of 1 through 3 aligned with clinician scores of “yes”.
Statistical Methods
Patient-clinician agreement
An independent T-test was used to compare the mean total symptom burden scores between patients and clinicians. We used weighted kappa statistics to evaluate the degree of agreement between patient and clinician scores. We report the expected agreement, observed agreement, kappa value, significance (with an alpha level of 0.05), and qualitative interpretation of the kappa value. The kappa statistic is measured on a scale of 0–1 with a kappa value of 0 denoting no agreement and a value of 1 denoting perfect agreement (17). We quantified kappa scores as having none (0–0.20), minimal (0.21–0.39), weak (0.40–0.59), moderate (0.60–0.79), strong (0.80–0.90), or almost perfect agreement (>0.90) (17).
Linear and logistic regression models
We selected one pair of completed, matched assessments per patient-clinician pair, using the pair of assessments completed furthest from the initiation of treatment. We used linear regression with robust standard errors to identify covariates associated with discordance, and logistic regression to identify covariates associated with discordance greater than 1. All covariates described above were included in each model. We verified normality of residuals for linear regression models. We used locally weighted scatterplot smoothing to assess linearity in the log-odds of continuous covariates versus the logistic outcome (18). Additionally, we evaluated logistic model goodness-of-fit using a Hosmer-Lemeshow test (19). For the logistic model, we collapsed race categories of Other and Unknown because of sparse data.
Generalized estimating equation models
Using all paired patient-clinician assessments, we used generalized estimating equation (GEE) models to assess associations between covariates described above and patient-clinician discordance. We used a GEE model with gaussian family and identity link to assess overall discordance, and binomial family and logit link to assess discordance greater than 1. We assessed quasi-likelihood under the independence model criterion to select correlation structures, and chose exchangeable correlation structures for both models (20,21). For the binomial model, we collapsed race categories of Other and Unknown because of sparse data.
Missing data
Following extensive review and conversations with on-site study team members and clinicians, we concluded that data missing from clinician assessments, in which some items were completed but others were not, was not missing at random, as negative findings were typically not recorded. Resultantly, we used single imputation of zeros to address missing clinician data (22). Data missing from patient assessments totaled less than 10%, and no more than 5% in any single variable, and we used complete-case analysis to analyze data after single imputation of clinician data.
Sensitivity Analyses
Our primary analysis assessed kappa statistics with single imputation of missing provider data. We also evaluated kappa statistics using a complete-case approach, and with unweighted analyses. Results from sensitivity analyses are reported in Supplemental table 2.
Results
Participants
From June 3, 2019 to July 20, 2020, a total of 678 patients receiving radiotherapy for primary breast cancer were assigned a total of 2,081 assessments during treatment. A total of 965 assessments were completed during treatment for a response rate of 46%. Overall, 842 (87%) patient assessments completed on-treatment were matched to corresponding provider on-treatment assessments, accounting for 376 unique patients. The patient cohort was predominantly White, non-Hispanic (64%), received treatment at a suburban regional facility rather than the urban main hospital campus (74%), and had a college/vocational school (23%) or graduate level education (24%). Nearly half of the patients also received chemotherapy prior to radiotherapy (49%). Characteristics of patients included in the cohort are outlined in Table 1.
Table 1.
Cohort Characteristics
| Variable | Subcategory | All patients (n=376) n (%) or median (IQR) |
|---|---|---|
| Radiation Type | Partial breast irradiation (PBI) | 53 (14%) |
| Whole breast irradiation (WBI) | 219 (58%) | |
| Post-mastectomy radiation/WBI with regional nodal irradiation | 104 (28%) | |
| Age (years) | ― | 54 (46–63) |
| Race | White | 239 (64%) |
| Asian/Indian | 40 (11%) | |
| Black or African American | 40 (11%) | |
| Hispanic/Latino | 30 (8%) | |
| Other | 11 (3%) | |
| Unknown | 16 (4%) | |
| Clinic Type | Main | 97 (26%) |
| Education | Less than a high school diploma | 3 (1%) |
| High school diploma | 44 (12%) | |
| College/Vocational school | 86 (23%) | |
| Graduate or professional school | 89 (24%) | |
| Unknown | 154 (41%) | |
| Employment | Yes | 180 (48%) |
| No | 103 (27%) | |
| Unknown | 93 (25%) | |
| Chemotherapy | Yes | 184 (49%) |
| Distance to treatment center (miles, n=370) | ― | 14 (8–22) |
| Socioeconomic status (n=369) | ― | 12 (5–19) |
There were several instances in which clinicians completed some but not all of the items available for clinicians to report (see methods). Overall, missing clinician data from the 842 pairs was as follows: dermatitis (n=94, 11%), fatigue (n=95, 11%), edema (n=107, 13%), pruritis (n=97, 12%), pain (n=14, 2%), and psychosocial concerns (n=145, 17%).
Patient-clinician agreement
Patients reported a higher mean total symptom burden score than clinicians (4.7 vs. 2.3, p<0.01). Figure 1 depicts all patient- versus clinician-reported acute radiotherapy toxicities, including recalibrated scores so that patient and clinician assessment scores are on the same scale. Calculated weighted kappa statistics for patient-clinician, symptom specific assessment pairs are outlined in Table 2. Patient-reported skin breakdown, dryness, and discoloration was paired with clinician-reported dermatitis, patient-reported skin itchiness was paired with clinician-reported pruritus, patient-reported breast enlargement was paired with clinician-reported edema, and patient-reported worry and nervousness was paired with clinician-reported psychosocial concerns. Patient-reported pain and fatigue were paired with clinician-reported pain and fatigue, respectively. Analyses showed that dermatitis, pruritis, pain, and edema pairs were classified as having minimal agreement between clinician and patient assessments. Fatigue and psychosocial patient-clinician pairs were found to have no agreement (Table 2).
Figure 1.
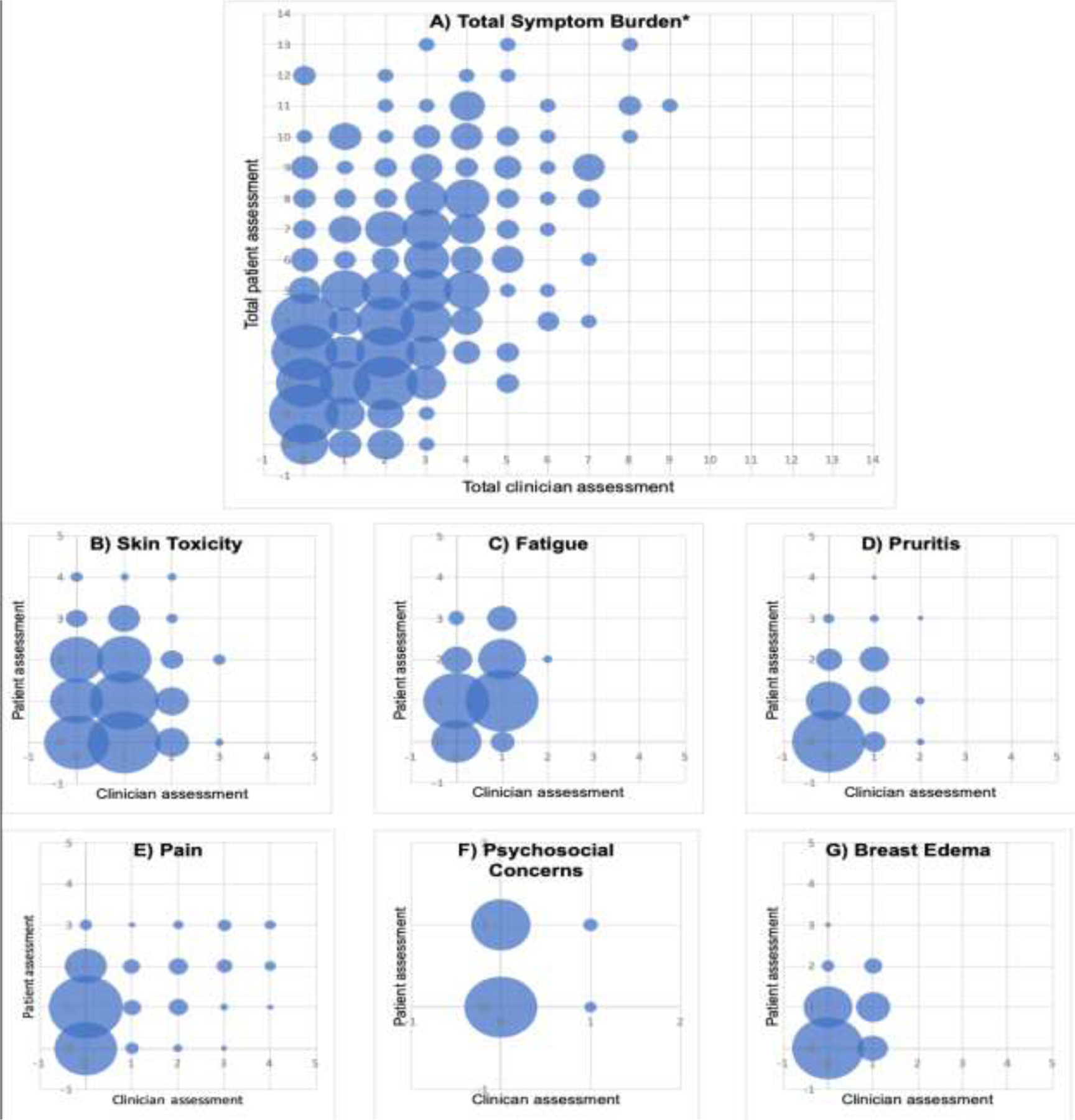
Patient-reported versus clinician-reported assessments of acute A) total symptom burden, B) skin toxicity, C) fatigue, D) pruritis, E) pain, F) psychosocial concerns, and G) breast edema during breast radiotherapy. *Total symptom burden equates to the sum of assessments scores from all symptoms listed.
Table 2.
Concordance between patient and clinician assessments by symptoms according to single, weighted kappa analysis
| Clinician Variable | Patient Variable | Agreement | Expected Agreement | Kappa | p-value | Interpretation of agreement* |
|---|---|---|---|---|---|---|
| Dermatitis Radiation | Skin Toxicity | 82.65% | 79.93% | 0.2478 | <0.001 | Minimal |
| Fatigue | Fatigue Composite | 77.87% | 73.43% | 0.1669 | <0.0001 | None |
| Pruritus | Itchy Skin | 86.96% | 83.00% | 0.2333 | <0.001 | Minimal |
| Pain Score | Pain Composite | 79.47% | 74.18% | 0.2047 | <0.0001 | Minimal |
| Psychosocial | Psychosocial | 59.04% | 57.59% | 0.0343 | 0.0718 | None |
| Breast edema | Breast enlargement | 66.40% | 55.48% | 0.2453 | <0.001 | Minimal |
Logistic and linear regression model results
Average discordance was greater than or equal to one point difference among 79 patients. Within the logistic regression model, the only variable that was associated with high patient-clinician discordance (average discordance greater than or equal to one point difference) was time at which the matched assessments were completed from the start of treatment. As treatment progressed, discordance increased. Survey pairs that were collected a week or greater from the start of treatment were associated with high discordance (OR 1.46, 95% CI 1.07, 1.99) compared to those initially collected (Table 3).
Table 3.
Results of the logistic regression model assessing variables associated with high patient-clinician discordance (average discordance ≥1 point difference)
| Variable | OR (95% CI) |
|---|---|
| Radiation Type | |
| Partial breast irradiation (PBI) | ― |
| Whole breast irradiation (WBI) | 1.12 (0.29–4.38) |
| Post-mastectomy radiation/WBI with regional nodal irradiation | 2 (0.13–3.39) |
| Age | 1.00 (0.97–1.03) |
| Race | |
| White, non-Hispanic | ― |
| Asian | 1.42 (0.53–3.81) |
| Black or African American | 0.58 (0.17–1.99) |
| Hispanic or Latino | 1.14 (0.36–3.63) |
| N/A or Other | 0.27 (0.03–2.30) |
| Clinic Type | |
| Main | ― |
| Regional | 0.84 (0.39–1.81) |
| Level of Education | |
| High school diploma or less | ― |
| College/Vocational school | 0.83 (0.25–2.72) |
| Graduate or professional school | 1.65 (0.54–4.99) |
| Unknown | 0.60 (0.15–2.35) |
| Work Status | |
| Not employed full-time | ― |
| Employed full-time | 0.74 (0.33–1.65) |
| Unknown | 1.52 (0.42–5.46) |
| Received Prior Chemotherapy | |
| No | ― |
| Yes | 1.02 (0.50–2.11) |
| Distance from Clinic | |
| Less than 15 miles | ― |
| 15 to 49 miles | 1.20 (0.59–2.45) |
| Greater than 49 miles | 1.34 (0.46–3.93) |
| Socioeconomic Status | 1.01 (1.00–1.02) |
| Week from Treatment Start | 1.46** (1.07–1.99) |
p-value <0.05
Within the linear regression model, in which discordance was treated as a continuous variable, assessment pairs from patients who identified as Black or African American were associated with a 0.13 point decrease in discordance (95% CI −0.25, −0.01) (Table 4). Time from the start of treatment was associated with increased discordance, with each week from the start of treatment resulting in a 0.10 point increase in discordance (95% CI 0.07, 0.12) (Table 4).
Table 4.
Results of the linear regression model assessing variables associated with patient-clinician discordance
| Variable | Coef (95% CI) |
|---|---|
| Radiation Type | |
| Partial breast irradiation (PBI) | ― |
| Whole breast irradiation (WBI) | −0.02 (−0.14–0.09) |
| Post-mastectomy radiation/WBI with regional nodal irradiation | −0.10 (−0.25–0.04) |
| Age | 0.00 (−0.01–0.001) |
| Race | |
| White | ― |
| Asian or Indian | 0.08 (−0.05–0.21) |
| Black or African American | −0.13** (−0.25-(−0.01)) |
| Hispanic or Latino | 0.06 (−0.09–0.21) |
| N/A | 0.05 (−0.17–0.27) |
| Other | −0.05 (−0.25–0.15) |
| Clinic Type | |
| Main | ― |
| Regional | −0.03 (−0.14–0.07) |
| Level of Education | |
| High school diploma or less | ― |
| College/Vocational school | −0.02 (−0.17–0.13) |
| Graduate or professional school | 0.09 (−0.06–0.24) |
| Unknown | −0.07 (−0.23–0.08) |
| Work Status | |
| Not employed full-time | ― |
| Employed full-time | −0.05 (−0.15–0.05) |
| Unknown | 0.03 (−0.11–0.16) |
| Received Neoadjuvant Chemotherapy | |
| No | ― |
| Yes | 0.00 (−0.09–0.09) |
| Distance from Clinic | |
| Less than 15 miles | ― |
| 15 to 49 miles | 0.03 (−0.06–0.11) |
| Greater than 49 miles | −0.02 (−0.17–0.13) |
| Socioeconomic Status | 0.001 (−0.001–0.003) |
| Week from Treatment Start | 0.10** (0.07–0.12) |
p-value <0.05
Generalized estimating equation model results
In the gaussian GEE model, which accounts for multiple sets of surveys for the same patient, patients who identified as Black or African American had discordance scores that were 0.15 points lower than patients who identified as White, non-Hispanic (95% CI −0.27, −0.02). No variables were associated with high discordance in the binomial GEE model.
Discussion
In a cohort of patients receiving radiotherapy for primary breast cancer, PRO-CTCAE-based patient assessments matched temporally to assessments for clinicians showed minimal to no agreement. Clinicians most often underreported the presence and/or severity of side effects compared to patients. Additionally, discordance between patient-reported side effects and clinician-reported side effects increased over the course of patients’ treatment. Upon adjustment for patient characteristic and treatment variables, matched patient-clinician assessments from patients who identified as Black or African American were more likely to show agreement between patient-reported and clinician-reported acute toxicity outcomes.
This finding is consistent with several prior studies during radiotherapy, but contributes to the limited data specific to breast cancer . A report from the NRG Oncology Radiation Therapy Oncology Group (RTOG) 1203 Study, a phase III trial comparing standard pelvic radiation to pelvic radiation delivered using intensity-modulated radiotherapy, found that clinicians tended to underreport acute and late adverse effects of treatment compared to patients (23). A phase II trial, in which patients with oropharyngeal cancer received de-intensified chemoradiation, also found that clinicians rated treatment toxicity severity lower than patients when using CTCAE and PRO-CTCAE items, respectively (24). Furthermore, similar to the results of the present study, this study also found that patient-clinician agreement declined over the course of patients’ treatment (24). Side effects of breast radiation tend to accumulate and worsen as patients’ treatment progresses, with peak symptom burden typically seen near the end of treatment (25–28). Additionally, discordance between patient and clinician assessments has been associated with increased symptom severity among patients with various types of cancer (29) and breast cancer specifically (30).
An important caveat to the comparison of PRO-CTCAE items and CTCAE items that was mentioned in both aforementioned trials, is that the domains assessed for a given symptom may vary between patients and clinicians (23,24). For example, the PRO-CTCAE items used to assess fatigue in our assessment asked patients to rate the severity of their fatigue (none, mild, moderate, severe, very severe) and how much the fatigue interfered with their usual daily activities (not at all, a little bit, somewhat, quite a bit, very much), whereas the CTCAE prompts clinicians to assess fatigue as one, encompassing domain. Unlike prior studies, we were able to account for the differences in the structure of the assessments by extrapolating composite symptom scores from symptom items with multiple domains based on a recent publication by Basch et al (16). When comparing the kappa statistic from patient-clinician pairs that used only patient-rated severity versus the patient composite score based on all domains, patient-clinician assessments of fatigue continued to show no agreement while patient-clinician assessments of pain went from no agreement to minimal agreement. These findings may suggest that the lack of patient-clinician agreement seen in previous studies is perhaps less a product of differences in the wording and structure of assessment tools used by patients and clinicians than previously thought, and more so a result of differences in patient-clinician symptom reporting (24).
A strength of this investigation was our ability to assess patient characteristics and treatment factors that may influence patient-clinician discordance. A prior study that assessed patient-clinician agreement of symptoms during chemotherapy found no association between concordance and patient age, sex, and disease characteristics (31). Within radiation oncology, a previously presented abstract suggested that older patient age and patient race of Black or African American were associated with increased discordance among patient and clinician reports of toxicity during radiotherapy for head and neck cancer (32). In general, there is ample evidence that clinicians underestimate pain experienced by patients who are Black or African American and that these patients receive less-adequate pain management (33,34), extending to the management of Black or African American patients with cancer (35–37). Interestingly, in our investigation patient identification as Black or African American was associated with slightly decreased patient-clinician discordance, although the effect size was small and therefore unlikely to translate to a change in patient care. Nonetheless, we observed no increase in discordance, despite differences in self-reported race/ethnicity. Several toxicity scales, including the CTCAE assessment, fail to account for variations in the presentation of skin toxicity that may be seem among patients of color compared to patients with less skin pigmentation (38). It is also possible that because the bias towards and underreporting of symptoms among patients who are Black or African American is well documented in the literature, that clinicians may be more aware of their potential biases in reporting symptoms among patients in this population and their evaluations of pain and additional symptoms may reflect this. In the future, qualitative interviews and analyses could be done to better understand the unique experiences of women of color who undergo radiotherapy and the clinicians awareness and understanding of their experience.
There are several limitations to our investigation including the single institution nature of the study. Furthermore, patient-reported worry and concern during treatment and clinical-reported psychosocial concerns, as well as patient-reported pain in the radiated area and clinician-reported pain were assessed using different instruments between clinicians and patients. Although we re-calibrated the scales used to evaluate these symptoms in our analysis, these assessments were not designed to be directly compared and have intrinsic differences that we may not have been completely able to account for. Additionally, the PRO-CTCAE and CTCAE assessments were designed to complement each other rather than be directly compared (39). Because the majority of the data were collected during the initial pilot implementation of an electronic patient-reported outcome assessment at a large, comprehensive cancer center, each patient was primarily seen and treated by one of only three radiation oncologists. Clinician characteristics and their influence on patient-clinician discordance could potentially be evaluated in a future study, involving a larger cohort of patients and clinicians. It is also important to note that the majority of the patient symptom assessments in our study reported low-severity symptoms. While these low-severity symptoms influence patient quality of life, they may not always necessitate clinician concern and intervention. Psychosocial concerns, such as nervousness and worry associated with treatment, have been shown to effect quality of life in women with breast cancer more than physical side effects (40). However, we found no agreement between clinicians and patients in terms of psychosocial concerns. This poses a clinical challenge as low-severity symptoms, fatigue, and psychosocial symptoms are less straight forward to manage. Further research is needed to determine how best to manage these symptoms so that they are addressed in a meaningful yet clinically efficient way.
Conclusion
For patients receiving radiotherapy for primary breast cancer, patient- and clinician- reporting of fatigue and psychosocial effects of treatment demonstrated no agreement while reporting of acute radiation dermatitis, pruritis, pain, and breast enlargement demonstrated only minimal agreement. Patient-clinician discordance increased as patients’ treatment progressed, as they developed more severe side effects. Patient-clinician assessment pairs that included assessments completed by patients who identified as Black or African American were associated with decreased discordance, however the effect size of this comparison was small and may not be clinically meaningful. Regardless, further confirmation and mechanism of reduced discordance among this population appears warranted. Further investigation is needed to determine if interventions to address lower severity symptoms can reduce overall symptom burden during radiotherapy and thereby improve quality of life.
Supplementary Material
Research Support:
This work is supported by an MSK Core Grant (P30 CA008748). Additional funding provided by the National Cancer Institute (K08 CA252640, E.F.G). Caroline King was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number TL1TR002371 and the National Institute on Drug Abuse of the National Institutes of Health under Award Number F30DA052972.
Funding:
This work is supported by an Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748). Additional funding provided by the National Cancer Institute (K08 CA252640, E.F.G). Caroline King was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number TL1TR002371 and the National Institute on Drug Abuse of the National Institutes of Health under Award Number F30DA052972.
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
Research data are stored in a secure, institutional database and will be shared upon request to the corresponding author.
References
- 1.Bryant AL, Coffman E, Phillips B, et al. Pilot randomized trial of an electronic symptom monitoring and reporting intervention for hospitalized adults undergoing hematopoietic stem cell transplantation. Support Care Cancer 2019. [DOI] [PMC free article] [PubMed]
- 2.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeksted C, Pappot H, Nissen A, et al. Feasibility and acceptability of electronic symptom surveillance with clinician feedback using the patient-reported outcomes version of common terminology criteria for adverse events (pro-ctcae) in danish prostate cancer patients. J Patient Rep Outcomes 2017;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluetz PG, Chingos DT, Basch EM, et al. Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (pro-ctcae). Am Soc Clin Oncol Educ Book 2016;35:67–73. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Dueck AC, Rogak LJ, et al. Feasibility of implementing the patient-reported outcomes version of the common terminology criteria for adverse events in a multicenter trial: Ncctg n1048. J Clin Oncol 2018:JCO2018788620. [DOI] [PMC free article] [PubMed]
- 6.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Natl Cancer Inst 2009;101:1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson TM, Li Y, Coffey CW, et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res 2012;21:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam E, Yee C, Wong G, et al. A systematic review and meta-analysis of clinician-reported versus patient-reported outcomes of radiation dermatitis. Breast 2020;50:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler KA, Mitchell SA, Basch E, et al. Content validity of anatomic site-specific patient-reported outcomes version of the common terminology criteria for adverse events (pro-ctcae) item sets for assessment of acute symptomatic toxicities in radiation oncology. Int J Radiat Oncol Biol Phys 2018;102:44–52. [DOI] [PubMed] [Google Scholar]
- 10.Anonymized for review.
- 11.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med 2018;378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plummer F, Manea L, Trepel D, et al. Screening for anxiety disorders with the gad-7 and gad-2: A systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry 2016;39:24–31. [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Colevas AD, Setser A, et al. Ctcae v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81. [DOI] [PubMed] [Google Scholar]
- 14.Health UDo, Services H. Common terminology criteria for adverse events (ctcae) version 4.0. National Institutes of Health, National Cancer Institute 2009;4. [Google Scholar]
- 15.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 2001;8:1153–7. [DOI] [PubMed] [Google Scholar]
- 16.Basch E, Becker C, Rogak LJ, et al. Composite grading algorithm for the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (pro-ctcae). Clin Trials 2020:1740774520975120. [DOI] [PMC free article] [PubMed]
- 17.McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen R Nonparametric methods for modeling nonlinearity in regression analysis. Annual Review of Sociology 2009;35:67–85. [Google Scholar]
- 19.Fagerland MW, Hosmer DW. A generalized hosmer–lemeshow goodness-of-fit test for multinomial logistic regression models. The Stata Journal 2012;12:447–453. [Google Scholar]
- 20.Cui J Qic program and model selection in gee analyses. The Stata Journal 2007;7:209–220. [Google Scholar]
- 21.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57:120–125. [DOI] [PubMed] [Google Scholar]
- 22.King C, Englander H, Priest KC, et al. Addressing missing data in substance use research: A review and data justice-based approach. Journal of addiction medicine 2020;14:454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung AR, Pugh SL, Klopp AH, et al. Improvement in patient-reported outcomes with intensity-modulated radiotherapy (rt) compared with standard rt: A report from the nrg oncology rtog 1203 study. J Clin Oncol 2020:JCO1902381. [DOI] [PMC free article] [PubMed]
- 24.Falchook AD, Green R, Knowles ME, et al. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngol Head Neck Surg 2016;142:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the uk fast-forward trial. Radiother Oncol 2016;120:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchant TE, Bosley C, Smith J, et al. A phase iii trial comparing an anionic phospholipid-based cream and aloe vera-based gel in the prevention of radiation dermatitis in pediatric patients. Radiat Oncol 2007;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taunk NK, Haffty BG, Chen S, et al. Comparison of radiation-induced fatigue across 3 different radiotherapeutic methods for early stage breast cancer. Cancer 2011;117:4116–24. [DOI] [PubMed] [Google Scholar]
- 28.West K, Schneider M, Wright C, et al. Radiation-induced oesophagitis in breast cancer: Factors influencing onset and severity for patients receiving supraclavicular nodal irradiation. J Med Imaging Radiat Oncol 2020;64:113–119. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson TM, Reeve BB, Dueck AC, et al. Application of a bayesian graded response model to characterize areas of disagreement between clinician and patient grading of symptomatic adverse events. J Patient Rep Outcomes 2018;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyrop KA, Deal AM, Reeve BB, et al. Congruence of patient- and clinician-reported toxicity in women receiving chemotherapy for early breast cancer. Cancer 2020;126:3084–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the national cancer institute common terminology criteria for adverse events: Results of a questionnaire-based study. Lancet Oncol 2006;7:903–9. [DOI] [PubMed] [Google Scholar]
- 32.Falchook AD, Green R, Fleming ME, Amdur RJ, Mendenhall J, Grilley-Olson J, Hayes NN, Weiss J, Reeve B, Basch E, Chera BS Factors associated with discordance between patient and physician reported toxicity during radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2015;93:S36–S37. [Google Scholar]
- 33.Staton LJ, Panda M, Chen I, et al. When race matters: Disagreement in pain perception between patients and their physicians in primary care. J Natl Med Assoc 2007;99:532–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the united states. Pain Med 2012;13:150–74. [DOI] [PubMed] [Google Scholar]
- 35.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994;330:592–6. [DOI] [PubMed] [Google Scholar]
- 36.Cleeland CS, Gonin R, Baez L, et al. Pain and treatment of pain in minority patients with cancer. The eastern cooperative oncology group minority outpatient pain study. Ann Intern Med 1997;127:813–6. [DOI] [PubMed] [Google Scholar]
- 37.Anderson KO, Richman SP, Hurley J, et al. Cancer pain management among underserved minority outpatients: Perceived needs and barriers to optimal control. Cancer 2002;94:2295–304. [DOI] [PubMed] [Google Scholar]
- 38.Shumway DA, Kapadia N, Walker EM, et al. Development of an illustrated scale for acute radiation dermatitis in breast cancer patients. Pract Radiat Oncol 2020. [DOI] [PubMed]
- 39.Basch E, Reeve BB, Mitchell SA, et al. Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (pro-ctcae). J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams PA, Cao S, Yang D, et al. Patient-reported outcomes of the relative severity of side effects from cancer radiotherapy. Support Care Cancer 2020;28:309–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in a secure, institutional database and will be shared upon request to the corresponding author.


