Abstract
Background:
Decipher is a genomic classifier (GC) prospectively validated post-prostatectomy. Herein, we validate the performance of the GC in pre-treatment biopsy samples within the context of three randomized phase III high-risk definitive radiotherapy trials.
Methods:
A pre-specified analysis plan (NRG-GU-TS006) was approved to obtain formalin-fixed paraffin-embedded tissue from biopsy specimens from the NRG biobank from patients enrolled on the NRG/RTOG 9202, 9413, and 9902 phase III randomized trials. After central review, the highest-grade tumors were profiled on clinical-grade whole-transcriptome arrays and GC scores were obtained. The primary objective was to validate the independent prognostic ability for the GC for distant metastases (DM), and secondary for prostate cancer-specific mortality (PCSM) and overall survival (OS) with Cox univariable (UVA) and multivariable analyses (MVA).
Results:
GC scores were obtained on 385 samples, of which 265 passed microarray quality control (69%) and had a median follow-up of 11 years (interquartile range, 9, 13). In the pooled cohort, on UVA, the GC was shown to be a prognostic factor for DM (per 0.1 unit; sHR 1.29, 95%CI 1.18-1.41, p<0.001), PCSM (sHR 1.28, 95%CI 1.16-1.41, p<0.001), and OS (HR 1.16, 95%CI 1.08-1.22, p<0.001). On MVA, the GC (per 0.1 unit) was independently associated with DM (sHR 1.22, 95%CI 1.09-1.36), PCSM (sHR 1.23, 95%CI 1.09-1.39), and OS (HR 1.12, 95%CI 1.05-1.20) after adjusting for age, PSA, Gleason score, cT-stage, trial, and randomized treatment arm. GC had similar prognostic ability in patients receiving short-term or long-term androgen-deprivation therapy (ADT) but the absolute improvement in outcome varied by GC risk.
Conclusions:
This is the first validation of a gene expression biomarker on pre-treatment prostate cancer biopsy samples from prospective randomized trials and demonstrates an independent association of GC score with DM, PCSM, and OS. High-risk prostate cancer is a heterogeneous disease state and GC can improve risk stratification to help personalize shared decision-making.
Keywords: prostate cancer, gene expression, Decipher, radiation, hormonal therapy
Introduction
Approximately 30% of newly diagnosed localized prostate cancer cases present with high-risk disease, defined as the presence of grade group 4-5, clinical T3-T4, and/or a pretreatment PSA ≥20 ng/mL.1 These patients have an increased risk of recurrence, metastasis, and death compared to lower risk disease and represents a potentially lethal form of the disease. However, long-term follow-up of randomized trials has demonstrated that >70% of men with high-risk prostate cancer after definitive radiotherapy (RT) and long-term androgen-deprivation therapy (ADT) will never develop metastatic disease.2 Thus, high-risk prostate cancer is a heterogeneous disease state.
While the use of clinicopathologic prognostic variables, such as grade group or PSA, help risk-stratify, they have minimal ability to further risk stratify within a given NCCN risk group. A recent study of men with high-risk prostate cancer demonstrated that the use of the NCCN high versus very-high risk sub-categorization, or the use of CAPRA, had below 60% accuracy to discriminate which high-risk men would develop distant metastases.3 The low performance of routine clinicopathologic variables motivated multiple retrospective studies to incorporate the Decipher prognostic genomic classifier (GC) to improve risk stratification.4,5 These studies have demonstrated that the addition of GC significantly and independently improved prognostication and discrimination within men with high-risk prostate cancer.6
In high-risk men, the current guideline concordant treatment options include external beam RT, potential for a brachytherapy boost, variable duration of ADT from 1 to 3 years, and variable use of prophylactic nodal RT.7 Furthermore, recent evidence has suggested potential benefit from the addition of next-generation androgen receptor signaling inhibitors (ARSIs), such as abiraterone acetate, in men with high-risk disease.8 However, given the current inability to accurately risk stratify patients into those with biologically lethal disease from those that will have a more indolent disease trajectory, clinicians are forced to use generic one-size-fits-all approaches that inherently over- and under-treat many men.
Herein, we present the results of the first validation of any gene expression biomarker from pre-treatment biopsy samples in men with prostate cancer treated on prospective phase III randomized trials. Tissue from three randomized phase III high-risk definitive radiotherapy NRG Oncology trials, RTOG 9202, 9413, and 99029–11, were pooled to assess if biopsy derived Decipher GC was independently prognostic for clinically meaningful endpoints.
Methods
Translational Science Project
Approval for this translational science project and statistical analysis plan was granted from the Cancer Therapy Evaluation Program Core Correlative Sciences Committee (CTEP CCSC) through the NCI to access archival biopsy specimens from men treated on NRG Oncology/RTOG 9202, 9413, and 9902. The study obtained IRB approval (Quorum #33234). These trials were identified by searching the NRG Oncology phase III randomized trial portfolio of men treated with RT for high-risk prostate cancer that had available tissue biospecimens and long-term follow-up for oncologic events. Details of each of the three trials can be found in the Supplementary Appendix 1.
GC score generation
After CTEP CCSC approval, the NRG biobank retrieved available pre-treatment diagnostic biopsy samples. Central pathology review was conducted (JPS), and the biopsy specimen with highest-grade tumor focus was selected for microdissection. At least 0.5 mm of linear length of tumor was required and 1-10 unstained slides were used for microdissection or alternatively, tissue blocks were sampled with a 1.5 mm diameter punch tool. RNA extraction from formalin-fixed paraffin embedded tumor tissue, cDNA amplification, oligonucleotide microarray hybridization and microarray quality control (QC) were all conducted in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory (Decipher Biosciences, San Diego, CA, USA) as previously described.12,13 Samples that passed all criteria were included in the final analysis.
GC scores were calculated based on the locked GC model and scores were generated on a scale of 0 to 1 (see Supplementary Appendix 2). The continuous GC scores were generated and then linked to the clinical trial database at the NRG Oncology Statistics and Data Management Center. Categorical GC analysis used the cut-points of 0.23 and 0.32 to define low, intermediate, and high GC risk groups (see Supplementary Appendix 2).
The age of the tissue in this cohort (15-30 years) naturally depresses the GC scores.12,14 To account for this, we utilized the GC score distributions observed in a large cohort of NCCN high risk with prospectively collected GC (n=2,286) to adjust the thresholds for categorical GC analysis of our archival tissue samples1. This resulted in ~30% to have low GC scores, as is observed with prospective samples of high risk prostate cancer whereas in this study 63% of patients would have had a low GC score using the original cut-points for the commercial assay which were originally discovered on tissue that was 5-10 years old. The commercial cut-points would underestimate the proportion of GC high risk in this study.
Treatment
Full details of the treatment received can be found in the study publications.9–11 Briefly, men treated on each of the phase III trials received external beam RT with conventional fractionation to doses of 65-70.2 Gy. Whole pelvis RT was used in all patients of these trials except for two arms of NRG/RTOG 9413. Combined androgen blockade was used in all trials. NRG/RTOG 9202 control arm and NRG/RTOG 9413 (all arms) utilized ADT for 4 months, NRG/RTOG 9202 experimental arm used 28 months, and RTOG 9902 (both arms) used 24 months. In NRG/RTOG 9902, the experimental arm also received paclitaxel, estramustine, and oral etoposide.
Endpoints
The primary objective of this project was to assess the ability of the GC to be independently associated with time to the development of distant metastasis (DM) on multivariable analysis. Secondary endpoints included time to prostate cancer specific mortality (PCSM) and death of any cause (OS). All endpoints were defined per the trial protocol with the exception of PCSM. PCSM was defined after NRG Oncology central review of cause of death. Cases where the index cancer was deemed to be the cause of death were assigned as death from prostate cancer. Exploratory endpoints included GC’s ability to prognosticate time to biochemical failure (BF) and metastatic free survival (MFS). Due to the available tissue, the final sample size limits the evaluation of GC’s predictiveness in response to duration of ADT as an exploratory aim.
Statistical Analysis
Summary statistics such as medians/interquartile ranges (IQRs) and counts/proportions were reported for continuous and categorical clinical or pathological variables, respectively. Comparative analyses between groups were performed using either Fisher’s exact test or chi-square test for categorical variables and Wilcoxon rank-sum test or Kruskal-Wallis test for continuous variables. The Kaplan-Meier method was used to estimate the survival rate for OS where GC risk groups were compared with a log-rank test. The cumulative incidence method was used to estimate rates of DM, PCSM, BF in the presence of competing risks (death without the event), and GC risk group estimates were compared using Gray’s test.
Univariable and multivariable analyses (UVA, MVA) of Fine and Gray models (for DM, PCSM, and BF with death without the event as a competing risk) and Cox proportional hazards (PH) models (for OS) for GC were constructed. MVAs were adjusted by age, pre-treatment PSA, T-stage (T3-4 vs. T1-2) and Gleason sum (8-10 vs. <8) and using randomization arms as the strata variable to account for study and treatment heterogeneity. We report hazard ratios (HR) and subdistribution hazard ratios (sHR) with their 95% confidence intervals (CI) for Cox PH and Fine and Gray models, respectively. In addition, GC effects from univariable models fit within study were aggregated using a two-stage individual patient data meta-analysis approach and a random-effects meta-analysis model. No model-based feature selection was performed as these variables were all that were consistently available across the three trials and the groupings followed the trials’ original publications.9–11
Sensitivity analyses were also conducted on all samples that yielded a gene expression result to ensure that bias was not introduced from the inclusion of only samples that passed microarray quality control. Exploratory analyses included subgroup analysis of ADT duration (i.e., 4-month as short-term and 24/28-month as long-term ADT). Additional sensitivity analyses were carried out to further solidify the prognostic performance of GC in this study, including using trials as the strata variable and repeating analyses with the inclusion of 120 patients that had GC scores but failed microarray quality metrics. Due to the exploratory nature, no multiple testing adjustment was used. All testing was performed at a significance level of 0.05 with two-sided tests. Statistical analyses were performed using R, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The three phase III trials contained 3,197 patients combined (Figure 1). Of which 677 patients had tissues that remained available after previous translational science projects. Of these patients, there was tissue of sufficient quality to obtain GC scores in 385 unique patient samples (57%), which were 16-28 years old. Of those, 265 (69%) passed microarray quality control (QC). There were no relevant patient, tumor, or follow-up differences from samples that passed or failed QC (Supplementary Table 1).
Figure 1.

CONSORT diagram of NRG GU-TS006.
The final cohort of 265 patients had a median age of 69, 20% were self-reported African American and 77% were self-reported white (Table 1). The median pre-treatment PSA was 25.8 ng/mL (IQR 12.8-45.3), 40% had a cT3-T4, and 45% had grade group 4-5 disease. The median follow-up was 11 years (IQR 8.99-13.1). The final cohort was comprised of 26%, 43%, and 31% patients from NRG/RTOG 9202, 9413, and 9902, respectively.
Table 1.
Baseline characteristics
| Variables | Total | RTOG 9202 | RTOG 9413 | RTOG 9902 | p-value |
|---|---|---|---|---|---|
| Total | 265 | 70 (26.4) | 114 (43.0) | 81 (30.6) | |
| Age | |||||
| Median (Q1, Q3) | 69 (63, 73) | 70 (66, 73) | 69 (65, 74) | 65 (60, 71) | <0.001a |
| Race, n (%) | |||||
| African American | 52 (19.6) | 11 (15.7) | 22 (19.3) | 19 (23.5) | 0.671b |
| White | 203 (76.6) | 57 (81.4) | 86 (75.4) | 60 (74.1) | |
| Other | 10 (3.8) | 2 (2.9) | 6 (5.3) | 2 (2.5) | |
| Pre-treatment PSA (ng/mL) | |||||
| Median (Q1, Q3) | 25.8 (12.8, 45.3) | 29.4 (15.6, 51.3) | 23.5 (13.2, 39.6) | 25.8 (9, 45.3) | 0.156a |
| <10 | 50 (18.9) | 7 (10.0) | 22 (19.3) | 21 (25.9) | 0.058b |
| 10-20 | 47 (17.7) | 17 (24.3) | 21 (18.4) | 9 (11.1) | |
| >20 | 168 (63.4) | 46 (65.7) | 71 (62.3) | 51 (63.0) | |
| T-stage, n (%) | |||||
| T1 | 18 (6.8) | 10 (8.8) | 8 (9.9) | 0.003b | |
| T2 | 140 (52.8) | 33 (47.1) | 65 (57.0) | 42 (51.9) | |
| T3 | 101 (38.1) | 32 (45.7) | 38 (33.3) | 31 (38.3) | |
| T4 | 6 (2.3) | 5 (7.1) | 1 (0.9) | ||
| Gleason, n (%) | |||||
| <7 | 43 (16.2) | 21 (30.0) | 22 (19.3) | <0.001c | |
| 7 | 103 (38.9) | 25 (35.7) | 49 (43.0) | 29 (35.8) | |
| 8-10 | 119 (44.9) | 24 (34.3) | 43 (37.7) | 52 (64.2) | |
| Follow-up (year) | |||||
| Median (Q1, Q3) | 11 (8.99, 13.1) | 19.2 (17.2, 20.2) | 10.7 (4.68, 17.4) | 10.7 (9.13, 12) | <0.001a |
Kruskal-Wallis test
Fisher’s exact test
Chi-squared test
Box dot plots show the distribution of GC scores by the total cohort, each of the three trials, and by Gleason score <6, 7, 8-10 (Figure 2). There was substantial heterogeneity of GC scores within a given trial, and both across and within a given Gleason score.
Figure 2.

Box plots of the continuous GC score distribution with A) the final QC pass cohort (n=265), B) within each of the three phase III trials, and C) by Gleason Score.
Univariable Analysis
On meta-analysis, the GC score was statistically significantly associated with time to DM (sHR 1.29, 95%CI 1.18-1.41), p<0.001), PCSM (sHR 1.28, 95%CI 1.16-1.41, p<0.001), and OS (HR 1.16, 95%CI 1.08-1.22, p<0.001) (Figure 3). There was no apparent heterogeneity of the GC effect across any of the three trials for DM (I2 0%), PCSM (I2 0%), or OS (I2 0%).
Figure 3.
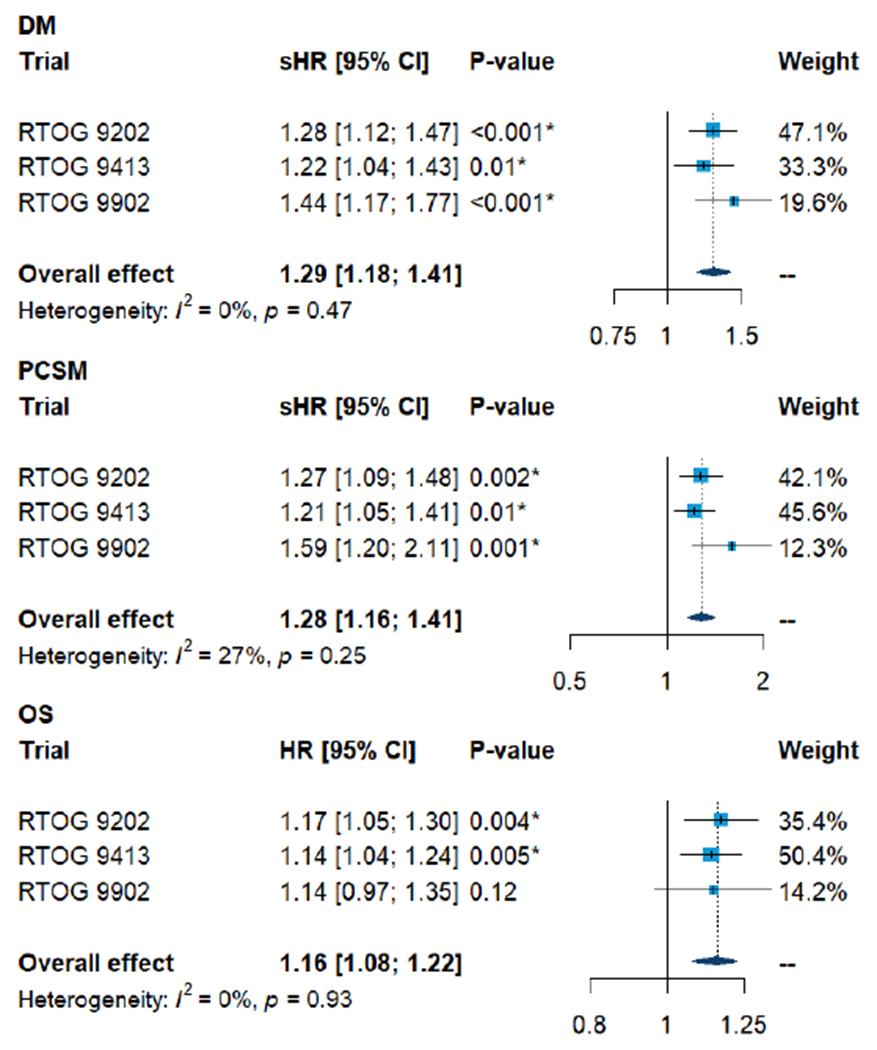
Univariable prognostic effect of GC on distant metastasis (DM), prostate cancer specific mortality (PCSM), and overall survival (OS) within each and the pooled cohort of three RTOG trials.
The cumulative incidence of DM by the GC risk groups (Figure 4 and Supplementary Figure 1) demonstrated that GC was prognostic. The estimated rates of DM for GC low, intermediate, and high at 5 years were 3%, 10% and 15%, at 10 years were 6%, 15% and 26% and at 15 years were 10%, 15% vs 36%, respectively (p<0.001). Similarly, GC risk groups were prognostic for PCSM (p<0.001) with 10-year PCSM rates being 7%, 16% and 22% and 15-year being 7%, 19% and 32%, and OS (p=0.002) with 10-year survival being 65%, 58% vs 49%, and 15-year being 49%, 29% vs 22% for GC Low vs Intermediate and High, respectively. Examining the threshold for intensification (GC>0.85) utilized in the prospective NRG-GU009 high-risk trial (NCT04513717), the estimated DM rates at 5 and 10 years were 29% and 42% for patients above the threshold in this study.
Figure 4.

Cumulative incidence estimates of distant metastasis, prostate cancer specific mortality, and Kaplan-Meier estimates of overall survival by GC Low (<0.23), Intermediate (0.23-0.32) High (>0.32) risk groups.
Multivariable Analysis
On multivariable analysis (MVA), adjusting for age, PSA, Gleason score, cT-stage, trial and treatment arm, GC as a continuous score was independently prognostic for DM (sHR 1.22, 95%CI 1.09-1.36, p<0.001), PCSM (sHR 1.23, 95%CI 1.09-1.39, p<0.001), and OS (HR 1.12, 95%CI 1.05-1.20, p<0.001) (Table 2). Additionally, univariable analysis and categorical GC results can be found in Supplementary Tables 2–4. For example, for DM GC High (57% of patients) had a MVA sHR of 3.45 (95% CI 1.42-8.36, p=0.006) compared to GC Low, a larger effect than that observed with high grade Gleason 8-10 (GG4-5) disease (45% of patients), which had an HR of 2.62 (95% CI 1.51-4.53, p<0.001) compared to Gleason <8 (GG1-3).
Table 2.
Multivariable models
| Variable | DM | PCSM | OS | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI), P-value | ||||||
|
| ||||||
| GC score | 1.22 (1.09 - 1.36) | <0.001* | 1.23 (1.09 - 1.39) | <0.001* | 1.12 (1.05 - 1.20) | 0.001* |
| Age | 1.00 (0.96 - 1.04) | 0.98 | 1.00 (0.96 - 1.04) | 0.97 | 1.07 (1.04 - 1.10) | <0.001* |
| Log2 pre-treatment PSA | 0.99 (0.80 - 1.22) | 0.91 | 0.96 (0.78 - 1.18) | 0.68 | 1.01 (0.88 - 1.15) | 0.90 |
| T3-T4 vs. T1-T2 | 1.52 (0.89 - 2.62) | 0.13 | 1.40 (0.80 - 2.43) | 0.24 | 1.19 (0.85 - 1.67) | 0.30 |
| Gleason 8-10 vs. <8 | 2.46 (1.41 – 4.31) | 0.002* | 1.31 (0.73 – 2.36) | 0.36 | 1.40 (0.99 – 1.99) | 0.06 |
Hazard ratios of genomic classifiers were per 0.1 unit increased. Strata = original arm. Death as a competing risk for DM and PCSM.
Further sensitivity analyses to ensure that bias was not introduced from the inclusion of only samples that passed microarray quality control were conducted using all patients with available GC scores (n=385) demonstrated similar results (Supplementary Figure 2). In multivariable analysis, GC was similarly a prognostic variable for DM (sHR 1.23 [1.11-1.36] vs 1.22 [1.09-1.36]), PCSM (sHR 1.27, 95%CI 1.13-1.42 vs 1.23, 95%CI 1.09-1.39), and OS (HR 1.11, 95%CI 1.05-1.19 vs 1.12, 95%CI 1.05-1.20) for the total GC available cohort and those that passed QC, respectively. GC also was shown to be independently associated with MFS (Supplementary Figure 2). The GC+Clinical Variables also had better Area Under the Curve (AUC) than Clinical Variables alone at both 5 and 10 years for DM (10 year – 77.8 vs. 71.8), PCSM (10 year – 68.6 vs 63.4), and OS (10 year- 72.9 vs. 71.5) (Supplementary Table 5).
GC score also remained independently prognostic in patients treated with short-term ADT (n=143; DM, GC sHR 1.15, 95%CI 1.01-1.32) and in those treated with long-term ADT (n=122; DM, GC sHR 1.28, 95%CI 1.07-1.52). Unfortunately, due to the availability of tissue samples, this project was underpowered for appropriately sized interaction effect analyses of GC score and duration of ADT treatment. However, exploratory comparisons of short- and long-term ADT by GC were examined (Supplementary Figure 3). For example, at 10 years the DM estimates were 31% for short-term vs 20% for long-term ADT in GC High patients (an absolute benefit of 11%) but were 7% vs 4% in GC Low patients (an absolute benefit of 3%).
Discussion
The current study represents the first validation of any tissue-based gene expression biomarker in the context of a phase III randomized trial from pre-treatment biopsy tissue in localized prostate cancer. In men with primarily NCCN high-risk disease the Decipher GC was independently prognostic for all clinical endpoints tested, including clinically meaningful endpoints such as distant metastasis, death from prostate cancer, and overall survival. The prognostic effect of the GC was similar across each of the phase III trials with no observed heterogeneity of the treatment effect across trials. This data represents an important milestone for the personalization of prostate cancer care.
NCCN high-risk prostate cancer represents a potentially lethal form of the disease. However, despite the ‘high-risk’ moniker, the majority of men will die of other causes with standard of care treatment. This point was nicely demonstrated by SPCG-4, a phase III randomized trial of watchful waiting versus radical prostatectomy.15 At 18-years post-randomization, for men with high-risk disease randomized to watchful waiting, an estimated 35% of men died of other-causes without developing metastatic disease or are still alive without evidence of distant metastases. Thus, there remains remarkable clinical heterogeneity within this disease state. There is also molecular heterogeneity in high-risk prostate cancer, which has been captured in multiple studies that demonstrate that there is substantial genomic diversity within high-risk prostate cancer. However, current guidelines recommend a one-size fits all approach despite this known clinical and molecular heterogeneity.7
The most commonly used guideline concordant treatment for high-risk prostate cancer is the use of radiotherapy plus long-term ADT.16 Despite the efficacy of this approach, ≥15% of men will still develop distant metastases. Unfortunately, our standard clinicopathologic variables and risk groups are unable to accurately risk stratify patients within a given risk group. Thus, over- and under-treatment have become accepted in the management of localized prostate cancer.17 The current study however demonstrates that with the use of a biopsy tissue-based GC, we now have the ability to more accurately prognosticate patient outcomes, even within a given risk group.
Previously, Spratt and colleagues have reported the ability to combine clinical, pathologic, and data from the GC to create the clinical-genomic risk groups.3 They demonstrated that not only did the clinical-genomic risk groups improve prognostication and discrimination, with prospective data they showed 67% of men would be re-classified to a new NCCN risk group and treatment algorithm. The evidence of this can also be realized from the current study where the GC was able to identify men at lower and higher risks of metastasis and death. For example, patients with GC Low had a 3% risk of metastasis at 5-years post-treatment as compared to those with GC High had 15%. A near 1 in 6 risk of developing metastases by 5-years with standard treatment is very high and clearly these men need novel treatment approaches.
The ability to calculate a patient’s absolute risk of recurrence more accurately is critical as it can further the personalization of care. The benefit of long-term ADT consistently has a relative benefit, depending on the endpoint, of 30-60%. However, a man with a baseline risk of metastasis with short-term ADT of 10% will derive an absolute 10-year benefit of only 3-6% from the use of long-term ADT. It may be reasonable to reduce the duration of ADT for these men with low GC especially in those with co-morbidities such as cardiovascular disease, dementia or cognitive decline which could be worsened by prolonged hormonal therapy. In contrast, a man with an absolute 10-year risk of metastasis of 30% will derive a 9-18% absolute benefit, which is very large. This assumes that the GC is solely prognostic but given that this study was limited by a small sample size, an interaction of GC score by duration of ADT treatment and metastasis (i.e., GC as a predictive biomarker) cannot be ruled out.
There are several limitations in this study. All available tissue samples from the NRG biobank were collected from these three trials. However, they represent the minority of patients enrolled on these phase III trials as tissue was not mandatory to be collected for correlative research. The sample size that passed all QC metrics (69% of those that made it to microarray, 42% of the total number of available tumor samples) was lower than with current prospective clinical use of the GC test (>98% pass rate). This is likely due to the tissue being 15-25 years old at the time of genomic profiling, which results in a depression of the GC scores due to the decreased quality and fidelity of RNA that is observed for older archival tissue samples. Current clinically-used cut-points are relevant to prospectively collected tissue and are different from the cut-points in this study of archival tissue. Nevertheless, categorical analysis of either the cut-points appropriate for these aged, archival samples or the commercial use cut-points demonstrated consistently, that patients with lower GC risk harbor significantly more favorable prognosis tumor, while those with higher GC risk harbor tumors with a very poor prognosis (i.e., nearly 5 times higher risk for DM, nearly 2 times for PCSM and OS), despite receipt of standard of care therapy by these men.
Currently, the Decipher GC is available in the US for the majority of localized prostate cancer patients and is also available for use in men with non-metastatic prostate cancer at diagnosis or after prostatectomy. The present study adds level 1 biomarker evidence to the prognostic ability of the GC from three phase III randomized trials. The results from this study and others were the motivation for the currently active and enrolling parallel phase III trial, PREDICT-RT (NRG-GU009, NCT04513717). Given the established, and now validated, prognostic power of the Decipher GC, this trial is randomizing men with lower 2/3rd GC score to radiotherapy + 24 months of ADT vs radiotherapy + 12 months of ADT. In men with the upper 1/3rd of GC scores or node positive disease, men are randomized to radiotherapy + 24 months of ADT +/− intensification with apalutamide. Now that the GC is established as a critical prognostic variable, we are excited to best determine how men should be treated based on GC scores.
Supplementary Material
Acknowledgments:
We would like to thank the support of the NCI for the conduct of this randomized trial. We thank Gerald Hank, MD (deceased) for his work as the PI on NRG/RTOG 9202. Additional Support was provided by The Baldwin-Politi Distinguished Chair, The Kevin and Mary Speicher Charitable Trust, the Cleo and Paul Schimmel Charitable Foundation, Geoffrey Freeman and Family
Funding:
This study was conducted with the support of NIH-R01 CA240582-01. This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology Statistics and Data Management Center), U24CA196067 (NRG Specimen Bank), and UG1CA189867 (NCORP), P30CA240139 from the National Cancer Institute, and a University of Miami Sylvester Comprehensive Cancer Center Professorship (AP) as well as Pfizer and Bristol Myers Squibb.
Conflicts of Interest
Drs Desai, Peters, Rabinovitch, Roach, Rodgers, Rosenthal Shipley and Zeitzer have nothing to declare. Dr Mendez-declares Payments directed to the institution to run clinical trials in prostate and gynecologic malignancies ACURA-CARO, Ride for Dad and AMOSO. Dr. Sandler declares Member, clinical trial steering committee at Janssen, Member, Board of Directors at ASTRO. Low value stock from inactive medical advisory board Radiogel. Dr. Hall declares Institutional Research, Meeting and Travel support from Elekta. Dr. Tran declares Grants or contracts from Astellas, Bayer and RefleXion Medical, Patent 9114158- Compounds and Methods of Use in Ablative Radiotherapy licensed to Natsar Pharm, Consulting fees from Noxopharm, Janssen-Taris Biomedical, Myovant and AstraZeneca, Support for attending meetings and/or travel RefleXion Medical, Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for NRG GU Translational Science Chair, ASTRO and RSNA. Dr. Davicioni declares Stock or stock options and Employee (formerly Decipher Biosciences, manufacturers of Decipher GC test) Veracyte, Inc. Dr. Dicker declares Grants or contracts from NCI, ASCO and NRG Oncology, Consulting fees from Janssen, Oncohost, Oranomed and CVS, Application No.PCT/US19/37487, Title: Doped BEO compounds for optically stimulated luminescence (OSL) and thermoluminescence (TL) radiation dosimetry, Stock or stock options Oncohost. Dr. Hartford member of the committee NRG Oncology Genitourinary Cancer Committee. Dr. Huang is Employed by Decipher, Veracyte. Stock or stock options Owned equity of Decipher, Veracyte. Dr. Efstathiou declares Consulting fees from Blue Earth Diagnostics, Boston Scientific, AstraZeneca, Genentech and Participation on a Data Safety Monitoring Board or Advisory Board for Merck, Roviant Pharma, Myovant Sciences, Janssen, Bayer Healthcare. Dr. Feng declares in the past 36 months, I have consulted for: Janssen, Bayer, PFS Genomics (this termed in April 2021 since the company has been acquired), Myovant Sciences, Roivant, Astellas, Foundation Medicine, Varian, Bristol Meyers Squibb, Exact Sciences, BlueStar Genomics, Novartis, and Tempus. I have also received stock options from SerImmune from serving on their scientific advisory board in 2020. This was a one-time advisory board meeting. I am a co-founder and advisor of Artera, a company focused on digital pathology biomarkers in prostate cancer. I am Chair of the Genitourinary Cancer Committee for NRG Oncology. My role is to help investigators design proposals for clinical trials, that are then evaluated by the NCI for funding. All funding decisions are made by the NCI – not by NRG or by myself. Dr. Nguyen funding NIH R01CA240582, Institutional Grants with Janssen, Bayer, Astellas, Consulting fees Janssen, Boston Scientific, Blue Earth, Astellas, Bayer, Cota, Myovant. Dr. Spratt declares Consulting fees from Boston Scientific, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Bayer, Blue Earth, GammaTile, Janssen, Novartis, Varian. Dr. Pollack declares Grants P30CA240139 from the National Cancer Institute, and a University of Miami Sylvester Comprehensive Cancer Center Professorship (AP). Past co-Chair of the RTOG/NRG GU Translational Research Program. Dr. Simko declares GenomeDX, Inc. paid fees to my institution (UCSF) to complete this work. Grant to my institution from NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing Statement
All data will be made available per the NCTN Data Archive rules. The link for the archive is: https://nctn-data-archive.nci.nih.gov/.
www.ClinicalTrials.gov: NRG/RTOG 9202-NCT00767286, NRG/RTOG 9413-NCT00769548, NRG/RTOG 9902-NCT00004054
References
- 1.Muralidhar V, Zhang J, Wang Q, et al. Genomic Validation of 3-Tiered Clinical Subclassification of High-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2019;105:621–7. [DOI] [PubMed] [Google Scholar]
- 2.Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol 2014;11:308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Birer SR, Jeffrey Karnes R, et al. Performance of clinicopathologic models in men with high risk localized prostate cancer: impact of a 22-gene genomic classifier. Prostate Cancer Prostatic Dis. 2020;23(4):646–653. doi: 10.1038/s41391-020-0226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spratt DE, Zhang J, Santiago-Jimenez M, et al. Development and Validation of a Novel Integrated Clinical-Genomic Risk Group Classification for Localized Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jairath NK, Dal Pra A, Vince R Jr., et al. A Systematic Review of the Evidence for the Decipher Genomic Classifier in Prostate Cancer. Eur Urol 2021;79:374–83. [DOI] [PubMed] [Google Scholar]
- 6.Tosoian JJ, Birer SR, Jeffrey Karnes R, et al. Performance of clinicopathologic models in men with high risk localized prostate cancer: impact of a 22-gene genomic classifier. Prostate Cancer Prostatic Dis 2020;23:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw 2021;19:134–43. [DOI] [PubMed] [Google Scholar]
- 8.Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447–460. doi: 10.1016/S0140-6736(21)02437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26:2497–504. [DOI] [PubMed] [Google Scholar]
- 10.Lawton CA, DeSilvio M, Roach M 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys 2007;69:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal SA, Hunt D, Sartor AO, et al. A Phase 3 Trial of 2 Years of Androgen Suppression and Radiation Therapy With or Without Adjuvant Chemotherapy for High-Risk Prostate Cancer: Final Results of Radiation Therapy Oncology Group Phase 3 Randomized Trial NRG Oncology RTOG 9902. Int J Radiat Oncol Biol Phys 2015;93:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng FY, Huang HC, Spratt DE, et al. Validation of a 22-Gene Genomic Classifier in Patients With Recurrent Prostate Cancer: An Ancillary Study of the NRG/RTOG 9601 Randomized Clinical Trial. JAMA Oncol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen PL, Martin NE, Choeurng V, et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis 2017;20:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdueva D, Wing M, Schaub B, Triche T, Davicioni E. Quantitative expression profiling in formalin-fixed paraffin-embedded samples by affymetrix microarrays. The Journal of molecular diagnostics : JMD 2010;12:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal V, Ma X, Hu JC, Barbieri CE, Nagar H. Trends in Diagnosis and Disparities in Initial Management of High-Risk Prostate Cancer in the US. JAMA Network Open 2020;3:e2014674–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


