Abstract
Background
Melatonin, zinc, and multivitamins are among most recommended supplements in the fight against coronavirus disease 2019 (COVID-19). We aimed to examine the efficacy and safety of this association in the treatment of COVID-19 and COVID-like illnesses.
Methods
We conducted a multicenter prospective, randomized, double-blind, controlled trial. Patients with no medical history consulting the emergency department for covid and covid-like illness and who were not hospitalized were included. Patients were assigned in a 1:1 ratio to the treatment or the placebo group. The primary outcome was studying the effectiveness of zinc multivitamin supplement and melatonin in the treatment of COVID and -like illnesses symptoms’ according to the time from randomization to clinical improvement. The pre-specified secondary outcomes were date of disappearance of symptoms present on admission, appearance of an adverse effect due to the administration of the treatment, number of patients developing complications, requiring hospitalization, requiring respiratory support.
Results
One hundred sixty four patients were eligible for the study and were randomized to either the treatment group or the placebo group. Overall, 128 of the 164 patients had a PCR for SARS-CoV-2, yielding a positive PCR result in 49.1% of them. Regarding the disappearance of all initial presenting symptoms: on the 5th day of the follow-up, there was a significant difference between the two groups with a p value 0.04;On the 10th day, there was a significant difference too with p value of 0.038. There were no significant differences between the two groups in recovery during the 15th day of follow-up p>0.5. Finally, 100% of patients fully recovered in the treatment group vs 98.8% in the placebo group. No severe adverse events were reported throughout the trial.
Conclusions
Our results showed that daily doses of Melatonin, zinc and vitamins did significantly reduce the duration of symptoms accelerating its disappearance among patients consulting with COVID-19 or COVID-19 like illness.
Keywords: Melatonin, zinc, vitamin C, vitamin D, Covid-19, emergency department
Introduction
In 2019, a new coronavirus (SARS-CoV-2) infection emerged and spread rapidly. This led the World Health Organization (WHO) to declare coronavirus disease-2019 (COVID-19) a Public Health Emergency of International Concern (PHEIC) on 30 January 2020, and to characterize the outbreak as a pandemic on 11 March 2020. SARS-CoV-2 has since caused distressing economic, health and social impacts worldwide1.
In Tunisia, COVID-19 has thus far been responsible for over 1 million cases and 20,000 fatalities2. Despite intensive efforts, there have been no effective remedies for this life-threatening virus. Added to that, vaccinations were not widely accessible in low-income countries, and their efficacy declines over time and may be compromised against new Covid-19 virus lineages.3 Taken together, there remains an unmet need for simple, accessible, low cost, harmless and effective pharmacological agents.
As consequence, fearful of getting infected and seriously ill, people tended to take combined supplements that have antiviral and immunomodulatory activities in the early stage of the disease or as a preventive measure4 based on the commonly affected processes in viral infections .
Melatonin, zinc and multivitamins, especially Vitamin C and Vitamin D were among the most recommended supplements. Numerous investigations have reported wide spectrum physiological and pharmacological roles of melatonin as it modulates cellular functions via many processes damping inflammatory activity, so being considered as a powerful antioxidant and anti-inflammatory agent5 On the other hand, melatonin has an immunomodulatory effect since it is known that it can prevent a possible cytokine storm, and other severe symptoms that may develop in the event of viral invasion6
Zinc, the second most abundant trace metal in the human body after iron, also plays a critical role in antiviral immunity by up-regulating metallothioneins (MT) expression exerting numerous antioxidant and antiviral effects7 . Vitamins especially vitamin C and D reducing the levels of pro-inflammatory cytokines are also suggested to have a supportive role in treating viral infections such as the covid-198. Vitamin C increases antiviral cytokines and free radical formation. It also attenuates the hyperactivation of immune cells.9Vitamin D improves the physical barrier against viruses and stimulates the production of antimicrobial peptides.9Pharmacies recorded severe shortages in supplements, immunity boosters,vitamins, minerals and other drugs10. But These products taken separately have not proven to be very effective. This led us to test their effectiveness when they are co-used .
For our study, we chose to discuss the efficacy in terms of rapid improvements without complications and safety of the association: melatonin, zinc and multivitamins supplements, mainly vitamins C and D in the treatment of COVID-19 and COVI-like illnesses, regulating their prescription.
Material and Methods
This was a multicenter, double-blind, randomized, placebo-controlled trial. The study was approved by the Ethics Committee of the Faculty of Medicine of sousse. Patients provided written informed consent before participation. Patients were recruited from three emergency departments. The following formula was used to estimate the minimum sample size: n = [3.84 * ( (P * Q) / i²)] with P = the prevalence of COVID-19 illness = 0.12, Q = 1-P = 0.88, i = 0.05, or 162 patients.
They were enrolled from 2 January 2023, to 4 February 2023. The final follow-up was on 4 March 2023. Patients aged less than 60 years with no previous medical history consulting the emergency department for covid and covid-like illness and who were not hospitalized were included. Those with known allergies or who were likely to develop severe side effects from the study drugs and those who refused to consent were excluded. Pregnant women were not included. For all the included patients, the diagnosis of COVID-19 was confirmed by positive reverse transcriptase–polymerase chain reaction test from analysis of nasopharyngeal swab specimens, or a rapid test. Patients were assigned in a 1:1 ratio to the treatment group or the placebo group. The randomization list was created using a computer-generated code. A staff member who had no role in the study managed the randomization. The treatment group received two pills in the morning: The first was a multivitamin pill containg: 10 mcg Provitamin A; Vitamin B (0.65 mgB1, 0.7 mg B2, 8mg B3, 3mg B5, 0.7 mg B6, 25 mcg B8, 100 mcg B9, 1.25 mcg B12); 40 mg Vitamin C; 2.5 mcg Vitamin D; 6 mg vitamin E and minerals. The second pill contained 25 mg of Zinc. Another pill of 2 mg of melatonin was taken at night. Patients from the placebo group received three similar pills containing 3.5 mg magnesium stearate and 346.5 mg of microcrystalline cellulose. The pills were identical in color, taste, smell, consistency, and container. They were prepared by XEn Plus laboratory members and labeled by a staff member who did not participate in the study.
Patients and investigators remained blinded to randomization until the final analysis. After consenting, patients were examined by an emergency resident. They were asked about the common Covid-19 and Covid-like illness symptoms: fever, headache, asthenia/fatigue, sputum expectoration, anosmia, chills, skin rash, diarrhea, sore throat, abdominal pain, cough, vomiting, chest pain, hemoptysis, joint pain, ageusia, dyspnea, muscle pain and conjunctivities11.
On physical examination vital signs were checked: blood pressure, pulse rate, respiration rate, body temperature, glycemic index, oxygen saturation, height and weight. These symptoms were assessed using a scale from 0 to 3 (not at all, slight, a lot, awful) and they were monitored via telecommunication. A follow-up was carried by the clinical research associate for each included patient on day 1, day 10, day 15 and day 30. Compliance with treatment, the date of disappearance of symptoms, side effects, adverse events, hospitalization, respiratory assistance requirement and death were mentioned accordingly.
The primary outcome was studying the effectiveness of zinc multivitamin supplement and melatonin in the treatment of covid and covid-like illnesses symptoms’ according to the time from randomization to clinical improvement. The pre-specified secondary outcomes were the date of disappearance of symptoms present on admission, the appearance of an adverse effect due to the administration of the treatment, the number of patients developing complications, the number of participants requiring hospitalization due to COVID- infection, and the number of participants requiring respiratory support or hospitalization. The number of participants was included on the basis of feasibility, based on resources, capacity of emergency research staff and facility, and available patients having a phone for the tele follow-up, in line with current recommendations12. Statistical analyses were performed with IBM-SPSS software, version 20.0. The significance level was set at 2-sided α = .05. The differences between the two groups were tested by the fisher exact test.
Results
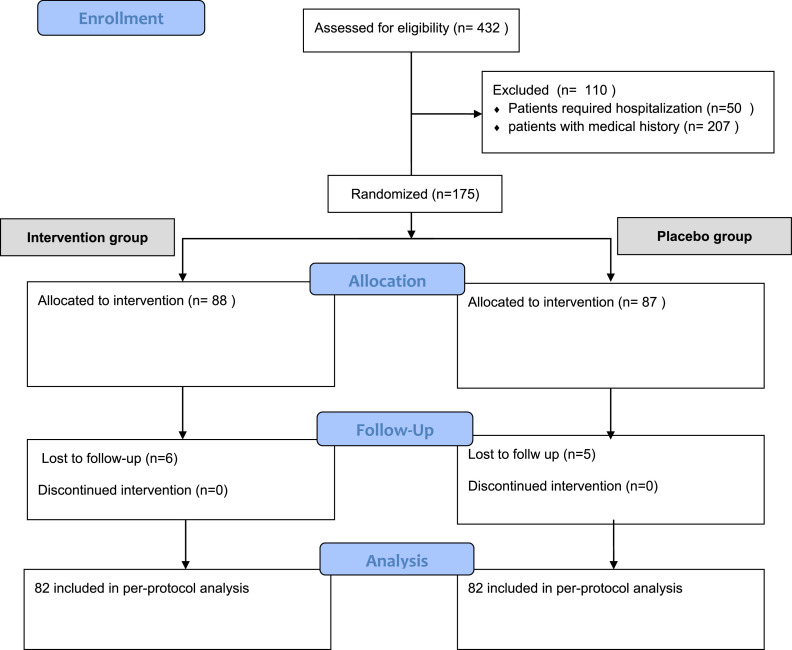
A total of 164 patients were eligible for the study and were randomized to either the treatment group or the placebo group. Patients were not eligible for inclusion due to the following reasons: had previous medical history, refused to participate, were pregnant or lactating during the study period, did not respond to phone calls or provided an unreachable number. Of the 82 patients who were randomized to the treatment group 32 (39%) were males with a male-female sex ratio: 0.64 (Table1 ). In the placebo group 47 (57.3%) were females. The mean (SD) was 35.6± 8.4 in the treatment group and 34.3±9.9 in the placebo group. Baseline characteristics including sex, age, and BMI were similar between the two groups.
Table 1.
Demographic characteristics of participants.
| GROUPE A | GROUPE B | P | ||
|---|---|---|---|---|
| Age | Mean ±IC | 35,6±8,4 | 34,3±9,9 | 0,370 |
| Gender | Male | 32 (39) | 35 (42,7) | 0,634 |
| Female | 50 (61) | 47 (57,3) |
Overall, 128 of the 164 patients had a PCR for SARS-CoV-2 and 36 patients had a rapid test at the time of enrollment and 49.1% of them had a positive PCR result.
On initial physical examination, the mean (SD) heart rate was 81.83 (11.9) brats per minute in the treatment group and was 83.62 (11.3) bpm in the placebo group. There was no significant difference between the two groups. Patients in the treatment group had a mean (SD) respiratory rate of 14.82 (1.79) cycles per minute (cpm) and those in the placebo group had a mean respiratory rate of 14.74 (1.57) cpm. Mean (SD) pulse oxygen saturation level in the treatment group and in the placebo group were 99.26 (0.994) and 99.41 (0.733) respectively with no significant difference between the two groups.
The most frequent reasons for consultation were headache (69.5% in the treatment group vs 69.9 in the placebo group), asthenia/fatigue (57.3% vs 63.4%) joint pain (51.2% vs 43.9%) and muscle pain (51.2% vs 47.6%). 76 patients presented with cough . Only one patient complained of skin rash. More than the third of patients presented with abdominal symptoms as abdominal pain, vomiting and diarrhea. Dyspnea was found in 7.3% of patients in both groups . Baseline characteristics of both groups are shown in Table2 . There was no significant heterogeneity between the two groups .
Table 2.
Clinical presentation on admission .
| GROUPE A | GROUPE B | P | |
|---|---|---|---|
| fever | 37 (45,1) | 38 (46,3) | 0,875 |
| headache | 57 (69,5) | 54 (65,9) | 0,616 |
| asthenia.fatigue | 47 (57,3) | 52 (63,4) | 0,425 |
| sputum | 6 (7,3) | 4 (4,9) | 0,514 |
| Anosmia | 21 (25,6) | 32 (39) | 0,066 |
| chills | 18 (22) | 24 (29,3) | 0,283 |
| Skin rash | 1 (1,2) | 0 | 0,316 |
| diarrhea | 21 (25,6) | 21 (25,6) | - |
| odynophagia | 16 (19,8) | 21 (25,6) | 0,372 |
| Abdominal pain | 12 (14,6) | 13 (15,9) | 0,828 |
| Cough | 31 (37,8) | 36 (43,9) | 0,427 |
| Vomiting | 10 (12,2) | 9 (11) | 0,807 |
| Chest pain | 6 (7,3) | 11 (13,4) | 0,200 |
| hemoptysis | 4 (4,9) | 1 (1,2) | 0,173 |
| Joint pain | 42 (51,2) | 36 (43,9) | 0,348 |
| ageusia | 16 (19,5) | 19 (23,5) | 0,540 |
| dyspnea | 6 (7,3) | 6 (7,3) | - |
| Muscle pain | 42 (51,2) | 39 (47,6) | 0,639 |
| Conjunctives | 2 (2,4) | 1 (1,4) | 0,560 |
On analytical analysis, there was a significant difference between the treatment group and the placebo group (29.3%) regarding the disappearance of all the initial presenting symptoms of covid-19 and covid-like illness on the 5th day with a p value of 0.041 (Table3 ): In the treatment group 46.3% of patients did not present with any more symptoms by day 5 versus 29.3% from the placebo group who were still symptomatic . On the 10th day of follow-up, statistical analysis revealed a significant difference between the two groups with a p value of 0.038: Sixty-six patients (80.5%) in the treatment group showed a complete recovery and only 55 (67.1%) patients recovered completely in the placebo group. There were no significant differences between the treatment group and the placebo group in recovery during the 15th day of follow-up p>0.5. And finally, there were no between-group differences with respect to the components of the primary outcome on 30th day recovery, 100% of patients fully recovered in the treatment group vs 98.8% in the placebo group. No patient required hospitalization or ventilation. The treatment was well tolerated and no severe adverse events were reported throughout the trial.
Table 3.
Disappearance of all the symptoms.
| Treatment Group | Placebo Group | P | |
|---|---|---|---|
| D5 | 38 (49,4%) | 24 (32,9%) | 0,041 |
| D10 | 66 (80,5%) | 55 (67,1%) | 0,038 |
| D15 | 76 (92,7%) | 68 (82,9%) | 0,05 |
| D30 | 82 (100%) | 81 (98,8%) | 0,316 |
Discussion
Since December 2019, the COVID-19 outbreak has resulted in a huge economic deterioration and exceptional uncontrolled health crisis throughout the globe13. Although several vaccines are now available, only few data have been released regarding the efficacy and safety of vaccines in humans, not to mention that the long-term adequacy of those vaccines still remains as an open address14. Consequently, the world is eagerly awaiting a panacea of treatment options for COVID-19. Frightened by this virus, people sought simple, accessible, effective, low cost15, harmless and previously used treatments16. As consequence, people resorted to the widely use of nutrient supplements17 known for their antiviral and immunomodulatory activities for the treatment of COVID, maily in its early stage, and even as a preventive measure.
In this randomized, double-blind, placebo-controlled clinical trial, daily doses of melatonin, zinc and multivitamins supplements (vitamin C and D) did significantly reduce the duration of COVID-19 and COVID-like illnesses symptoms and reduced its burden among non-hospitalized patients considered healthy. To our knowledge, this is the first randomized clinical trial to demonstrate these findings.
In our study, we used specific exclusion criteria that limited the population being studied to a healthy subgroup of the population. Patients were younger than 60, had no PMH, half had negative PCR, and all had mild disease. These factors have the potential to diminish treatment effect size. We found a significant difference between the treatment and control groups, despite the potential constraint on effect size.
Melatonin (N-acetyl-5-methoxytryptamine), a bioactive molecule18with highly divergent actions, has documented positive effects in alleviating acute respiratory stress induced by virus, bacteria, radiation, etc19 20 21through various pathways suggesting its utility in buffering against Covid -19 especially in the initial ‘cytokine storm’22. This main pineal gland hormone exerts anti-inflammatory effects by reducing pro-inflammatory cytokines and elevating the level of anti-inflammatory cytokines23. The anti-inflammatory effect of melatonin cooperates with its anti-oxydant actions by up-regulating anti-oxidative enzymes and down-regulating pro-oxidative enzymes18. Melatonin also acts as a ‘free radical scavenger’ interacting directly with free radicals24. A clinical trial conducted in a hospital in Iran in 202225 including 74 hospitalized patients with confirmed mild to moderate COVID-19showed that the adjuvant use of melatonin improved clinical symptoms and time of discharge of COVID-19 patients and contributed to a faster return of patients to baseline health. Compared with the control group, the clinical symptoms as well as the level of C-reactive protein and the pulmonary involvement in the intervention group had significantly improved. Hasan, Z.T., et al. research study26 showed that melatonin, a multifunctional molecule, may help to reduce thrombosis, sepsis, and mortality in COVID-19 patients with severe coronavirus infection.
But melatonin only may be not enough to treat patients infected with Sars-Cov2, in a multicenter retrospective observational study involving 58 562 adult patients hospitalized for COVID-19, melatonin use (N = 272) at a mean daily dose of 2.6 mg was not associated with reduced mortality.27 However, the failure of melatonin in this may reflect the severity of the cases, and/or the inadequacy of the dose used, rather than the need for other substances to be used at the same time
Zinc, one of the most abundant and important trace metals in the human body, known as ‘the metal of life’28 influences growth and affects the development and the integrity of the immune system having an impact on key immunity mediators, such as enzymes, thymic peptides and cytokines, explaining the paramount importance of zinc status on the regulation of the immune system29 . This essential micronutrient has interestingly several anti-viral properties and is important in defending against respiratory viral infections and regulating the immune response in the respiratory tract30. Since the outbreak of the coronavirus pandemic, several studies have been conducted to evaluate the role of zinc in the treatment COVID-19 infection when combined with other treatments or used solely.
A recently published placebo-controlled study, evaluating the efficacy of zinc in adult patients with COVID-19 infection, showed that oral zinc can decrease 30-day death, ICU admission rate and can shorten symptom duration31. Another placebo-controlled study evaluating intravenous zinc injections in hospitalized covid-19 patients, showed that all hospitalized patients had zinc deficiency however, they could not assess the primary outcome of whether zinc injections reduced the level of oxygenation in non-ventilated or improved the PaO2/FiO2 ratio because the study did not reached its target enrollment due to the marked reduction in patient hospitalizations32. Another 20-week study with a multi-component over-the-counter (OTC) “core formulation” regimen including zinc and other vitamins demonstrated their effectiveness in treating mild to moderate symptoms of early-stage unspecified flu-like illness (presumably also COVID) at 2 doses/day33. However, a recent study including 214 patients showed that treatment with high-dose zinc gluconate, ascorbic acid, or a combination of the two supplements did not significantly decrease the duration of symptoms compared with standard of care34.
Multivitamins, playing crucial roles in immune system, emerged as top candidates for protection and treatment against an array of different viruses including the coronavirus. Vitamins C and D are the leading ones; they gained the most attention during the COVID-19 pandemic17. Vitamin C, a potent antioxidant, contributes to immune defense by supporting various cellular functions of both the innate and adaptive immune system. This vitamin is able, as consequence, to prevent and even treat respiratory and systemic infections. Several studies during the covid pandemic have proven its utility in curing and preventing the infection. A clinical trial conducted on 120 hospitalized critically ill patients infected with COVID-19 demonstrated the potential of vitamin C supplementation in enhancing the survival duration of critically ill patients35. An open-label controlled trial performed in Pakistan on 150 patients with COVID-19 infection, showed that the group receiving the IV vitamin C in addition to the standard therapy became symptom-free earlier and reported a shorter duration of hospitalization compared with the control group, while no significant difference was found in the need for mechanical ventilation and mortality36.
Vitamin D also has beneficial proprieties and plays crucial roles in the treatment of viral infections including COVID-19. Via its active metabolite 1,25 (OH)2D, vitamin D, as a facilitator of immunocompetence regarding innate and adaptive immunity, has a potential role in modulating the pathophysiological aspects of the cytokine storm in the covid-19 infection37. A pilot randomized clinical study conducted in Reina Sofia University Hospital including 76 hospitalized patients demonstrated that the administration of a high dose of vitamin D active form significantly reduced the need for ICU treatment38. In another multicenter controlled study in Brazil including 240 hospitalized patients with COVID-19 who were moderately to severely ill, Howbeit showed that a single dose of vitamin D, compared with placebo, did not significantly reduce hospital length of stay39. These results are somewhat consistent with those reported by Elamir, Y.M., et al., who conducted a similar open-label trial examining the effects of vitamin D on the hospital length of stay in hospitalized patients with COVID-19, showing no significant difference between the two groups40.
Overall, melatonin, zinc and multivitamins share similarities as they exert effects on several immune-cell types and modulate inflammatory reaction41. They have a synergistic action for the treatment Covid-19 and Covid-like illness. Yet, only few studies have investigated this available association of treatment which is based on hormones minerals and vitamins. This new potential therapeutic choice would be of a great help for physicians as it presents an available low coast and effective treatment especially in low-income countries42.
Despite, these encouraging results the current study suffered from several limitations. These included the small cohort size and the failure to study different possible mechanisms responsible for the beneficial effects of nutrients and to measure biomarkers of inflammation, coagulation, cardiac and renal injures. In addition, the relative contributions of each active ingredient remain unknown. Added to that, the short follow-up time may hide late side effects. The absence of patients with comorbidities or significant disease makes it difficult to generalize the results.
Our study contained no dose stratification, so it is not possible to identify a dose-response relationship. There was any baseline measurement of serum vitamin C, vitamin D, or zinc. One might expect a patient whose baseline vitamin D level was low to gain more benefit from vitamin D supplementation than a patient whose vitamin D level is already adequate.
Further studies are warranted to better understand the efficacy and tolerability of this association besides studying mechanisms of actions of the products.
Conclusion
Among non-hospitalized patients presenting to the emergency department with Covid-19 and Covid-like illness, daily doses of Melatonin, zinc and multivitamin compared with placebo, did significantly reduce the duration of symptoms accelerating its disappearance without developing complications or necessitating hospitalization or experiencing adverse events . The findings do support the use of this association for the treatment of COVID-19.
Uncited Link
Table 4.
Findings of the reviewed studies
| Study Citation | Patient's characteristics | Severity of disease | Outcomes | Components | ||||
|---|---|---|---|---|---|---|---|---|
| Age | Comorbidities | Component | Form | Dose | Duration | |||
| Farnoosh, et al25 | 50.7±14.4 | Hypertension 33.3% Diabetes 25% Rheumatic disease 4.2% Cardiovascular disease 12.5% Cancer |
Mild to moderate | Clinical improvement of symptoms and laboratory parameters | Melatonin | Oral | 3mg*3/day | 14 days |
| Hasan ZT, et al 26 | 56.3±7.3 | Hypertension 53.2% Ischemic heart disease 15.8% Asthma 29.7% |
Severe | thrombosis, sepsis, and mortality rate | Melatonin | Oral | 10 mg | 14 days |
| Marina Sánchez-Rico, et al 27 | 18–70 71–80 81+ |
diabetes mellitus diseases of the circulatory system diseases of the respiratory system neoplasms and diseases of the blood |
NA | in-hospital all-cause mortality | Melatonin | Oral | 2.61 mg | |
| Ben Abdallah S et al 31 | 54.2 ± 17.3 | NA | Moderate | *For inpatients: death, need for ICU admission and length of ICU and hospital stay at 30-day *For outpatients: appearance of new symptoms, the need for hospitalization, need for ICU admission, and survival status |
Zinc | Oral | 25 mg | 15 days |
| Oneel Patel, et al32 | 59.8 ± 16.8 | Hypertension Diabetes Chronic cardiovascular disease Chronic respiratory Disease Cirrhosis Hepatic Failure |
Moderate to severe | In non‐ventilated patients was assessed as the level of oxygenation expressed as oxygen flow (in liters/min) required to maintain blood oxygen levels (SpO2) above 94% and the worst (lowest) PaO2/FiO2 ratio in ventilated patients | Zinc | IV | 0.24 mg/kg/day | 7 days |
| Margolin, et al33 | 45.2 (14.6) | Diabetes Hypertension Dyslipidemia Asthma |
Mild Outpatients |
the number of days required to reach a 50% reduction in symptoms | Vitamin C Zinc |
Oral | *8000 mg of ascorbic acid (to be divided over 2-3 times per day) *50 mg of zinc |
10 days |
| Majidi, et al 35 | 59.42± 15 | Severe | Survival duration | Vitamin C | IV | 500 mg | 14 days | |
| Poona Kumari, et al 36 | 52 ± 11 | Moderate to severe | *Days to be symptom-free * Days spent in the hospital * Need for mechanical ventilation |
Vitamin C | IV | |||
| Castillo ME, et al38 | 53.14 +/- 10.77 | Lung disease Diabetes mellitus Hypertension Cardiovascular disease |
Moderate to Severe | Rate of ICU admission and deaths | Vitamin D | Oral | 0.532 mg 0.266 mg 0.266 mg |
Day1 Day3 Day7 |
| Igor H Murai, et al39 | 56.2 [14.4] | Moderate to severe | length of stay in hospital | Vitamin D | Oral | 200 000 IU | 1 day | |
Fig 1.
. Flow chart
Conflict of interest
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.explore.2023.06.009.
Appendix. Supplementary materials
References
- 1.Abrams EM, Szefler SJ. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020 Jul 1;8(7):659–661. doi: 10.1016/S2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunisia COVID - Coronavirus Statistics - Worldometer [Internet]. [cited 2023 Mar 7]. Available from: https://www.worldometers.info/coronavirus/country/tunisia/
- 3.Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. The Lancet. 2022 Mar 5;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M M, Mj T, R J, A A, R H. Self-medication during Covid-19 pandemic: challenges and opportunities. Drugs Ther Perspect Ration Drug Sel Use [Internet] 2020;36(12) doi: 10.1007/s40267-020-00785-z. https://pubmed.ncbi.nlm.nih.gov/33041621/ [cited 2023 Mar 7]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitimus DM, Popescu MR, Voiculescu SE, Panaitescu AM, Pavel B, Zagrean L, et al. Melatonin's Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules. 2020 Aug 20;10(9):1211. doi: 10.3390/biom10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskologlu IC, Erdag E, Sayiner S, Abacioglu N, Sehirli AO. Melatonin and REGN-CoV2 combination as a vaccine adjuvant for Omicron variant of SARS-CoV-2. Mol Biol Rep. 2022;49(5):4061–4068. doi: 10.1007/s11033-022-07419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read SA, Parnell G, Booth D, Douglas MW, George J, Ahlenstiel G. The antiviral role of zinc and metallothioneins in hepatitis C infection. J Viral Hepat. 2018;25(5):491–501. doi: 10.1111/jvh.12845. [DOI] [PubMed] [Google Scholar]
- 8.Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021 Jan;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae M, Kim H. The Role of Vitamin C, Vitamin D, and Selenium in Immune System against COVID-19. Molecules. 2020 Nov 16;25(22):5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamulka J, Jeruszka-Bielak M, Górnicka M, Drywień ME, Zielinska-Pukos MA. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients. 2020 Dec 27;13(1):54. doi: 10.3390/nu13010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coronavirus [Internet]. [cited 2023 Mar 7]. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_3
- 12.Bacchetti P. Current sample size conventions: Flaws, harms, and alternatives. BMC Med. 2010 Mar 22;8(1):17. doi: 10.1186/1741-7015-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín-Blanco C, Zamorano M, Lizárraga C, Molina-Moreno V. The Impact of COVID-19 on the Sustainable Development Goals: Achievements and Expectations. Int J Environ Res Public Health. 2022 Dec 5;19(23):16266. doi: 10.3390/ijerph192316266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharif N, Alzahrani KJ, Ahmed SN, Efficacy Dey SK. Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bello IB, Akinnawo EO, Akpunne BC, Mopa-Egbunu A. Knowledge of COVID-19 and preventive measures on self-medication practices among Nigerian undergraduates. Cogent Arts Humanit. 2022 Dec 31;9(1) [Google Scholar]
- 16.Shaffer L. Lots of long COVID treatment leads, but few are proven. Proc Natl Acad Sci U S A. 2022 Sep 6;119(36) doi: 10.1073/pnas.2213524119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlieg-Boerstra B, de Jong N, Meyer R, Agostoni C, De Cosmi V, Grimshaw K, et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy. 2022 May;77(5):1373–1388. doi: 10.1111/all.15136. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020 Jun 1;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SH, Cao XJ, Liu W, Shi XY, Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res. 2010;48(2):109–116. doi: 10.1111/j.1600-079X.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 20.Yip HK, Chang YC, Wallace CG, Chang LT, Tsai TH, Chen YL, et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J Pineal Res. 2013 Mar;54(2):207–221. doi: 10.1111/jpi.12020. [DOI] [PubMed] [Google Scholar]
- 21.Melatonin Alleviates Radiation-Induced Lung Injury via Regulation of miR-30e/NLRP3 Axis - PubMed [Internet]. [cited 2023 Mar 7]. Available from: https://pubmed.ncbi.nlm.nih.gov/30755784/
- 22.Anderson G, Reiter RJ. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev Med Virol. 2020 May;30(3):e2109. doi: 10.1002/rmv.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habtemariam S, Daglia M, Sureda A, Selamoglu Z, Gulhan MF, Nabavi SM. Melatonin and Respiratory Diseases: A Review. Curr Top Med Chem. 2017;17(4):467–488. doi: 10.2174/1568026616666160824120338. [DOI] [PubMed] [Google Scholar]
- 24.Reiter RJ, Ma Q, Sharma R. Treatment of ebola and other infectious diseases: melatonin “goes viral. Melatonin Res. 2020 Mar 10;3(1):43–57. [Google Scholar]
- 25.Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. Arch Med Res. 2022 Jan;53(1):79–85. doi: 10.1016/j.arcmed.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan ZT, Atrakji DMQYMAA, Mehuaiden DAK. The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2022 Jan;114:79–84. doi: 10.1016/j.ijid.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez-Rico M, de la Muela P, Herrera-Morueco JJ, Geoffroy PA, Limosin F, Hoertel N, et al. Melatonin does not reduce mortality in adult hospitalized patients with COVID-19: a multicenter retrospective observational study. J Travel Med. 2022 May 31;29(3):taab195. doi: 10.1093/jtm/taab195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur K, Gupta R, Saraf SA, Saraf SK. Zinc: The Metal of Life. Compr Rev Food Sci Food Saf. 2014 Jul;13(4):358–376. doi: 10.1111/1541-4337.12067. [DOI] [PubMed] [Google Scholar]
- 29.Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002 Aug;56(3):S20–S23. doi: 10.1038/sj.ejcn.1601479. Suppl. [DOI] [PubMed] [Google Scholar]
- 30.Zinc and Respiratory Viral Infections: Important Trace Element in Anti-viral Response and Immune Regulation - PMC [Internet] 2023 Mar 7 doi: 10.1007/s12011-021-02859-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8349606/ [cited]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben Abdallah S, Mhalla Y, Trabelsi I, Sekma A, Youssef R, Bel Haj Ali K, et al. Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. Clin Infect Dis. 2023 Jan 13;76(2):185–191. doi: 10.1093/cid/ciac807. [DOI] [PubMed] [Google Scholar]
- 32.Patel O, Chinni V, El-Khoury J, Perera M, Neto AS, McDonald C, et al. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J Med Virol. 2021 May;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolin L, Luchins J, Margolin D, Margolin M, Lefkowitz S. 20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment. J Evid-Based Integr Med. 2021;26 doi: 10.1177/2515690X211026193. 2515690 × 211026193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection. JAMA Netw Open. 2021 Feb 12;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majidi N, Rabbani F, Gholami S, Gholamalizadeh M, BourBour F, Rastgoo S, et al. The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.717816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, et al. The Role of Vitamin C as Adjuvant Therapy in COVID-19. Cureus. 12 (11):e11779. [DOI] [PMC free article] [PubMed]
- 37.Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19. Eur J Endocrinol. 2020 Nov;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020 Oct;203 doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021 Mar 16;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elamir YM, Amir H, Lim S, Rana YP, Lopez CG, Feliciano NV, et al. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone. 2022 Jan;154 doi: 10.1016/j.bone.2021.116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrao S, Mallaci Bocchio R, Lo Monaco M, Natoli G, Cavezzi A, Troiani E, et al. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients. 2021 Apr 12;13(4):1261. doi: 10.3390/nu13041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker T. Critical care in low-income countries. Trop Med Int Health TM IH. 2009 Feb;14(2):143–148. doi: 10.1111/j.1365-3156.2008.02202.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.