Abstract
Steroidogenic acute regulatory protein (StAR) plays a critical role in the regulation of progesterone (P4) production. Resveratrol (RSV), a natural polyphenol, has beneficial effects on reproductive function. However, its effects on StAR expression and P4 production in human granulosa cells remain undetermined. In this study, we showed that treatment of RSV upregulated StAR expression in human granulosa cells. G protein-coupled estrogen receptor (GPER) and ERK1/2 signaling were involved in RSV-stimulated StAR expression and P4 production. In addition, the expression of a transcriptional repressor, Snail, was downregulated by RSV, which contributed to the RSV-induced inductions of StAR expression and P4 production.
Keywords: Granulosa cells, Progesterone, Resveratrol, Snail, StAR
1. Introduction
Steroidogenesis is one of the pivotal functions of the ovary. Progesterone (P4) is known as the pregnancy hormone in females and also plays a significant role during the normal menstruation cycle. To ensure a normal pregnancy, P4 production by the corpus luteum is required until the placenta becomes competent to take over the role of P4 synthesis and production. Inadequate P4 production can lead to luteal phase deficiency, which is one of the major causes of female infertility [1]. Steroid hormones are produced from cholesterol. Human steroidogenic acute regulatory protein (StAR) is synthesized as a 37-kDa preprotein in the cytoplasm and then imported into mitochondria, where it is processed to yield a 30-kDa mature protein by cleavage of a N-terminal mitochondrial import sequence. StAR-mediated transportation of cholesterol from the outer to the inner membrane of the mitochondria acts as a vital step for steroidogenesis [2,3]. Granulosa cells, including cumulus cells and mural granulosa cells, are located in the ovarian follicles and are essential for normal oocyte development and steroid hormone production. In the ovary, the expression of StAR in granulosa cells is primarily regulated by follicle-stimulating hormone (FSH) and luteinizing hormone (LH) through the cAMP-PKA signaling pathway [4].
Resveratrol (RSV), a stilbenoid natural polyphenol, exists in more than 70 types of plants but is highly concentrated in the skin of red grapes. RSV has been reported to have various health benefits, especially in the prevention of chronic diseases, due to its antioxidant and anti-inflammatory properties [5]. Increasing evidence from both in vitro experiments and in vivo studies using animals and humans reveals the beneficial effects of RSV on both male and female reproductive function [6,7]. However, because of its hormetic property and the uses of different experimental models and conditions, several controversial results regarding the biological effects of RSV are observed [8]. RSV treatment increases P4 production in bovine granulosa and cumulus cells, as well as in cultured rabbit ovarian fragments [9–11]. In pigs, P4 production is stimulated by RSV in granulosa cells, but it is suppressed by RSV in cultured ovarian follicles [12,13]. Notably, regardless of the effects of RSV on P4 production, thus far, the regulatory role of RSV in StAR expression in human granulosa cells is unclear. We have reported that epigallocatechin-3-gallate, a green tea polyphenol, stimulates StAR expression and P4 production in human granulosa cells [14]. Recent studies show that RSV protects human granulosa cells from oxidative stress [15,16]. However, whether the StAR expression and P4 production are affected by RSV in human granulosa cells remains unknown.
The present study was designed to examine the effect of RSV on StAR expression and P4 production in human granulosa cells. To make the experiments more technically feasible, especially for those gene silencing and overexpression, we used KGN, a human granulosa-like tumor cell line, as our major in vitro model because this cell line retains many normal physiological characteristics of granulosa cells, including steroidogenesis [17]. In addition, primary human granulosa-lutein (hGL) cells obtained from patients undergoing in vitro fertilization (IVF) were also used to investigate the effects and related underlying molecular mechanisms of RSV on StAR expression and P4 production in human granulosa cells.
2. Methods
2.1. Antibodies and reagents
The p-ERK1/2 (#9106), ERK1/2 (#9102), p-AKT (#9271), AKT (#9272), Snail (#3895), and Slug (#9585) antibodies were purchased from Cell Signaling Technology. The StAR (#sc-166821) and α-tubulin (#sc-23948) antibodies were purchased from Santa Cruz Biotechnology. The StAR anti-body only detects the mature form of StAR (30-kDa). The GPER (#ab154069) antibody was purchased from Abcam. The resveratrol was obtained from Sigma. The G-15 and U0126 were obtained from Cayman.
2.2. Cell culture
The human granulosa cell tumor-derived cell line, KGN [17], was kindly provided by Professor Aaron Hsueh in the Department of Obstetrics and Gynecology at Stanford University. The primary human granulosa-lutein (hGL) cells were purified by density centrifugation from follicular aspirates collected from women undergoing oocyte retrieval, as previously described [18]. The using of clinical samples received approval and was carried out in accordance with the approved guidelines from the Zhengzhou University Research Ethics Board. Both KGN and primary hGL cells were cultured in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C in Dulbecco’s Modified Eagle Medium/nutrient mixture F-12 Ham medium (DMEM/F-12; Gibco) supplemented with 10% FBS (HyClone), 100 U/mL of penicillin and 100 μg/mL of streptomycin sulfate (Boster). Individual primary cultures were composed of cells from one individual patient. Each experiment was repeated at least three times, and each time used cells derived from different patients or different passages.
2.3. Reverse transcription quantitative real-time PCR (RT-qPCR)
Total RNA was extracted with TRIzol (Invitrogen) according to the manufacturer’s instructions. RNA (1 μg) was reverse-transcribed into first-strand cDNA with the iScript Reverse Transcription Kit (Bio-Rad Laboratories). Each 20 μL qPCR reaction contained 1X SYBR Green PCR Master Mix (Applied Biosystems), 60 ng of cDNA, and 250 nM of each specific primer. The primers used were 5′-AAA CTT ACG TGG CTA CTC AGC ATC-3′ (sense) and 5′-GAC CTG GTT GAT GCT CTT G-3′ (antisense) for steroidogenic acute regulatory protein (StAR) and 5′-GAG TCA ACG GAT TTG GTC GT-3′ (sense) and 5′-GAC AAG CTT CCC GTT CTC AG-3′ (antisense) for GAPDH. The qPCR was performed on an Applied Biosystems QuantStudio 12K Flex system equipped with 96-well optical reaction plates. The specificity of each assay was validated by melting curve analysis and agarose gel electrophoresis of the PCR products. All of the RT-qPCR experiments were run in triplicate, and a mean value was used to determine the mRNA levels. RNase-free water and mRNA without RT were used as negative controls. Relative quantification of the mRNA levels was performed using the comparative Ct method with GAPDH as the reference gene and using formula 2−ΔΔCt.
2.4. Western blot analysis
Cells were lysed in cell lysis buffer (Cell Signaling Technology) supplemented with a protease inhibitor cocktail (Sigma). The protein concentration was measured by the BCA protein assay kit (Thermo Scientific). Samples with an equal amount of protein were separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Bio-Rad Laboratories). After 1 h blocking at room temperature with 5% non-fat dry milk in Tris-buffered saline (TBS), the membranes were incubated overnight at 4 °C with primary antibodies diluted in 5% non-fat milk/TBS. Following primary antibody incubation, the membranes were washed with TBS and subsequently incubated with appropriate HRP-conjugated secondary antibodies (Bio-Rad Laboratories). The immunoreactive bands were detected with an enhanced chemiluminescence kit and imaged with a ChemiDoc MP Imager (Bio-Rad Laboratories).
2.5. Small interfering RNA (siRNA) transfection and Snail overexpression
To knock down endogenous GPER, ERK1, ERK2, or Snail, KGN cells were transfected with 50 nM ON-TARGETplus SMARTpool siRNA targeting for the specific gene (Dharmacon) using Lipofectamine RNAiMAX (Invitrogen). The siCONTROL NON-TARGETING pool siRNA (Dharmacon) was used as the transfection control. To overexpress Snail, KGN cells were transfected with 1 μg empty pCMV vector or vector encoding a full-length of human Snail gene (GeneChem) using Lipofectamine 3000 (Invitrogen).
2.6. ELISA assay for progesterone
Progesterone (P4) levels were measured using an ELISA Kit (#582601, Cayman) as per the manufacturer’s instructions. The interassay CV and intraassay CV for P4 ELISA were <10%. The analytical sensitivity of P4 ELISA was 10 pg/mL. P4 levels in culture media were normalized to protein concentrations from corresponding cell lysates.
2.7. MTT assay
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma) assay was used to determine cell viability. Cells were seeded in 24-well plates (1 × 104 cells/well in 500 μL of medium) and treated with vehicle control (DMSO) or resveratrol. MTT was added to a final concentration of 0.5 mg/mL, the cells were incubated for 4 h, and the medium was removed. DMSO was added to each well, and absorbances were measured at 570 nm using a microplate reader.
2.8. Statistical analysis
The results are presented as the mean ± SEM of at least three independent experiments. All statistical analyses were analyzed by the PRISM software. Multiple comparisons were analyzed by one-way ANOVA, followed by Tukey’s multiple comparison tests. For experiments involving only two groups, data were analyzed by t test. A significant difference was defined as p < 0.05. Values that are statistically different from one another ( p < 0.05) are indicated by different letters. The values with any common letter are not significantly different.
3. Results
3.1. RSV upregulates StAR expression in both KGN and hGL cells
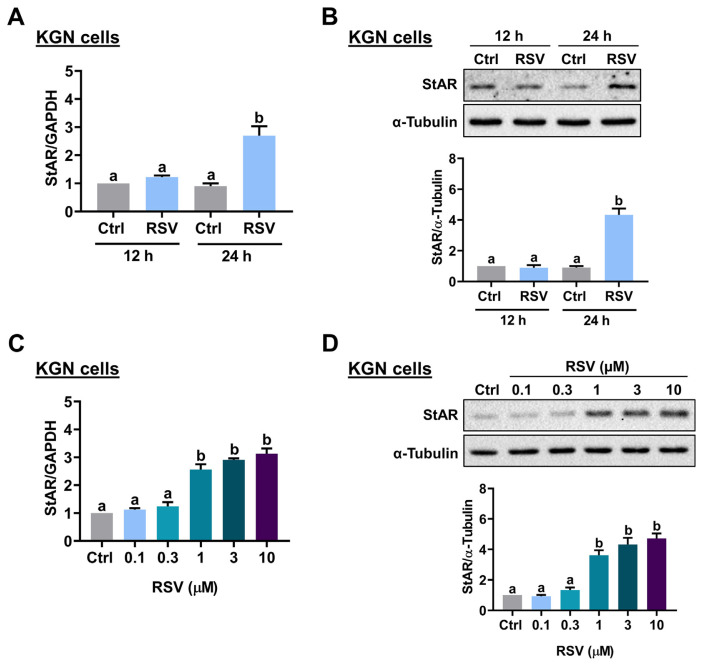
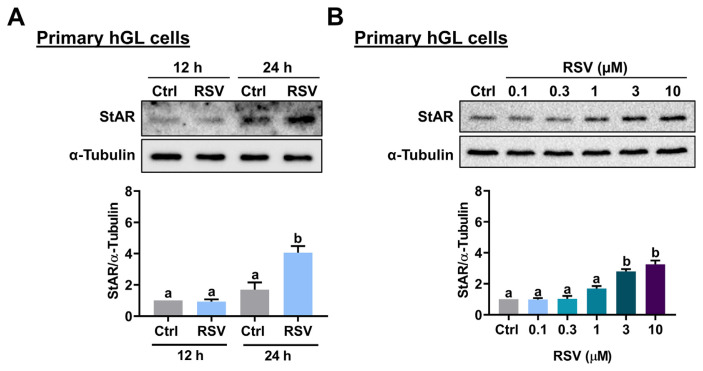
To determine the effect of RSV on StAR expression, KGN cells were treated with 10 μM RSV for 12 and 24 h, and the mRNA levels of StAR were analyzed by RT-qPCR. As shown in Fig. 1A, 12 h of RSV treatment did not affect the mRNA levels of StAR. However, the mRNA levels of StAR were significantly upregulated after 24 h of RSV treatment. Western blot analysis showed a similar effect that 24 h of RSV treatment upregulated StAR protein levels in KGN cells (Fig. 1B). In mice and rats, the peak concentrations of plasma levels of RSV after RSV administration were 32 μM and 8.1 μM, respectively [19,20]. In humans, after 1 h of a single dose of oral ingestion of RSV (5 mg and 1 g), the plasma RSV concentration could reach 0.6 μM and 137 μM, respectively [21]. Thus, next, we examined the effect of different concentrations of RSV on StAR expression in KGN cells. While treatment of 0.1 or 0.3 μM RSV had no significant effect, StAR mRNA levels were significantly upregulated by exposure to 1, 3, or 10 μM RSV with a comparable effect (Fig. 1C). A similar stimulatory effect of RSV on StAR protein levels was observed by the Western blot analysis (Fig. 1D). Therefore, 1 μM RSV was used for the following experiments for KGN cells. To further confirm the stimulatory effect of RSV on StAR expression in human granulosa cells, primary hGL cells purified from follicular aspirates of IVF patients were used. As shown in Fig. 2A, treatment with 10 μM RSV for 24 h upregulated protein levels of StAR in primary hGL cells. Similar to the results obtained from KGN cells, RSV stimulated StAR protein levels in a dose-dependent manner with a significant effect observed by the treatment of 3 or 10 μM RSV (Fig. 2B). Therefore, 3 μM RSV was used for the following experiments for primary hGL cells.
Fig. 1.
RSV stimulates StAR expression in KGN cells. A and B, Cells were treated with 10 μMRSV for 12 and 24 h. ThemRNA(A) and protein (B) levels of StAR were examined by RT-qPCR and Western blot, respectively. C and D, Cells were treated with 0.1, 0.3, 1, 3, or 10 μMRSV for 24 h. The StARmRNA levels (C) and protein levels (D) were examined by RT-qPCR and Western blot, respectively. The results are expressed as the mean ± SEM of at least three independent experiments. Values that are statistically different from one another ( p < 0.05) are indicated by different letters.
Fig. 2.
RSV stimulates StAR expression in primary hGL cells. A, Cells were treated with 10 μM RSV for 12 and 24 h. The protein levels of StAR were examined by Western blot. B, Cells were treated with 0.1, 0.3, 1, 3, or 10 μM RSV for 24 h. The StAR protein levels were examined by Western blot. The results are expressed as the mean ± SEM of at least three independent experiments. Values that are statistically different from one another ( p < 0.05) are indicated by different letters.
3.2. RSV upregulates StAR expression by acting through GPER
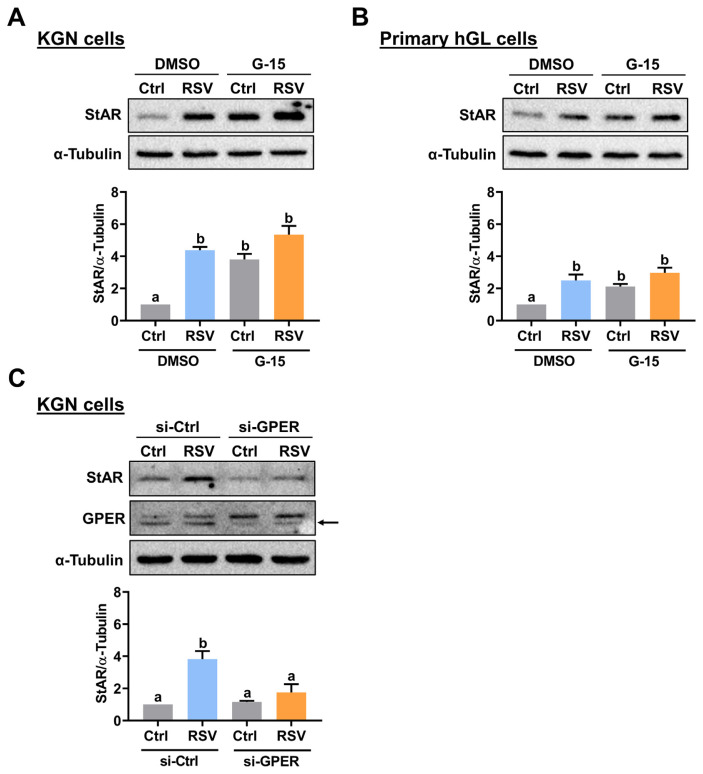
RSV inhibits voltage-gated potassium channels by binding to the G protein-coupled estrogen receptor (GPER), which is also known as GPR30 [22]. To examine whether GPER is involved in RSV-stimulated StAR expression, a GPER selective antagonist G-15 was used to block the function of GPER [23]. As shown in Fig. 3A, pretreatment with G-15 increased basal StAR protein levels, which may be attributed to the off-target effect of the pharmacological inhibitor. Nevertheless, the stimulatory effect of RSV on StAR protein levels in KGN cells was attenuated by pretreatment with G-15. Similarly, inhibition of GPER attenuated the stimulatory effect of RSV on StAR protein levels in primary hGL cells (Fig. 3B). To avoid the off-target effect from the pharmacological inhibitor, the involvement of GPER in RSV-induced upregulation of StAR expression was further confirmed by using a siRNA-mediated knockdown approach. As shown in Fig. 3C, transfection with GPER-specific siRNA significantly downregulated endogenous GPER protein levels. The siRNA-mediated knockdown of GPER did not affect the basal protein levels of StAR but attenuated the RSV-upregulated StAR protein levels.
Fig. 3.
GPER mediates RSV-induced StAR expression. A and B, KGN (A) and primary hGL (B) cells were pretreated with vehicle control (DMSO) or 1 μM G-15 for 1 h, and then treated with RSV (1 μM for KGN cells and 3 μM for primary hGL cells) for 24 h. The StAR protein levels were examined by Western blot. C, KGN cells were transfected with 50 nM control siRNA (si-Ctrl) or GPER siRNA (si-GPER) for 48 h, and then treated with 1 μM RSV for 24 h. The StAR and GPER protein levels were examined by Western blot. The results are expressed as the mean ± SEM of at least three independent experiments. Values that are statistically different from one another ( p < 0.05) are indicated by different letters.
3.3. ERK1/2 signaling mediates RSV-upregulated StAR expression
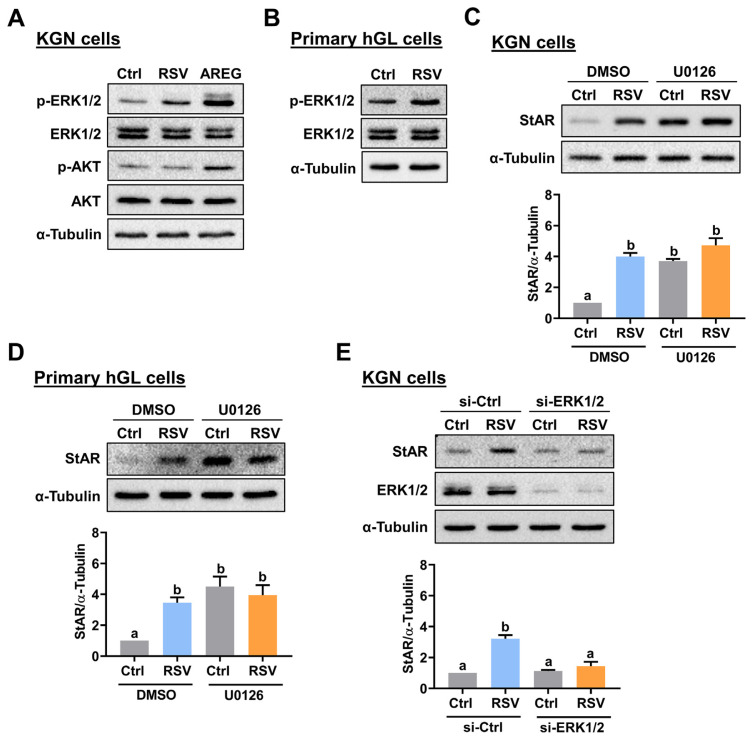
Activated GPER can lead to the activation of MEK/ERK1/2 and PI3K/AKT signaling pathways [24]. To examine whether RSV activates these signaling pathways, KGN cells were treated with RSV for 10 min. As shown in Fig. 4A, RSV treatment activated ERK1/2 but not AKT signaling in KGN cells. Cell lysates obtained from KGN cells treated with amphiregulin, an epidermal growth factor receptor (EGFR) ligand, were used as positive controls for the activations of ERK1/2 and AKT signaling pathways [25]. Similarly, treatment with RSV also activated ERK1/2 in hGL cells (Fig. 4B). Next, we used a specific MEK inhibitor, U0126, to determine whether the ERK1/2 signaling pathway is required for RSV-induced upregulation of StAR expression. As shown in Fig. 4C and D, due to the off-target effect, pretreatment with U0126 increased the basal protein levels of StAR, which is consistent with our previous study [26]. However, inhibition of ERK1/2 signaling blocked the RSV-induced upregulation of StAR protein levels in both KGN and primary hGL cells. To further confirm the involvement of ERK1/2 signaling in RSV-induced StAR expression and prevent the off-target effect of the MEK inhibitor, the expression of endogenous ERK1 and ERK2 were knocked down by co-transfecting KGN cells with ERK1 and ERK2 siRNAs. As shown in Fig. 4E, the knockdown of ERK1/2 blocked the stimulatory effect of RSV on StAR protein levels in KGN cells. Taken together, these results indicate that the RSV-induced upregulation of StAR expression is mediated by the ERK1/2 signaling.
Fig. 4.
Activation of ERK1/2 signaling is required for the RSV-induced StAR expression. A, KGN cells were treated with 1 μM RSV for 10 min. The levels of phosphorylated and total forms of ERK1/2 and AKT were determined by Western blot. KGN cells treated with 10 ng/mL amphiregulin (AREG) for 10 min were used as positive controls for the activations of ERK1/2 and AKT signaling pathways. B, Primary hGL cells were treated with 3 μM RSV for 10 min. The levels of phosphorylated and total forms of ERK1/2 were determined by Western blot. C and D, KGN (C) and primary hGL (D) cells were pretreated with vehicle control (DMSO) or 1 μM U0126 for 1 h, and then treated with RSV (1 μM for KGN cells and 3 μM for primary hGL cells) for 24 h. The StAR protein levels were examined by Western blot. E, KGN cells were transfected with 50 nM control siRNA (si-Ctrl) or ERK1+ERK2 siRNAs (si-ERK1/2) for 48 h, and then treated with 1 μM RSV for 24 h. The StAR and ERK1/2 protein levels were examined by Western blot. The results are expressed as the mean ± SEM of at least three independent experiments. Values that are statistically different from one another ( p < 0.05) are indicated by different letters.
3.4. RSV stimulates StAR expression by suppressing the expression of transcriptional repressor Snail
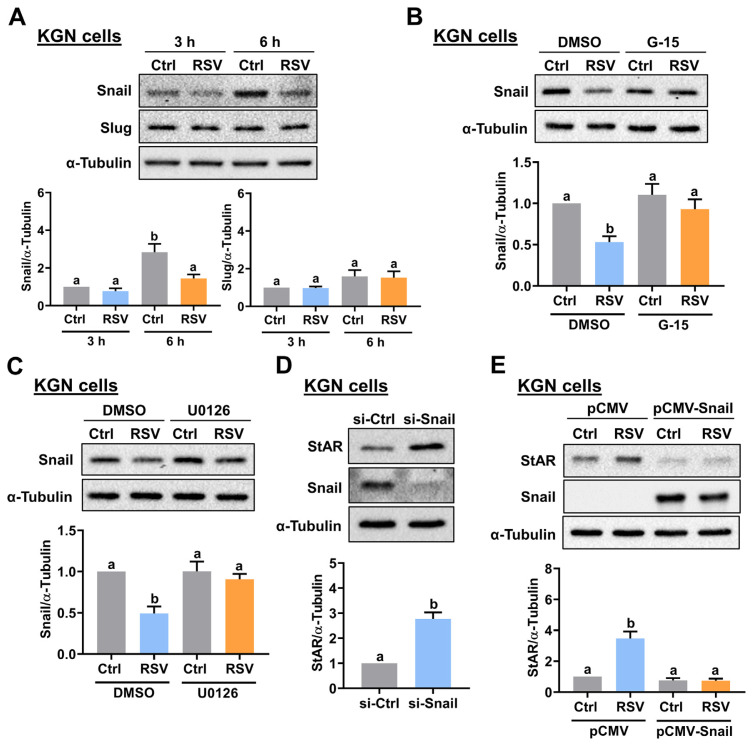
We have shown that transforming growth factor beta-1 (TGF-β1) can inhibit StAR expression in human granulosa cells [27]. In addition, RSV suppresses TGF-β1-induced epithelial-to-mesenchymal transition (EMT) by inhibiting the expression of transcriptional repressors, Snail and Slug [28]. Next, we were interested in determining whether the expression of Snail and Slug can be affected by RSV and contributes to the RSV-stimulated StAR expression in human granulosa cells. As shown in Fig. 5A, treatment of KGN cells with RSV down-regulated Snail protein levels without affecting the protein levels of Slug. We noticed that the basal protein levels of Snail were upregulated through the increase in culture time. However, we do not know the exact factor that caused this result. Nevertheless, the inhibitory effect of RSV on Snail protein levels was blocked by the inhibition of GPER and ERK1/2 signaling (Fig. 5B and C). To further confirm the role of Snail in the regulation of StAR expression, gain- and loss-of-function approaches were applied. As shown in Fig. 5D, the siRNA-mediated knockdown of Snail upregulated the protein levels of StAR in KGN cells. In addition, the overexpression of Snail blocked the stimulatory effect of RSV on StAR protein levels (Fig. 5E).
Fig. 5.
Downregulation of Snail is required for RSV-induced StAR expression. A, KGN cells were treated with 1 μM RSV for 3 and 6 h. The protein levels of Snail and Slug were examined by Western blot. B and C, KGN cells were pretreated with vehicle control (DMSO), 1 μM G-15 (B) or 1 μM U0126 (C) for 1 h, and then treated with 1 μM RSV for 6 h. The Snail protein levels were examined by Western blot. D, KGN cells were transfected with 50 nM control siRNA (si-Ctrl) or Snail siRNA (si-Snail) for 48 h. The StAR and Snail protein levels were examined by Western blot. E, KGN cells were transfected with 1 μg control vector (pCMV) or vector containing Snail (pCMV-Snail) for 48 h, and then treated with 1 μM RSV for 24 h. The protein levels of StAR and Snail were examined by Western blot. The results are expressed as the mean ± SEM of at least three independent experiments. Values that are statistically different from one another (p < 0.05) are indicated by different letters.
3.5. GPER/ERK1/2-mediated downregulation of Snail contributes to the RSV-stimulated P4 production
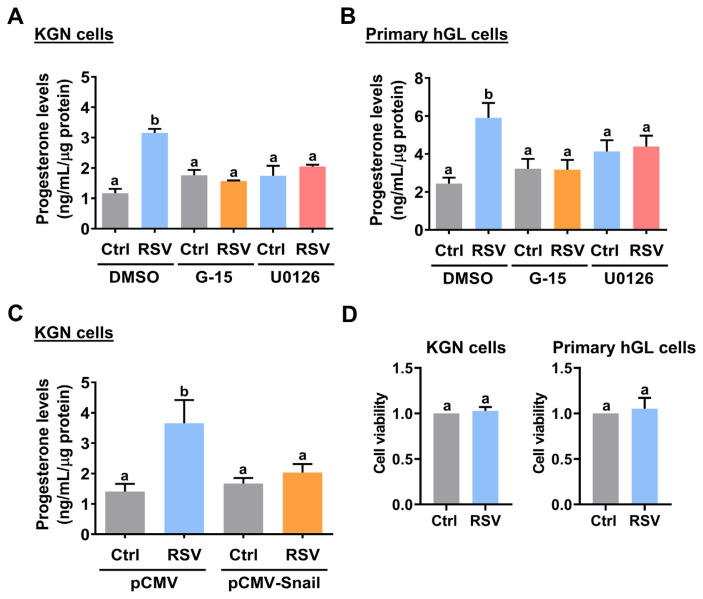
Given the important role of StAR protein in the regulation of P4 production, we next examined the effect of RSV on P4 production. ELISA results showed that KGN cells stimulated with RSV increased the production of P4. This stimulatory effect was blocked by the inhibition of GPER and ERK1/2 signaling (Fig. 6A). Similarly, the stimulatory effect of RSV on P4 production was also observed in primary hGL cells. Consistent with the results obtained from KGN cells, activations of GPER and ERK1/2 signaling were required for RSV-induced P4 production in primary hGL cells (Fig. 6B). Moreover, in KGN cells, the RSV-stimulated P4 production was attenuated by the overexpression of Snail (Fig. 6C). Next, we used MTT assay to determine whether the effect of RSV on P4 production could be influenced by changes in cell viability. MTT assay showed that 24 h treatment of RSV did not significantly influence the cell viability in both KGN and primary hGL cells (Fig. 6D).
Fig. 6.
GPER/ERK1/2-mediated downregulation of Snail contributes to the RSV-stimulated P4 production. A and B, KGN (A) and primary hGL (B) cells were pretreated with vehicle control (DMSO), 1 μM G-15, or 1 μM U0126 for 1 h, and then treated with RSV (1 μM for KGN cells and 3 μM for primary hGL cells) for 36 h. Progesterone levels in culture media were examined using ELISA. C, KGN cells were transfected with 1 μg control vector (pCMV) or vector containing Snail (pCMV-Snail) for 48 h, and then treated with 1 μM RSV for 36 h. Progesterone levels in culture media were examined using ELISA. D, KGN (left panel) and primary hGL (right panel) cells were treated with 1 μM and 3 μM RSV for 36 h, respectively. The cell viability was examined by MTT assay. The results are expressed as the mean ± SEM of at least three independent experiments. Values that are statistically different from one another ( p < 0.05) are indicated by different letters.
4. Discussion
Increasing evidence from both in vitro and in vivo studies suggests that dietary polyphenols, including RSV, play an important role in the prevention of chronic diseases and have beneficial effects on reproductive health [29]. These benefits can be attributed to the suppressive effects of RSV in oxidative stress and inflammation. In a context-dependent manner, RSV regulates physiological functions through numerous signaling pathways. However, only a few molecular mechanisms have been delineated that mediate the function of RSV in the ovary [30]. Although the differential effects of RSV on ovarian P4 production have been reported in different animal models, whether RSV affects P4 production in human granulosa cells remains undefined. In KGN cells, some phytoestrogens, including RSV, can upregulate StAR expression [31]. In the present study, we showed that RSV upregulated StAR expression and P4 production in both KGN and primary hGL cells. The stimulatory effects of RSV on StAR expression and P4 production were mediated by GPER, ERK1/2, and Snail signaling.
Because RSV has a chemical structure similar to that of some estrogens, it is considered a natural phytoestrogen [32]. It is known that GPER mediates non-genomic estrogen signaling [33]. Some plant-derived polyphenolic compounds, including RSV, can bind to GPER [34]. Using a selective GPER antagonist, G-15, and siRNA-mediated knockdown approach, our results revealed that the action of GPER was involved in RSV-induced StAR expression in human granulosa cells. Signaling through GPER occurs via transactivation of the EGFR. This effect can be achieved by activating metalloproteinases which cleave pro-heparin-binding-epidermal growth factor (HB-EGF) and release free HB-EGF. HB-EGF then binds and activates EGFR leading to downstream activation of signaling molecules, such as ERK1/2 [35,36]. Our recent study demonstrates that HB-EGF induces upregulation of StAR expression and P4 production in both KGN and primary hGL cells and these effects require the EGFR-mediated activation of the ERK1/2 signaling pathway [26]. In the present study, our results showed that activation of ERK1/2 signaling was involved in RSV-stimulated StAR expression and P4 production in both KGN and primary hGL cells. Therefore, it is possible that transactivation of the EGFR is also involved in the stimulatory effects of RSV on StAR expression and P4 production in human granulosa cells. Further investigation could be conducted to test this hypothesis.
Snail and Slug are transcriptional repressors [37]. They play a critical role during the EMT by suppressing E-cadherin expression through binding to the E-boxes in its proximal promoter [38]. The members of the TGF-β superfamily, such as TGF-β1, activins, and growth differentiation factor-8, can stimulate Snail and Slug expression [39–42]. Notably, our research group has shown that all these factors can downregulate StAR expression in human granulosa cells [27,43,44]. However, whether Snail or Slug participates in the StAR expression is unknown. In the present study, we showed that only the expression of Snail but not that of Slug was downregulated by RSV in human granulosa cells. Using gain- and loss-of-function approaches, we demonstrated that downregulation of Snail was required for the RSV-induced StAR expression. We do not know whether Snail can bind directly to the StAR promoter. To date, many transcription factors have been identified to regulate the expression of StAR in gonads [45]. Among them, steroidogenic factor-1 (SF-1) is the most known transcription factor that mediates the expression of StAR [46]. E-box has been identified in the SF-1 promoter and is required for its expression [47]. Therefore, it is possible that RSV reduces Snail expression, which stimulates SF-1 expression and that contributes to the upregulation of StAR expression. Thus, the investigation of whether Snail can affect the expression of SF-1 and subsequently contribute to the regulation of StAR in human granulosa cells will be of great interest.
5. Conclusions
In summary, the present study, for the first time, reports that the StAR expression and P4 production in human granulosa cells can be upregulated in response to the RSV treatment. The stimulatory effects of RSV on StAR expression and P4 production are mediated by GPER/ERK1/2 signaling. In addition, RSV-induced downregulation of the Snail transcriptional repressor is required for RSV-induced StAR expression and P4 production. This study increases the understanding of the biological function of RSV and provides a new mechanism for the regulation of ovarian steroidogenesis, which could help to develop therapeutic methods for disorders of ovarian steroidogenesis.
Acknowledgments
This work was supported by operating grants (32070848 to Lanlan Fang and 32170868 to Jung-Chien Cheng) from the National Natural Science Foundation of China. This work was also supported by the Henan Health Science and Technology Innovation Program for Distinguished Young Scholars from the Henan Health Commission (YXKC2022027) and Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (QNCXTD2023011) to Lanlan Fang. The graphical abstract was created with BioRender.com. We have been granted a license to use this figure in journal publications.
Funding Statement
This work was supported by operating grants (32070848 to Lanlan Fang and 32170868 to Jung-Chien Cheng) from the National Natural Science Foundation of China. This work was also supported by the Henan Health Science and Technology Innovation Program for Distinguished Young Scholars from the Henan Health Commission (YXKC2022027) and Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (QNCXTD2023011) to Lanlan Fang.
Footnotes
Ethics statements
The using of clinical samples received approval and was carried out in accordance with the approved guidelines from the Zhengzhou University Research Ethics Board.
Author’s contribution
Tinglin Song: Data curation, Formal analysis, Investigation, Writing - original draft. Jiaye Chen: Data curation; Formal analysis. Sizhu Yang: Formal analysis. Boqun Liu: Formal analysis. Lingling Zhang: Resource. Qian Zhang: Resource. Jung-Chien Cheng: Funding acquisition, Project administration, Writing - review & editing. Lanlan Fang: Funding acquisition, Project administration, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no competing interests.
References
- 1. Practice committees of the American society for reproductive M the society for reproductive E infertility. Diagnosis and treatment of luteal phase deficiency: a committee opinion. Fertil Steril. 2021;115:1416–23. doi: 10.1016/j.fertnstert.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 2. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–22. [PubMed] [Google Scholar]
- 3. Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–44. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 4. Selvaraj V, Stocco DM, Clark BJ. Current knowledge on the acute regulation of steroidogenesis. Biol Reprod. 2018;99:13–26. doi: 10.1093/biolre/ioy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koushki M, Amiri-Dashatan N, Ahmadi N, Abbaszadeh HA, Rezaei-Tavirani M. Resveratrol: a miraculous natural compound for diseases treatment. Food Sci Nutr. 2018;6:2473–90. doi: 10.1002/fsn3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasquariello R, Verdile N, Brevini TAL, Gandolfi F, Boiti C, Zerani M, et al. The role of resveratrol in mammalian reproduction. Molecules. 2020:25. doi: 10.3390/molecules25194554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Novakovic R, Rajkovic J, Gostimirovic M, Gojkovic-Bukarica L, Radunovic N. Resveratrol and reproductive health. Life. 2022:12. doi: 10.3390/life12020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: a literature review. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balazi A, Sirotkin AV, Foldesiova M, Makovicky P, Chrastinova L, Makovicky P, et al. Green tea can supress rabbit ovarian functions in vitro and in vivo. Theriogenology. 2019;127:72–9. doi: 10.1016/j.theriogenology.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 10. Wang F, Tian X, Zhang L, He C, Ji P, Li Y, et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril. 2014;101:577–86. doi: 10.1016/j.fertnstert.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 11. Kolesarova A, Capcarova M, Maruniakova N, Lukac N, Ciereszko RE, Sirotkin AV. Resveratrol inhibits reproductive toxicity induced by deoxynivalenol. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47:1329–34. doi: 10.1080/10934529.2012.672144. [DOI] [PubMed] [Google Scholar]
- 12. Sirotkin A, Alexa R, Kadasi A, Adamcova E, Alwasel S, Harrath AH. Resveratrol directly affects ovarian cell sirtuin, proliferation, apoptosis, hormone release and response to follicle-stimulating hormone (FSH) and insulin-like growth factor I (IGF-I) Reprod Fertil Dev. 2019;31:1378–85. doi: 10.1071/RD18425. [DOI] [PubMed] [Google Scholar]
- 13. Sirotkin A, Kadasi A, Balazi A, Kotwica J, Alwasel S, Harrath AH. The action of benzene, resveratrol and their combination on ovarian cell hormone release. Folia Biol. 2020;66:67–71. [PubMed] [Google Scholar]
- 14. Fang L, Guo Y, Li Y, Jia Q, Han X, Liu B, et al. Epigallocatechin-3-gallate stimulates StAR expression and progesterone production in human granulosa cells through the 67-kDa laminin receptor-mediated CREB signaling pathway. J Cell Physiol. 2022;237:687–95. doi: 10.1002/jcp.30538. [DOI] [PubMed] [Google Scholar]
- 15. Moreira-Pinto B, Costa L, Felgueira E, Fonseca BM, Rebelo I. Low doses of resveratrol protect human granulosa cells from induced-oxidative stress. Antioxidants. 2021:10. doi: 10.3390/antiox10040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nie Z, Hua R, Zhang Y, Zhang N, Zhang Y, Li Q, et al. Resveratrol protects human luteinised granulosa cells against hydrogen peroxide-induced oxidative injury through the Sirt1. Reprod Fertil Dev. 2021;33:831–40. doi: 10.1071/RD21069. [DOI] [PubMed] [Google Scholar]
- 17. Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–45. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 18. Fang L, Yu Y, Li Y, Wang S, Zhang R, Guo Y, et al. Human chorionic gonadotropin-induced amphiregulin stimulates aromatase expression in human granulosa-lutein cells: a mechanism for estradiol production in the luteal phase. Hum Reprod. 2019;34:2018–26. doi: 10.1093/humrep/dez171. [DOI] [PubMed] [Google Scholar]
- 19. Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br J Cancer. 2004;90:736–44. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bottner M, Christoffel J, Jarry H, Wuttke W. Effects of long-term treatment with resveratrol and subcutaneous and oral estradiol administration on pituitary function in rats. J Endocrinol. 2006;189:77–88. doi: 10.1677/joe.1.06535. [DOI] [PubMed] [Google Scholar]
- 21. Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, et al. Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med. 2015;7:298ra117. doi: 10.1126/scitranslmed.aaa7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong WH, Chen JC, He YL, Xu JJ, Mei YA. Resveratrol inhibits K(v)2.2 currents through the estrogen receptor GPR30-mediated PKC pathway. Am J Physiol Cell Physiol. 2013;305:C547–57. doi: 10.1152/ajpcell.00146.2013. [DOI] [PubMed] [Google Scholar]
- 23. Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–7. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qie Y, Qin W, Zhao K, Liu C, Zhao L, Guo LH. Environmental estrogens and their biological effects through GPER mediated signal pathways. Environ Pollut. 2021;278:116826. doi: 10.1016/j.envpol.2021.116826. [DOI] [PubMed] [Google Scholar]
- 25. Fang L, Yu Y, Zhang R, He J, Sun YP. Amphiregulin mediates hCG-induced StAR expression and progesterone production in human granulosa cells. Sci Rep. 2016;6:24917. doi: 10.1038/srep24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng JC, Han X, Meng Q, Guo Y, Liu B, Song T, et al. HB-EGF upregulates StAR expression and stimulates progesterone production through ERK1/2 signaling in human granulosa-lutein cells. Cell Commun Signal. 2022;20:166. doi: 10.1186/s12964-022-00983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-beta1 downregulates StAR expression and decreases progesterone production through Smad3 and ERK1/2 signaling pathways in human granulosa cells. J Clin Endocrinol Metab. 2014;99:E2234–43. doi: 10.1210/jc.2014-1930. [DOI] [PubMed] [Google Scholar]
- 28. Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, et al. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ly C, Yockell-Lelievre J, Ferraro ZM, Arnason JT, Ferrier J, Gruslin A. The effects of dietary polyphenols on reproductive health and early development. Hum Reprod Update. 2015;21:228–48. doi: 10.1093/humupd/dmu058. [DOI] [PubMed] [Google Scholar]
- 30. Jozkowiak M, Hutchings G, Jankowski M, Kulcenty K, Mozdziak P, Kempisty B, et al. The stemness of human ovarian granulosa cells and the role of resveratrol in the differentiation of MSCs-A review based on cellular and molecular knowledge. Cells. 2020:9. doi: 10.3390/cells9061418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solak KA, Wijnolts FMJ, Nijmeijer SM, Blaauboer BJ, van den Berg M, van Duursen MBM. Excessive levels of diverse phytoestrogens can modulate steroidogenesis and cell migration of KGN human granulosa-derived tumor cells. Toxicol Rep. 2014;1:360–72. doi: 10.1016/j.toxrep.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–43. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metabol. 2009;20:409–16. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 34. Luo J, Liu D. Does GPER really function as a G protein-coupled estrogen receptor in vivo? Front Endocrinol. 2020;11:148. doi: 10.3389/fendo.2020.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–26. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 37. Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 38. Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiong S, Klausen C, Cheng JC, Leung PC. Activin B promotes endometrial cancer cell migration by down-regulating E-cadherin via SMAD-independent MEK-ERK1/2-SNAIL signaling. Oncotarget. 2016;7:40060–72. doi: 10.18632/oncotarget.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yi Y, Cheng JC, Klausen C, Leung PCK. Activin A promotes ovarian cancer cell migration by suppressing E-cadherin expression. Exp Cell Res. 2019;382:111471. doi: 10.1016/j.yexcr.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 41. Zhao J, Klausen C, Xiong S, Cheng JC, Chang HM, Leung PC. Growth differentiation factor 8 induces SKOV3 ovarian cancer cell migration and E-cadherin down-regulation. Cell Signal. 2016;28:1615–22. doi: 10.1016/j.cellsig.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 42. Cheng JC, Chang HM, Leung PC. Transforming growth factor-beta1 inhibits trophoblast cell invasion by inducing Snail-mediated down-regulation of vascular endothelial-cadherin protein. J Biol Chem. 2013;288:33181–92. doi: 10.1074/jbc.M113.488866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fang L, Chang HM, Cheng JC, Yu Y, Leung PC, Sun YP. Growth differentiation factor-8 decreases StAR expression through ALK5-mediated Smad3 and ERK1/2 signaling pathways in luteinized human granulosa cells. Endocrinology. 2015;156:4684–94. doi: 10.1210/en.2015-1461. [DOI] [PubMed] [Google Scholar]
- 44. Chang HM, Cheng JC, Huang HF, Shi FT, Leung PC. Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells. Mol Cell Endocrinol. 2015;412:290–301. doi: 10.1016/j.mce.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 45. Stocco DM, Selvaraj V. Yet another scenario in the regulation of the steroidogenic acute regulatory (STAR) protein gene. Endocrinology. 2017;158:235–8. doi: 10.1210/en.2016-1874. [DOI] [PubMed] [Google Scholar]
- 46. Sugawara T, Kiriakidou M, McAllister JM, Holt JA, Arakane F, Strauss JF., 3rd Regulation of expression of the steroidogenic acute regulatory protein (StAR) gene: a central role for steroidogenic factor 1. Steroids. 1997;62:5–9. doi: 10.1016/s0039-128x(96)00152-3. [DOI] [PubMed] [Google Scholar]
- 47. Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–37. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]