Despite a progressive increase in the complexity of pediatric heart transplant recipients, post-transplant survival has continued to improve over the last 3 decades.1 This improvement may be attributed to advances in the pre-transplant, peri-transplant, and long-term management of transplant recipients. Although the fundamental role of heart transplantation as the only accepted therapy for end-stage heart failure remains the same, there have been significant changes in the recipient profile over time with advances in congenital heart disease surgery, in particular for children with single ventricle disease, and the development and widespread use of ventricular assist devices for children awaiting heart transplantation.2,3 In the 23rd annual ISHLT Registry report published last year, we described changes in the donor profile over the previous 3 decades.4 We also described donor characteristics associated with post-transplant survival at 1 year, at 5 years conditional upon surviving 1 year, and freedom from cardiac allograft vasculopathy (CAV). The goal of this focused report is to describe the changes in recipient characteristics over the last 3 decades, to describe trends in outcomes in recipients with specific characteristics, and to identify important recipient, donor, and transplant characteristics that were associated with post-transplant survival at 1 year, at 5 years conditional upon surviving 1 year, and freedom from CAV conditional on survival to discharge.
This 24th annual pediatric heart transplant report is based on data submitted to the Thoracic Organ Transplant Registry on 15,726 pediatric heart transplants through June 30, 2018. In response to a changing regulatory environment, the ISHLT Registry is undergoing an update in data acquisition and the pediatric cohort examined in this report is, therefore, the same as that examined in the 2019 and 2020 annual reports.1,4 We refer the reader to the 2019 report for additional core analyses not directly related to the focus of this year’s report.
Statistical methods
Data collection, conventions, and statistical methods
The 2021 International Thoracic Organ Transplant Registry report, as in past years, was developed using data submitted to the Registry from national and multinational transplant collectives as well as individual transplant centers. Between 2010 and 2018, 210 centers performing heart transplants in pediatric recipients contributed data to the Registry.1
This report presents an overview of characteristics of pediatric recipients of deceased donor heart alone transplants and their association with outcomes, with a particular focus on how the recipient profile has changed over time. The results seek to provide as granular detail as possible from data retained in the ISHLT Registry for transplants performed through June 30, 2018 with follow-up as of November 2, 2018. In addition to the data presented within the primary manuscript, extended analyses are presented in the online slide sets (https://ishltregistries.org/registries/slides.asp). The ISHLT web site also contains slide sets for previous annual reports. This report references specific online e-slides when particular data are discussed but not shown due to space limitations. E-slide H(p) numbers refer to the online pediatric heart transplant slides.
The ISHLT Registry website (https://ishlt.org/research-data/registries/ttx-registry#data-fields-look-up-tables-forms) provides detailed spreadsheets of the data elements collected in the Registry. The Registry required submission of core donor, recipient, and transplant procedure variables at the time of transplantation and at yearly follow-up, with low rates of missing data. Nevertheless, data quality depends on the accuracy and completeness of reporting. Missing data may be significantly higher for Registry variables that rely on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
Unless otherwise specified, analyses of heart transplants do not include combined heart-lung transplant data. The Registry does not capture the exact occurrence date for most secondary outcomes (e.g., CAV), but it does capture specific time periods during which an event occurred (i.e., between the first and the second-year annual follow-up visits). For the report’s analyses, the midpoint between annual follow-ups is used as a proxy for the event date. There are specific conventions in reporting secondary outcomes and other follow-up information if some recipients have died. Some analyses are limited to surviving patients to reduce the possibility of underestimating event rates or other outcomes. For time-to-event rates and cumulative morbidity rates, follow-up of recipients not experiencing the event of interest is censored at the last time the recipient was reported not to have had the event, either the most recent annual follow-up or the time of re-transplantation. Time-to-event graphs (e.g., survival graphs) are truncated when the number of subjects at risk becomes <10.
Focus theme: Changing recipient characteristics
Recipient characteristics
A comparison of baseline characteristics between pediatric recipients during the years 1992 to 2000, 2001 to 2009, and 2010 to 2018 is shown in Table 1 and eSlides H(p) 4 to 7. In each successive era, the number of pediatric heart transplant recipients increased in Europe, North America, and Other regions as heart transplantation became more widely available (eSlide H[p] 4–7). The proportion of pediatric heart transplants performed in North American centers has remained stable at ~68%, while the proportion of pediatric transplants performed in Europe declined from 29% during 1992 to 2000 to 25% during 2010 to 2018, and the proportion of those contributed by Other regions of the world to the Registry increased from 3% to 7.5% during this time.
Table 1.
Distribution of Recipient Characteristics by Transplant Era (Transplants: January 1992 – June 2018)
| Jan 1992–Dec 2000(n = 3,666) | Jan 2001–Dec 2009(n = 4,476) | Jan 2010–Jun 2018(n = 5,307) | p-value | |
|---|---|---|---|---|
| Age (years) | 6 (0 – 17) | 7 (0–17) | 7 (0 – 17) | 0.0005 |
| Age <1 year | 25.5% | 22.7% | 21.9% | 0.0003 |
| Male | 58.2% | 54.6% | 54.5% | 0.0009 |
| BMI (kg/m2) | 19.9 (16.1 – 30.0) | 20.9 (16.3 – 32.4) | 21.1 (16.4–31.7) | <0.0001 |
| PRA ≥20% | 6.7% | 15.1% | 23.8% | <0.0001 |
| PRA ≥80% | 1.3% | 5.1% | 5.9% | <0.0001 |
| History of malignancy | 3.2%a | 2.4% | 2.2% | 0.0653 |
| Pre-transplant dialysis | 1.9%a | 4.1% | 3.3% | 0.0002 |
| Bilirubin (mg/dl) | 0.8 (0.2 – 3.2)a | 0.7 (0.2 – 3.4) | 0.6 (0.2 – 2.9) | <0.0001 |
| Creatinine (mg/dl) | 0.6 (0.3 – 1.3)a | 0.5 (0.2 – 1.4) | 0.4 (0.2–1.1) | <0.0001 |
| GFR (ml/min/1.73 m2)c | 80.7 (39.6 – 127.2)a | 82.6 (39.4 – 138.8) | 94.0 (50.8 – 164.2) | <0.0001 |
| PVR (woods unit) | 3.0 (0.8 – 11.8)a | 3.0 (0.8 – 12.2) | 2.6 (0.6 – 11.8) | 0.0046 |
| Inotrope use | 42.2%a | 49.6% | 50.5% | <0.0001 |
| PGE use, age <1 yeard | 32.2%b | 11.6% | 8.7% | <0.0001 |
| ECMO use | 4.4%b | 8.6% | 4.4% | <0.0001 |
| MCS use: | ||||
| - None | - | 86.3% | 74.3% | <0.0001 |
| - VAD | - | 9.8% | 20.0% | |
| - TAH | - | 0.2% | 0.3% | |
| - BIVAD | - | 3.7% | 5.4% | |
| Ventilator use | 17.4% | 21.2% | 16.6% | <0.0001 |
| Hospitalized | 64.7% | 69.3% | 72.2% | <0.0001 |
BMI, body mass index; PRA, panel reactive antibody; GFR, Glomerular Filtration Rate; PVR, pulmonary vascular resistance; PGE, prostaglandin; ECMO, extracorporeal membrane oxygenation; MCS, mechanical circulatory support; VAD, ventricular assist device; TAH, total artificial heart.
Summary statistics excluded transplants with missing data.
Continuous factors are expressed as median (5th – 95th percentiles).
Comparisons for categorical variables were made using the chi-square statistic.
Comparisons for continuous variables were made using the Wilcoxon test.
Based on April 1994 – December 2000 transplants.
Based on April 1995 – December 2000 transplants.
GFR was estimated using the Cockcroft-Gault formula.
Percentage was calculated using recipients <1 year with known PGE use as the denominator.
The median recipient age was 6 years in the 1990s and increased to 7 years during 2001 to 2018, in part because infants <1-year old represent a lower proportion (22%) of pediatric recipients in the 2010s compared to the 1990s (25.5%) (Table 1). The median recipient age has been consistently lower in North America compared to Europe and Other regions since the 1990s (eSlide H[p] 11). This may be explained by a relatively higher percentage of infant recipients <1-year old in North America (~30%) compared to Europe (~10%) and Other regions of the world (<5%).1 The sex distribution among pediatric recipients has become more equal during the last 3 decades as the percentage of male recipients has decreased from 58.2% during 1992 to 2000 to 54.5% of recipients during 2010 to 2018 (Table 1). Irrespective of the region or era, the donor and the recipient were sex-matched in approximately 50% of all transplants, suggesting perhaps that donor and recipient sex-matching is not an important consideration in pediatric heart transplantation (eSlide H[p] 10). There was an increase in the recipient median body mass index (BMI) from 19.9 kg/m2 in the 1990s to 21.1 kg/m2 during 2010 to 2018 (Table 1), in part because of an increase in median age, since older children tend to have a higher BMI. Notably, however, the median BMI of pediatric recipients in North America has been consistently higher than that of recipients in Europe and Other regions despite a lower median age (eSlide H[p] 11). This finding is probably related to the well-documented childhood obesity epidemic in the United States, where children with congenital or acquired heart disease have similar rates of overweight and obesity compared to age-matched peers.5
The percentage of recipients with blood type O has increased from 41% in the 1990s to 44% in the most recent era, those with blood type B has increased from 11% to 14%, and the percentage of recipients with blood type A has decreased from 43% to 38% (eSlide H[p] 4). The trends in recipient blood type over this time frame vary by region, and the differences are probably due to changes in the population distribution of blood types in each region (eSlide H[p] 8)
Children receiving heart transplants in the most recent era were sicker than in the earlier eras. The percentage of recipients hospitalized at the time of transplant increased from 65% during 1992 to 2000 to 72% during 2010 to 2018, while those on inotropes at the time of transplant increased from 42% to 51% during the same time (Table 1). The percentage of recipients with previous cardiac surgery increased from 29% during 1992 to 2000 to 55% during 2010 to 2018 (eSlide H[p] 5), with a decrease in the percentage of infants supported on prostaglandin infusion from 32% to 9% during this time (Table 1). The percentage of recipients supported on a ventricular assist device increased from 13.5% during 2001 to 2009 to 25% during 2010 to 2018. The increased use of ventricular assist devices may explain some of the other trends over time in recipient characteristics, such as changes in sensitization, cardiac hemodynamics, and end-organ function. For example, the percentage of children with a panel of reactive antibodies (PRA) ≥20% increased from 7% during 1992 to 2000 to 24% during 2010 to 2018; 6% of recipients had PRA ≥80% during 2010 to 2018 (Table 1). In contrast, there has been a gradual decline in median pulmonary artery pressure, median pulmonary artery wedge pressure, and median pulmonary vascular resistance over the same time (eSlide H[p] 6). The median estimated glomerular filtration rate and median total bilirubin have also improved in successive eras (Table 1). It is notable, however, that despite widespread use of durable ventricular assist devices, 17% of all recipients during 2010 to 2018 were still supported on a ventilator, 4.4% were supported by extracorporeal membrane oxygenator (ECMO), and 3.3% were on dialysis at the time of transplant.
There were regional differences in the distribution of dilated cardiomyopathy, congenital heart disease, and re-transplantation as indications for transplant (Figure 1 and eSlide H[p] 9). In Europe, >50% of pediatric recipients had heart transplantation for dilated cardiomyopathy, and only ~25% of recipients had congenital heart disease as their diagnosis. In contrast, dilated cardiomyopathy and congenital heart disease were equally prevalent diagnoses in transplant recipients in North America, each in ~40%. In Other regions of the world, dilated cardiomyopathy was the most prevalent diagnosis where it was the diagnosis in >70% of transplant recipients in 2010s whereas <10% of all recipients had congenital heart disease. These regional differences are partly due to infants <1-year old contributing a much higher percentage of recipients in North America, an age group for which congenital heart disease is the most common diagnosis. These differences may in turn be due to regional difference in the availability of donor hearts for infants, and perhaps due to regional differences in clinical approach to complex congenital heart disease in infants. Another potential factor may be that dilated cardiomyopathy is usually the primary indication for heart transplantation at new centers, which may explain the preponderance of such patients in Other regions of the world with many newer centers.
Figure 1.
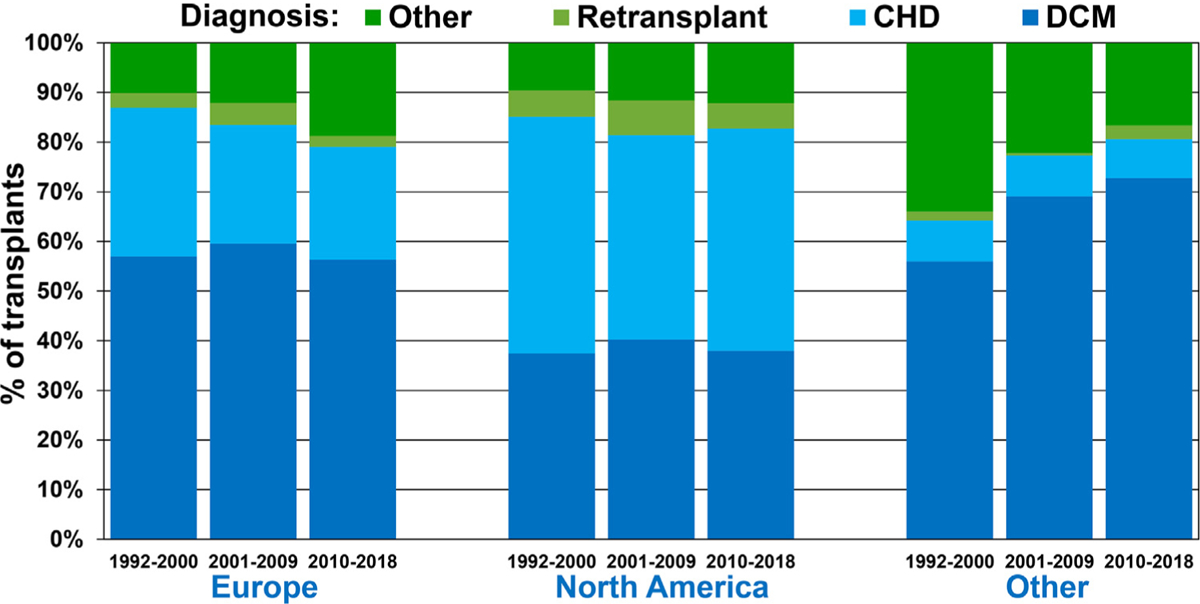
Recipient diagnosis by location and era (January 1992-June 2018).
Survival analysis
One-year survival
Overall, 1-year post-transplant survival improved from 87% during 2000 to 2005 to 92% during 2012 to 2017 (eSlide H[p] 14). We examined the associations between recipient characteristics and 1-year survival during 2000 to 2017, focusing on how 1-year survival has changed in recipients stratified by specific characteristics. In all regions, overall 1-year survival among pediatric recipients was highest during 2012 to 2017 (Figure 2 and eSlide H[p] 15), however, the improvement did not reach statistical significance in recipients in Europe or Other regions, likely due to low statistical power. Stratified by recipient age <1 year, 1 to 10 years, and 11 to 17 years, there was improved 1-year survival during 2000 to 2017 in all age groups (Figure 3 and eSlide H[p] 17). Among these recipients, 1-year survival was lowest in infant recipients <1-year old and highest in children 11 to 17 years old. One-year survival in recipients transplanted during 2012 to 2017 was 89% in infants <1-year old, 92% in children 1 to 10 years old, and 94% in children 11 to 17 years old. One-year survival, stratified by region and recipient age, is illustrated on eSlide H[p] 16).
Figure 2.
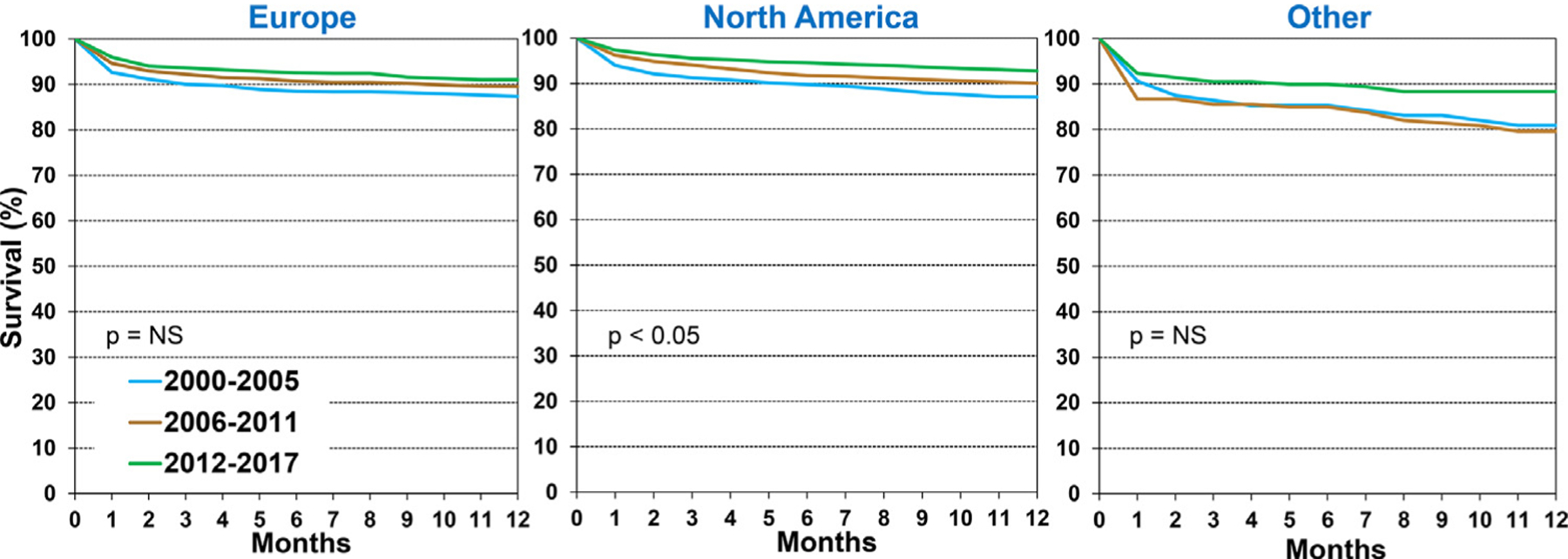
Kaplan-Meier survival within 12-months by location and era (January 2000-June 2017).
Figure 3.
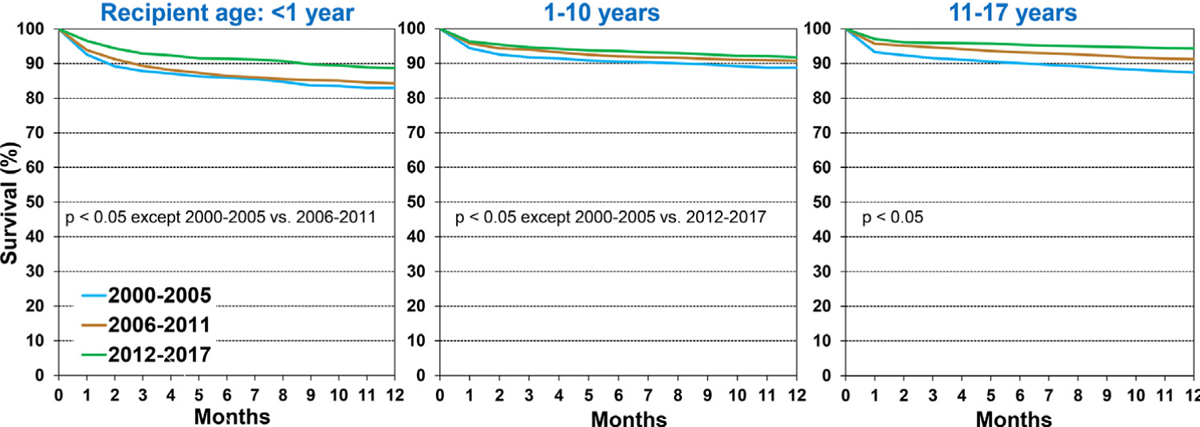
Kaplan-Meier survival within 12-months by recipient age and era (January 2000-June 2017).
Recipients transplanted for dilated cardiomyopathy and congenital heart disease in 2012 to 2017 had higher survival compared to those transplanted in previous eras. Recipients of re-transplantation in the 2012 to 2017 era had a similar improvement in survival, although the difference with previous eras did not reach statistical significance, likely due to a smaller sample size (Figure 4 and eSlide H[p] 18). One-year survival among those transplanted in 2012 to 2017 was 95%, 88% and 91% in recipients with a diagnosis of dilated cardiomyopathy, congenital heart disease and re-transplantation, respectively. In an analysis stratified by mechanical circulatory support at transplant, 1-year survival improved significantly in recipients supported with a durable ventricular assist device/total artificial heart, and in those not requiring any mechanical circulatory support, but not among those supported on ECMO (eSlide H[p] 19). When stratified by kidney function, a significant improvement in 1-year survival was demonstrable only among children with estimated glomerular filtration rate >60 ml/min/ 1.73m2. Although recent recipients with chronic kidney disease (those with estimated glomerular filtration rate <30 and 30–60 ml/min/1.73m2) appeared to have better outcomes compared to earlier-era recipients, this improvement did not reach significance (eSlide H[p] 20). One-year survival was lower among transplant recipients on dialysis at the time of transplant vs those not on dialysis (eSlide H[p]21), and higher among those who received a transplant with a past history of malignancy (vs. the remaining recipients), probably because recipients with a history of malignancy had chemotherapy-induced cardiomyopathy whereas the remaining patients formed a heterogeneous group that included higher-risk recipients with congenital heart disease (eSlide H[p] 21).
Figure 4.
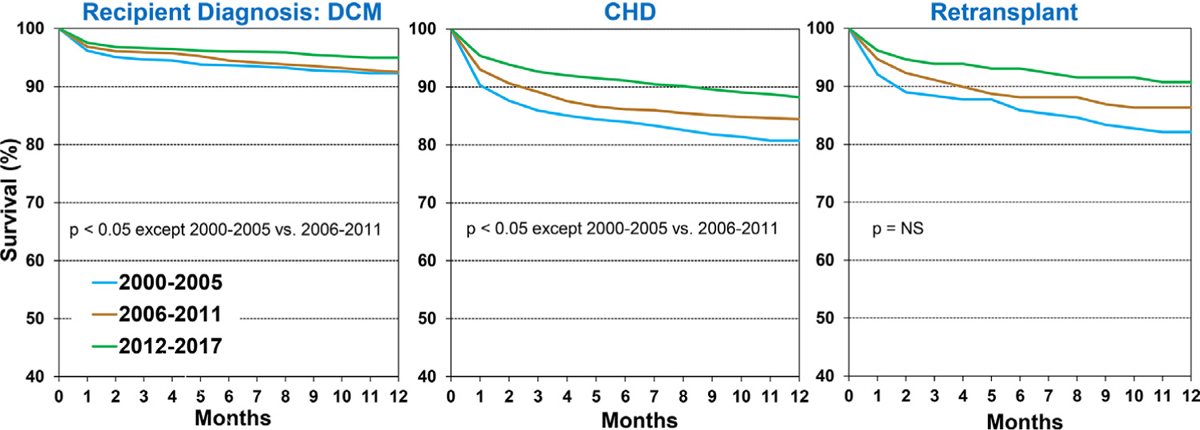
Kaplan-Meier survival within 12-months by recipient diagnosis and era (January 2000-June 2017).
Five-year survival conditional on surviving to 1 year
We next assessed how 5-year survival in recipients who survived the first post-transplant year has changed over time. There was no difference in 5-year conditional survival between recipients who were transplanted during 1996 to 2001 (87%) and 2002 to 2007 (88%); however, 5-year conditional survival in pediatric recipients transplanted during 2008 to 2013 (90.5%) was significantly higher (Figure 5 and eSlide H[p] 23). When transplant recipients were stratified by region, 5-year conditional survival improved significantly only in North America during this time period (eSlide H[p] 24). Stratified by region and age, in both North America and Europe, 5-year conditional survival was higher in transplant recipients 1 to 10 years old compared to recipients 11 to 17 years old (eSlide H[p] 25). Conditional survival in North America among infant recipients <1-year old was almost identical to those 1 to 10 years old (eSlide[p] 25).
Figure 5.
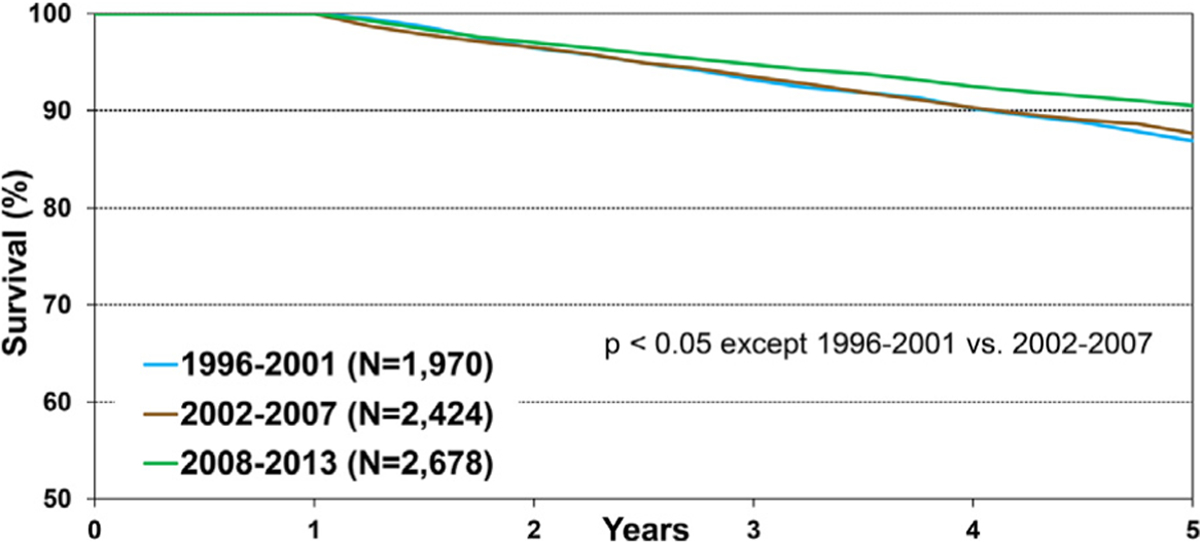
Kaplan-Meier survival within 5 years conditional on survival to 1 year by era (January 1996-June 2013).
Overall, 5-year conditional survival improved in all 3 age groups (<1 year, 1–10 years, and 11–17 years) when compared between 1996 to 2000, 2002 to 2007, and 2008 to 2013, albeit at different rates (Figure 6 and eSlide H[p] 26). Among transplant recipients of the most recent era evaluated (2008–2013), 5-year conditional survival was 91.2% in infant recipients <1-year-old, 92.3% in recipients 1 to 10 years old, and 88% in recipients 11 to 17 years old at transplant. The lower conditional survival in older, adolescent recipients may be explained by a potentially higher prevalence of medication non–adherence in this age group. Interestingly, 5-year conditional survival improved over time in recipients with a diagnosis of dilated cardiomyopathy but not in those with a diagnosis of congenital heart disease (Figure 7 and eSlide H[p] 27). Furthermore, 5-year conditional survival improved over time in recipients with a GFR≥60 ml/min/1.73 m2 but not in other GFR groups (eSlide H[p] 28). These findings may suggest that the improvement in conditional survival has been uneven across recipients with different baseline risk profiles; however, these comparisons are not adjusted for other factors. Five-year conditional survival was not different between recipients stratified by dialysis at transplant or history of malignancy (eSlide H[p] 29).
Figure 6.
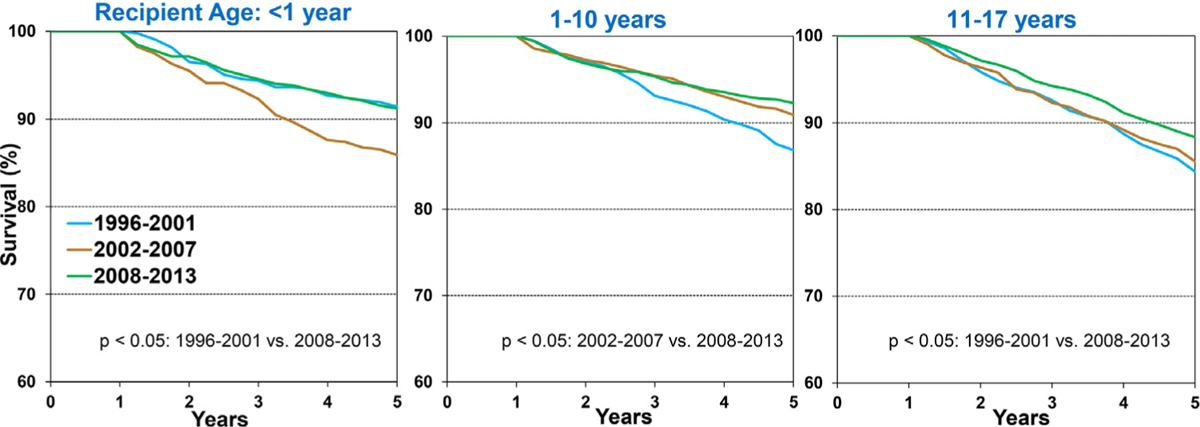
Kaplan-Meier survival within 5-years conditional on survival to 1 year by recipient age and era (transplants: January 1996-June 2013). (slide 26)
Figure 7.
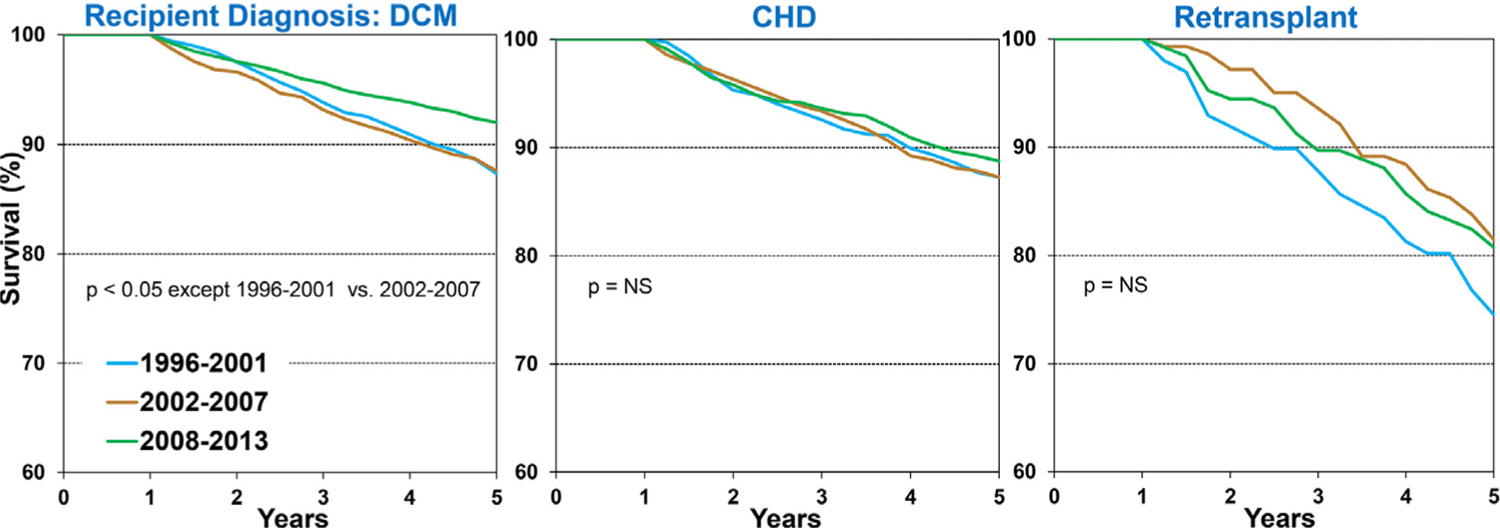
Kaplan-Meier survival within 5-years conditional on survival to 1 year by recipient diagnosis and era (transplants: January 1996-June 2013). (slide 27)
Freedom from CAV
We examined trends in freedom from CAV in children who survived the initial transplant hospitalization, and explored whether these trends differed among recipients stratified by specific characteristics. Overall, there was no difference in freedom from CAV among pediatric recipients who underwent a transplant during 1996 to 2001, 2002 to 2007, and 2008 to 2013 (Figure 8 and eSlide H[p] 18). Furthermore, no improvement in freedom from CAV is seen in recipients when stratified by age (eSlide H[p] 32) or diagnosis (eSlide H[p] 33).
Figure 8.
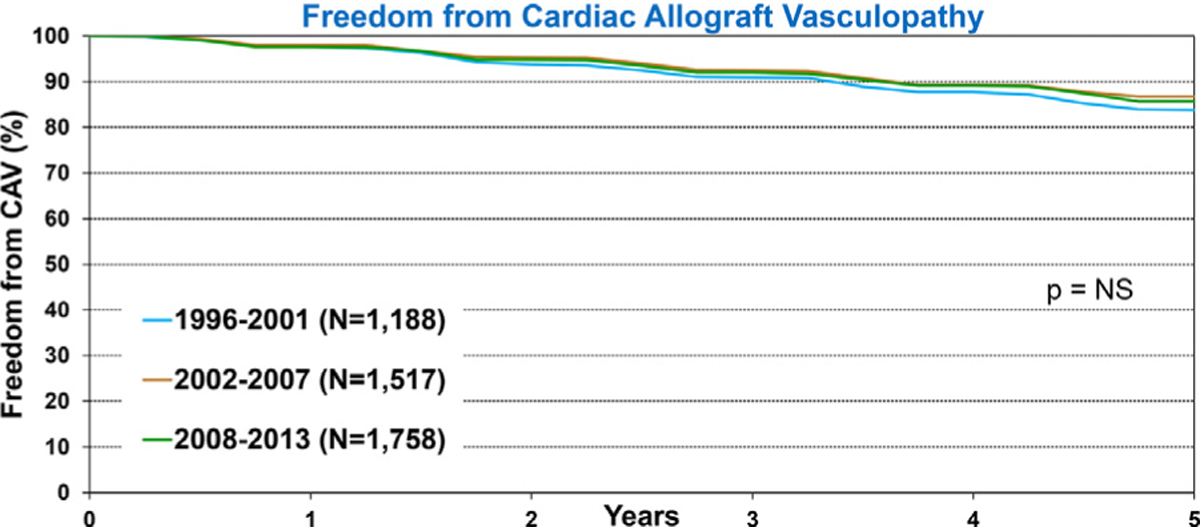
Freedom from CAV conditional on survival to discharge by era (transplants: January 1996-June 2013).
Multivariable analyses
We next performed multivariable Cox regression analyses to evaluate risk factors for 1-year mortality, 5-year mortality conditional upon surviving the first post-transplant year, and risk of developing CAV conditional on survival to discharge. Covariates included in the multivariable models are listed in Supplemental Table 1. Statistically significant categorical risk factors associated with higher 1-year mortality in transplant recipients between 2000 and 2017 were female sex, a diagnosis other than dilated cardiomyopathy (congenital heart disease, re-transplant, or other), prior cardiac surgery, extracorporeal membrane oxygenation support, ventilator support, and cerebrovascular accident (vs head trauma) as the donor cause of death (Figure 9 and eSlide H[p] 36). Although durable ventricular assist device support was not an independent risk factor for 1-year mortality (vs no mechanical circulatory support), device implantation was captured in the broader category of prior cardiac surgery, which was a mortality risk factor. Continuous risk factors associated with higher 1-year mortality were younger recipient age, with age <1 year associated with the highest risk (Figure 10 and eSlide H[p] 39), kidney disease (with progressively higher risk associated with lower glomerular filtration rate) (Figure 11A and eSlide H[p] 41), hepatic dysfunction assessed as elevated serum bilirubin (Figure 11B and eSlide H[p] 43), higher PRA (Figure 12 and eSlide H[p] 44), longer allograft ischemic time (Figure 13 and eSlide H[p] 45), and lower transplant center volume during the preceding 3 years (Figure 14 and eSlide H[p] 46). We explored interactions of era of transplant with recipient diagnosis (eSlide H[p] 37), age (eSlide H[p] 40) and glomerular filtration rate (eSlide H[p] 42) for 1-year mortality, and found a borderline significant era effect (p=0.06), and no other significant interactions, suggesting that the improvement in 1-year survival over time has not been modified by these recipient factors.
Figure 9.
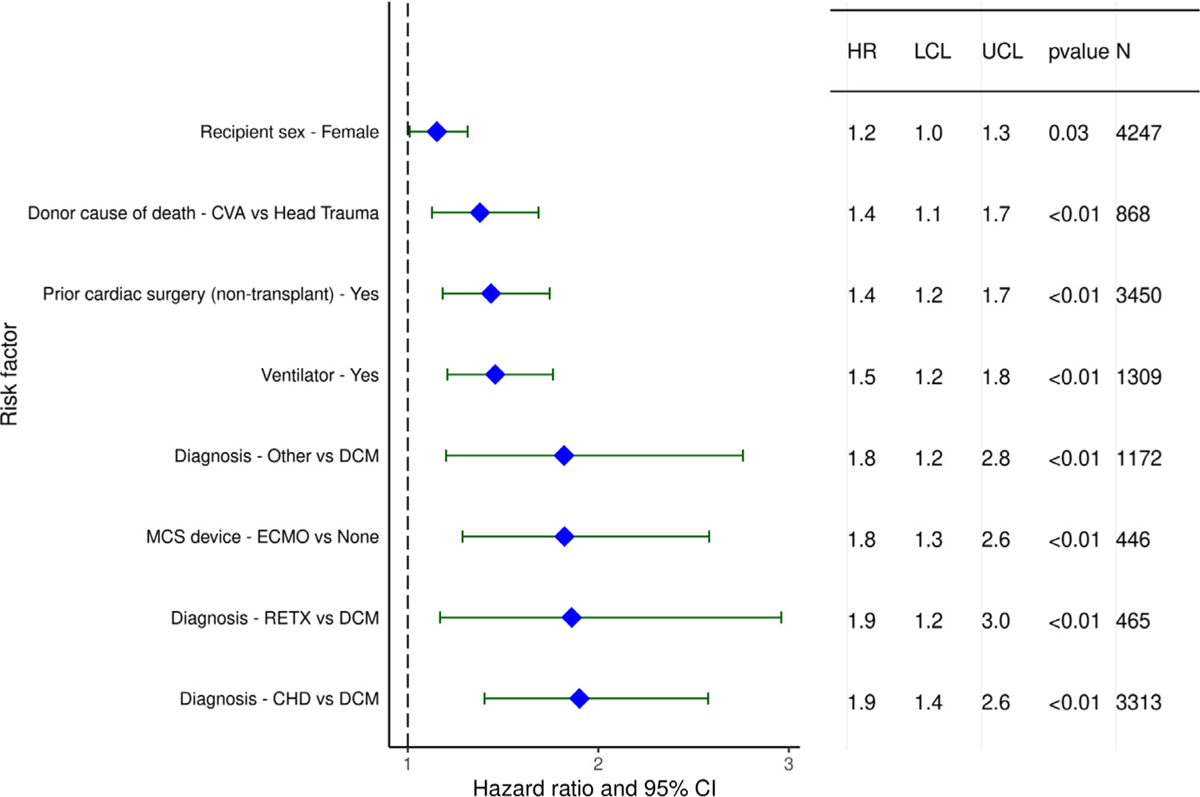
Statistically significant categorical risk factors for 1-year mortality with 95% confidence limits (January 2000-June 2017, n = 9,376).
Figure 10.
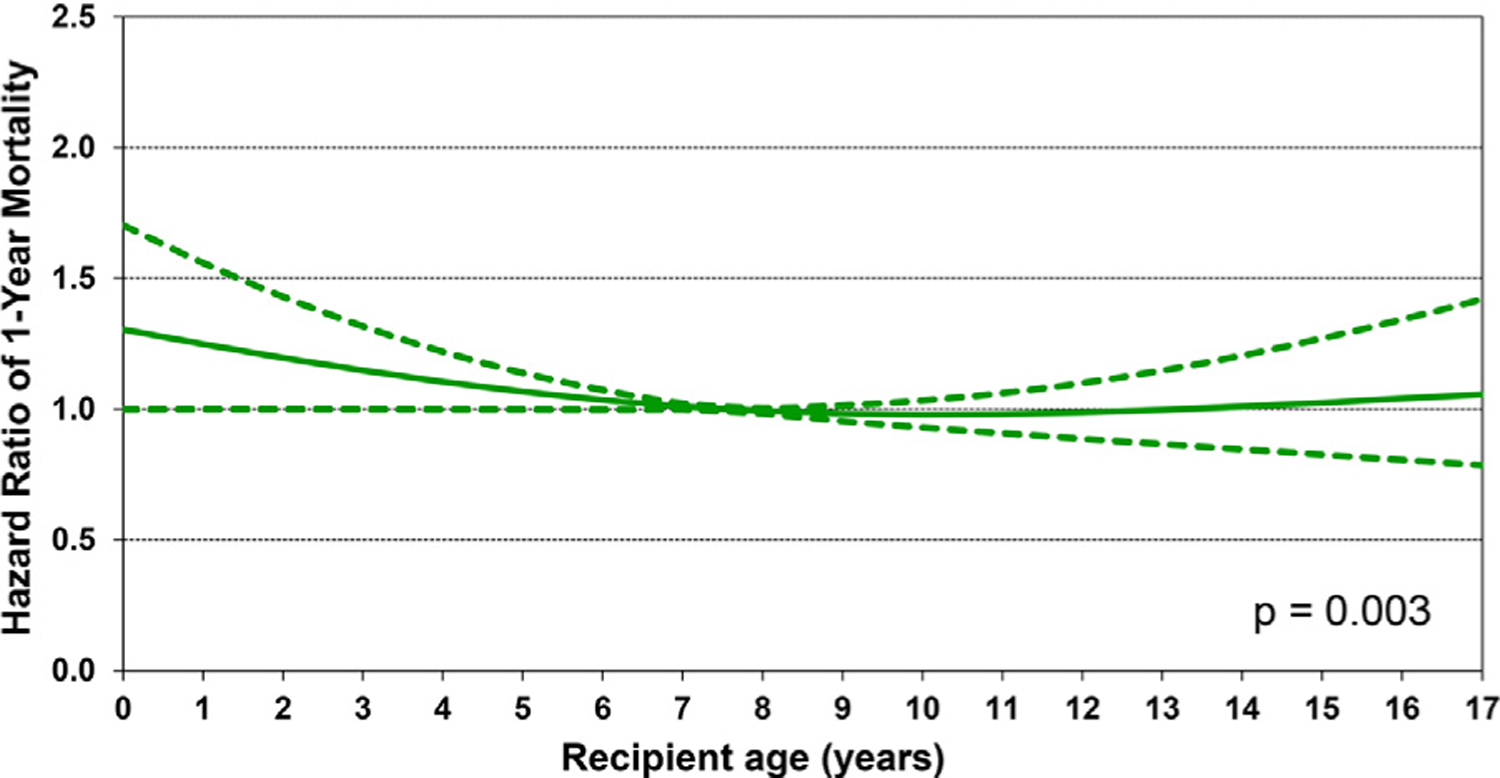
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient age (January 2000-June 2017; n = 9,376).
Figure 11.
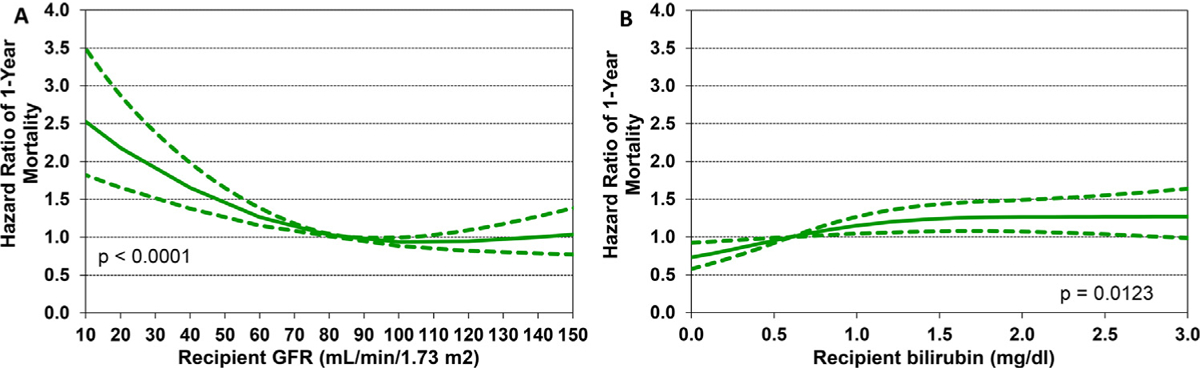
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient (A) glomerular filtration rate (GFR) and (B) bilirubin (January 2000-June 2017; n = 9,376). GFR was estimated using the modified Schwartz formula.
Figure 12.
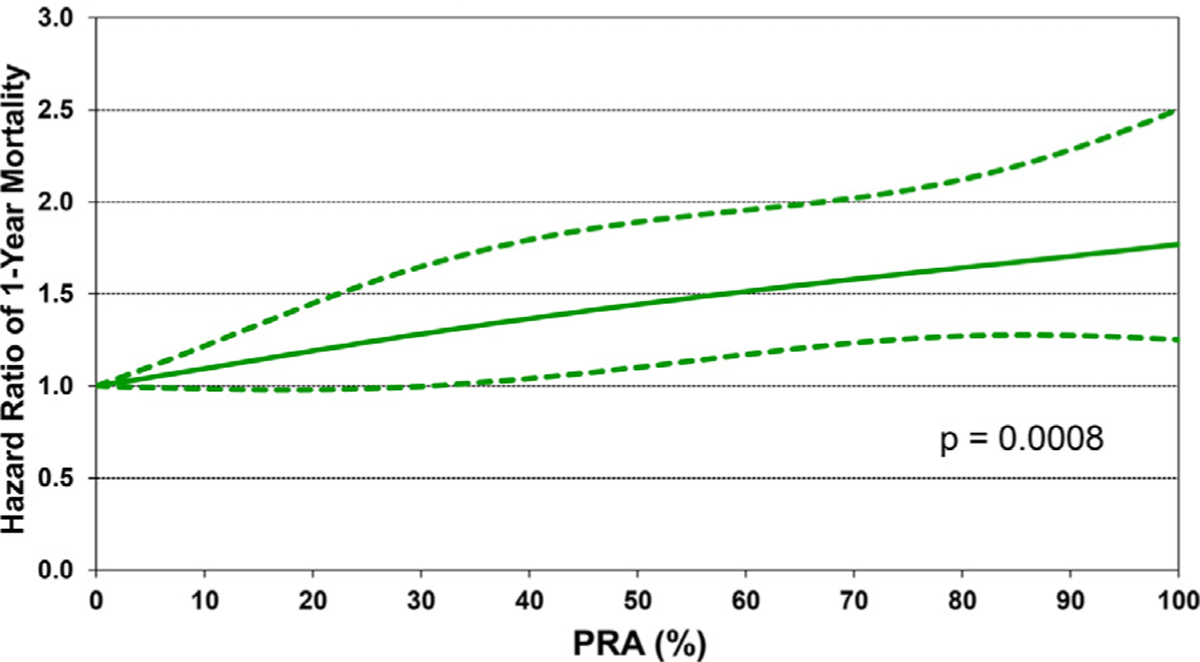
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by PRA (January 2000-June 2017; n = 9,376).
Figure 13.
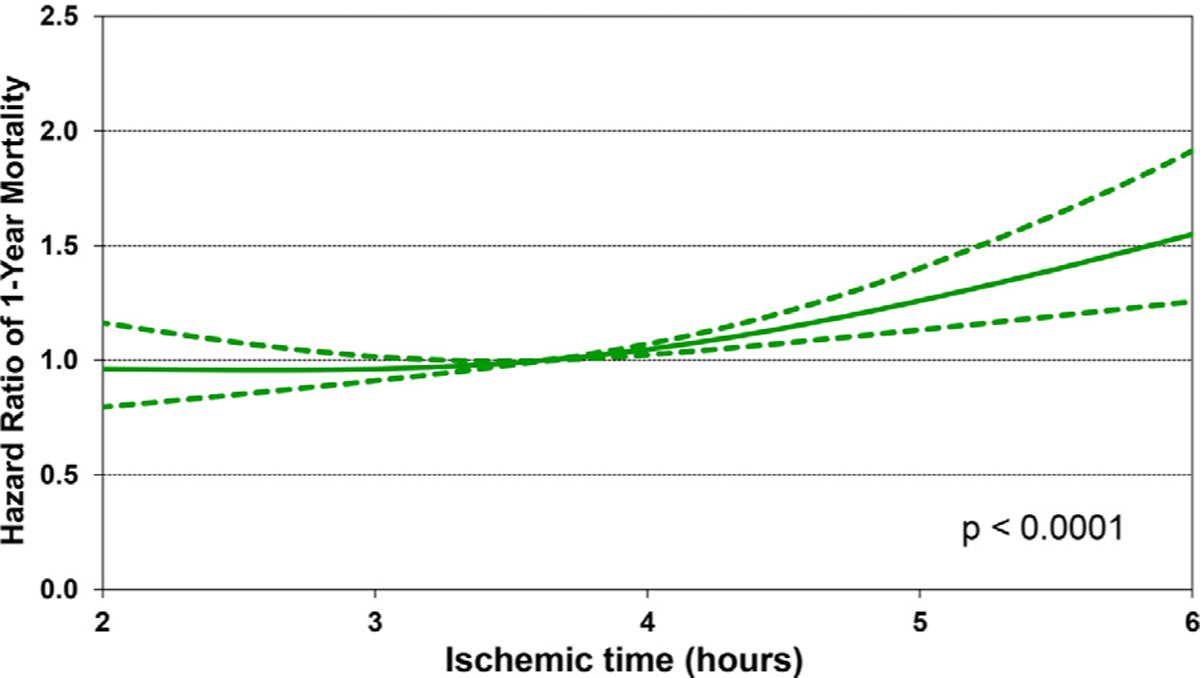
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by ischemic time (January 2000-June 2017; n = 9,376).
Figure 14.
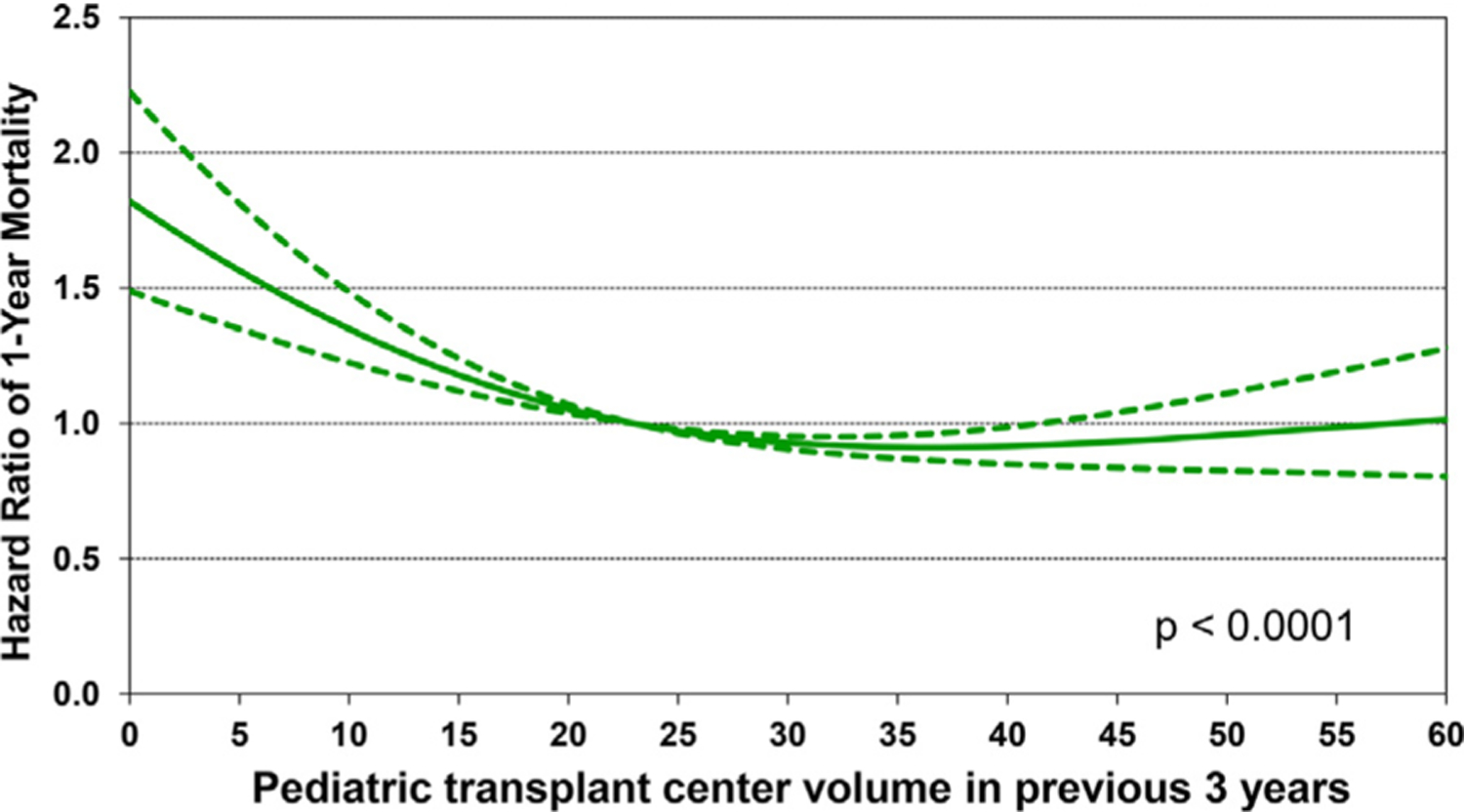
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by center volume in the previous 3 years (January 2000-June 2017; n = 9,376).
Recipient variables associated with higher 5-year mortality, conditional upon surviving the first year, included female sex, diagnosis of re-transplantation, and prior cardiac surgery (Figure 15 and eSlide H[p]48). Earlier era was a risk factor; however, the interaction of era of transplant with cardiac diagnosis was not significant (eSlide H[p] 49). Continuous variables associated with conditional 5-year mortality included recipient age (with a U-shaped relationship between hazard ratio and age at transplant, Figure 16 and eSlide H[p] 51) and higher PRA at transplant (Figure 17 and eSlide H[p] 53). The persistence of younger age as a risk factor for mortality beyond the first post-transplant year, in particular for infants <1year old, suggests that the purported immunologic advantage associated with this age, which eventually results in the best median survival (~24.5 years) in heart transplant recipients of any age group, takes longer than 5 years to manifest.1 The risk associated with older (adolescent) age may be mediated by medication noncompliance, which is more common in this age group. Furthermore, many adolescents receive hearts from adult donors which may increase their risk of developing CAV.
Figure 15.
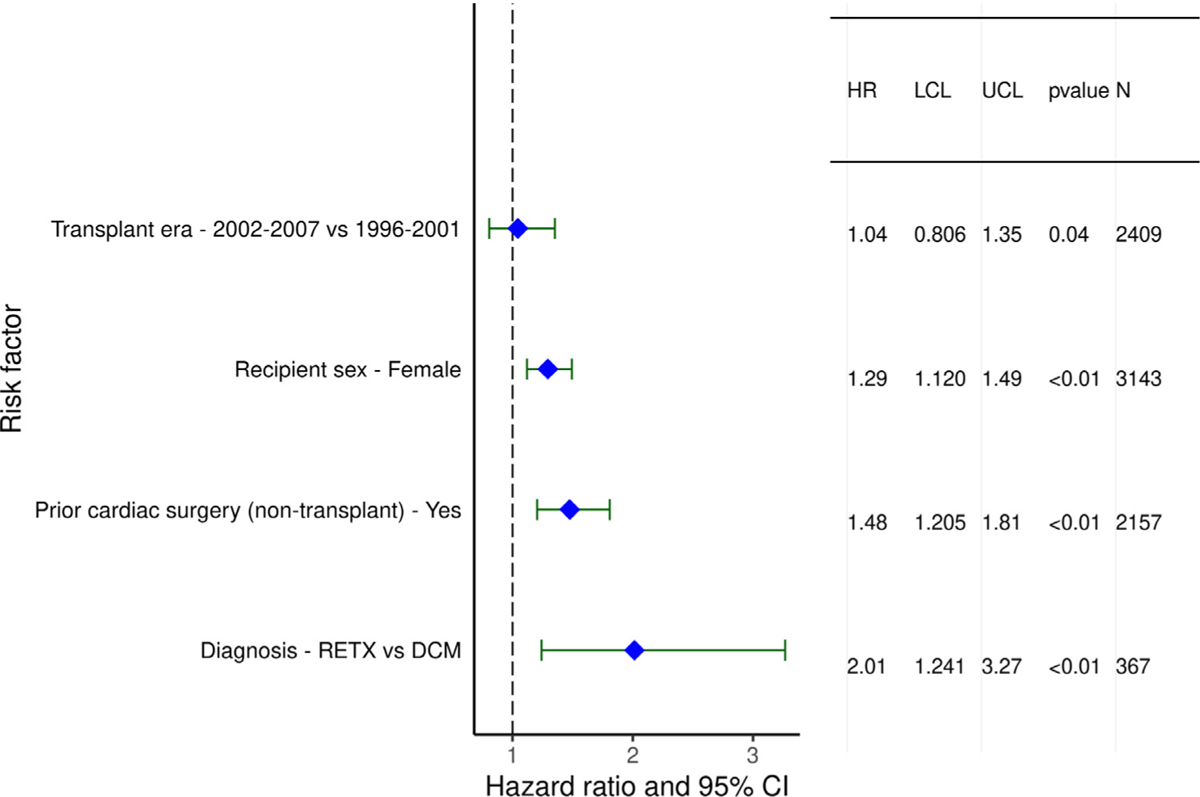
Statistically significant categorical risk factors for 5-year mortality conditional on survival to 1 year with 95% confidence limits (January 1996-June 2013; n = 7,022).
Figure 16.
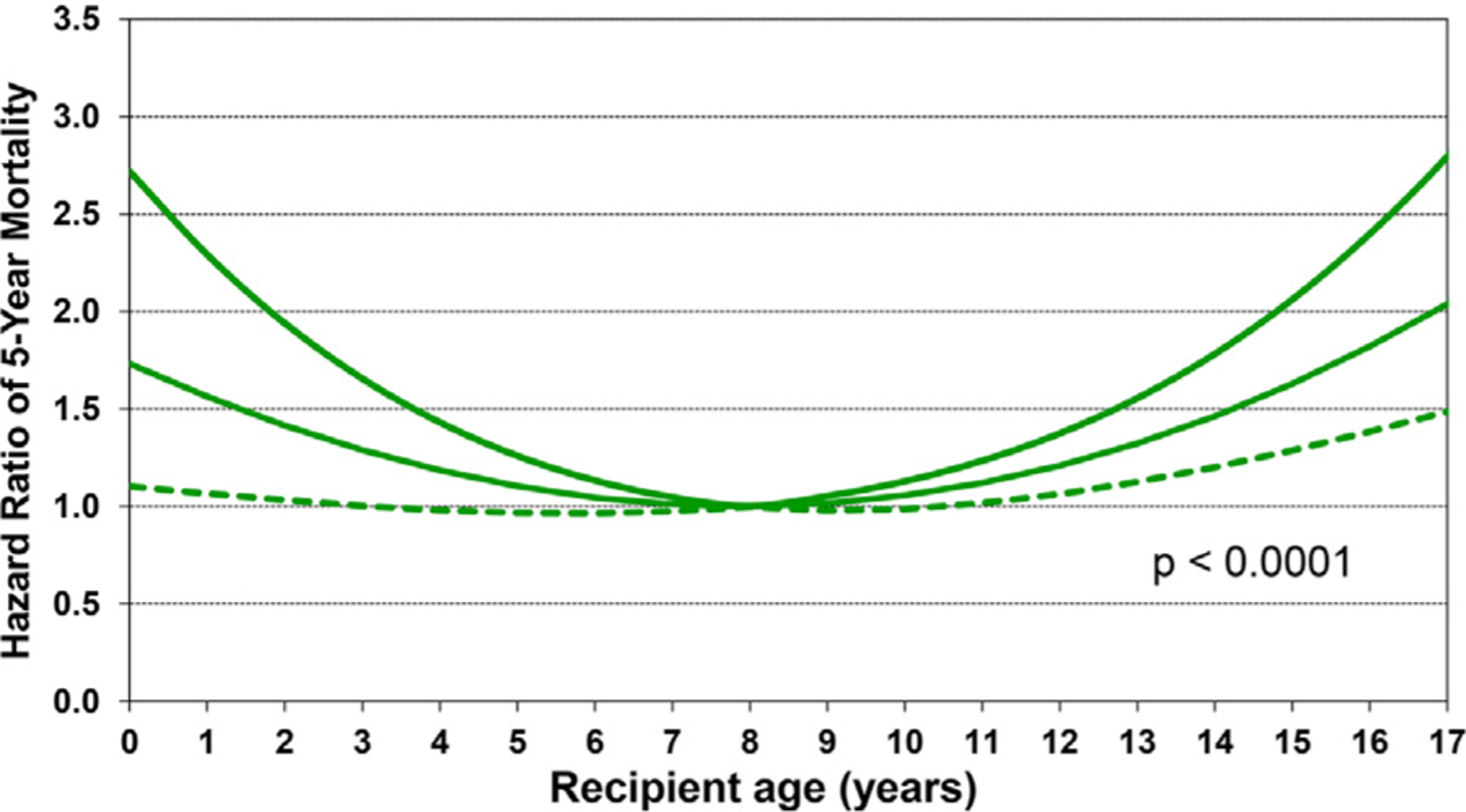
Multivariable hazard ratio plot for 5-year mortality conditional on survival to 1 year with 95% confidence limits, by recipient age (January 1996-June 2013; n = 7,022).
Figure 17.
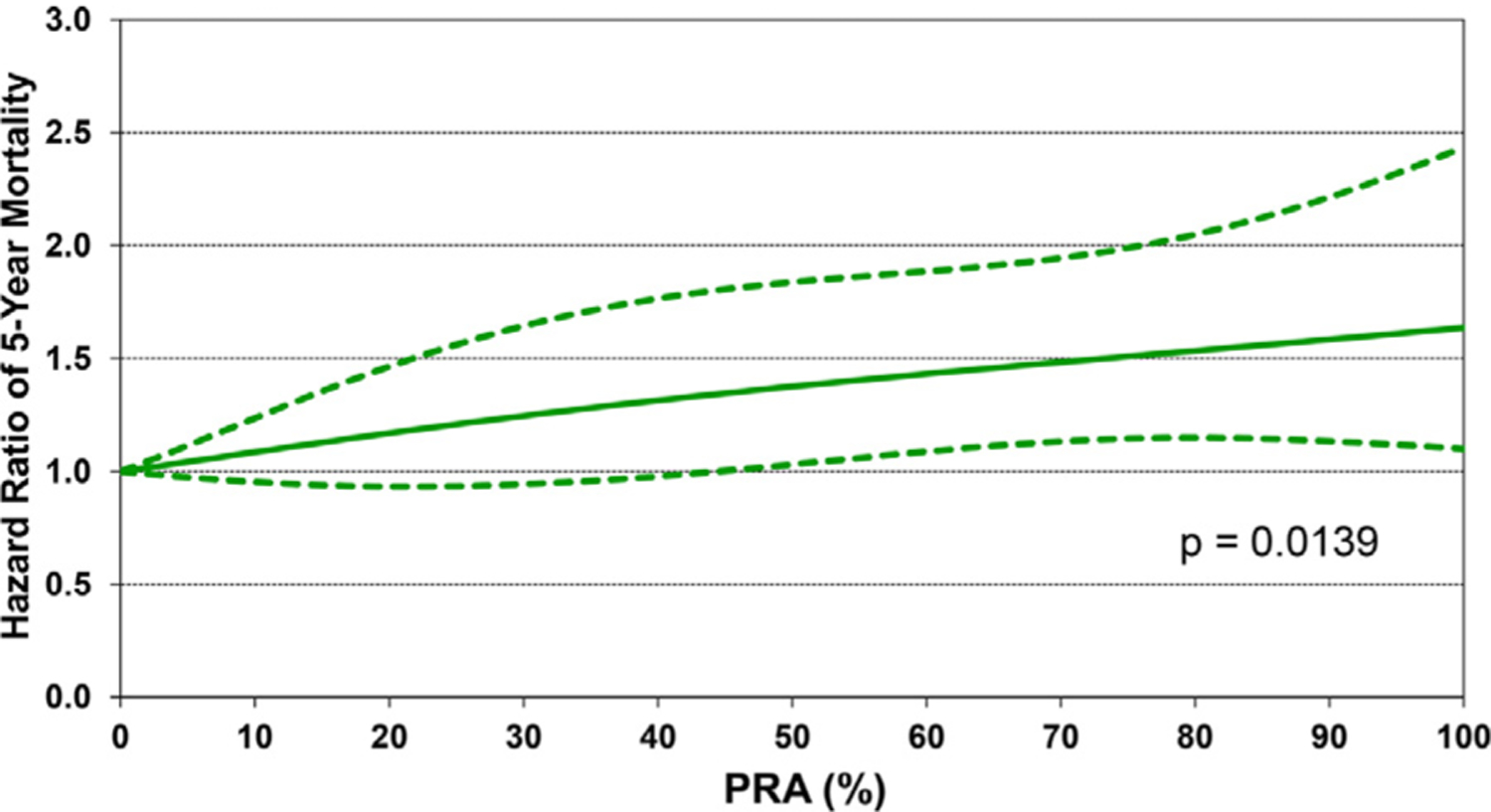
Multivariable hazard ratio plot for 5-year mortality conditional on survival to one year with 95% confidence limits, by recipient PRA (January 1996-June 2013; n = 7,022).
Statistically significant variables associated with developing CAV during the first 5 years after transplant in recipients who survived to hospital discharge were similar to risk factors for 5-year conditional mortality and included categorical variables of transplant in an earlier era, female sex, re-transplantation, and prior non–transplant cardiac surgery (Figure 18 and eSlide H[p] 55), as well as continuous variables such as older age (Figure 19 and eSlide H[p] 57) and higher PRA (eSlide H[p] 58). The association with higher PRA may be due to an increased risk of antibody-mediated rejection in such patients. Being supported on a ventilator at transplant was associated with lower risk CAV, however, this association is difficult to explain and is likely a proxy for an unmeasured confounder.
Figure 18.
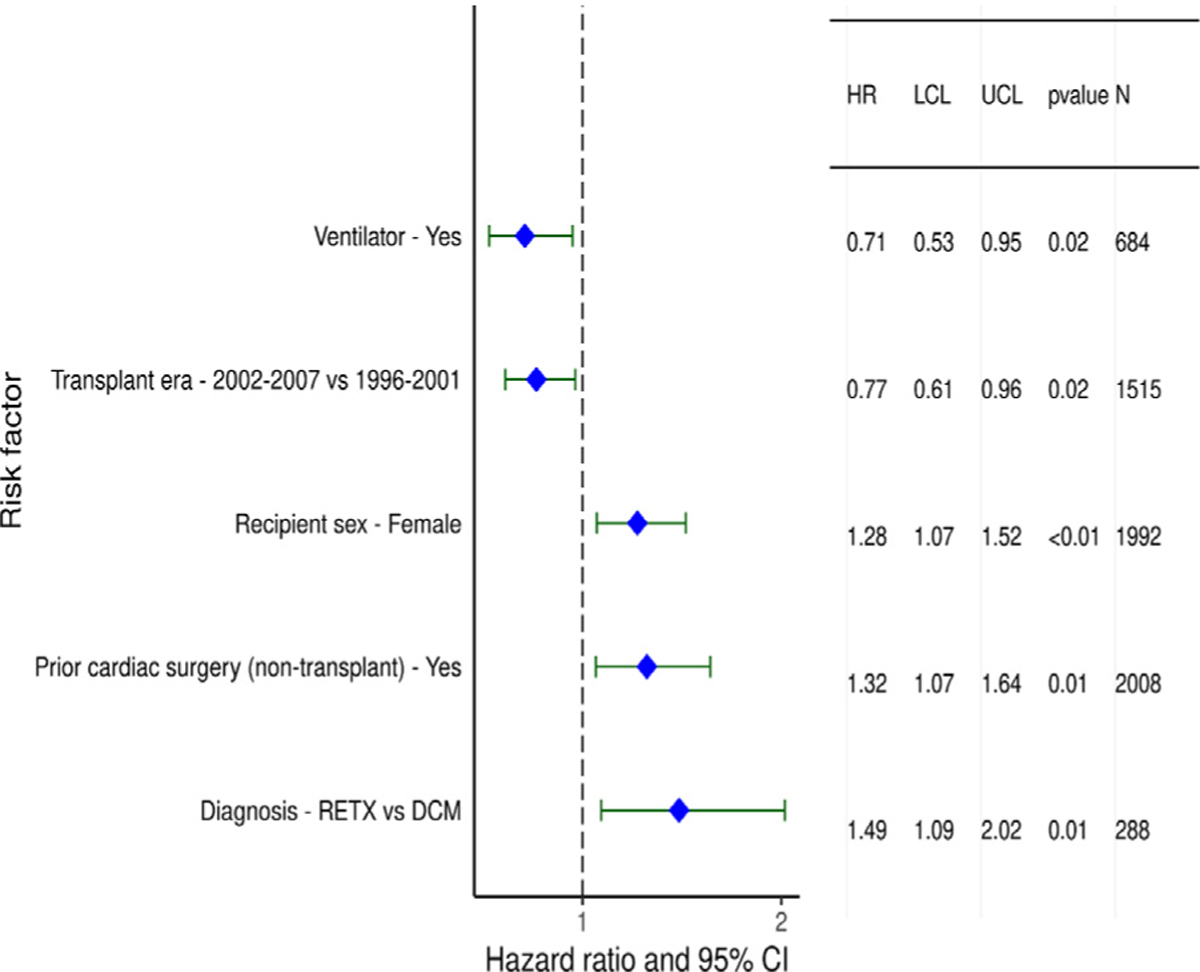
Statistically significant categorical risk factors for CAV conditional on survival to discharge with 95% confidence limits (January 1996-June 2013; n = 4,455).
Figure 19.
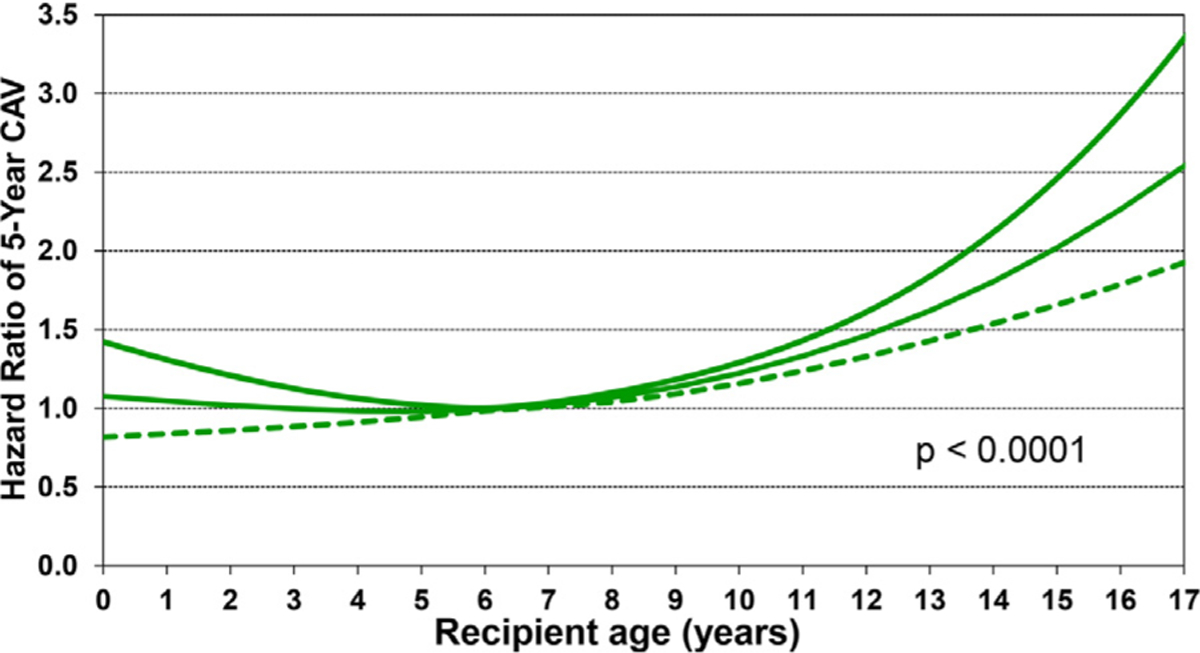
Multivariable hazard ratio plot for 5-year CAV conditional on survival to discharge with 95% confidence limits, by recipient age (January 1996-June 2013; n = 4,455).
Conclusion
In this focused report, we present changes in baseline characteristics over the past 3 decades in pediatric heart transplant recipients. The median pediatric recipient age has increased over time, primarily because more transplants are being performed in children >1-year-old. Despite the widespread use of durable ventricular assist devices in the current era, over two-thirds of pediatric recipients receive a transplant while hospitalized, a majority while supported on inotropes, and 1 in 6 while supported on a ventilator. The optimal care of these complex patients requires dedicated multidisciplinary teams, including those with specific medical and surgical expertise. It is heartening to know that their efforts have made a difference, as both unadjusted and adjusted survival have improved. As described previously in ISHLT reports, immunosuppressive protocols have also changed significantly during these years and have likely contributed to improved post-transplant outcomes.1
We present multivariable models for 1-year mortality, 5-year mortality conditional upon surviving 1 year, and risk of developing CAV conditional upon survival to discharge. In the 2009 ISHLT Registry report, Taylor et al made a key observation that “the simple act of identifying a potential risk factor may have an immediate effect upon its future predictive ability as clinicians quickly modify behavior in regard to the identified risk factor. This may eventually (and hopefully) lead to neutralization of the risk factor.”6 It may also be that some of the associations identified are a proxy for risk factors that are captured differently in subsequent analyses. For example, previous Registry reports identified variables associated with 1-year mortality such as being hospitalized before transplant, or transfusion before transplant, that are not in the current model but are likely captured differently as markers of heart failure severity and congenital heart disease.7,8 For this report, we used several interaction terms to explore whether recipients with specific characteristics had improvements in survival to a different degree across transplant eras, but these interactions were not significant, which suggests that improved outcomes are due to broad advances in transplant expertise and systems of care rather than mostly identification and mitigation of specific high risk targets.
The ISHLT Thoracic Registry remains the largest resource of patient data for thoracic transplant recipients. In this annual report, we have addressed some questions of potential interest to the pediatric heart transplant community which we hope will stimulate discussion and research ideas within the community.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Lyna Cherikh, United Network of Organ Sharing Research Summer Intern, for her assistance with preparing the figures/table for the manuscript and reviewing the manuscript.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose. Daniel C. Chambers received travel support from Astellas Pharma, Inc, and serves as a consultant and speaker for Roche Ltd; Kiran K. Khush serves as a consultant and speaker for CareDx, Inc; Josef Stehlik serves as a consultant for Medtronic, received research support from Natera and received funding from ISHLT; Michael Perch receives research funding from Roche, travel support from Boeringer-Ingel-heim, and is a speaker for Mallinckrodt, Glaxo Smith Kline, and Astra-Zeneca; Wida S. Cherikh, Aparna Sadavarte, and Alice Toll received funding from ISHLT; Don Hayes, Jr.; Michael O. Harhay; Eileen Hsich; Luciano Potena; and Tajinder P. Singh do not have any relevant disclosures.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2021.07.022.
References
- 1.Rossano JW, Singh TP, Cherikh WS, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: twenty-second pediatric heart transplantation report 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 2010;362:1980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales DLS, Adachi I, Peng DM, et al. Fourth annual pediatric interagency registry for mechanical circulatory support (pedimacs) report. Ann Thorac Surg 2020;110:1819–31. [DOI] [PubMed] [Google Scholar]
- 4.Singh TP, Hsich E, Cherikh WS, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: 23rd pediatric heart transplantation report 2020; focus on deceased donor characteristics. J Heart Lung Transplant 2020;39:1028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele JM, Preminger TJ, Erenberg FG, et al. Obesity trends in children, adolescents, and young adults with congenital heart disease. Congenit Heart Dis 2019;14:517–24. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the international society for heart and lung transplantation: twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant 2009;28:1007–22. [DOI] [PubMed] [Google Scholar]
- 7.Boucek MM, Edwards LB, Keck BM, Trulock EP, Taylor DO, Hertz MI. Registry of the international society for heart and lung transplantation: eighth official pediatric report–2005. J Heart Lung Transplant 2005;24:968–82. [DOI] [PubMed] [Google Scholar]
- 8.Dipchand AI, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: seventeenth official pediatric heart transplantation report–2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:985–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


