This 39th annual international adult heart transplant report focuses on transplantation for heart disease with restrictive physiology, that is, restrictive cardiomyopathies and hypertrophic cardiomyopathy, based on data submitted to the International Society for Heart and Lung Transplantation (ISHLT) Thoracic Organ Transplant (TTX) Registry between January 1992 and June 2018. Restrictive cardiomyopathy (RCM) refers to a heterogeneous group of cardiomyopathies that includes amyloid, sarcoid, radiation and/or chemotherapy-induced cardiomyopathy, and other types of heart disease that all share restrictive physiology, and reduced or normal diastolic and systolic volumes in 1 or both ventricles.1 Hypertrophic cardiomyopathy (HCM) is classified separately according to the World Heart Federation and MOGE(S) classification but will be included in this report because RCM and HCM share restrictive physiology, and may represent a different phenotypic expression of the same genotype (i.e., autosomal dominant inherited mutations in the desmin, alpha B crystalline, alpha-actinin 2, or beta-myosin heavy chain 7 genes).2
The ISHLT Registry is currently updating the processes involved in data acquisition from contributing transplant centers and collectives. Therefore, the source data utilized for this report are the same as in the 2019 to 2021 annual reports, including 108,034 adult heart transplant recipients, with 4,306 who underwent a transplant for restrictive physiology.3–5 Further information regarding a detailed description of the entire cohort and additional core analyses not related to this year’s focus theme are available in the 2019 annual ISHLT report for adult heart transplantation.3
Statistical methods
Data collection, conventions, and statistical methods
The 2022 ISHLT International TTX Registry report, as in past years, was developed using data submitted to the Registry from national and multinational transplant collectives and individual transplant centers. Since the Registry’s inception, 481 adult heart transplant centers have contributed approximately 3-quarters of the worldwide thoracic transplant activity.
This report focuses on adult heart transplant recipients transplanted for restrictive physiology, and includes HCM, amyloidosis, sarcoidosis, radiation and/or chemotherapy-induced cardiomyopathy, and “other” restrictive cardiomyopathies such as endocardial fibrosis. The estimated glomerular filtration rate was calculated using Cockcroft-Gault formula. Baseline characteristics at the time of transplant and their associations with post-transplant outcomes were compared, when possible, over 3 different eras: January 1992-December 2000, January 2001-December 2009, and January 2010-June 2018. Induction immunosuppression and maintenance immunotherapy at the time of hospital discharge from index heart transplantation were reviewed, with the analysis limited to patients alive at the time of discharge. The results reported herein seek to provide as granular detail as possible with data retained in the ISHLT International TTX Registry for transplants through June 30, 2018.
In addition to the data presented within the primary manuscript, extended analyses are shown in the online slide sets (https://ishltregistries.org/registries/slides.asp). The ISHLT website also contains slide sets for previous annual reports. This report references specific online e-slides when particular data are discussed but not shown due to space limitations, with eSlide H(a) numbers refer to the online adult heart transplant slides.
The ISHLT International TTX Registry website (https://ishlt.org/research-data/registries/ttx-registry) provides detailed spreadsheets of the data elements collected in the Registry. The Registry requires submission of core donor, recipient, and transplant procedure variables at baseline (i.e., at the time of transplantation) and annual follow-up. These variables have low rates of missingness. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that rely on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
Unless otherwise specified, analyses of combined heart-lung transplants are not included in analyses of heart transplants. The Registry does not capture the exact occurrence date for most secondary outcomes (e.g., cardiac allograft vasculopathy), but it does capture the window of occurrence. For the report’s analyses, we use the mid-point between the annual follow-ups as a surrogate for the event date (i.e., the event occurred between the first and the second annual follow-up visits). On the follow-up where a death is reported, some under-reporting of secondary outcomes and other information is highly probable. Thus, some analyses are restricted to include only surviving recipients to reduce the potential for underestimating event rates or other outcomes. For time-to-event analyses, we censored the follow-up of recipients who did not experience the event of interest at the last time the recipient was reported not to have had the event, which would either be the most recent annual follow-up or the time of re-transplantation. We truncated time-to-event graphs (e.g., survival graphs) when the number of individuals at risk was less than 10. Previous Registry reports provide additional details regarding specific donor and recipient characteristics and outcomes.
Recipient characteristics
As shown in Table 1 and eSlide H(a) 4, the number of adults with restrictive heart disease who underwent heart transplantation increased over time and nearly tripled from 1992–2000 to 2010–2018. HCM remained the most common type of heart disease, and endocardial fibrosis was the least likely. Transplantation for amyloid, sarcoid, and chemotherapy and/or radiation-induced cardiomyopathy also increased over time, with fewer patients labeled as idiopathic or other restrictive cardiomyopathies. As shown in Table 2 and eSlide H(a) 5–7, the majority of transplants for restrictive heart disease were done in Europe (n = 1946) and North America (n = 2115) as compared to “Other” regions (n = 245). When comparing the 3 different eras: 1992–2000, 2001–2009 to 2010–2018, there was a slight decline in the proportion of transplants done for restrictive heart disease in Europe and a minimal increase in North America and “Other” regions. Most recipients were men with a median age of 47 years in the earlier era (1992–2000), increasing to 52 years in the most recent era (2010–2018), perhaps reflecting a rise in transplantation for amyloidosis, which predominantly affects older patients. The percent of sensitized recipients with panel reactive antibody (PRA) ≥20% increased over time from 3.5% during 1992–2000, to 19.1% during 2010–2018, and those with PRA ≥80% from 0.6% during 1992–2000 to 3.6% during 2010–2018. There was an increase in recipient history of malignancy from 5% during 1992–2000 to 11.5% during 2010–2018, which could not be fully explained by the less marked increase in the proportion of patients with radiation and/or chemotherapy-induced RCM. There was no significant change over time in the prop proportion of patients with prior cardiac surgery (approximately 25%), about 1 of 3 had a history of smoking, and very few were on dialysis prior to transplantation.
Table 1.
Diagnosis Categories Among Patients Transplanted for Restrictive Heart Disease by era (Transplants: January 1992-June 2018)
| January 1992-December 2000 (n = 760) | January 2001-December 2009 (n = 1417) | January 2010-June 2018 (n = 2129) | p-value | |
|---|---|---|---|---|
| Hypertrophic cardiomyopathy | 352 (46.3%) | 752 (53.1%) | 1065 (50.0%) | <0.0001 |
| Amyloid | 53 (7.0%) | 114 (8.0%) | 299 (14.0%) | |
| Sarcoid | 25 (3.3%) | 50 (3.5%) | 133 (6.2%) | |
| Radiation/chemotherapy | 9 (1.2%) | 26 (1.8%) | 53 (2.5%) | |
| Other restrictive cardiomyopathy | 321 (42.2%) | 475 (33.5%) | 579 (27.2%) | |
| – Idiopathic | 203 (26.7%) | 240 (16.9%) | 266 (12.5%) | |
| – Endocardial Fibrosis | 9 (1.2%) | 12 (0.8%) | 16 (0.8%) | |
| – Other | 109 (14.3%) | 223 (15.7%) | 297 (14.0%) |
Continuous factors are expressed as median (5th−95th percentiles).
Summary statistics included transplants with non-missing data.
Diagnosis categories (with “Other RCM” combined) were compared using the chi-square statistic using single p-value.
Table 2.
Recipient Characteristics by era (RCM/HCM Transplants: January 1992-June 2018)
| January 1992-December 2000 (n = 760) | January 2001-December 2009 (n = 1417) | January 2010-June 2018 (n = 2129) | p-value | |
|---|---|---|---|---|
| Geographic Location | <0.0001 | |||
| – Europe | 47.8% | 49.5% | 41.4% | |
| – North America | 46.7% | 46.1% | 52.0% | |
| – Other | 5.5% | 4.4% | 6.6% | |
| Age (years) (continuous) | 47.0 (22.0–63.0) | 49.0 (22.0–65.0) | 52.0 (23.0–68.0) | <0.0001 |
| Female | 38.7% | 44.7% | 42.2% | 0.0233 |
| Weight (kg) | 69.1 (48.4–96.0) | 72.0 (49.9–100.0) | 75.0 (51.3–105.9) | <0.0001 |
| Height (cm) | 170.0 (152.4–185.0) | 170.2 (154.0–186.0) | 170.2 (154.0–188.0) | 0.036 |
| BMI (kg/m2) | 23.7 (17.9–31.8) | 24.7 (18.7–32.8) | 25.5 (19.1–34.2) | <0.0001 |
| ABO blood type | 0.0881 | |||
| – A | 46.8% | 42.6% | 41.0% | |
| – AB | 4.0% | 5.0% | 4.4% | |
| – B | 11.0% | 13.8% | 14.0% | |
| – O | 38.2% | 38.5% | 40.6% | |
| PRA ≥ 20% | 3.5% | 7.6% | 19.1% | <0.0001 |
| PRA ≥ 80% | 0.6% | 1.5% | 3.6% | 0.0017 |
| CMV antibody positive | 57.5% | 53.9% | 54.4% | 0.5138 |
| History of malignancy | 5.0%a | 6.5% | 11.5% | <0.0001 |
| History of smoking | - | 31.2%b | 33.3% | 0.4412 |
| Pre-transplant dialysis | 0.8%a | 2.4% | 3.0% | 0.1206 |
| Prior cardiac surgery | 22.0%a | 25.7% | 27.5% | 0.1348 |
| Hospitalized | 59.2% | 47.7% | 54.9% | 0.0005 |
| Inotrope use | 43.6% | 43.9% | 44.5% | 0.9403 |
| ECMO use | 0% | 0.4% | 0.9% | 0.1422 |
| IABP use | 2.2% | 2.8% | 6.9% | <0.0001 |
| Ventilator use | 2.2% | 1.6% | 0.9% | 0.1609 |
| MCS use | <0.0001 | |||
| – BIVAD | - | 2.0% | 1.3% | |
| – TAH | - | 0.7% | 2.2% | |
| – VAD | - | 6.7% | 14.5% | |
| – None | - | 90.6% | 82.1% | |
| Bilirubin (mg/dl) | 0.9 (0.3–2.7)a | 0.9 (0.3–2.9) | 0.8 (0.3–2.4) | <0.0001 |
| Creatinine (mg/dl) | 1.2 (0.7–2.2)a | 1.1 (0.7–2.2) | 1.2 (0.7–2.0) | 0.1459 |
| GFR (mL/min/1.73m2)c | 69.3 (34.6–142.8)a | 71.7 (37.4–139.4) | 74.7 (39.5–142.6) | 0.0202 |
| PCW mean (mmHg) | 20.0 (8.0–32.0)a | 20.0 (8.0–30.0) | 18.0 (7.0–30.0) | 0.0008 |
| PA mean (mmHg) | 30.0 (16.0–50.0)a | 28.0 (15.0–45.0) | 27.0 (14.0–43.0) | <0.0001 |
| PVR (woods unit) | 2.5 (0.7–6.5)a | 2.3 (0.8–5.8) | 2.2 (0.6–5.1) | 0.0212 |
Continuous factors are expressed as median (5th–95th percentiles).
Summary statistics included transplants with known/non-missing data.
BMI, body mass index; BIVAD, biventricular assist device; CMV, cytomegalovirus; ECMO, extracorporeal membrane oxygenation; GFR, Glomerular Filtration Rate; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; PCW, pulmonary capillary wedge; PA, pulmonary artery pressure; PVR, pulmonary vascular resistance; PRA, panel reactive antibody; TAH, total artificial heart; VAD, ventricular assist device.
Based on April 1994-December 2000 transplants.
Based on July 2004-December 2009 transplants.
GFR was estimated using the Cockcroft-Gault formula.
At the time of transplantation, nearly half of the patients transplanted for restrictive heart disease were hospitalized, with 44% being on inotropes for hemodynamic support. Mechanical circulatory support was relatively uncommon and notable for a gradual increase in usage of durable ventricular assist devices, from 6.7% during 2001–2009 to 14.5% during 2010–2018, and an increase in the use of intra-aortic balloon pumps from 2.2% in 1992–2000 to 6.9% in 2010–2018. Mechanical ventilation at the time of transplantation was rare. Although patients with restrictive heart disease are at risk for significant pulmonary hypertension as well as abnormal renal and liver function due to congestion and/or due to concomitant systemic disease, the majority of patients that were transplanted for restrictive heart disease had normal renal function, normal liver function, and average PVR <3 Wood units at the time of transplant.
The type of heart disease requiring transplantation for restrictive physiology varied based on age, era, and location. The majority of patients 18–39 years old had HCM (58.8%), and very few (≤2%) had amyloid, sarcoid, or radiation and/or chemotherapy-induced RCM (Figure 1, eSlide H(a) 8). For recipients older than 40 years of age, there were relatively fewer recipients with HCM and more with amyloidosis. For instance, among 60–69 years old recipients, there were 24.2% with amyloid, and 40.5% with HCM. Among patients 70 years or older, the majority had amyloid (65.4%), only 18.5% had HCM, and 14.8% had radiation/chemotherapy-induced cardiomyopathy. With respect to changes in the distribution of transplants for restrictive heart disease over time (Figure 2, eSlide H(a) 12), there was a decrease in the proportion of patients whose diagnosis was listed as ‘Other RCM’, the proportion of patients transplanted for amyloid and sarcoid increased over the 3 eras, and HCM remained the dominant diagnostic category. The proportion of patients transplanted for the specific restrictive heart disease type also varied based on location (Figure 3, eSlide H(a) 14). In Europe the majority had HCM (54%), with very few having amyloid (4%), sarcoid (<1%) or radiation and/or chemotherapy induced heart disease (<1%). In North America, 47% had HCM, 18% amyloid, 10% sarcoid, and 3% had radiation and/or chemotherapy induced heart disease. Among those transplanted in regions other than Europe or North America, the type of heart disease distribution was more similar to Europe than North America.
Figure 1.
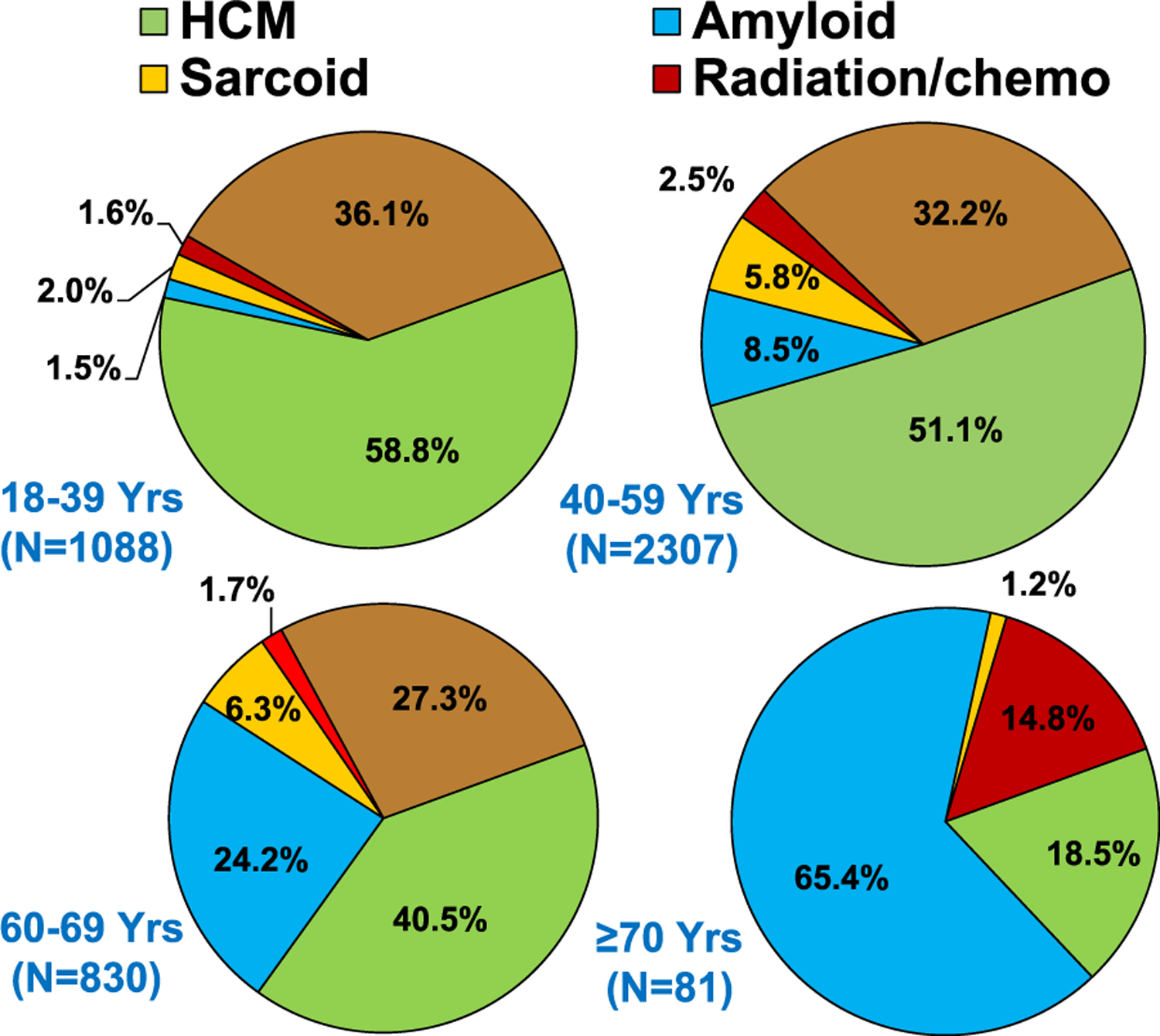
Diagnosis distribution by age group (transplants: January 1992-June 2018).
Figure 2.
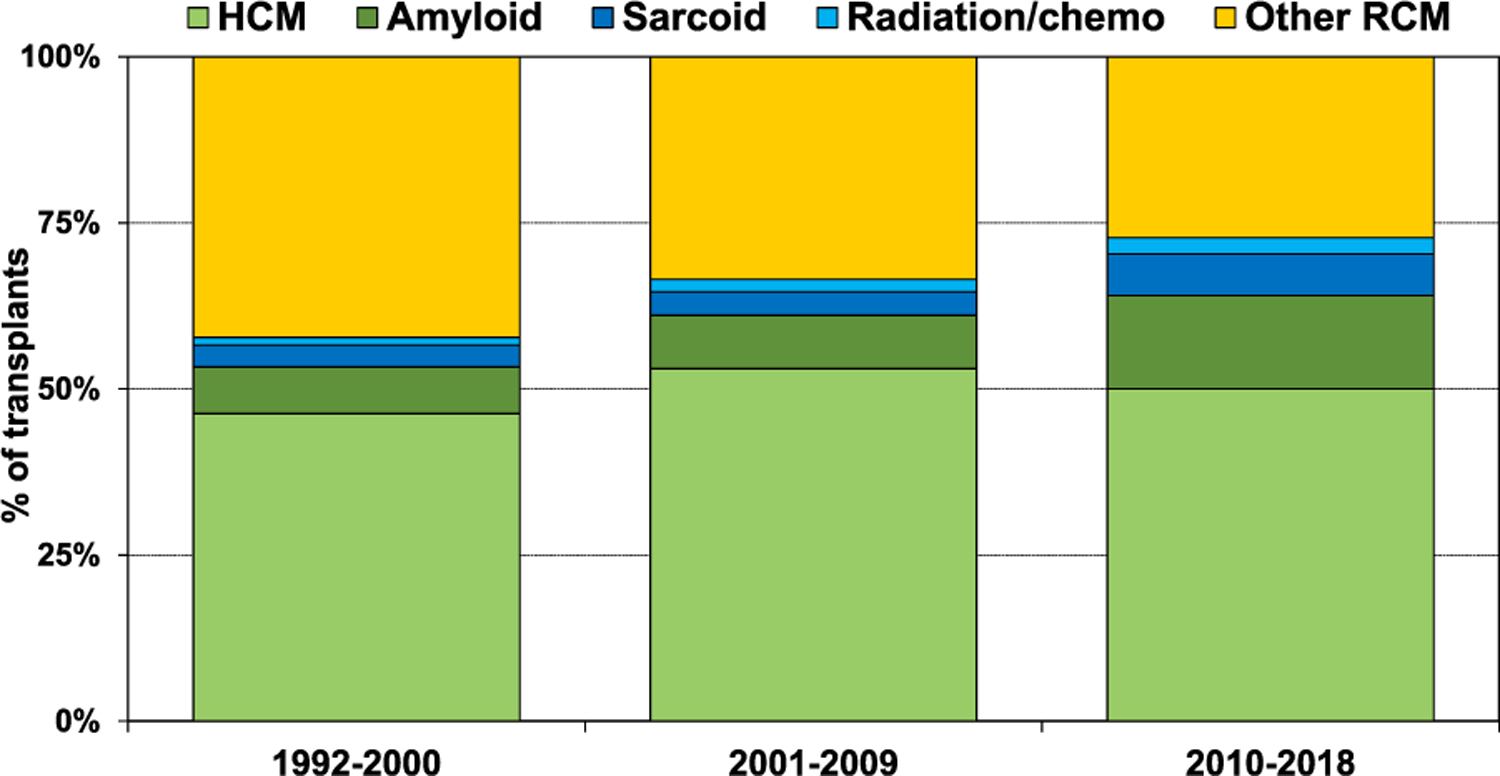
Diagnosis distribution by era (transplants: January 1992-June 2018).
Figure 3.
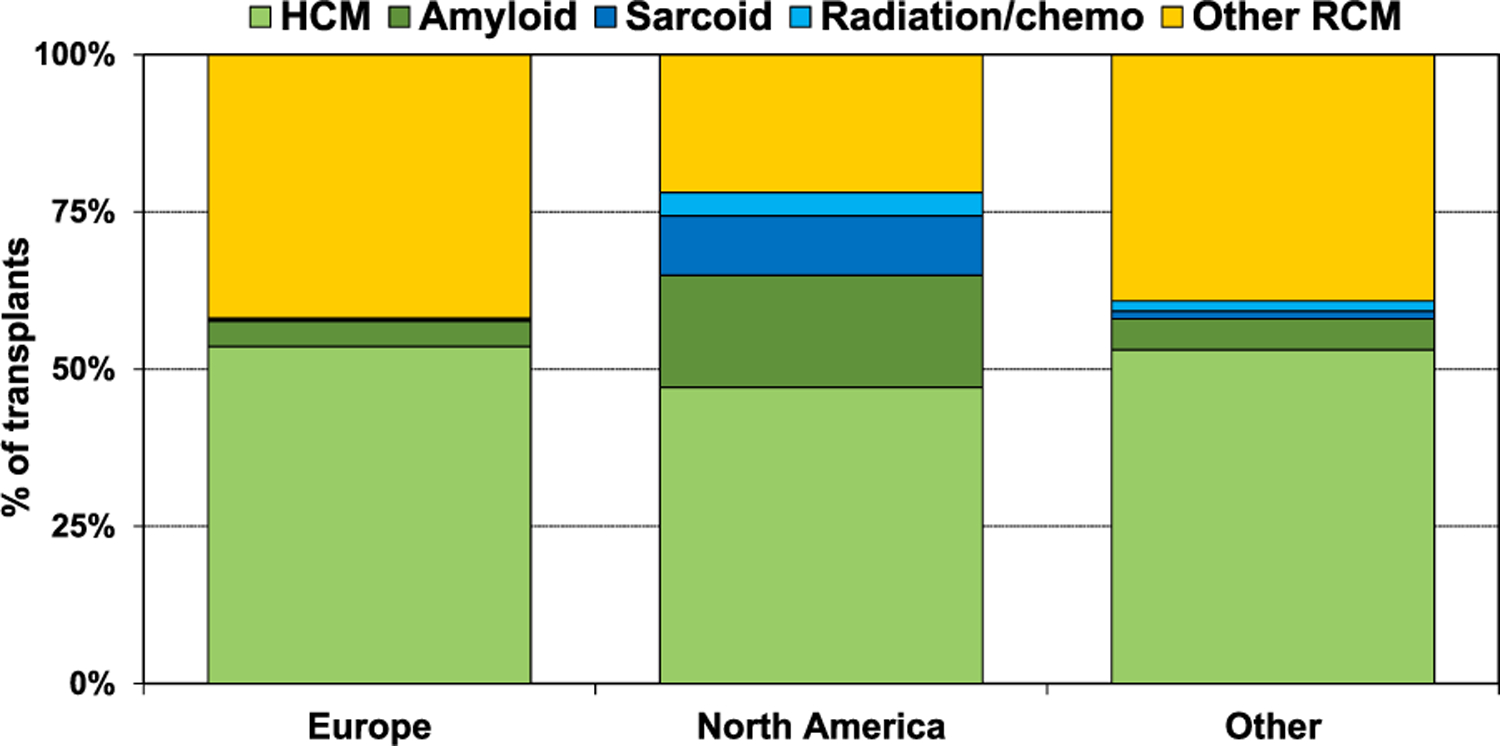
Diagnosis distribution by geographic region (transplants: January 1992-June 2018).
Immunosuppression, Rejection, and Cardiac Allograft Vasculopathy (CAV)
Induction therapy was used in 50% of the recipients, with IL-2 receptor antagonists used as often as polyclonal anti-thymocyte and/or anti-lymphocyte globulin therapy (eSlide H(a)16). Maintenance therapy usually consisted of calcineurin inhibitor (97%), mycophenolate mofetil (79%), and steroids (81%) (eSlide H(a) 17). One-year risk of rejection after transplant hospital discharge decreased from 22% during 2005–2009 to 11.8% during 2010–2018 (Figure 4, eSlide H(a) 18). Among patients transplanted in 1992–2017, nearly 25% developed cardiac allograft vasculopathy (CAV) within 5 years post-transplantation and almost 50% by 10 years following transplantation (Figure 5, eSlide H(a) 27). There was no significant difference in freedom from CAV between the patients by etiology of restrictive heart disease.
Figure 4.
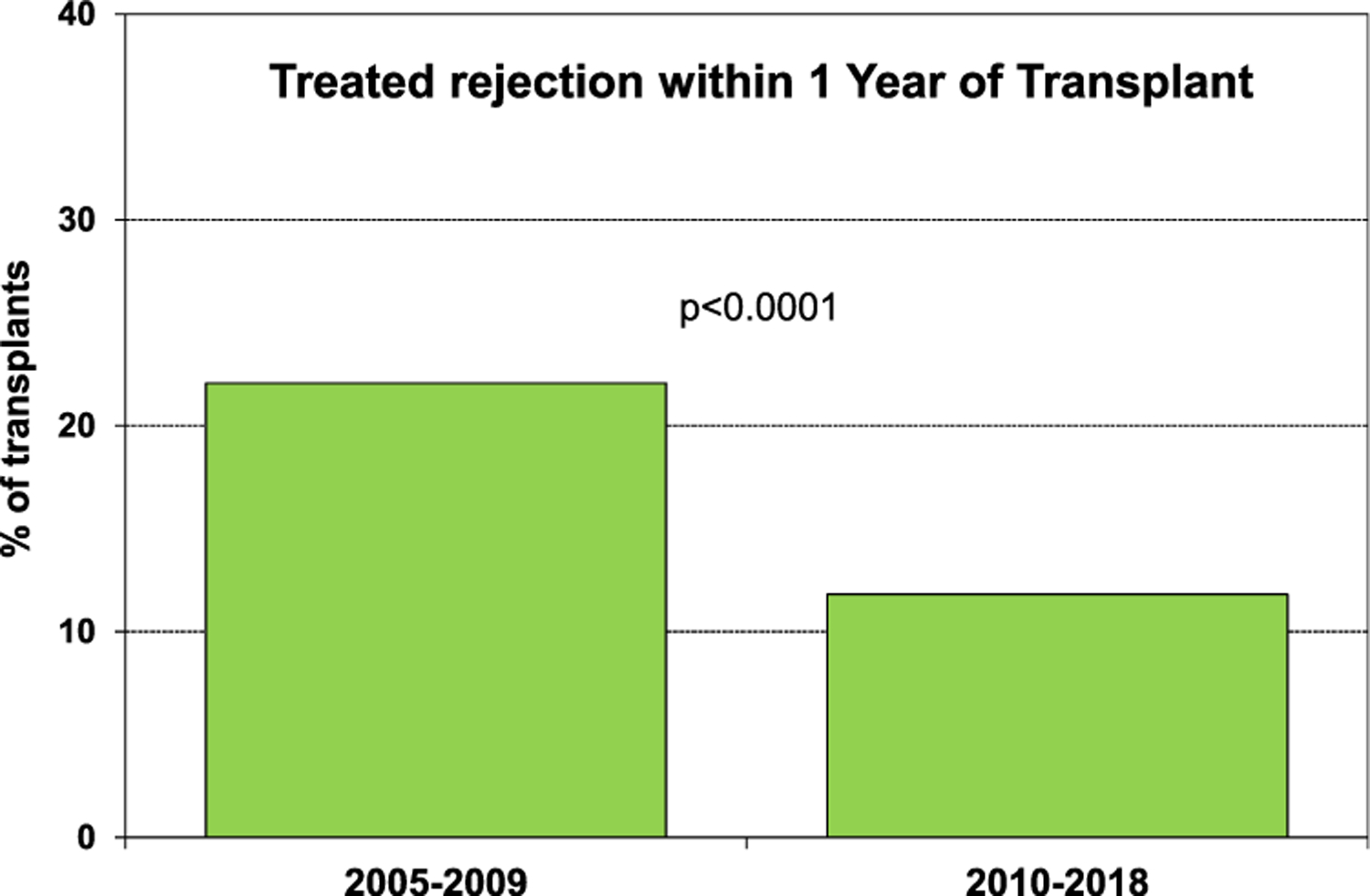
Percent of recipients who experienced rejection between discharge and 1 year follow-up by era (transplant follow-ups: January 2005-June 2018).
Figure 5.
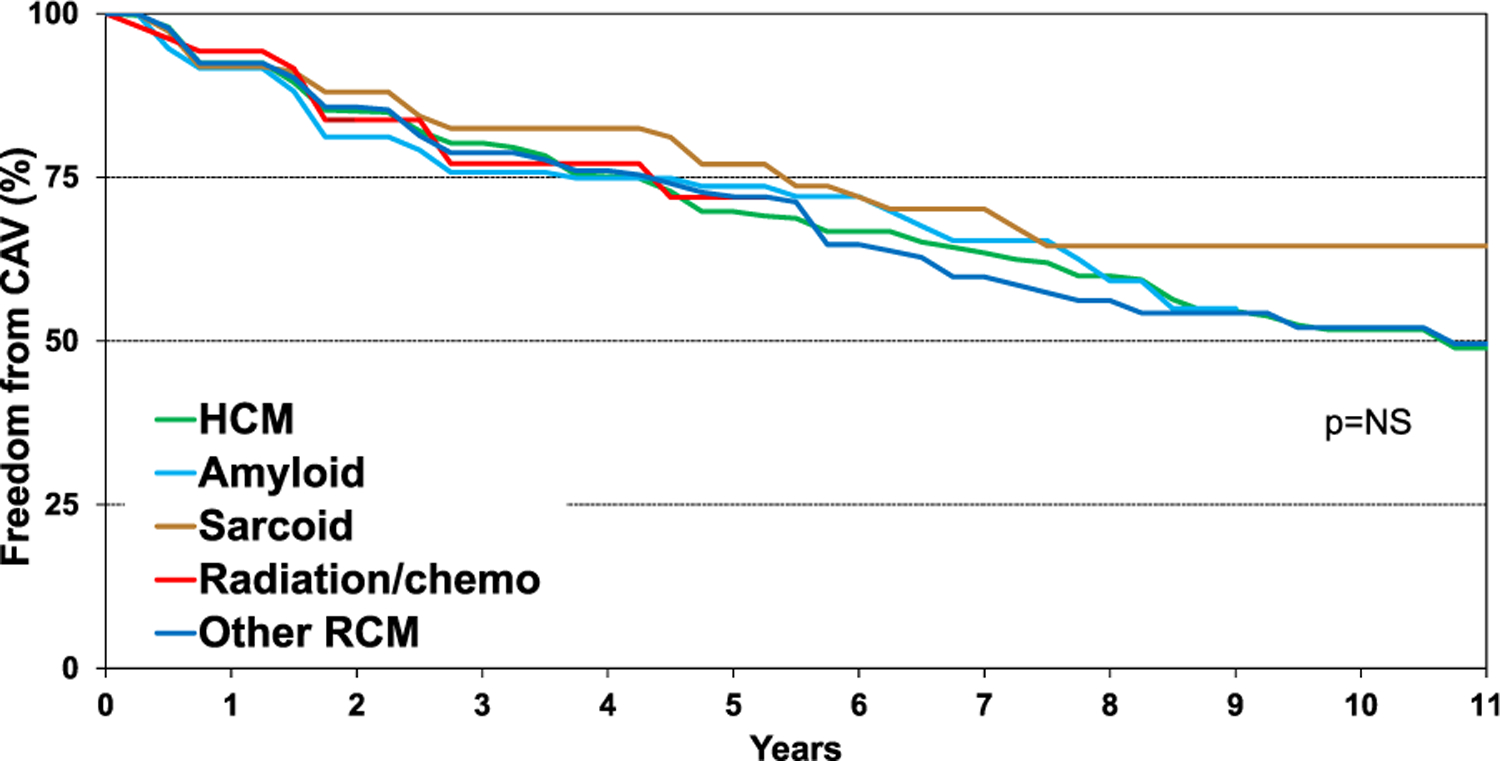
Freedom from cardiac allograft vasculopathy (CAV) by diagnosis (transplants: January 1992-June 2017).
Survival
There were significant differences in post-transplant survival between recipients transplanted for restrictive heart disease and those transplanted for other etiologies (eSlide H(a) 28), a finding consistent with other reports.6–10 There were also important differences in survival among subgroups of patients transplanted for different types of restrictive heart disease (Figure 6, eSlide H(a) 29).
Figure 6.
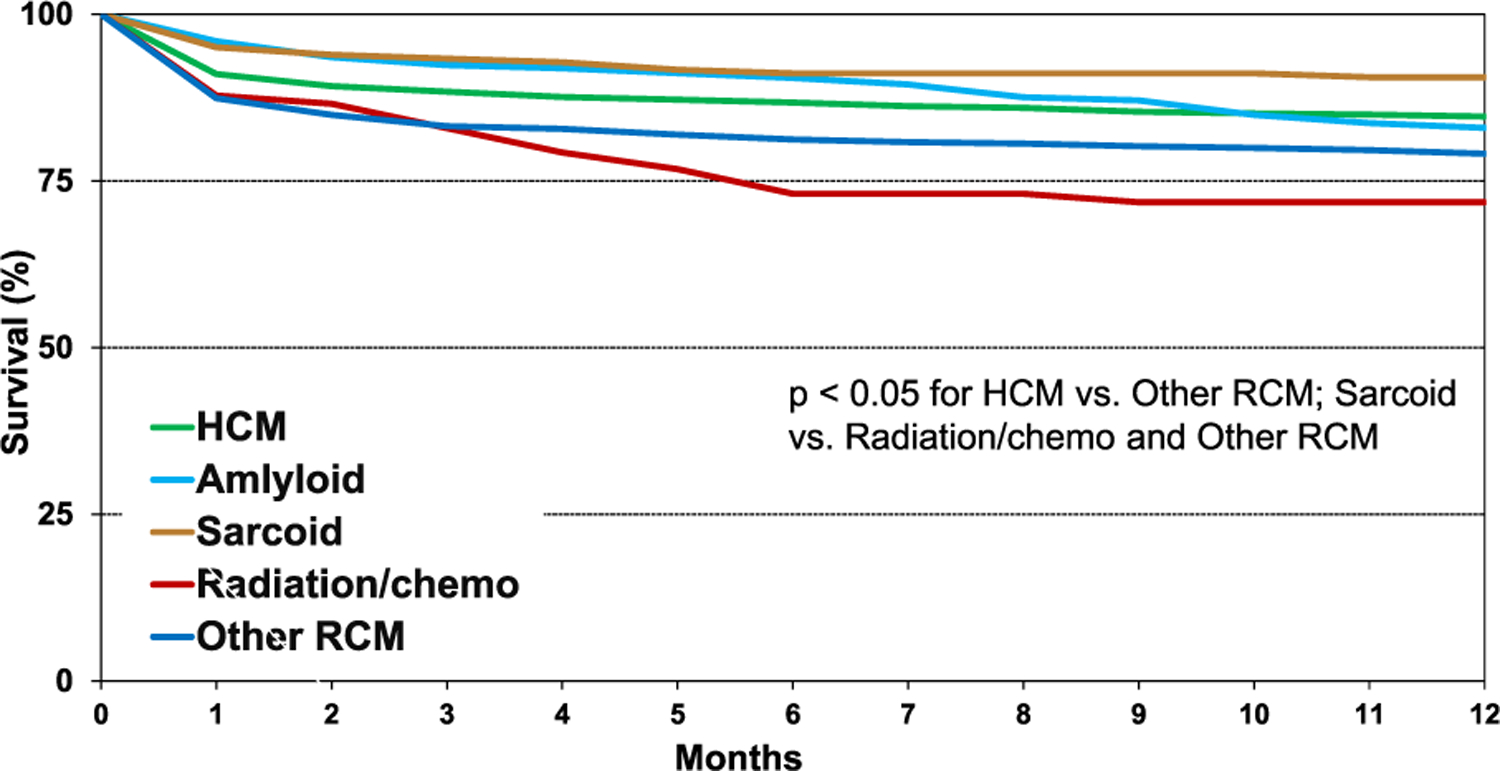
Kaplan-Meier survival within 12 months by diagnosis (transplants: January 1992-June 2017).
One-year survival
When comparing unadjusted survival between HCM, amyloid, sarcoid, radiation and/or chemotherapy, and “other” RCM (eSlide H(a) 29), recipients with sarcoidosis had the highest 1 year survival, and those with radiation and/or chemotherapy-induced cardiomyopathy had the lowest (90.6% vs 71.8%, p < .05). Patients with HCM had a 1 year survival of 84.6%, amyloid 82.9%, and “other” RCM 79.1%. Infection, graft failure, and multi-organ failure were the leading causes of death within the first year (Figure 7, eSlide H(a) 24). Infection as the cause of death was more common among those with amyloid (29.7%) and sarcoid (43.8%), likely due to the need for additional immunotherapy (eSlide H(a) 21–22). When comparing eras, patients in the most recent era had higher 1 year survival than those in the earliest era (Figure 8A, eSlide H(a) 30). Transplant recipients in North America had higher 1 year survival than those in Europe (88.1% vs 77.0%, p < .05), which may reflect regional differences in the underlying cause for RCM (Figure 8B, eSlide H(a) 30). Europe transplanted a higher percentage of “other” RCM patients compared to North America (41.9% vs 21.9%), but a lower percentage of patients with amyloidosis (4.0 % vs 17.8%), (eSlide H(a) 14). There was no difference in survival by sex (Figure 9A, eSlide H(a) 31). Survival of patients transplanted at age 18–39 years old was higher compared to those 40–59 years or 60–69 years old at transplant (Figure 9B, eSlide H (a) 31). Estimated glomerular filtration rate (eGFR) was associated with survival, with significantly higher mortality among those with eGFR <30 ml/min/1.73 m2 at transplant compared to ≥60 ml/min/1.73 m2 at transplant (Figure 10, eSlide H(a) 32).
Figure 7.
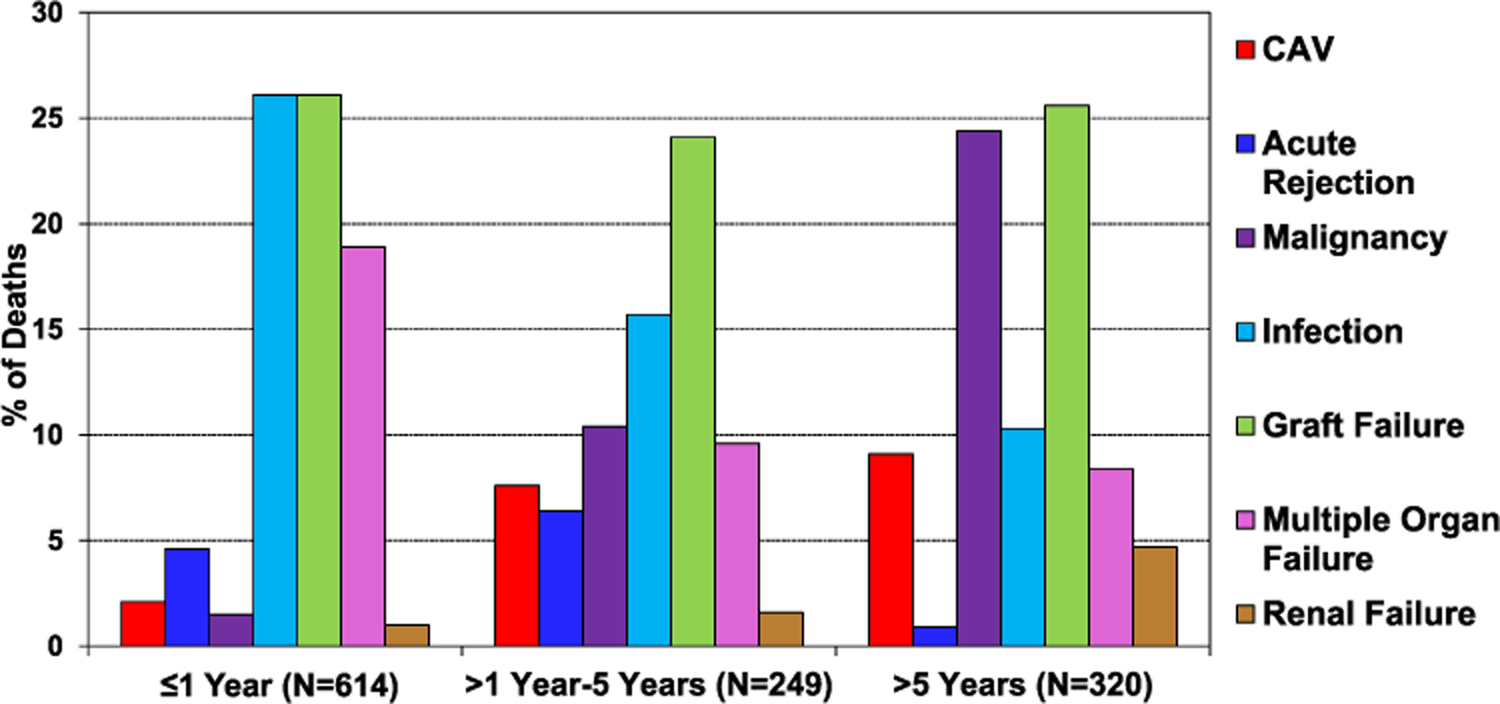
Relative incidence of leading causes of death (transplant deaths: January 2010-June 2018).
Figure 8.
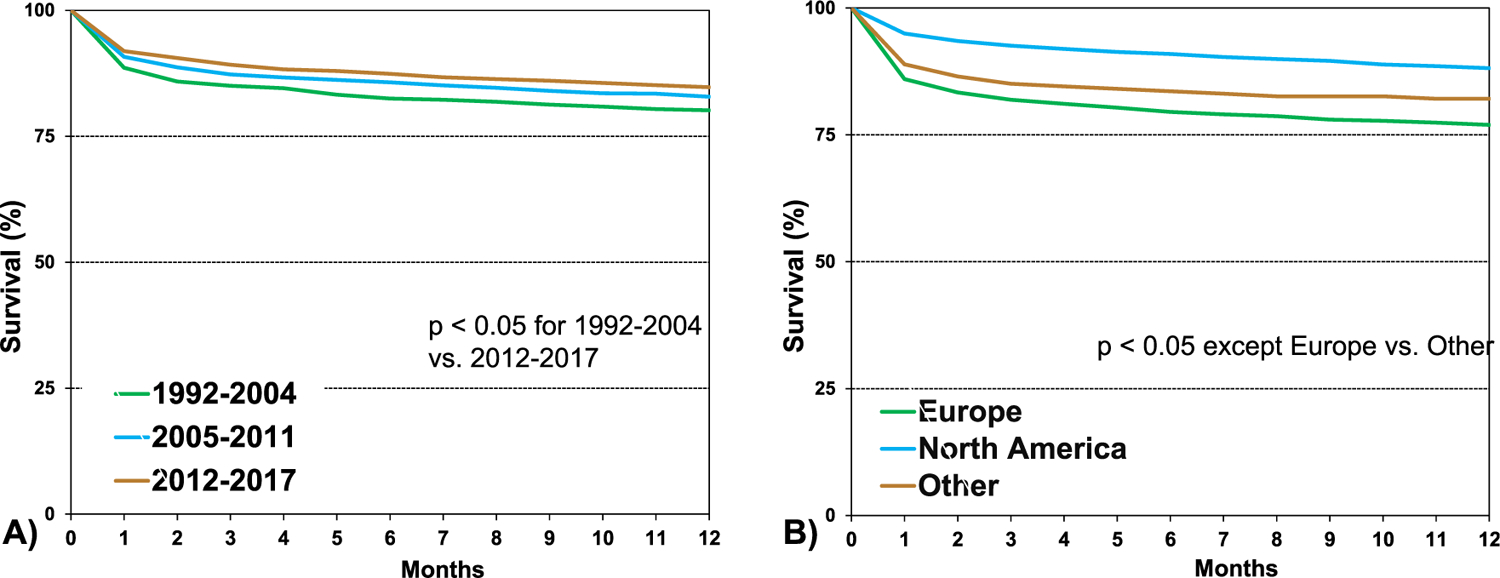
Kaplan-Meier survival within 12 months by (A) era and (B) geographic region (transplants: January 1992-June 2017).
Figure 9.
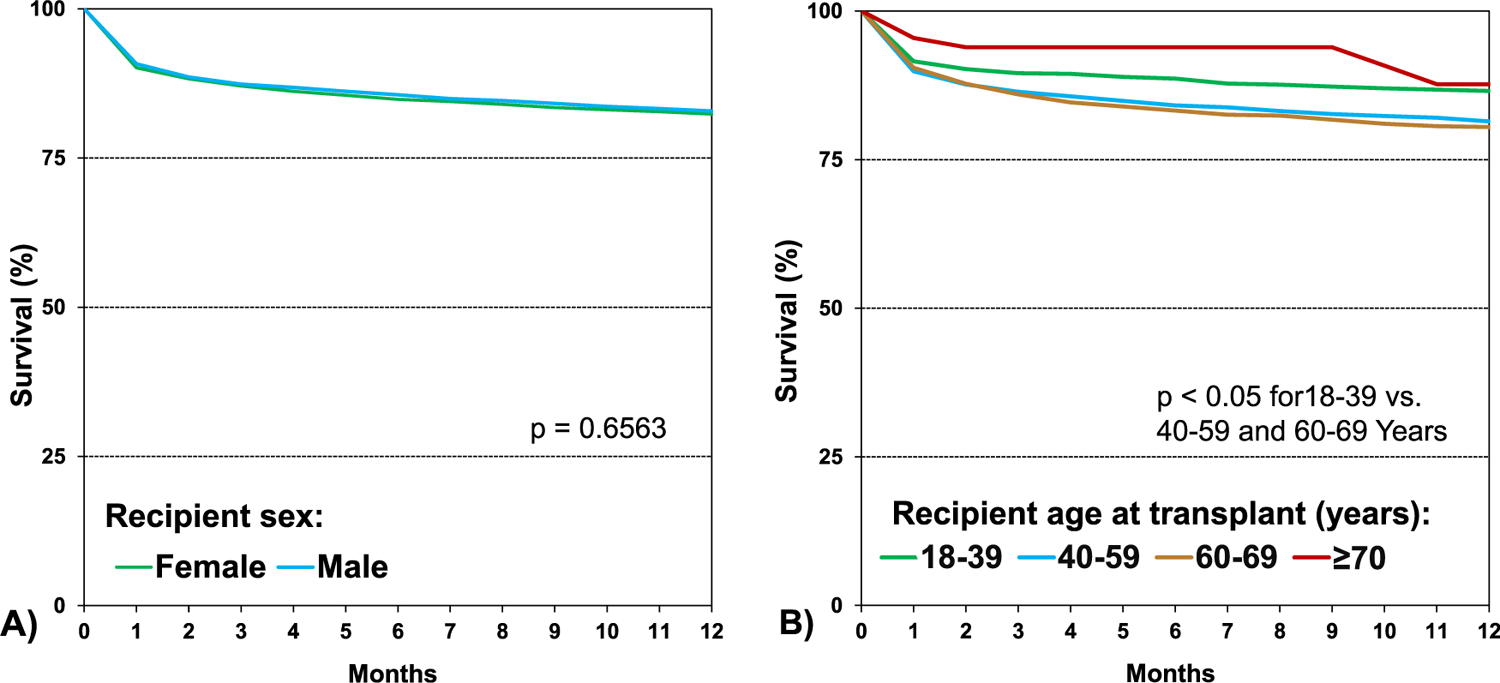
Kaplan-Meier survival within 12 months by recipient (A) sex and (B) age (years) (transplants: January 1992-June 2017).
Figure 10.
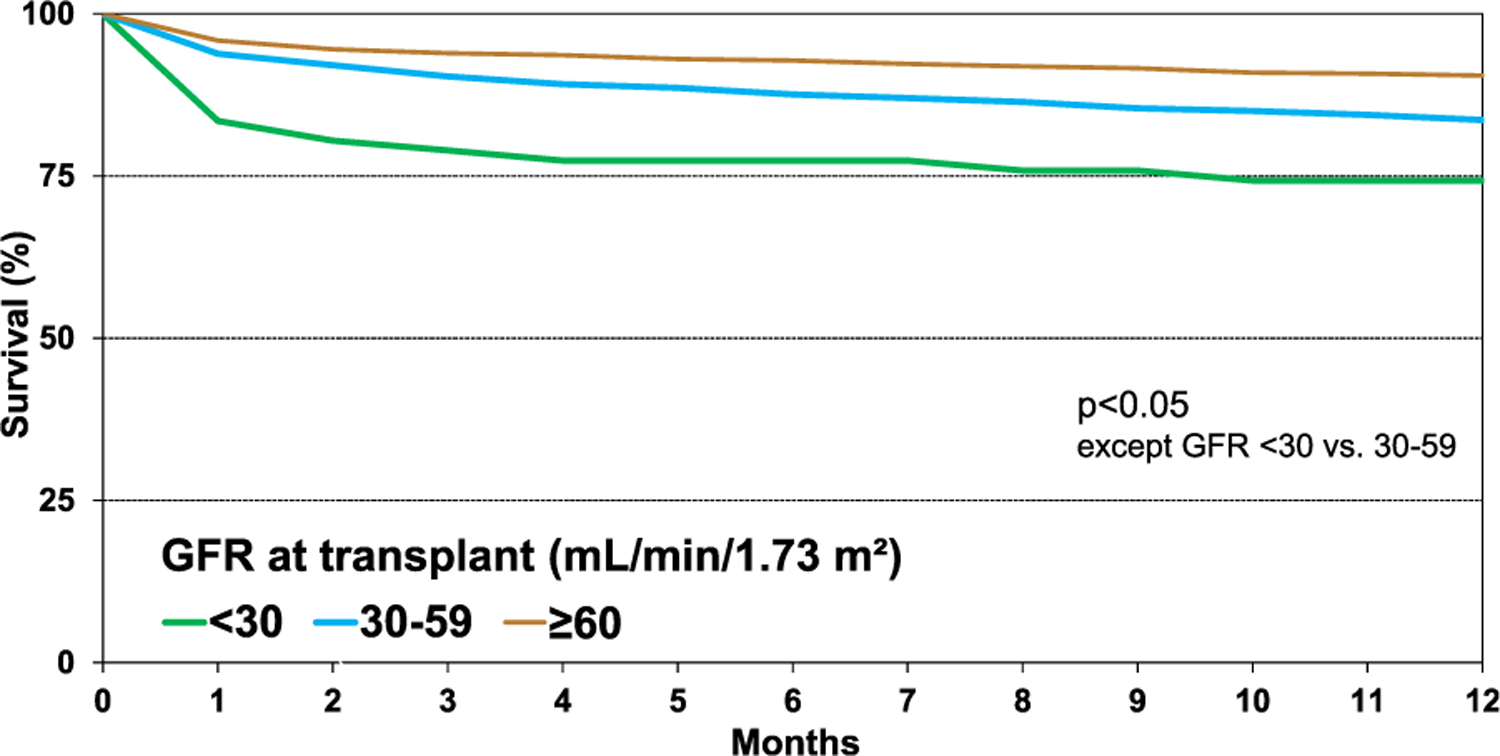
Kaplan-Meier survival within 12 months by recipient GFR (transplants: January 1994-June 2017). GFR was estimated using the Cockcroft-Gault formula.
Five-year survival, conditional on survival to 1 year
When comparing unadjusted survival between HCM, amyloid, sarcoid, radiation and/or chemotherapy, and “other” RCM patients (Figure 11, eSlide H(a) 34), those with sarcoidosis had the highest 5 year conditional survival, and those with a history of radiation and/or chemotherapy had the lowest (94.0% vs 71.8%, p = .0011). Patients with HCM had a 5 year survival of 92.1%, “other” RCM patients 85.7%, and those with amyloid 74.3%. Graft failure and infection remained the leading causes of death 5 years post-heart transplantation (eSlide H(a) 25) There was no difference in 5 year conditional survival among transplants stratified by era, location, sex, or age (eSlide H(a) 35–36). Pulmonary hypertension as reflected by PVR did not affect 5 year conditional survival, but severe renal function, defined as initiation of dialysis, renal transplant, or creatinine >2.5 mg/dl within 1 year post-transplant, was associated with significantly lower survival (Figure 12, eSlide H(a) 37).
Figure 11.
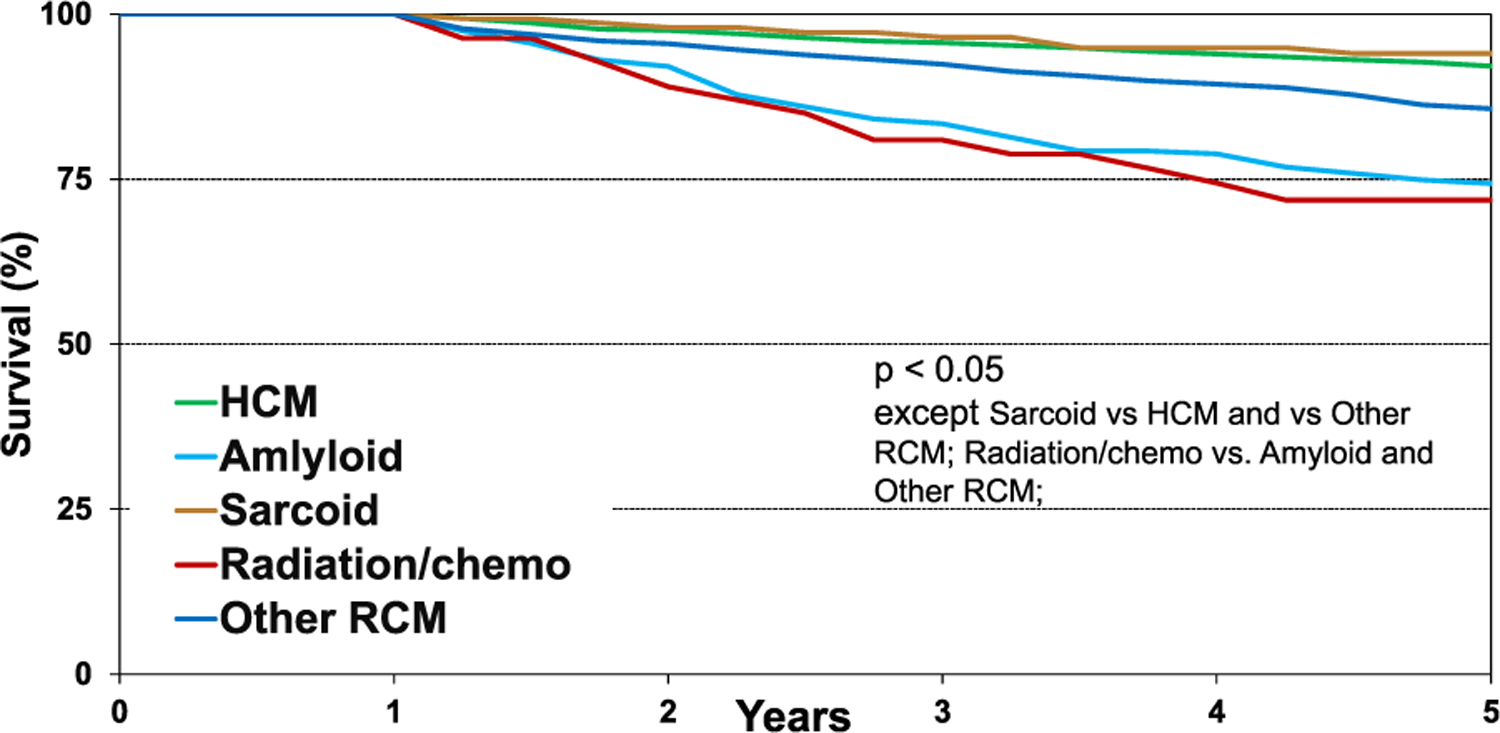
Kaplan-Meier survival within 5 years conditional on survival to 1 year by diagnosis (transplants: January 1992-June 2013).
Figure 12.
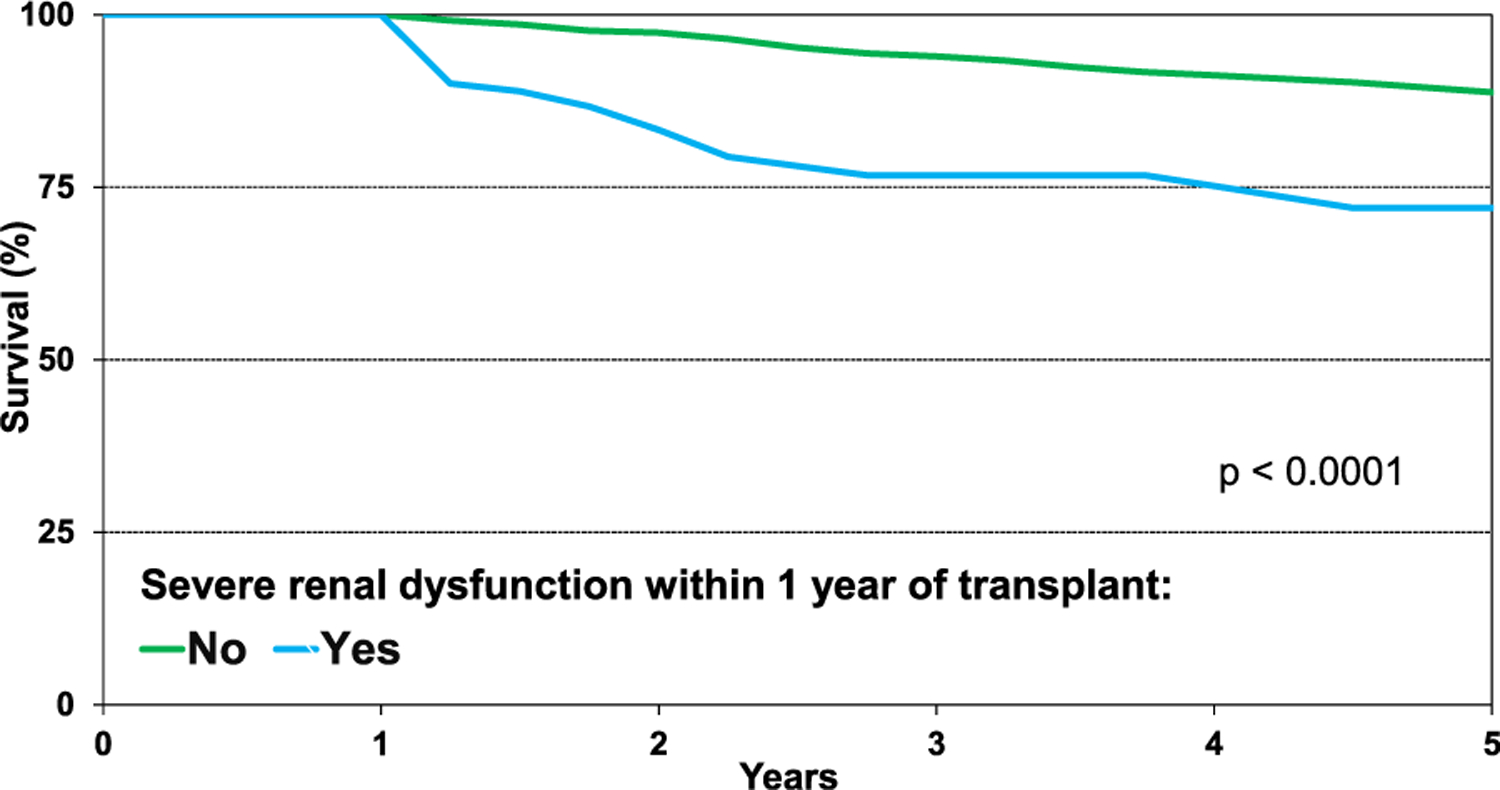
Kaplan-Meier survival within 5 years conditional on survival to 1 year by severe renal dysfunction within 1 year (transplants: January 1992-June 2013). Severe renal dysfunction was calculated as creatinine >2.5 mg/dl, dialysis, or renal transplant.
Causes of death beyond 5 years
Among patients transplanted for restrictive heart disease, there were 320 deaths reported beyond 5 years between January 2010 and June 2018. The leading causes of death were graft failure and malignancy (Figure 7, eSlide H(a) 24), which were among more common causes of death in patients with a history of HCM, amyloidosis, sarcoidosis, and “other” RCM (eSlide H(a) 20–23). Although CAV and renal failure were less common causes of death, their incidence increased over time, while the relative incidence of rejection, infection, and multi-organ failure as the cause of death decreased over time (Figure 7, eSlide H(a) 24)
Multivariable analyses
Multivariable proportional hazards regression analyses were utilized to identify independent risk factors associated with mortality at the time of transplant-but cannot establish causality. Covariates included in the multivariable models are listed in Supplemental Table 1. Risk factors may differ over time, and those at the time of transplant best predict mortality closest to the time of surgery. Therefore, specific associations observed among these data, especially beyond 1 year survival, should be interpreted with caution.
Multivariable analysis was notable for the lower risk in 1 year mortality following transplant in the more recent eras compared to earliest era (Figure 13, eSlide H(a) 39). Patients with a history of radiation and/or chemotherapy and “other” RCM had a higher risk of mortality when compared to HCM. Prior cardiac surgery, dialysis, and mechanical ventilation also increased mortality risk when compared to recipients without these attributes. When assessing continuous variables, 1 year mortality increased with increasing values for recipient bilirubin at the time of transplant (Figure 14A, eSlide H(a) 41). The risk of 1 year mortality increased with worsening renal function at the time of transplant (Figure 14B, eSlide H (a) 42). One-year mortality risk of pulmonary hypertension based on pulmonary vascular resistance (PVR) increased as the value of PVR rose (Figure 14C, eSlide H(a) 43). Donor age also affected 1 year mortality significantly with the risk increasing for older donor (Figure 15A, eSlide H(a) 44). Center volume affected survival with higher 1 year mortality for centers with smaller transplant volume in the previous 3 years (Figure 15B, eSlide H(a) 45).
Figure 13.
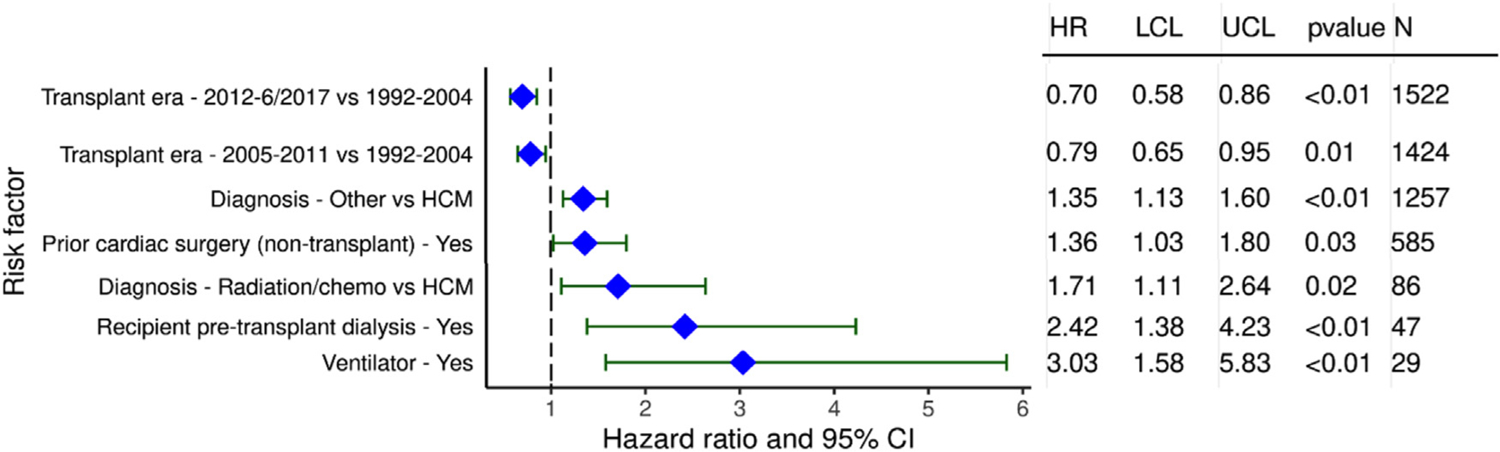
Statistically significant categorical risk factors for 1 year mortality with 95% confidence limits (transplants January 1992-June 2017).
Figure 14.
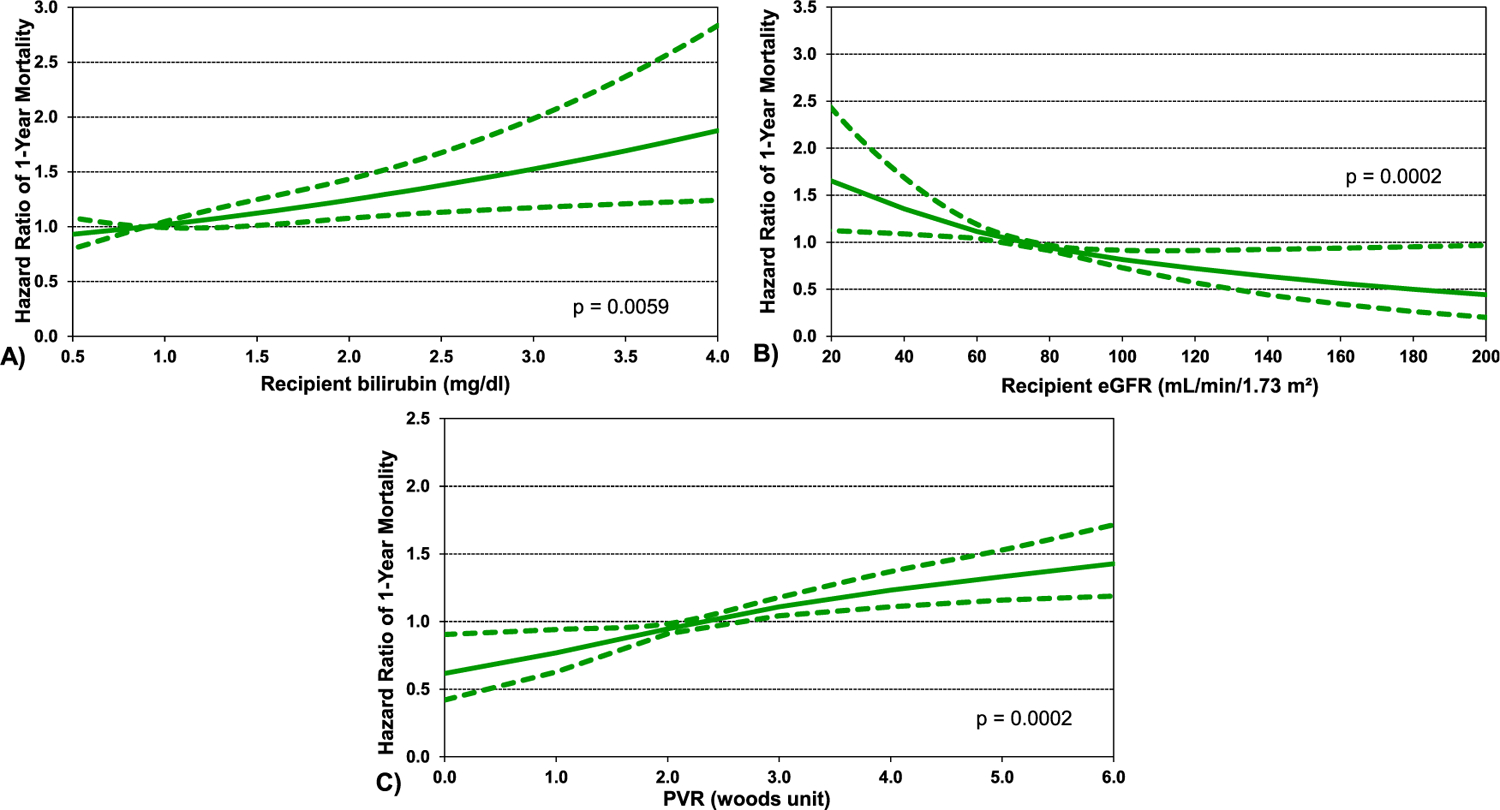
Multivariable hazard ratio plot for 1 year mortality with 95% confidence limits, by (A) bilirubin, (B) GFR, and (C) PVR (transplants: January 1992-June 2017; n = 4292).
Figure 15.
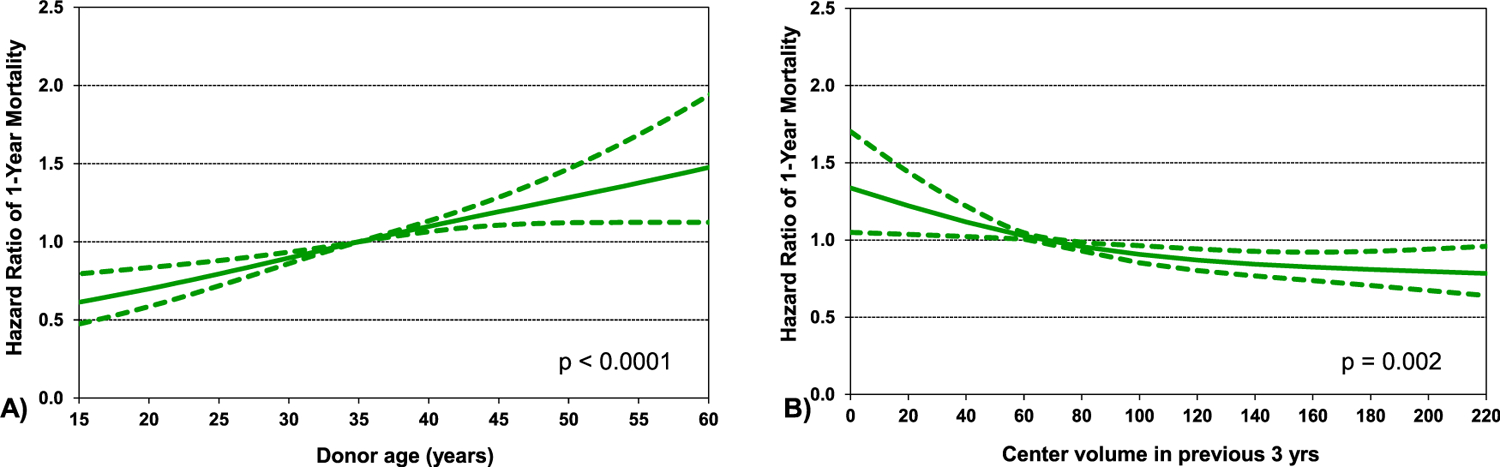
Statistically significant risk factors for 1 year mortality with 95% confidence limits, by (A) donor age (years), and (B) center volume in the previous 3 years (transplants: January 1992-June 2017; n = 4292).
Risk factors for 5 year mortality conditional to survival to 1 year post heart transplantation were notable for significant differences based on the type of heart disease (Figure 16, eSlide H(a) 47). When compared to HCM, “other” RCM had an HR of 1.98 (95% CI 1.50–2.62, p < .01), amyloid had a HR of 4.5 (95% CI 3.27–6.19, p < .01), and radiation and/or chemotherapy a HR of 4.63 (95% CI 2.46–8.70, p < .01). Mean pulmonary artery pressure (PAP) was the only continuous variable significantly associated with 5 year mortality, with the risk increasing as mean PAP increased (eSlide H(a) 49).
Figure 16.
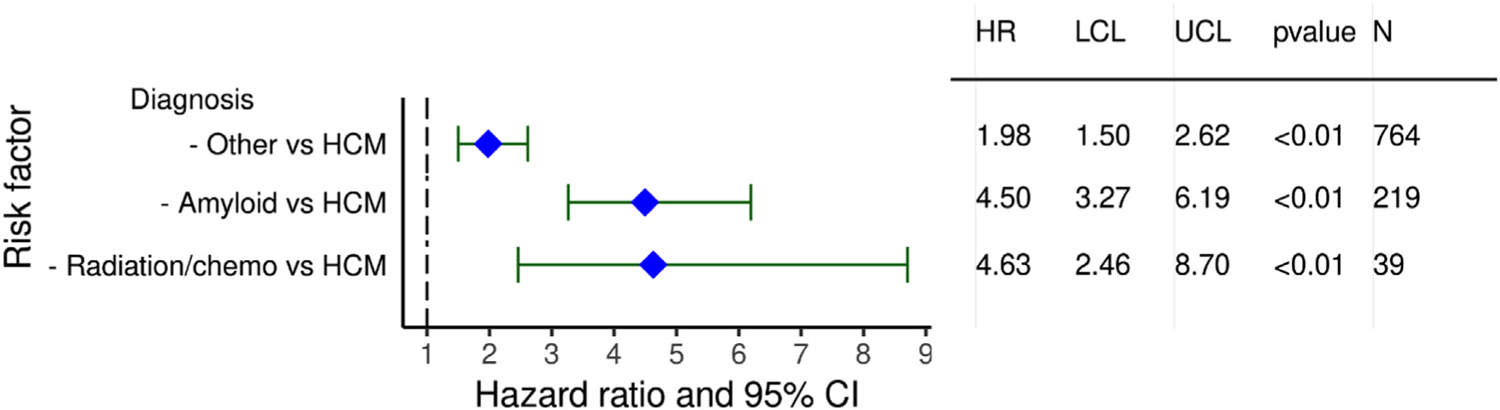
Statistically significant categorical risk factors for 5 year mortality conditional on survival to 1 year with 95% confidence limits (transplants January 1992-June 2013; n = 2490).
Discussion
This 2022 ISHLT Adult Heart Transplantation Report focused on recipients who underwent heart transplantation for HCM, amyloid, sarcoid, radiation and/or chemotherapy, and other types of RCM. We found that the number of patients transplanted for restrictive heart disease increased over time, few patients were bridged to transplant with mechanical circulatory support, and we describe differences in survival based on the underlying type of heart disease. While patients with restrictive cardiac physiology suffer from symptomatic heart failure,11 they represent a relatively small proportion of patients who receive heart transplant. This may be partly explained by the lower frequency of restrictive heart disease prevalence compared to ischemic and non-ischemic cardiomyopathy, but is also due to a lower access to transplant and a higher mortality on the waitlist. This appears to persist even after adjustments in allocation rules, for example, in the United States after 2018.12,13 Few patients with restrictive heart disease were bridged to transplant with mechanical circulatory support. As previously suggested, this likely reflects a significantly higher risk of adverse events after durable left ventricular assist device implant in patients with restrictive heart disease compared to patients with dilated and ischemic cardiomyopathy, including bleeding, right ventricular failure, renal failure, pump thrombosis and mortality.14–17
Restrictive heart disease has been identified as a risk factor for post-transplant mortality.6–10 Our report studied the effects of the restrictive disease type and patient characteristics on post-transplant survival and other outcomes. At first glance, it may appear surprising that type of heart disease pre-transplant was associated with such distinct differences in mortality after heart transplantation. However, when reviewing differences in age and treatment for each heart disease and their associations with systemic disease, differences in post-transplant mortality risk appear logical and likely. For instance, HCM is typically not associated with systemic extracardiac disease, and patients listed for transplant are often young, therefore the outcomes in this population tend to be good and similar to dilated cardiomyopathy. Sarcoidosis, on the other hand, is a systemic disease, yet with similarly favorable post-transplant survival (eSlide H(a) 29 and Figure 11, eSlide H(a) 34), likely due to the aligned effect of immunotherapy treatment post-heart transplantation on prevention of rejection and sarcoidosis (eSlide H(a) 20 and 22). Amyloid cardiomyopathy is a subcategory affecting older patients who often have comorbidities and systemic disease affecting many organs. Short term transplant survival is favorable but intermediate- and long-term survival is lower compared to the previously mentioned subgroups. Lastly, patients with a history of radiation and/or chemotherapy had the lowest survival compared to the other restrictive cardiomyopathies. This is likely related to the increased risk of surgery after chest radiation, complications from radiation-induced lung disease, and risk of post-transplant malignancy attributable to pre-transplant cancer and its treatment18–20
Limitations
The limitations of this study include possible inaccuracies of the clinical cardiac phenotype of restrictive heart disease pre-transplantation (to include the relatively large group of patients in the ‘other RCM’ category), limited granularity of data collected, including the type of amyloidosis, and potential errors in data entry which exists for all large databases. Only a few single center studies have assessed pathologic correlation of clinical diagnosis in restrictive heart disease, and most noted a high correlation except for the diagnosis of sarcoidosis, which often was misdiagnosed.21–24 Therefore, it is likely that sarcoidosis in our analysis may be an underrepresentation of those who actually received a transplant for sarcoidosis. Transthyretin amyloidosis, and some other types of amyloidosis were not well known in the earlier eras leading to possible under-representation of this entity or assignment to the ‘other RCM’ category as well. Distinguishing chemotherapy induced cardiomyopathy from those with concomitant radiation would be of importance, although the majority of these patients in our cohort likely did have radiation induced heart disease rather than chemotherapy induced disease, since most chemotherapeutic agents cause dilated rather than restrictive cardiomyopathy.
Conclusions
In this 2022 ISHLT Adult Heart Transplantation Report we describe a large cohort of patients who received transplant for restrictive heart disease. HCM was the most common reason for transplantation especially among young recipients while amyloidosis was more common among older recipients. Ventricular assist devices and intra-aortic balloon pumps were used more frequently over time to bridge to transplant but remained overall infrequent when compared to inotrope support. Transplant candidate selection was notable for most patients having normal renal function, normal bilirubin, and median pulmonary vascular resistance <2.5 Wood units at time of transplantation, underscoring the importance of optimizing hemodynamics and reducing mortality risk associated with pulmonary hypertension and right ventricular dysfunction.25 One-year survival was highest among recipients with sarcoid, HCM, and amyloidosis and lowest among those with radiation and/or chemotherapy-induced cardiomyopathy. Five-year survival conditional on survival to 1 year was highest in sarcoid and HCM while radiation and/or chemotherapy-induced cardiomyopathy and amyloidosis were lower. Lower survival with radiation and/or chemotherapy and amyloid cardiomyopathy underscores the importance of careful attention to patient selection and timing of transplant.
Supplementary Material
Disclosure statement
Michael Perch received an institutional research grant from Roche, Ambu, Zambon, consultant fees from Takeda, Zambon, PulmonX, Mallinkrodt, GSK, Novartis, Boeringer-Ingelheim. Michael Harhay received consulting fees from Trinity life sciences. Luciano Potena received consulting fees from Biotest, Novartis, Takeda, Sandoz and CareDx. Andreas Zuckermann Andreas Zuckermann served on the speakers bureau of Paragonix, Mallinckrodt, Medtronik and Franz Kohler Chemie, and received research grants from Biotest and Xvivo. Josef Stehlik received consulting fees for Medtronic, Natera, Sanofi-Aventis, Transmedics and research support from Natera; Wida S. Cherikh, Aparna Sadavarte, Kelsi Lindblad received funding from ISHLT; Don Hayes, Eileen Hsich and Tajinder P. Singh do not have any relevant financial disclosures.
The authors wish to thank Ms. Lyna Cherikh, United Network of Organ Sharing Research Intern, for her assistance with preparing the figures/table for the manuscript and reviewing the manuscript.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2022.07.018.
References
- 1.Seferovic PM, Polovina M, Bauersachs J, et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:553–76. [DOI] [PubMed] [Google Scholar]
- 2.Arbustini E, Narula N, Dec GW, et al. The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: endorsed by the World Heart Federation. J Am Coll Cardiol 2013;62:2046–72. [DOI] [PubMed] [Google Scholar]
- 3.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khush KK, Hsich E, Potena L, et al. International Society for H and Lung T. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-eighth adult heart transplantation report-2021; focus on recipient characteristics. J Heart Lung Transplant 2021;40:1035–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khush KK, Potena L, Cherikh WS, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics. J Heart Lung Transplant 2020;39:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg 2011;92:520–7. discussion 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsich EM, Blackstone EH, Thuita LW, et al. Heart transplantation: an in-depth survival analysis. JACC Heart Fail 2020;8:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson J, Ohlsson M, Hoglund P, Ekmehag B, Koul B, Andersson B. The International Heart Transplant Survival Algorithm (IHTSA): a new model to improve organ sharing and survival. PLoS One 2015; 10:e0118644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi JR, Cheng A, Ising M, Lenneman A, Birks E, Slaughter MS. Heart transplant survival based on recipient and donor risk scoring: a UNOS database analysis. ASAIO J 2016;62:297–301. [DOI] [PubMed] [Google Scholar]
- 10.Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg 2011;92:914–21. discussion 921–2. [DOI] [PubMed] [Google Scholar]
- 11.Charron P, Elliott PM, Gimeno JR, et al. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J 2018;39:1784–93. [DOI] [PubMed] [Google Scholar]
- 12.Hsich EM, Rogers JG, McNamara DM, et al. Does survival on the heart transplant waiting list depend on the underlying heart disease? JACC Heart Fail 2016;4:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loyaga-Rendon RY, Fermin D, Jani M, et al. Changes in heart transplant waitlist and posttransplant outcomes in patients with restrictive and hypertrophic cardiomyopathy with the new heart transplant allocation system. Am J Transplant 2021;21:1255–62. [DOI] [PubMed] [Google Scholar]
- 14.Chouairi F, Mullan CW, Sen S, et al. Impact of the new heart allocation policy on patients with restrictive, hypertrophic, or congenital cardiomyopathies. PLoS One 2021;16:e0247789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler CC, Helmers MR, Smood B, et al. The modified US heart allocation system improves transplant rates and decreases status upgrade utilization for patients with hypertrophic cardiomyopathy. J Heart Lung Transplant 2021;40:1181–90. [DOI] [PubMed] [Google Scholar]
- 16.Griffin JM, DeFilippis EM, Rosenblum H, et al. Comparing outcomes for infiltrative and restrictive cardiomyopathies under the new heart transplant allocation system. Clin Transplant 2020;34:e14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sreenivasan J, Kaul R, Khan MS, et al. Left ventricular assist device implantation in hypertrophic and restrictive cardiomyopathy: a systematic review. ASAIO J 2021;67:239–44. [DOI] [PubMed] [Google Scholar]
- 18.Al-Kindi SG, Oliveira GH. Heart transplantation outcomes in radiation-induced restrictive cardiomyopathy. J Card Fail 2016; 22:475–8. [DOI] [PubMed] [Google Scholar]
- 19.Saxena P, Joyce LD, Daly RC, et al. Cardiac transplantation for radiation-induced cardiomyopathy: the Mayo Clinic experience. Ann Thorac Surg 2014;98:2115–21. [DOI] [PubMed] [Google Scholar]
- 20.Batra J, DeFilippis EM, Golob S, et al. Impact of pretransplant malignancy on heart transplantation outcomes: contemporary united network for organ sharing analysis amidst evolving cancer therapies. Circ Heart Fail 2022;15:e008968. [DOI] [PubMed] [Google Scholar]
- 21.Raeisi-Giglou P, Rodriguez ER, Blackstone EH, Tan CD, Hsich EM. Verification of heart disease: implications for a New Heart Transplantation Allocation System. JACC Heart Fail 2017;5:904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts WC, Roberts CC, Ko JM, Filardo G, Capehart JE, Hall SA. Morphologic features of the recipient heart in patients having cardiac transplantation and analysis of the congruence or incongruence between the clinical and morphologic diagnoses. Medicine (Baltimore) 2014;93:211–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luk A, Metawee M, Ahn E, Gustafsson F, Ross H, Butany J. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can J Cardiol 2009;25:e48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu FY, Song LF, Liu L, et al. Clinicopathologic analysis of dilated heart in cardiac transplant recipients. Zhonghua Bing Li Xue Za Zhi 2007;36:796–800. [PubMed] [Google Scholar]
- 25.Bellettini M, Frea S, Pidello S, et al. Pretransplant right ventricular dysfunction is associated with increased mortality after heart transplantation: a hard inheritance to overcome. J Card Fail 2022;28:259–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


