In recent years, we have seen innovations in organ procurement and organ preservation, post-transplant management, and the use of non-transplant treatments for advanced heart and lung diseases. These developments continue to influence our decisions on recipient selection and transplantation. This focused report aims to document the changes that took place in the pediatric lung transplant recipient profile over the years and to identify important trends in recipient characteristics and their impact on post-transplant outcomes. This 24th annual Pediatric Lung Transplant Report is based on data submitted to the International Society for Heart and Lung Transplantation (ISHLT) International Thoracic Organ Transplant (TTX) Registry on 2,323 pediatric recipients of deceased donor lung transplants between January 1, 1992, and June 30, 2018. In response to a changing regulatory environment, the ISHLT Registry is undergoing an update in data acquisition, and the patient cohort examined in this Report is, therefore, derived from the same data source or datasets as that examined in the 2019 annual Reports.1–4 We refer the reader to the 2019 Report on pediatric lung transplantation for a detailed description of the baseline characteristics of the cohort and additional core analyses not directly related to the focus on the donor explored in this years Report. The Registry slide set available online (https://ishlt.org/research-data/registries/ttx-registry/ttx-registry-slides) provides more detail, additional analyses, and other information not included in this printed Report.
Statistical methods
Data collection, conventions, and statistical methods
Since the Registry’s inception, 481 heart transplant centers, 260 lung transplant centers, and 184 heart-lung transplant centers have reported data to the Registry. This years Report presents an overview of recipient characteristics and post-transplant outcomes, focusing on how the recipient profile has changed over time. The results reported herein seek to provide as granular detail as possible, with data retained in the ISHLT TTX Registry for transplants through June 30, 2018. With the current Report examining the same patient cohort as the 2019 Report,1 an overview of donor and recipient characteristics and outcomes is presented throughout those specific Reports for heart and lung transplantation in adults and children.1–4 In the 23rd annual ISHLT Registry Report on pediatric lung transplantation,5 we described changes in the donor profile over the previous 3 decades. In last years Report,5 we also described associations of donor characteristics with post-transplant survival at 1 year, at 5 years conditional upon surviving 1 year, and freedom from bronchiolitis obliterans syndrome (BOS). The goal of this year’s focused Report is to describe the changes in recipient characteristics over these years, to describe the trends in these outcomes in recipients with specific characteristics, and to identify important recipient and transplant characteristics that were associated with post-transplant survival at 1 year, at 5 years conditional upon surviving 1 year, and freedom from BOS conditional upon surviving to transplant discharge. This year’s pediatric lung transplant Report refers to specific online e-slides when particular data are discussed but not shown due to space limitations; eSlide L(p) refers to the online pediatric lung transplant slides.
The ISHLT TTX Registry website (https://ishlt.org/research-data/registries/ttx-registry) provides detailed spreadsheets of the data elements collected in the Registry. The Registry required submission of core donor, recipient, and transplant procedure variables around the time of transplantation and at yearly follow-up, and these variables, therefore, have low rates of missing data. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that rely on voluntary reporting. However, the Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
For this year’s Report, analyses of pediatric lung transplants do not include data for combined heart-lung or lung with other organ transplants. In addition, the Registry does not capture the exact occurrence date for most secondary outcomes (e.g., BOS), but it does capture the event within a period of time (i.e., between the first and the second-year annual follow-up visits). For the Report’s analyses, we use the mid-point between the annual follow-ups as a surrogate for the event date. On the follow-up where a death is reported, some under-reporting of secondary outcomes and other information is highly probable. Thus, to reduce the potential of underestimating event rates or other outcomes, we restrict some analyses to include only surviving recipients. For time-to-event analyses, we censor the follow-up of recipients who do not experience the event of interest at the last time the recipient was reported not to have had the event, which would either be the most recent annual follow-up or the time of retransplantation. We truncate time-to-event graphs (e.g., survival graphs) when the number of individuals at risk becomes <10.
The Registry Steering Committee selected recipient characteristics as the theme for the 2021 Report. To build upon last year’s Report on donor characteristics,5 this year’s Report concentrated on changes in the characteristics of the children who undergo lung transplantation, to further improve our understanding of donorrecipient matching to enhance outcomes for pediatric lung transplant recipients.
There is a paucity of information in the medical literature on changes in recipient characteristics for children undergoing lung transplantation One recent single-center study examined adult lung transplant recipient characteristics from January 1990 to January 2019.6 The authors reported that although 5-year survival had significantly improved in both the 1999–2008 and 2009–2018 eras compared to 1990–1998, there was no difference in survival comparing the 2 most recent eras.6 Noteworthy changes in the modern era included pulmonary fibrosis now being the leading indication for adult lung transplantation, median recipient age significantly increasing, and intraoperative mechanical circulatory support use significantly increasing.6 Our report, along with the companion Adult Lung TTX Report published in this issue of the journal,7 provides insight into significant changes in adult lung transplantation over the past 30 years in a large cohort of patients transplanted at multiple institutions in different regions. This year’s Registry Report, focusing on recipient characteristics expands upon previous work by Elgharably and colleagues6 and builds upon recent Registry Reports,1–2,5,7 and provides novel data in pediatric lung transplantation.
Recipient characteristics
With an objective to assess changes in recipient characteristics over time, we examined associations of these changes with outcomes. Table 1 (eSlides L(p) 4–6) outlines the characteristics of pediatric lung transplant recipients between January 1992 and June 2018 by era, unless otherwise noted in the table. For our analysis, we defined the eras as January 1992 to December 2000 (N = 592), January 2001 to December 2009 (N = 824), and January 2010 to June 2018 (N = 907). Locations of transplant centers were divided into Europe, North America, and Other regions (e.g., South America, Asia, the Middle East, Australia, and others). By era, there has been an increase in pediatric lung transplants performed in Europe and Other regions. In North America, the number increased between the first and second era but declined in the third era.
Table 1.
Recipient Characteristics by Era (Transplants: January 1992 to June 2018)
| Jan 1992-Dec 2000 (N = 592) | Jan 2001-Dec 2009 (N = 824) | Jan 2010-Jun 2018 (N = 907) | p-value | |
|---|---|---|---|---|
| Geographic Location: | ||||
| - Europe | 188 (31.8%) | 312 (37.9%) | 395 (43.6%) | <0.0001 |
| - North America | 391 (66.0%) | 457 (55.5%) | 417 (46.0%) | |
| - Other | 13 (2.2%) | 55 (6.7%) | 95 (10.5%) | |
| Age (yr) | 13 (0 – 17) | 14 (1 – 17) | 14.0 (1 – 17) | <0.0001 |
| Male | 43.1% | 41.6% | 390 (43.0%) | 0.8071 |
| Weight (kg) | 47.0 (40.0 – 70.4) | 47.6 (40.3 – 70.0) | 48.0 (40.0 – 70.3) | 0.6623 |
| Height (cm) | 160.0 (144.0 – 173.0) | 160.0 (149.0 – 175.0) | 158.0 (147.3 – 175.3) | 0.5553 |
| BMI (kg/m2) | 18.4 (15.7 – 26.9) | 18.9 (16.1 – 25.1) | 18.8 (15.7 – 26.4) | 0.5297 |
| Blood type: | ||||
| - A | 41.9% | 41.3% | 44.8% | 0.463 |
| - AB | 5.5% | 4.7% | 4.5% | |
| - B | 10.4% | 12.9% | 10.1% | |
| - O | 42.1% | 41.1% | 40.6% | |
| PRA ≥ 20% | 3.2% | 3.9% | 13.0% | <0.0001 |
| PRA ≥ 80% | 1.7% | 2.7% | 1.0% | 0.2741 |
| CMV antibody positive | 26.7% | 34.1% | 38.0% | 0.0042 |
| EBV antibody positive | 44.4%a | 54.4% | 52.7% | 0.4293 |
| Hep B antibody positive | 1.2%b | 4.7% | 4.1% | 0.0583 |
| Hep C antibody positive | 0.8%b | 0.9% | 0.2% | 0.3872 |
| History of malignancy | 2.7%b | 5.5% | 5.2% | 0.172 |
| Previous lung surgery | 29.2%b | 19.6% | 14.6% | 0.003 |
| Ventilator use | 36.6% | 37.1% | 42.2% | 0.0615 |
| ECMO use | 18.8% | 14.2% | 19.6% | <0.0001 |
| Hospitalized | 1.3% | 1.2% | 9.8% | 0.1921 |
| Bilirubin (mg/dl) | 0.4 (0.1 – 1.4)b | 0.3 (0.1 – 1.1) | 0.3 (0.1 – 1.2) | <0.0001 |
| Creatinine (mg/dl) | 0.5 (0.2 – 1.1)b | 0.5 (0.2 – 1.0) | 0.4 (0.1 – 0.8) | <0.0001 |
| GFR (ml/min/1.73 m2)c | 105.3 (57.1 – 175.9)b | 113.6 (65.7 – 211.3) | 134.0 (69.9 – 293.0) | <0.0001 |
| PCW mean (mm Hg) | 9.0 (1.0 – 22.0)b | 10.0 (4.0 – 20.0) | 11.0 (5.0 – 21.0) | <0.0001 |
| PA mean (mm Hg) | 50.0 (20.0 – 80.0)b | 30.0 (17.0 – 71.0) | 30.0 (13.0 – 75.0) | 0.0095 |
| PVR (Wood units) | 4.2 (2.2 – 18.8)b | 3.6 (2.0 – 10.0) | 3.9 (2.0 – 16.5) | <0.0001 |
| FEV1% predicted | 27.0 (15.0 – 81.0) | 26.0 (13.0 – 79.0) | 26.0 (12.0 – 81.0) | <0.0001 |
| FVC% predicted | 42.0 (20.0 – 89.0) | 40.0 (20.0 – 85.0) | 40.0 (15.0 – 81.0) | <0.0001 |
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein Barr virus; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GFR, Glomerular Filtration Rate; PA, pulmonary artery pressure; PCW, pulmonary capillary wedge; PRA, panel reactive antibody; PVR, pulmonary vascular resistance.
Continuous factors are expressed as median (5th to 95th percentiles).
Summary statistics included transplants with known/nonmissing data.
Based on Oct 1999 to Dec 2000 transplants.
Based on Apr 1994 to Dec 2000 transplants.
GFR was estimated using the modified Schwartz formula.
The median recipient age of pediatric lung transplant recipients has increased over time from 13 years old in January 1992 to December 2000 to 14 years old in January 2010-June 2018 (Table 1) (eSlides L(p) 4–6). More children with a panel of reactive antibodies ≥ 20% underwent a lung transplant in the last era. During this same time period, more children on extracorporeal membrane oxygenation (ECMO) underwent a lung transplant, which may partly explain the increase in allosensitization during this time period with increased risk of bleeding and need for blood product transfusions with ECMO. Over time, fewer children with previous lung surgery underwent a lung transplant, which could suggest lung transplantation was considered before other surgical procedures, such as pleurodesis, or that fewer patients with a history of lung surgery were evaluated for transplant.
Figure 1 (eSlides L(p) 7) illustrates trends of recipient blood type between January 1992 and June 2018 by location and era, with Other regions having more variation over time, which may reflect transplant programs initiation in new parts of the world. Figure 2 (eSlides L(p) 8) shows the recipient diagnosis distribution by location and era. In the most recent era, compared to 1992 to 2000, the number of lung transplants in children with cystic fibrosis (CF) declined in both Europe and Other regions while remaining unchanged in North America. Notably, surfactant deficiencies and pulmonary vascular disorders were grouped into the Other category. Although we do not have specific data on the reasons for the observed reduction of CF patients undergoing lung transplantation, it may be that this decline is related to the introduction of cystic fibrosis transmembrane conductance regulator (CFTR) modulators. A study examining data from the United States and the United Kingdom CF registries found that CFTR modulator therapies were associated with a significantly lower need for organ transplantation.8 With advancements in CF care and increased use of CFTR modulator therapy, we anticipate a continued decline or delay in the need for lung transplantation for both pediatric and adult patients.
Figure 1.

Recipient blood type distribution by location and era (transplants: January 1992 to June 2018).
Figure 2.

Recipient diagnosis distribution by location and era (transplants: January 1992 to June 2018).
The distribution of donor-recipient sex matching by location and era observed in Figure 3 (eSlides L(p) 9) shows a rise in female donors in Europe compared to North America and Other regions. We reported an increase in female donors occurring specifically in Europe in last year’s Registry report.1 With more female donors in Europe, there has been an increase in donor-recipient sex matching for females and reduced for male recipients.
Figure 3.

Donor-recipient sex distribution by location and era (transplants: January 1992 to June 2018).
Figure 4 (eSlides L(p) 10) shows median recipient age by location, median recipient body mass index (BMI) by location, and overall median recipient estimated glomerular filtration rate (eGFR). Median recipient age (Figure 4A) (eSlides L(p) 10) and BMI (Figure 4B) (eSlides L(p) 10), varied over time across all 3 regions (Europe, North America, and Other). Figure 4C (eSlides L(p) 10) shows overall eGFR increasing over time. The eGFR was estimated using the modified Schwartz formula. Caution needs to be applied to eGFR results as this could be overestimated in children, especially if there is an increase in body weight without a proportional increase in serum creatinine.
Figure 4.

Median recipient (A) age (transplants: January 1992 to June 2018), (B) BMI (transplants: January 1992 to June 2018), and (C) and Glomerular Filtration Rate (GFR) by geographic location (transplants: January 1992 to June 2018). GFR was estimated using the modified Schwartz formula.
Survival
Survival within 12 months of transplantation
To determine how recipient characteristics influenced short-term post-lung transplant survival in pediatric lung transplant recipients, we used univariate analysis with Kaplan-Meier curves to assess survival within 12 months of lung transplantation in children between January 2000 and June 2017. The cohorts were divided into 3 eras: 2000–2005, 2006–2011, and 2012–2017. Survival for pediatric lung transplant recipients within 12 months was highest in the most recent era compared to the 2000–2005 era (Figure 5) (eSlides L(p) 12). We expanded our analysis and added location, which demonstrated that the improvement in 12-month survival for 2012–2017 was most pronounced in Europe (Figure 6) (eSlides L(p)).
Figure 5.

Kaplan-Meier survival within 12 months by era (transplants: January 2000 to June 2017).
Figure 6.
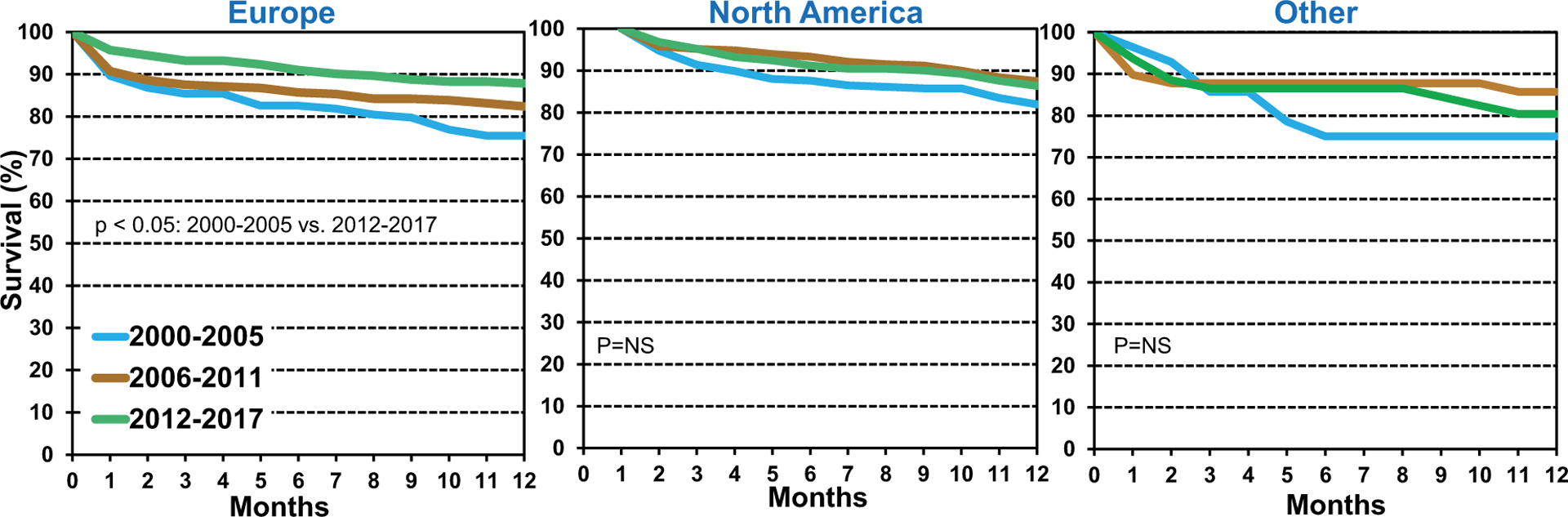
Kaplan-Meier survival within 12 months by location and era (transplants: January 2000 to June 2017).
We next explored whether age influenced survival within 12 months of lung transplant for children but found no statistically significant association by location and recipient age (Figure 7) (eSlides L(p) 14). Notably, there were no lung transplants in children between 0 and 5 years of age in the Other region between January 2000 and June 2017. Survival in 11–17-year-old lung transplant recipients has significantly improved over time compared to the 2000–2005 and 2012–2017 eras Figure 8 (eSlides L(p) 15). Historically, adolescent lung transplant recipients have had poorer outcomes than younger children and adults,9 so it is reassuring there appears to be an improvement in the most recent era for this vulnerable patient population.
Figure 7.

Kaplan-Meier survival within 12 months by location and recipient age (transplants: January 2000 to June 2017).
Note: There were no transplants in recipients 0 to 5 years in “Other” location.
Figure 8.

Kaplan-Meier Survival within 12 months by era and recipient age (transplants: January 2000 to June 2017).
We next explored the impact of recipient diagnosis on 12-month survival and found that CF had the highest survival (Figure 9A) (eSlides L(p) 16). Retransplant had the lowest survival during the first post-transplant year as compared to CF and Other diagnoses. Given the lack of outcome data on pediatric lung retransplantation with the last published article analyzing children in the United States transplanted from May 1988 to May 2008,10 we have identified an area that needs further analysis as more children undergo lung retransplantation.
Figure 9.

Kaplan-Meier survival within 12 months by recipient (A) diagnosis and (B) glomerular filtration rate (GFR) (transplants: January 2000 to June 2017). GFR was estimated using the modified Schwartz formula.
Due to the median recipient eGFR increasing over time as outlined in Figure 4C (eSlides L(p) 10), we explored the effect of recipient eGFR on the 12-month survival. Survival was significantly higher in children with an eGFR ≥ 90 ml/min/1.73 m2 (Figure 9B) (eSlides L(p) 16). In additional analyses, we found that bilateral/ double lung transplants in children were associated with increased survival (eSlides L(p) 17), and recipient history of malignancy was not associated with lower post-transplant survival (eSlides L(p) 17).
Survival within 5 years of transplantation conditional on survival to 1 year
To explore the effect of recipient characteristics on longer-term post-lung transplant survival in children, we used univariate analysis with Kaplan-Meier survival curves to determine survival within 5 years of transplantation conditional on survival to 1 year. The cohorts were divided into 3 eras: 1996–2001, 2002–2007, and 2008–2013. Figure 10 (eSlides L(p) 19) shows a longer-term survival advantage associated with pediatric recipients who underwent a lung transplant in the most recent era compared to the 1996–2001 era. Unlike our short-term analysis, when we included location to the survival analysis by era, we found no differences between Europe, North America, and Other regions (Figure 11) (eSlides L(p) 20).
Figure 10.

Kaplan-Meier survival within 5 years conditional on survival to 1 year by era (transplants: January 1996 to June 2013).
Figure 11.

Kaplan-Meier survival within 5 years conditional on survival to 1 year by location and era (transplants: January 1996 to June 2013).
Note: * There were no transplants during 1996 to 2001 in “Other” location.
To determine if age influenced long-term survival of pediatric lung transplant recipients, we analyzed recipient age by location and found no difference in survival by age in Europe or North America (Figure 12) (eSlides L(p) 21). Notably, there were 72 lung transplants performed in patients 11 to 17 years of age and no transplants in recipients <11 years of age in Other regions between January 1996 and June 2013, so that data was not included in our analysis. Moreover, we examined long-term survival by recipient age and era, and found no significant differences over time, as illustrated in Figure 13 (eSlides L(p) 22).
Figure 12.
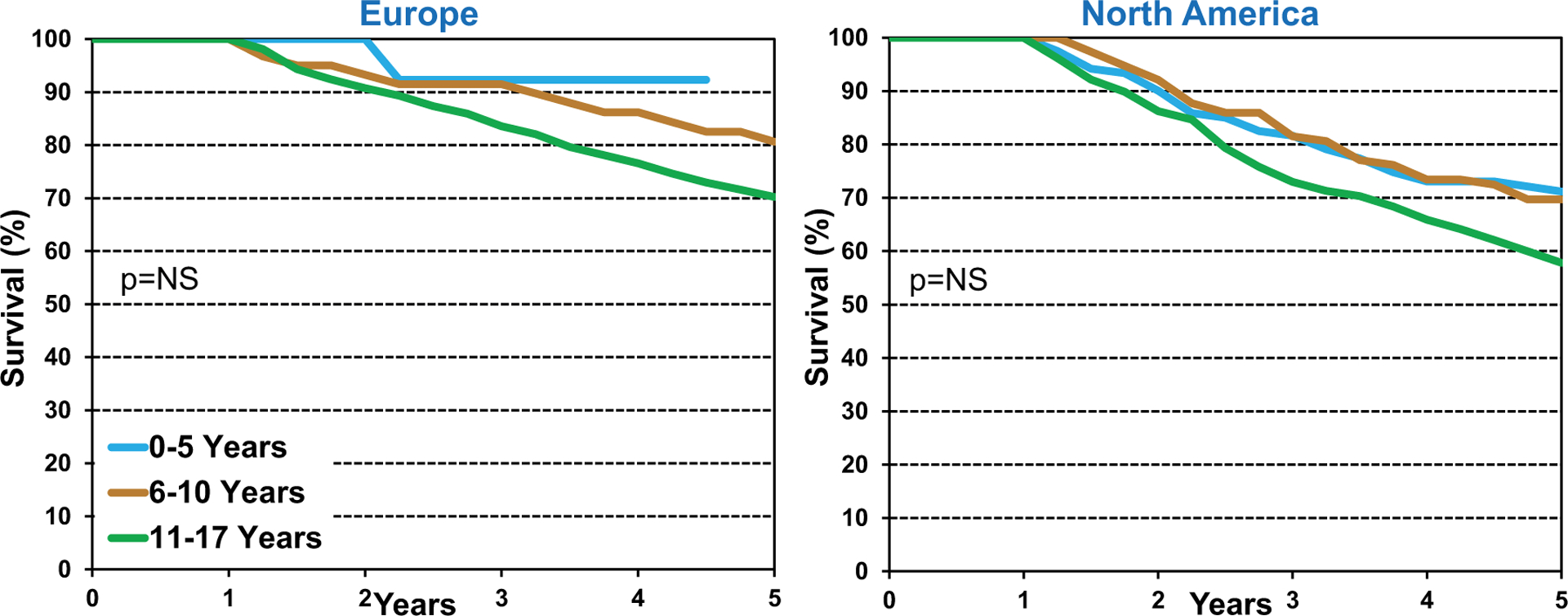
Kaplan-Meier survival within 5 years conditional on survival to 1 year by location and recipient age (transplants: January 1996 to June 2013).
Note: There were 72 transplants in recipients 11 to 17 years and no transplants in recipients <11 years in “Other” location.
Figure 13.

Kaplan-Meier survival within 5 years conditional on survival to 1 year by recipient age and era (transplants: January 1996 to June 2013).
Since we identified associations between recipient diagnosis (Figure 9A) (eSlides L(p) 16) and recipient eGFR (Figure 9B) (eSlides L(p) 16) and short-term post-transplant survival, we next explored if there was an effect on long-term survival (5-year survival conditional on survival to one year) by both of these recipient characteristics. We found no difference in long-term survival by recipient diagnosis (Figure 14A) (eSlides L(p) 23) or recipient eGFR (comparing eGFR < 90 ml/min/1.73 m2 and eGFR ≥ 90 ml/min/1.73 m2) (Figure 14B) (eSlides L(p) 23). As with our shortterm analysis, we also examined the effect of bilateral/double lung transplant (eSlides L(p) 24) and recipient history of malignancy (eSlides L(p) 24) in pediatric lung transplant recipients, and neither of these variables were associated with survival.
Figure 14.

Kaplan-Meier survival within 5 years conditional on survival to 1 year by recipient (A) diagnosis and (B) glomerular filtration rate (GFR) (transplants: January 1996 to June 2013). GFR was estimated using the modified Schwartz formula
Freedom from bronchiolitis obliterans syndrome conditional on survival to discharge
Due to the effect of bronchiolitis obliterans syndrome (BOS) on long-term survival after lung transplantation,11–12 we examined freedom from BOS conditional on survival to discharge by era and recipient age between January 1996 and June 2013 as depicted in Figure 15 (eSlides L(p) 26). The cohorts were divided into 3 eras: 1996–2001, 2002–2007, and 2008–2013. Figure 15A (eSlides L(p) 26) shows no difference in freedom from BOS conditional on survival to discharge across the 3 eras. Conversely, our analysis examining the effect of age found that children under 5 years of age had increased freedom from BOS compared to older children (Figure 15B) (eSlides L(p) 26).
Figure 15.

Freedom from bronchiolitis obliterans syndrome (BOS) conditional on survival to discharge by (A) era and (B) recipient age (transplants: January 1996 to June 2013).
Multivariable cox analyses
We next performed multivariable Cox proportional hazards regression analyses to identify independent risk factors for and potential risk factors associated with mortality within 12 months of transplantation, within 5 years of transplantation conditional on survival to 1 year, and BOS within 5 years conditional on survival to discharge in pediatric lung transplant recipients. Covariates included in the multivariable models are listed in Supplemental Table 1. These analyses establish independent associations between risk factors and outcomes but cannot establish causality. It is important to acknowledge that categorical and continuous risk factors for post-transplant mortality will differ by time since transplant, and long-term data reflect patients transplanted in earlier eras, and the findings may not apply to current conditions. Therefore, specific associations observed among these data should be interpreted with caution and are better explored in more detailed analyses of Registry data.
Assessing children who underwent lung transplantation between January 2000 and June 2017, our 1-year mortality analysis found the only statistically significant categorical risk factors were ventilator use and procedure type (single vs bilateral/double lung transplant) (Figure 16) (eSlides L(p) 28). Therefore, clinicians need to remain aware of the risk of lower survival in children on mechanical ventilation at the time of transplant or undergoing a single lung transplant. Similar to last year, this years analysis found donor age to be a significant risk factor for 1-year mortality, with a higher risk observed in younger and older donors (Figure 17) (eSlides L(p) 30).
Figure 16.

Statistically significant categorical risk factors for 1-year mortality with 95% confidence limits (transplants: January 2000 to June 2017; N = 1,681).
Note: None of the interaction terms (era*recipient age, era*diagnosis, era*GFR) were significant
Figure 17.

Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by donor age (transplants: January 2000 to June 2017; N = 1,681).
When we investigated longer-term post-lung transplant survival among children who underwent lung transplant between January 1996 and June 2013, we found that the only statistically significant categorical risk factor was transplant era (2008–6/2003 vs 1996–2001) (eSlides L(p) 32). Thus, it is reassuring that outcomes appear to be improving over time. The only continuous variable found to be significant was recipient age, with higher mortality among teenage recipients (Figure 18) (eSlides L(p) 34). Our findings support that both recipient and donor characteristics influence outcomes substantiate the importance of the work performed for this years and last years Registry Report1 as we attempt to optimize donor-recipient matching for lung transplantation, especially in children.
Figure 18.
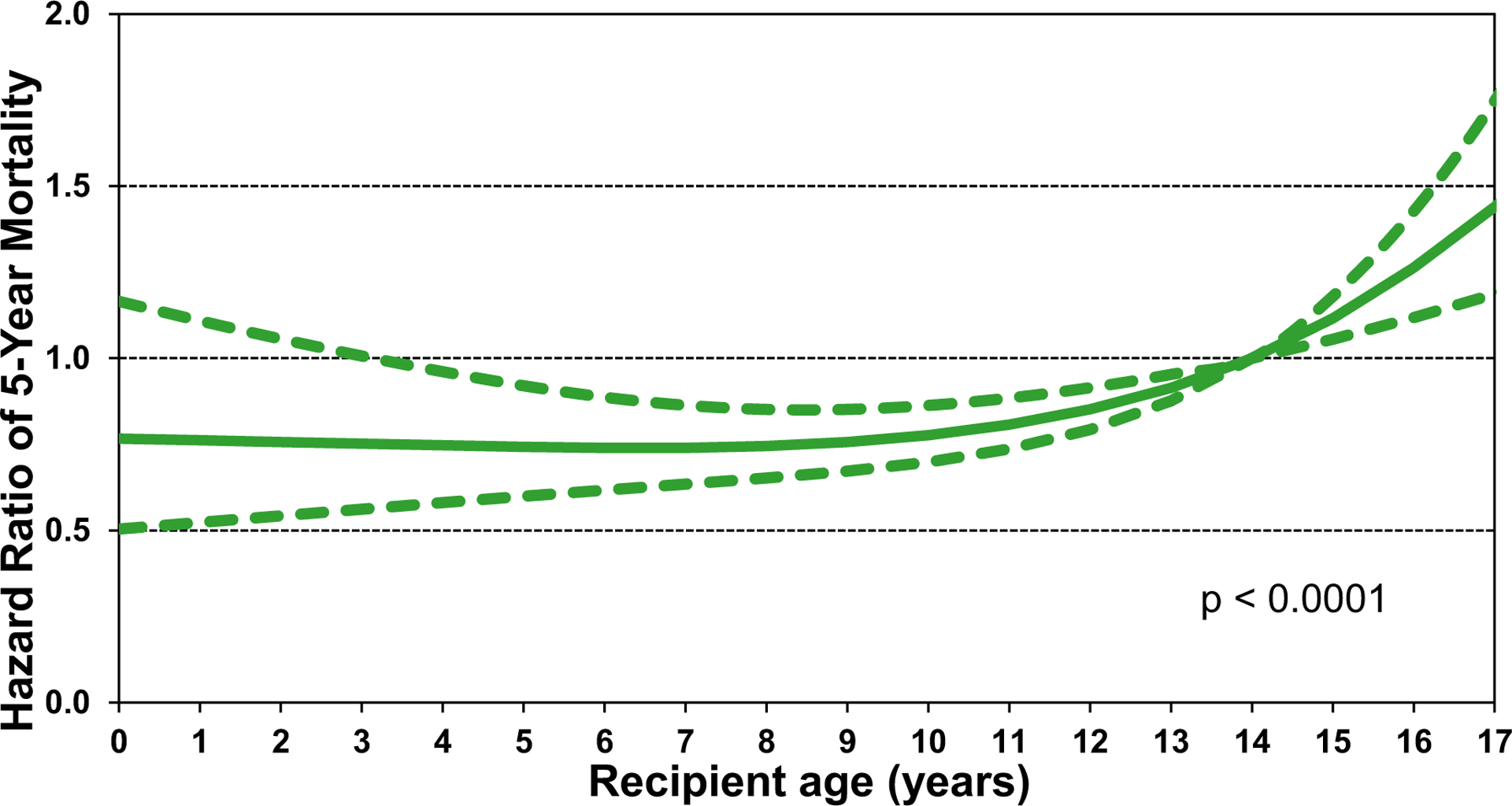
Multivariable hazard ratio for 5-year mortality conditional on survival to 1 year with 95% confidence limits, by recipient age (transplants: January 1996 to June 2013; N = 1,226).
When we investigated risk factors for BOS within 5 years (conditional on survival to discharge) among children who underwent lung transplantation between January 1996 and June 2013, we found that older recipient age was significantly associated with increased risk of BOS (Figure 19) (eSlides L(p) 37). Surprisingly, there is little data in the medical literature addressing the effect of recipient age on BOS development in pediatric lung transplant recipients. Our finding may provide insight into the observed inferior outcomes reported in adolescent lung transplant recipients, compared to younger children and adults.9 However, further research is needed to better understand this and to improve longterm outcomes.
Figure 19.

Multivariable hazard ratio plot for bronchiolitis obliterans syndrome (BOS) within 5 years conditional on survival to discharge with 95% confidence limits, by recipient age (transplants: January 1996 to June 2013; N = 666).
Conclusions
Building upon the last two annual Reports,1,5 the 2021 ISHLT TTX Registry Report on pediatric lung transplantation provides an update on key factors related to recipient characteristics. As we witness continued improvements in children’s survival after lung transplants, it is essential to examine all facets of the transplantation process to facilitate further advancements. Due to the relatively small sample size, these results should be interpreted cautiously, even if we found statistical significance. Despite this limitation, this year’s Report provides insight into important variables regarding recipient characteristics that can help clinicians with limited available evidence in this patient population. With the results presented here, we can see that key characteristics are evolving for children who undergo lung transplantation, and additional work is needed to continue advancing the field.
Supplementary Material
Acknowledgment
The authors wish to thank Ms. Lyna Cherikh, United Network of Organ Sharing Research Department Summer Intern, for her assistance with preparing the figures/table for the manuscript and reviewing the manuscript.
Disclosure statement
Daniel C. Chambers received travel support from Astellas Pharma, Inc, and serves as a consultant and speaker for Roche Ltd; Kiran K. Khush serves as a consultant and speaker for CareDx, Inc; Josef Stehlik serves as a consultant for Medtronic, receives research support from Natera, and received funding from ISHLT; Michael Perch receives research funding from Roche, travel support from Boeringer-Ingelheim, and is a speaker for Mallinckrodt, Glaxo Smith Kline, and Astra-Zeneca; For pediatric lung paper: Wida S. Cherikh, Aparna Sadavarte, and Sarah Booker received funding from ISHLT; Don Hayes, Jr.; Michael O. Harhay; Eileen Hsich; Luciano Potena; and Tajinder P. Singh do not have any relevant disclosures.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2021.07.018.
References
- 1.Hayes D Jr, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-second pediatric lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossano JW, Singh TP, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-second pediatric heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes D Jr, Harhay MO, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-third pediatric lung transplantation report - 2020; focus on deceased donor characteristics. J Heart Lung Transplant 2020;39:1038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgharably H, Ayyat KS, Okamoto T, et al. Evolution of recipient characteristics over 3 decades and impact on survival after lung transplantation. Transplantation 2021. 10.1097/TP.0000000000003756. [DOI] [PubMed] [Google Scholar]
- 7.Chambers DC, Zuckermann A, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 38th adult lung transplantation report - 2021; focus on recipient characteristics. J Heart Lung Transplant. 2021. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessonova L, Volkova N, Higgins M, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 2018;73:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paraskeva MA, Edwards LB, Levvey B, et al. Outcomes of adolescent recipients after lung transplantation: an analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant 2018;37:323–31. [DOI] [PubMed] [Google Scholar]
- 10.Scully BB, Zafar F, Schecter MG, et al. Lung retransplantation in children: appropriate when selectively applied. Ann Thorac Surg 2011;91:574–9. [DOI] [PubMed] [Google Scholar]
- 11.Kirkby S, Hayes D Jr.. Pediatric lung transplantation: indications and outcomes. J Thorac Dis 2014;6:1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes D Jr, Benden C, Sweet SC, Conrad CK. Current state of pediatric lung transplantation. Lung 2015;193:629–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


