ABSTRACT
M1 large circumferential aneurysms are clinically challenging because they cannot be treated by simple neck clipping and they may involve the lenticulostriate arteries (LSAs). Although some reports have described endovascular and direct surgical treatment of these aneurysms, the optimal treatment approach remains uncertain. We report a case involving a ruptured large M1 circumferential aneurysm that was treated with bypass-assisted trapping surgery and showed favorable outcomes. The patient was a 47-year-old man presenting with subarachnoid hemorrhage. Digital subtraction angiography revealed a large circumferential aneurysm in the right middle cerebral artery M1 segment with involvement of the lateral and medial LSAs. We successfully performed trapping surgery with the assistance of a superficial temporal artery (STA)-M2 bypass while preserving the medial and lateral LSAs. Although left hemiparesis caused by medial LSA thrombosis appeared in the early postoperative period, the patient showed good recovery from symptoms with rehabilitation and could independently perform daily activities at the five-month follow-up. The treatment of M1 large circumferential aneurysms should involve considerations for prevention of rebleeding, blood supply to the distal area, and preservation of perforating arteries. The treatment strategy for this challenging aneurysm should be planned based on the patient’s condition and individual anatomy.
Key Words: bypass, middle cerebral artery, circumferential aneurysm, lenticulostriate artery, trapping surgery
INTRODUCTION
Clinical management of large circumferential aneurysms, which are large fusiform aneurysms, is extremely challenging.1 In comparison with posterior circulation, large circumferential aneurysms are rarely found in the middle cerebral artery.2 Unlike usual saccular aneurysms, these aneurysms cannot be treated by simple neck clipping.3 Endovascular treatment with a flow-diverting stent has been reported, but this technique has been associated with hemorrhagic complications, and its long-term outcomes remain unclear.4-7 Trapping surgery with bypass assistance seems to be a radical treatment for ruptured cases, but the ischemic complications caused by perforating arteries remain an unsolved problem associated with this technique.8-11 Therefore, the treatment of this challenging aneurysm remains unestablished.
We report a case of a ruptured large M1 circumferential aneurysm with bypass-assisted trapping surgery that showed a favorable outcome.
CASE REPORT
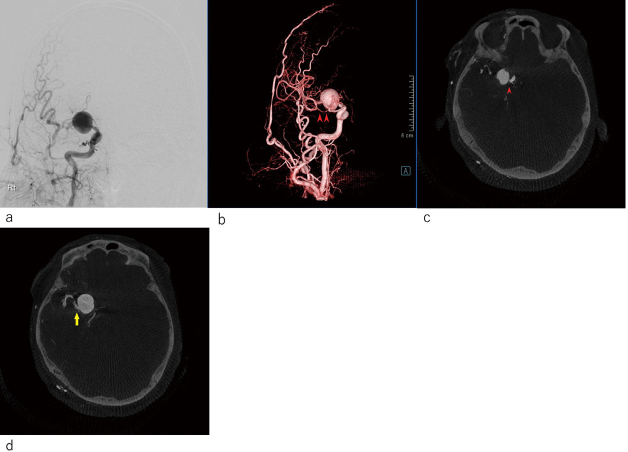
The patient was a 47-year-old man who presented with a chief complaint of severe headache. Head computed tomography at another hospital showed Fisher 3 subarachnoid hemorrhage. His Glasgow Coma Scale score was E3V4M6. Three-dimensional computed tomography angiography revealed a large circumferential aneurysm in the right middle cerebral artery (MCA) M1 segment. The patient was transferred to our hospital for surgical treatment. Digital subtraction angiography showed that the insular and cortical segments of the right MCA were enhanced in the delayed phase (Figure 1a). Three-dimensional digital subtraction angiography showed that the anterior temporal artery originated from the distal part of the M1 near the aneurysm (Figure 1b). Multiplanar reconstruction based on three dimensional digital subtraction angiography images also showed that some of the perforating arteries were involved. The medial lenticulostriate arteries (LSAs) had branched off just proximal to the aneurysm (Figure 1c), and the lateral LSA had also branched off just distal to the aneurysm (Figure 1d). However, no perforating artery was observed to arise from the aneurysmal wall. The maximum diameter of the aneurysm was 22 mm. We planned a bypass-assisted trapping surgery. A double-barrel STA-MCA bypass could not be applied since the superficial temporal artery (STA) frontal branch was hypoplastic. On the basis of the digital subtraction angiography findings, we estimated that the collateral blood flow in the left MCA area was sufficient. Therefore, we decided to perform trapping surgery with the assistance of the STA-M2 bypass while preserving the medial and lateral LSAs. The anterior temporal artery originating from the distal M1 near the aneurysm could be used as a flow outlet to avoid thrombosis of the lateral LSA (Figure 2).
Fig. 1.
Preoperative radiological examination
Fig. 1a: Digital subtraction angiography (DSA) showed that the insular segment and cortical segment of right middle cerebral artery were enhanced in the delayed phase.
Fig. 1b: Three-dimensional DSA (3-D DSA) showed that the anterior temporal artery originated from the distal part of M1 nearby aneurysm (arrowheads).
Fig. 1c: Slab multiplanar reconstruction (MIP) of 3-D DSA showed the medial lenticulostriate arteries (arrowhead) were branched off just proximal to the aneurysm.
Fig. 1d: The lateral lenticulostriate arteries (arrow) were branched off just distal to the aneurysm.
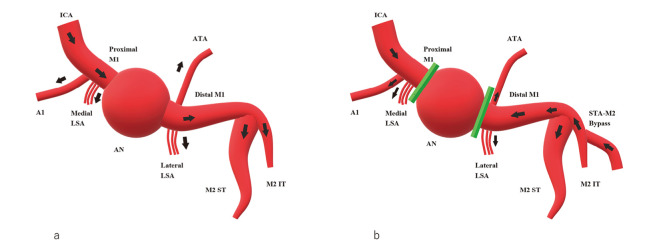
Fig. 2.
Schematic drawing of the surgery
(a) Preoperative and (b) postoperative hemodynamics are shown. The arrow shows the direction of blood flow. Preoperatively, the right internal carotid artery perfused the right anterior cerebral artery A1 portion and the middle cerebral artery, including the aneurysm. Postoperatively, the internal carotid artery supplied the right A1 and medial lenticulostriate arteries with antegrade blood flow. The aneurysm was trapped, and the distal M1 lesion, including the lateral lenticulostriate artery and anterior temporal artery, were supplied with retrograde blood flow of the superficial temporal artery-middle cerebral artery M2 portion bypass.
ICA: internal carotid artery
LSA: lenticulostriate artery
ATA: anterior temporal artery
ST: superior trunk
IT: inferior trunk
Operation
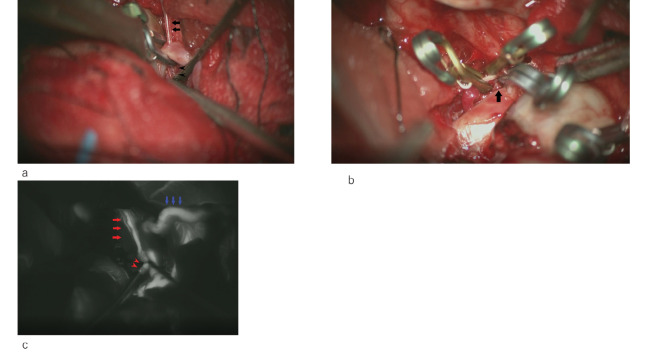
The operation was performed under general anesthesia with monitoring of the somatosensory evoked potentials of the right extremities and motor evoked potential of the right upper extremity. A right front temporal skin incision was made, and the STA parietal branch was harvested under a microscope. A front temporal craniotomy was then performed. After opening the dura mater, the distal Sylvian fissure was dissected under a microscope, the hematoma was irrigated and removed, and the M2 portion was exposed. Subsequently, we elevated the frontal lobe, secured the right internal carotid artery, and opened the lamina terminalis and Liliquist membrane to evacuate the cerebrospinal fluid. We elevated the more slacked frontal lobe and secured the hypoplastic right A1 portion. Internal carotid artery (ICA) bifurcation was identified at the same time, and the M1 proximal portion between the ICA terminalis and aneurysm was found to be very short. After securing the ICA and A1 and the M1 proximal portion, we dissected the proximal Sylvian fissure and exposed the M1-M2 bifurcation, M1 distal portion, and part of the anterior wall of the aneurysm. We then performed an STA-M2 (inferior trunk) bypass. The total clamp time of the MCA was 20 min 41s. The patency of the STA-M2 bypass was confirmed using microvascular Doppler. After completing the anastomosis, we applied a temporary clip to the ICA and dissected the aneurysm. During dissection, the aneurysm ruptured prematurely, and a temporary clip was applied to the right ICA and A1. We then applied a permanent clip for the M1 distal portion just distal to the aneurysm to obliterate retrograde flow from the STA-M2 bypass while preserving the anterior temporal artery and lateral LSA with careful manipulation (Figure 3a). The distal permanent clip stopped bleeding, and the aneurysm was dissected again. We barely applied a permanent clip to the very short M1 proximal portion while preserving the medial LSA with careful manipulation (Figure 3b). After trapping the aneurysm, we removed the temporary clip of the A1 and ICA. The total clamp time of the ICA was 9 min 21 s. No significant change in the somatosensory evoked potentials and motor evoked potential was noted during clamping. Indocyanine green video-angiography showed perfect patency of the STA-M2 bypass and blood flow to the M2 superior and inferior trunk, M1 distal portion, anterior temporal artery, and lateral LSA (Figure 3c).
Fig. 3.
Intraoperative view
Fig. 3a: Permanent clip for the M1 distal portion was applied just distal to the aneurysm to obliterate retrograde flow from the STA (superficial temporal artery)-M2 bypass to the aneurysm while preserving the anterior temporal (arrows) artery and lateral LSA (arrowheads).
Fig. 3b: Proximal permanent clip was barely applied to the very short M1 proximal portion while preserving medial LSA (arrow).
Fig. 3c: ICG video angiography showed perfect patency of the STA-M2 bypass (blue arrows) and retrograde blood flow to the M2 superior and inferior trunk, M1 distal portion, anterior temporal (red arrows) artery, and lateral LSA (arrowheads).
Postoperative course
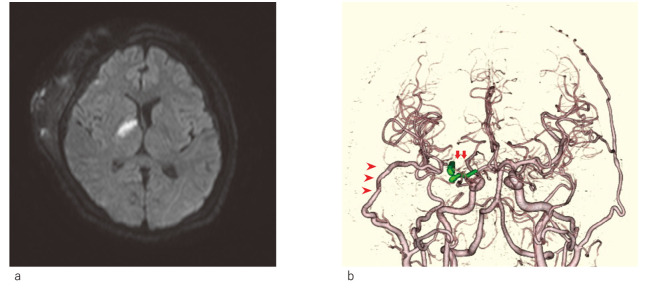
The patient showed no increase in hematoma on postoperative head computed tomography. Antiplatelet therapy was initiated with cilostazol (200 mg/day) and ozagrel sodium (80 mg/day) the day after the operation to prevent thrombosis of the medial LSA. However, the patient showed left hemiparesis (manual muscle test = 3) on day 3. The head magnetic resonance imaging diffusion-weighted image showed a fresh infarction of the right internal capsule (Figure 4a). Fortunately, the symptoms recovered well after rehabilitation for a while, and the patient did not show additional neurological symptoms. Three-dimensional computed tomography angiography showed excellent bypass patency and obliteration of the aneurysm (Figure 4b). The patient was discharged to the rehabilitation hospital on day 41 with a modified Rankin scale score of 2 and a Glasgow Coma Scale score of E4V5M6. At the 5-month follow-up evaluation at the outpatient clinic, the patient showed no neurological deficits and could perform daily activities independently.
Fig. 4.
Postoperative radiological examination
Fig. 4a: Postoperative diffusion-weighted imaging (DWI) showed fresh infarction of the right internal capsule.
Fig. 4b: Three-dimensional computed tomography angiography (3DCTA) showed perfect patency of the STA-M2 bypass (arrowheads) and complete obliteration of the aneurysm (arrows).
DISCUSSION
M1 large circumferential aneurysms are clinically challenging, and their treatment has been a topic of debate. The three major challenges in treating M1 large fusiform aneurysms can be summarized as prevention of rebleeding, ensuring blood supply to the distal area, and preservation of the perforating arteries. Simple clipping techniques cannot be applied to prevent rebleeding in cases with this aneurysm. Although endovascular treatments using a flow-diverting device have been reported for such cases, their long-term outcomes remain uncertain.12 Moreover, the use of a stent graft, including a flow-diverting device, in the acute phase of hemorrhagic stroke is regarded as an off-label use under the Japanese health insurance system. Staged ICA occlusion or proximal artery occlusion with the assistance of an extracranial - intracranial bypass has also been reported, but this approach is associated with the risk of rebleeding between the first extracranial - intracranial bypass operation and the second ICA occlusion surgery.13 A staged ICA occlusion with extracranial - intracranial bypass could be a potential treatment for unruptured cases, but we could not overcome the concerns regarding rebleeding. Therefore, we decided to perform a bypass-assisted trapping surgery in this case. Vascular reconstruction is necessary to ensure the blood supply to the distal area. The existing options for vascular reconstruction include STA-MCA low-flow bypass, STA- radial artery graft -M2 high-flow bypass, and direct M1 reconstruction with an interposition graft.3,9 High-flow bypass is a reliable option for ensuring sufficient blood supply to the entire MCA lesion. However, the procedure is somewhat complicated and more invasive than a low-flow bypass. Moreover, the handling of long interposition grafts through the submandibular tunnel is associated with some complications, and the failure of high-flow bypass can cause catastrophic outcomes. In our case, preoperative Magnetic Resonance Angiography did not show the right MCA lateral to the aneurysm, while angiography showed that the cortical MCA area was stained in the delayed phase. On the basis of these findings, we assumed that the collateral blood supply to the MCA cortical area was sufficient, and we chose the STA-M2 bypass as an insurance bypass procedure.
Even if an adequate extracranial - intracranial bypass is successfully performed and sufficient blood supply to the distal MCA lesion is acquired, thrombosis of the perforating artery can occur as a result of a blind alley when the aneurysm is trapped. Thus, an outlet for the M1 blood flow is necessary to avoid thrombosis of the perforating artery. In some cases, the anterior temporal artery and orbitofrontal artery can be used as an innate flow outlet, but their use depends on the individual anatomy, and these natural flow outlets may not be available in all patients. We propose that the optimal treatment to prevent thrombosis of the perforating artery is direct reconstruction of the original artery with an interposition graft. Successful reconstruction of M1 can result in antegrade blood flow without a blind alley and ensure sufficient blood flow to the distal MCA lesion.9 However, the operability of direct M1 reconstruction depends on the margin of the MCA after resection of the aneurysm. When we secured the proximal M1, we found that the proximal M1 was so short that end-to-end anastomosis could not be performed. Therefore, we gave up direct M1 reconstruction with an interposition graft and trapped the aneurysm under the assistance of the STA-M2 bypass. In our case, the anterior temporal artery was fortunately available as an outlet for reversal of the blood flow from the STA-M2 bypass, and thrombosis of the lateral LSA was avoided. However, the medial LSA, which was structurally preserved in the operation but was very close to the proximal clip, was thrombosed and the patient had an infarction. Although the patient subsequently recovered, the findings outline the importance of a flow outlet or sufficient margin of the blind alley to prevent thrombosis of the perforating artery. Bypass-assisted trapping of an aneurysm is a radical treatment for preventing rebleeding, but this procedure is frequently accompanied by thrombosis of the perforating artery. Therefore, the surgical strategy for M1 large fusiform aneurysms should be planned on the basis of individual anatomical characteristics and the patient’s condition.
CONCLUSION
We report a case of a large M1 fusiform aneurysm that was treated with bypass-assisted trapping surgery and showed favorable outcomes. This procedure is an optimal treatment for prevention of rebleeding, but a surgical strategy based on the individual anatomy should be planned to prevent thrombosis of the perforating artery.
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
DISCLOSURE
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
ETHICAL APPROVAL
No approval from the IRB was sought as this article was case report as per the ethical guidelines of Japan Neurosurgical Society.
INFORMED CONSENT
The authors obtained written informed consent from the patient and his family.
Abbreviations
- ICA
internal carotid artery
- LSA
lenticulostriate artery
- MCA
middle cerebral artery
- STA
superficial temporal artery
REFERENCES
- 1.Guo Y, Song Y, Hou K, Yu J. Intracranial Fusiform and Circumferential Aneurysms of the Main Trunk: Therapeutic Dilemmas and Prospects. Front Neurol. 2021;12:679134. doi: 10.3389/fneur.2021.679134. [DOI] [PMC free article] [PubMed]
- 2.Day AL, Gaposchkin CG, Yu CJ, Rivet DJ, Dacey RG Jr. Spontaneous fusiform middle cerebral artery aneurysms: characteristics and a proposed mechanism of formation. J Neurosurg. 2003;99(2):228–240. doi: 10.3171/jns.2003.99.2.0228. [DOI] [PubMed]
- 3.Lu X, Huang Y, Zhou P, Zhu W, Wang Z, Chen G. Cerebral revascularization for the management of complex middle cerebral artery aneurysm: A case series. Exp Ther Med. 2021;22(2):883. doi: 10.3892/etm.2021.10315. [DOI] [PMC free article] [PubMed]
- 4.Jeon P, Kim BM, Kim DI, et al. Reconstructive endovascular treatment of fusiform or ultrawide-neck circumferential aneurysms with multiple overlapping enterprise stents and coiling. AJNR Am J Neuroradiol. 2012;33(5):965–971. doi: 10.3174/ajnr.A2857. [DOI] [PMC free article] [PubMed]
- 5.Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Kallmes DF. Risk Factors for Hemorrhagic Complications following Pipeline Embolization Device Treatment of Intracranial Aneurysms: Results from the International Retrospective Study of the Pipeline Embolization Device. AJNR Am J Neuroradiol. 2015;36(12):2308–2313. doi: 10.3174/ajnr.A4443. [DOI] [PMC free article] [PubMed]
- 6.Kwak HS, Park JS, Koh EJ. Stent-assisted coil embolization on down-the-barrel view with spring-shaped microcatheter in patient with M1 ultrawide necked circumferential aneurysm. BMJ Case Rep. 2018;2018:bcr2017013597. doi: 10.1136/neurintsurg-2017-013597.rep. [DOI] [PMC free article] [PubMed]
- 7.Zanaty M, Chalouhi N, Tjoumakaris SI, Gonzalez LF, Rosenwasser R, Jabbour P. Flow diversion for complex middle cerebral artery aneurysms. Neuroradiology. 2014;56(5):381–387. doi: 10.1007/s00234-014-1339-x. [DOI] [PubMed]
- 8.Xu F, Xu B, Huang L, Xiong J, Gu Y, Lawton MT. Surgical Treatment of Large or Giant Fusiform Middle Cerebral Artery Aneurysms: A Case Series. World Neurosurg. 2018;115:e252–e262. doi: 10.1016/j.wneu.2018.04.031. [DOI] [PubMed]
- 9.Tayebi Meybodi A, Huang W, Benet A, Kola O, Lawton MT. Bypass surgery for complex middle cerebral artery aneurysms: an algorithmic approach to revascularization. J Neurosurg. 2017;127(3):463–479. doi: 10.3171/2016.7.JNS16772. [DOI] [PubMed]
- 10.Kalani MY, Zabramski JM, Hu YC, Spetzler RF. Extracranial-intracranial bypass and vessel occlusion for the treatment of unclippable giant middle cerebral artery aneurysms. Neurosurgery. 2013;72(3):428–435. doi: 10.1227/NEU.0b013e3182804381. [DOI] [PubMed]
- 11.Kivipelto L, Niemelä M, Meling T, Lehecka M, Lehto H, Hernesniemi J. Bypass surgery for complex middle cerebral artery aneurysms: impact of the exact location in the MCA tree. J Neurosurg. 2014;120(2):398–408. doi: 10.3171/2013.10.Jns13738. [DOI] [PubMed]
- 12.Cimflova P, Özlük E, Korkmazer B, et al. Long-term safety and efficacy of distal aneurysm treatment with flow diversion in the M2 segment of the middle cerebral artery and beyond. J Neurointerv Surg. 2021;13(7):631–636. doi: 10.1136/neurintsurg-2020-016790. [DOI] [PubMed]
- 13.Zhu W, Liu P, Tian Y, et al. Complex middle cerebral artery aneurysms: a new classification based on the angioarchitecture and surgical strategies. Acta Neurochir (Wien). 2013;155(8):1481–1491. doi: 10.1007/s00701-013-1751-8. [DOI] [PMC free article] [PubMed]