ABSTRACT
A 19-year-old woman presented with swelling of the left forehead without pain. She did not have any relevant past or family history. Computed tomography showed destruction of the outer cortex of the frontal bone. A solitary mass lesion with a fluid collection was detected with magnetic resonance imaging. Because the swelling of the left forehead had enlarged rapidly with osteolytic changes, surgical removal of the lesion was performed. The lesion appeared to be enveloped in a fibrous capsule. The soft lesion was removed from the frontal bone. The outer frontal bone was absent, although the inner frontal bone was preserved. Then, the frontal bone was resected with margins from the edge of the erosion. The dura mater under the lesion was intact. A cranioplasty was performed using titanium mesh. On histological examination, the trabecular bones revealed irregular shapes and arrangements, indicating fibrous dysplasia. There was a continuous high-cell-concentration pathological lesion outside the fibrous dysplasia. There were numerous cells, such as mononuclear cells, osteoclast-like multinucleated giant cells, foam cells, and red blood cells. The osteoclast-like multinucleated giant cells and other cells did not show significant nuclear atypia. Immunostaining with H3.3G34W was negative, and the ubiquitin-specific peptidase 6/Tre-2 gene showed no rearrangements. The histopathological diagnosis was secondary aneurysmal bone cyst with fibrous dysplasia. Additional postsurgical therapy was not performed. There has been no evidence of recurrence of the lesion for two years.
Key Words: aneurysmal bone cyst, skull, frontal bone, secondary, fibrous dysplasia
INTRODUCTION
Aneurysmal bone cyst (ABC) is benign bone tumors that account for 1–2% of all primary bone tumors.1 ABC is expansile osteolytic lesions containing thin-walled, blood-filled cystic cavities. Patients typically present with painless swelling, and the growth is often slow and gradual.2 ABC is commonly found in patients within the first two decades of life, and they are primarily located in the metaphyses of long tubular bones, vertebrae, or flat bones.1-4 ABC of the skull is very rare, accounting for 2–6% of all ABC.5 ABC is often classified as primary or secondary, with primary lesions in approximately 70% of cases, and the remaining 30% of cases occurring secondary to other lesions.6,7 Secondary ABC can occur with other bone tumors, including chondrosarcoma, chondroblastoma, osteoblastoma, giant cell tumor, chondromyxoid fibroma, nonossifying fibroma, and fibrous dysplasia, or as a result of trauma.6 A case of secondary ABC of the left frontal bone with fibrous dysplasia showing rapid expansion is presented along with an update on diagnosis and therapy.
CASE REPORT
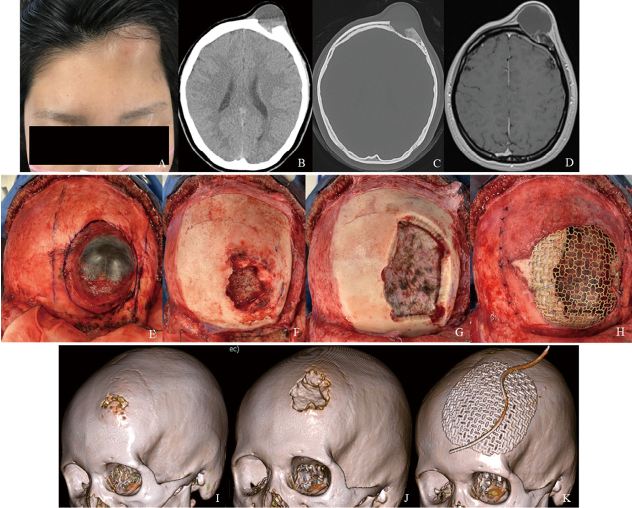
A 19-year-old woman presented with swelling of the left forehead without pain. She did not have any relevant past or family history. Computed tomography showed thickening of the frontal bone, and the lesion had the appearance of a ground-glass opacity (Fig. 1A-D). Magnetic resonance imaging showed a solitary mass lesion in the frontal bone with fluid collection containing a fluid-fluid level (Fig.1E and F). There were no apparent findings of invasion of the dura mater and edema around the brain. The lesion showed heterogeneous enhancement on contrast-enhanced T1-weighted images (Fig. 1G and H). The swelling of the left forehead enlarged rapidly with pain during the 2 weeks from the first magnetic resonance imaging (Fig.2A). Computed tomography showed new osteolytic changes and destruction of the outer cortex of the bone (Fig.2B and C). Magnetic resonance imaging showed increased fluid content (Fig. 2D).
Fig. 1.

CT and MRI findings before rapid expansion
Fig. 1A: Plain computed tomography shows thickening of the frontal bone.
Fig. 1B-D: Bone condition computed tomography shows the lesion as a ground-glass opacity (B: axial, C: coronal, and D: sagittal views).
Fig. 1E, F: Magnetic resonance images show a solitary mass lesion in the left frontal bone with fluid collection with a fluid-fluid level. There are no apparent findings of invasion of the dura mater and edema around the brain (E: T2-weighted, F: fluid-attenuated inversion recovery).
Fig. 1G, H: The lesion shows heterogenous enhancement on contrast-enhanced magnetic resonance T1-weighted images (G: axial, and H: sagittal views).
Fig. 2.
Findings of the lesion after rapid expansion, intraoperative photographs, and time-course changes of the skull bone
Fig. 2A: The swelling of the left forehead has enlarged rapidly over 2 weeks.
Fig. 2B, C: Computed tomography shows osteolytic changes and destruction of the outer cortex of the bone (B: plain, C: bone condition).
Fig. 2D: Magnetic resonance image shows increased fluid content.
Fig. 2E-H: Intraoperative photographs show that the lesion is enveloped in a fibrous capsule (E). After the soft lesion is removed, the frontal bone is eroded, but the inner bone is preserved (F). The frontal bone is resected with more than a 10-mm distance from the edge of the erosion (G). A cranioplasty is performed using titanium mesh (H).
Fig. 2I-K: 3D-skull computed tomography images show time-course changes of the skull bone (I: first visit, J: lesion expansion, and K: after surgery).
Prompt surgical removal of the lesion was performed for treatment and confirmation of the pathological diagnosis. The frontal skin was turned using a bicoronal skin incision onto the galea. The lesion appeared to be enveloped in a fibrous capsule (Fig. 2E). The mass contained dark fluid. After aspiration of the fluid, the soft lesion was removed from the frontal bone. The frontal bone was eroded, and the outer bone was absent, whereas the inner bone was preserved (Fig. 2F). Then, the frontal bone was resected with more than a 10-mm distance from the edge of the erosion (Fig. 2G). The dura mater under the lesion was intact. A cranioplasty was performed using titanium mesh and covered as much as possible with a vascularized pericranial flap (Fig. 2H). 3D-skull computed tomography showed the time-course changes of the skull bone from the first visit, with lesion expansion, and after surgery (Fig. 2I-K).
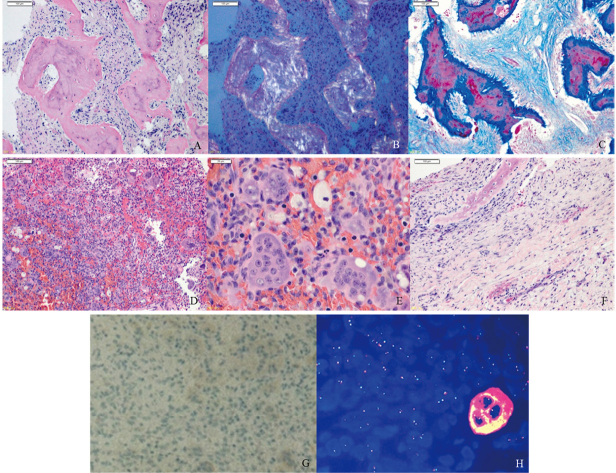
Histological examination showed an irregular shape and arrangement of the trabecular bones surrounded by spindle-shaped cells on hematoxylin and eosin staining (Fig. 3A). The trabecular bones showed apple-green sign by polarized light, which indicated osteoid (Fig. 3B). Shapey fibers were seen around the trabecular bones on Azan staining (Fig. 3C). These fibro-osseous findings indicated that the lesion was fibrous dysplasia. There was a high-cell-concentration pathological lesion outside of the fibrous dysplasia. There were numerous cells, such as mononuclear cells, osteoclast-like multinucleated giant cells, foam cells, and red blood cells on hematoxylin and eosin staining (Fig. 3D). The osteoclast-like multinucleated giant cells and other cells did not show significant nuclear atypia on hematoxylin and eosin staining (Fig. 3E). Reactive bone formations were seen in the fibrous capsule tissue on hematoxylin and eosin staining (Fig. 3F). Immunostaining with H3.3G34W was negative (Fig. 3G). Dual-color split-apart ubiquitin-specific peptidase 6 (USP6) fluorescence in situ hybridization probe was performed in paraffin-embedded tissues using probe on the centromeric (green) and telomeric (red) sides of the USP6 locus. Fluorescence in situ hybridization showed no split of the green-red probe signal (Fig. 3H). The cadherin11 was not examined in this case. The histopathological diagnosis was secondary ABC with fibrous dysplasia. Additional postsurgical therapy was not performed, and there has been no evidence of recurrence of the lesion for two years.
Fig. 3.
Histological and immunostaining findings
Fig. 3A: Surgical specimen stained with hematoxylin-eosin shows irregular shape and arrangement of the trabecular bones surrounded by spindle-shaped cells.
Fig. 3B: The trabecular bones show apple-green sign by polarized light.
Fig. 3C: Shapey fibers are seen around the trabecular bones on Azan staining.
Fig. 3D: There are numerous cells such as mononuclear cells, osteoclast-like multinucleated giant cells, foam cells, and red blood cells.
Fig. 3E: The osteoclast-like multinucleated giant cells and other cells do not show atypical findings.
Fig. 3F: Reactive bone formations are seen in the fibrous capsule tissue.
Fig. 3G: Immunostaining with H3.3G34W is negative.
Fig. 3H: Dual-color split-apart ubiquitin-specific peptidase 6 fluorescence in situ hybridization using probe on the centromeric (green) and telomeric (red) sides of the ubiquitin-specific peptidase 6 locus. Fluorescence in situ hybridization shows no split of the green-red probe signal which indicates no rearrangements of ubiquitin-specific peptidase 6.
DISCUSSION
The rare entity of ABC of the skull bone has been reported in the frontal,8 temporal,9,10 occipital,11 sphenoidal,12 clivus,13 and paranasal bones.14 Clinical presentations depend on the anatomical location. Convexity lesions usually present with local swelling, and neurological deficits are seldom present.8-11 Skull base lesions are more likely to present with focal neurological symptoms.12-14 An ABC is a honeycomb-shaped, cystic lesion composed of cyst walls and septum. The cyst cavities are filled with old blood and show a fluid-fluid level.15,16 Computed tomography findings include expansile lytic lesions with widening of diploic spaces and ground-glass opacity. Magnetic resonance imaging also shows a well-defined expansile mass lined by a T1 and T2 hypointense rim with septations that divide the mass into small cavities. The fluid-fluid levels are characteristic of ABC, but they are neither constant nor specific. Although an ABC is a benign bone tumor, the lesion sometimes destroys surrounding bone tissue and enlarges within several months or weeks. The pathogenesis of ABC is suggested to involve vascular occlusion of venous drainage of the ABC, or preexisting lesions of bone may initiate osseous arteriovenous fistulas inducing local circulatory disturbances.15,16
Histopathological diagnosis of ABC is based on the presence of blood-filled cystic spaces separated by fibrous septa. The fibrous septa are composed of fibroconnective tissue with occasional osteoclast-type giant cells and reactive new bone formation.17 The overlying bone can have erosions with cancellous bone replaced by fibrovascular tissue.16 Areas of hemorrhage are surrounded by fibroconnective tissue with areas of new bone formation.16 Numerous multinucleated giant cells may also be present, primarily near areas of hemorrhage.16 In terms of pathological diagnosis, it can be important to differentiate primary from secondary ABCs Many primary ABC show clonal chromosome band 17p13 translocation. This translocation leads to fusion of the cadherin11 gene with the USP6/Tre-2 gene. USP6/Tre-2 and/or cadherin11 rearrangements were detected in 69% of primary ABC. In contrast, USP6/Tre-2 and cadherin11 rearrangements were not found in secondary ABC.18 Split-apart of a green-red probe signal of fluorescence in situ hybridization indicates USP6 rearrangement. In our case, fluorescence in situ hybridization revealed no rearrangements of USP6, and which indicated the secondary ABC.
Gross total resection is the curative treatment of choice for skull ABC. Subtotal surgical resection or simple curettage has high recurrence rates, varying from 21% to 50%.5 However, gross total resection may be difficult to achieve when the lesion is large or when it involves the skull base. In such cases, partial resection or intralesional curettage with adjunctive therapy should be considered. Radiotherapy should seldom be selected because of the high recurrence rate and the risk of sarcomatous degeneration.5 Imaging findings of ABC are very similar to those of osteosarcoma, especially in cases showing cystic lesions with bone destruction.19 It is difficult to determine if the lesion contains malignant components by preoperative clinical and various imaging examinations. Therefore, a cystic lesion presenting with bone destruction and rapid expansion must be completely resected with adequate margins.19
In the present case, the features of the lesion were rapid expansion with osteolytic changes and the existence of numerous osteoclast-like multinucleated giant cells on pathological examination. These imaging and pathological findings are very similar to those of malignant bone tumors, especially osteosarcoma.19 The first critical differential diagnosis was malignant skull bone tumors. However, malignant bone tumors were excluded because there were no findings of proliferation of anaplastic or atypical cells. The second differential diagnoses included giant cell lesions, such as Langerhans cell histiocytosis, giant cell tumor, giant cell reparative granuloma, and aneurysmal bone cyst. Langerhans cell histiocytosis was excluded because of lack of eosinophilic infiltration, and negative both S-100 and CD1a immunostaining. On immunohistochemistry, giant cell tumors are mostly positive for H3.3G34W, although this case was negative.20 ABC and giant cell reparative granuloma have similar pathological and clinical profiles, and recently the two lesions are considered to be overlapped benign bone tumors.21
Skull bone lesions showing cystic and osteolytic findings on imaging examinations and the presence of multinucleated giant cells on pathology are not easy to diagnose distinctively. Therefore, these cases require careful follow-up to detect recurrence or malignant changes even if the gross total resection of the lesion is performed.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Takayuki Nojima (Kanazawa Medical University, Ishikawa, Japan) and Dr Yasuaki Nakashima (Mitsubishi Kyoto Hospital, Kyoto, Japan) for providing valuable pathological information.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
Abbreviations
- ABC
aneurysmal bone cyst
- USP6
ubiquitin-specific peptidase 6
REFERENCES
- 1.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;363:176–179. [PubMed]
- 2.Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921–2931. doi:. [DOI] [PubMed]
- 3.Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615–625. doi:. [DOI] [PubMed]
- 4.Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23(27):6756–6762. doi: 10.1200/JCO.2005.15.255. [DOI] [PubMed]
- 5.Guida F, Rapanà A, Conti C, Cagliari E, Civelli F, Trincia G. Cranial aneurysmal bone cyst: a diagnostic problem. With a review of the literature. Childs Nerv Syst. 2001;17(4–5):297–301. doi: 10.1007/s003810000414. [DOI] [PubMed]
- 6.Bonakdarpour A, Levy WM, Aegerter E. Primary and secondary aneurysmal bone cyst: a radiological study of 75 cases. Radiology. 1978;126(1):75–83. doi: 10.1148/126.1.75. [DOI] [PubMed]
- 7.Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012;20(4):233–241. doi: 10.5435/JAAOS-20-04-233. [DOI] [PubMed]
- 8.Hermann AL, Polivka M, Loit MP, Guichard JP, Bousson V. Aneurysmal bone cyst of the frontal bone - A radiologic-pathologic correlation. J Radiol Case Rep. 2018;12(7):16–24. doi: 10.3941/jrcr.v12i7.3344. [DOI] [PMC free article] [PubMed]
- 9.Gotecha S, Punia P, Chugh A, et al. A rare case of an aneurysmal bone cyst of the temporal bone. Asian J Neurosurg. 2020;15(3):699–702. doi: 10.4103/ajns.AJNS_125_19. [DOI] [PMC free article] [PubMed]
- 10.Kletke SN, Popovic S, Algird A, Alobaid A, Reddy KK. Aneurysmal bone cyst of the temporal bone presenting with headache and partial facial palsy. J Neurol Surg Rep. 2015;76(1):e18–e22. doi: 10.1055/s-0034-1390020. [DOI] [PMC free article] [PubMed]
- 11.Tse GH, Jiang FY, Radatz MWR, Sinha S, Zaki H. Skull base aneurysmal bone cyst presenting with hydrocephalus: progressive residuum obliterated by gamma knife stereotactic radiosurgery in a pediatric patient. J Neurosurg Pediatr. 2020;26(1):76–81. doi: 10.3171/2020.2.PEDS19755. [DOI] [PubMed]
- 12.Arocho-Quinones EV, Self S, Suchi M, Zwagerman NT, Lew SM. Spheno-orbital aneurysmal bone cyst in a 10-month-old infant. World Neurosurg. 2018;117:371–376. doi: 10.1016/j.wneu.2018.06.193. [DOI] [PubMed]
- 13.Ustabasioglu FE, Samanci C, Asik M, et al. Aneurysmal bone cyst of sphenoid bone and clivus misdiagnosed as chordoma: a case report. Brain Tumor Res Treat. 2015;3(2):115–117. doi: 10.14791/btrt.2015.3.2.115. [DOI] [PMC free article] [PubMed]
- 14.Hnenny L, Roundy N, Zherebitskiy V, Grafe M, Mansoor A, Dogan A. Giant aneurysmal bone cyst of the anterior cranial fossa and paranasal sinuses presenting in pregnancy: case report and literature review. J Neurol Surg Rep. 2015;76(2):e216–e221. doi: 10.1055/s-0035-1555017. [DOI] [PMC free article] [PubMed]
- 15.Jaffe HL. Aneurysmal bone cyst. Bull Hosp Joint Dis. 1950;11(1):3–13. [PubMed]
- 16.Lichtenstein L. Aneurysmal bone cyst; observations on fifty cases. J Bone Joint Surg Am. 1957;39-A(4):873–882. [PubMed]
- 17.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed]
- 18.Oliveira AM, Perez-Atayde AR, Inwards CY, et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004;165(5):1773–1780. doi: 10.1016/S0002-9440(10)63432-3. [DOI] [PMC free article] [PubMed]
- 19.Takeuchi Y, Sonobe S, Iwabuchi N, Yoshida M, Tominaga T. A case of telangiectatic osteosarcoma in the frontal bone. NMC Case Rep J. 2021;8(1):159–165. doi: 10.2176/nmccrj.cr.2019-0217. [DOI] [PMC free article] [PubMed]
- 20.Yang L, Zhang H, Zhang X, et al. Clinicopathologic and molecular features of denosumab-treated giant cell tumour of bone (GCTB): Analysis of 21 cases. Ann Diagn Pathol. 2022;57:151882. doi: 10.1016/j.anndiagpath.2021.151882. [DOI] [PubMed]
- 21.Zhou J, Zheng S, Zhou L, et al. Pathologic evaluation of the solid variant of aneurysmal bone cysts with USP6 rearrangement with an emphasis on the frequent diagnostic pitfalls. Pathol Int. 2020;70(8):502–512. doi: 10.1111/pin.12941. [DOI] [PubMed]