Abstract
Objective:
To evaluate risk factors for post-discharge sequelae in children and adolescents after hospitalization for acute COVID-19 or multisystem inflammatory syndrome in children (MIS-C).
Methods:
Multicenter prospective observational cohort study conducted in 25 U.S. pediatric hospitals. Patients < 21-years-old, hospitalized May 2020 to May 2021 for acute COVID-19 or MIS-C with follow-up 2–4 months after admission. We assessed readmissions, caregiver-reported persistent symptoms or activity impairment, and new morbidities identified by the Functional Status Scale. Multivariable regression was used to calculate adjusted risk ratios (aRR).
Results:
Of 358 eligible patients, 2–4 month survey data were available for 119/155 (76.8%) with acute COVID-19 and 160/203 (78.8%) with MIS-C. Thirteen (11%) patients with acute COVID-19 and 12 (8%) with MIS-C had a readmission. Thirty-two (26.9%) patients with acute COVID-19 had persistent symptoms (22.7%) or activity impairment (14.3%) and 48 (30.0%) patients with MIS-C had persistent symptoms (20.0%) or activity impairment (21.3%). For patients with acute COVID-19, persistent symptoms (aRR, 1.29[95% CI, 1.04–1.59]) and activity impairment (aRR, 1.37[95% CI, 1.06–1.78]) were associated with more organs systems involved. Patients with MIS-C and pre-existing respiratory conditions more frequently had persistent symptoms (aRR, 3.09[95% CI, 1.55–6.14]) and those with obesity more frequently had activity impairment (aRR, 2.52[95% CI, 1.35–4.69]). New morbidities were infrequent (9% COVID-19 and 1% MIS-C).
Conclusions:
Over one in four children hospitalized with acute COVID-19 or MIS-C experienced persistent symptoms or activity impairment for at least 2 months. Patients with MIS-C and respiratory conditions or obesity are at higher risk of prolonged recovery.
Keywords: post-acute COVID-19 syndrome, COVID-19 post-intensive care syndrome, critical care outcomes, SARS-CoV-2, multisystem inflammatory syndrome in children, MIS-C, COVID-19, pediatrics
Introduction
Since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged globally in 2020, it infected over 59 million U.S. persons including over 10.2 million children and adolescents.1 As of March 27, 2022, over 975,000 of them, including 1,374 children, died from Coronavirus Disease 2019 (COVID-19).1 Although hospitalization is less common in the very young than in the elderly, approximately 118,058 U.S. children were hospitalized for severe COVID-19 August 2020 to March 2022.2 Additional hospitalizations occurred due to a post-infectious complication of SARS-CoV-2, termed multisystem inflammatory syndrome in children (MIS-C).3 As of March 1, 2022, 7,459 U.S. cases of MIS-C were reported.2 Acute COVID-19 and MIS-C can lead to need for life-supporting interventions in children.4
Some adults infected with SARS-CoV-2 report prolonged symptoms with inability to return to their baseline health for months after a sometimes minimal initial illness.5 Post-COVID conditions (PCC) were evaluated across 16 studies of mostly hospitalized adults and identified persistent symptoms in a median of 72.5% of patients.6 Research evaluating PCC in children hospitalized for acute COVID-19 is limited.7 Pediatric studies of hospitalized children are limited to post-discharge outcomes identified by administrative data8, a multicenter study from Iran with follow-up 3 months post-discharge9, and a single-center study from Russia with follow-up 5 months post-discharge.10 Other than cardiac outcomes, data delineating post-hospitalization outcomes for patients with MIS-C are sparse, including one single-center study conducted in the United Kingdom11 and one population-based study in Sweden.12 The objectives of this study were to characterize the sequelae and recovery of U.S. children hospitalized with acute severe COVID-19 or MIS-C two or more months after hospital admission, and identify factors associated with ongoing symptoms or activity impairment.
Methods
We conducted a multicenter prospective observational cohort study in children and adolescents admitted for acute COVID-19 or MIS-C at 25 sites across the Overcoming COVID-19 network (Supplementary Appendix). We evaluated post-hospitalization outcomes and illness-associated complications. Informed consent was obtained by trained study staff from the patient’s legal guardian. Patient assent was also obtained, when possible, based on age, developmental capacity, and illness severity. This study was approved centrally by Boston Children’s Hospital’s Institutional Review Board and was reviewed by the Centers for Disease Control and Prevention (CDC).
We enrolled children and adolescents (< 21-years-old) hospitalized for acute COVID-19 (SARS-CoV-2 reverse transcriptase polymerase chain reaction or antigen test positive and admitted with symptoms suspected to be related to COVID-19) or MIS-C (fever, evidence of inflammation, multisystem [≥2] involvement (Supplemental Table 1), positive SARS-CoV-2 respiratory or antibody test results, and without another etiology) between May 12, 2020, and May 4, 2021.4 We excluded patients suspected of a nosocomial SARS-CoV-2 infection and in-hospital deaths. After October 27, 2020, we excluded patients with acute COVID-19 and pre-existing acquired immune compromise (Supplemental Table 1), limitations of life support due to poor prognosis, end-stage lung disease awaiting transplant, or requiring chronic mechanical ventilation support.
Patient and hospitalization characteristics were collected by chart review and interview. Patient data included demographics, acute symptoms, and comorbidities. Obesity was defined by national reference standards for body mass index if aged >2 years and was considered separately from other pre-existing conditions.13 Race and ethnicity data were collected by parental or patient report. Hospitalization data included organ system involvement based on the CDC MIS-C organ system involvement criteria (Supplemental Table 1), Intensive Care Unit (ICU) admission and duration, pediatric logistic organ dysfunction score (PELOD-2), organ failure support (invasive mechanical ventilation, vasopressor support, extracorporeal membrane oxygenation), impaired left ventricular ejection fraction defined as <55%, and hospital length of stay.14 Pre-illness Functional Status Scale (FSS) score was collected by patient/family interview.15
Clinical outcomes
Primary outcomes included persistent symptoms or activity intolerance 2–4 months (50–120 days) after hospitalization. For patients missing 2–4 month survey data, we reported 1-month (30–41 days) survey data, when available. Secondary outcomes were hospital readmission and new morbidity at hospital discharge and follow-up relative to pre-illness baseline. Data delineating ongoing symptoms and activity impairments were collected by telephone interview or online surveys at approximately 1 month and 3 months after enrollment. Due to variability in time of enrollment and follow-up interview(s), follow-up durations were reported relative to hospital admission. Caregivers were asked to respond based on their child’s symptoms during the seven days prior to survey completion. Questionnaires were based on symptoms and activity impairments previously reported in adult studies.16 New morbidity was defined as an increase of ≥ 3 points in the total FSS score or ≥ 2 points in a domain-specific score compared with pre-illness FSS.15 FSS and hospital readmission data were collected by caregiver interview and electronic health record review. All data were entered using standardized forms by trained study personnel into Boston Children’s Hospital’s REDCap database.17
Statistical Analyses
Descriptive statistics included frequency (proportion) for categorical variables and median (interquartile range [IQR]) for continuous variables. Fisher’s exact and Wilcoxon rank sum tests were used to compare variables between patients with acute COVID-19 and MIS-C. We conducted univariate analyses to identify variables associated with the two outcomes: persistent symptoms or impaired activity 2–4 months after hospitalization. Pre-existing respiratory disease and obesity were evaluated separately in the models due to prior reports of their association with PCC.10,18 Based on univariate results, variables associated (p<0.09) with 2–4 months outcomes were included in multivariable Poisson regression models using robust variance estimates to determine risk ratios. We conducted sensitivity analyses including 30-day survey data for patients with resolution of symptoms and activity impairments at 30-days but missing 60-day outcomes. We report adjusted risk ratios (aRR) and risk differences (aRD) and 95% confidence intervals (CI). P-values less than 0.05 were considered statistically significant. All analyses were conducted using R software, version 4.0.2 (R Project for Statistical Computing, Vienna).
Results
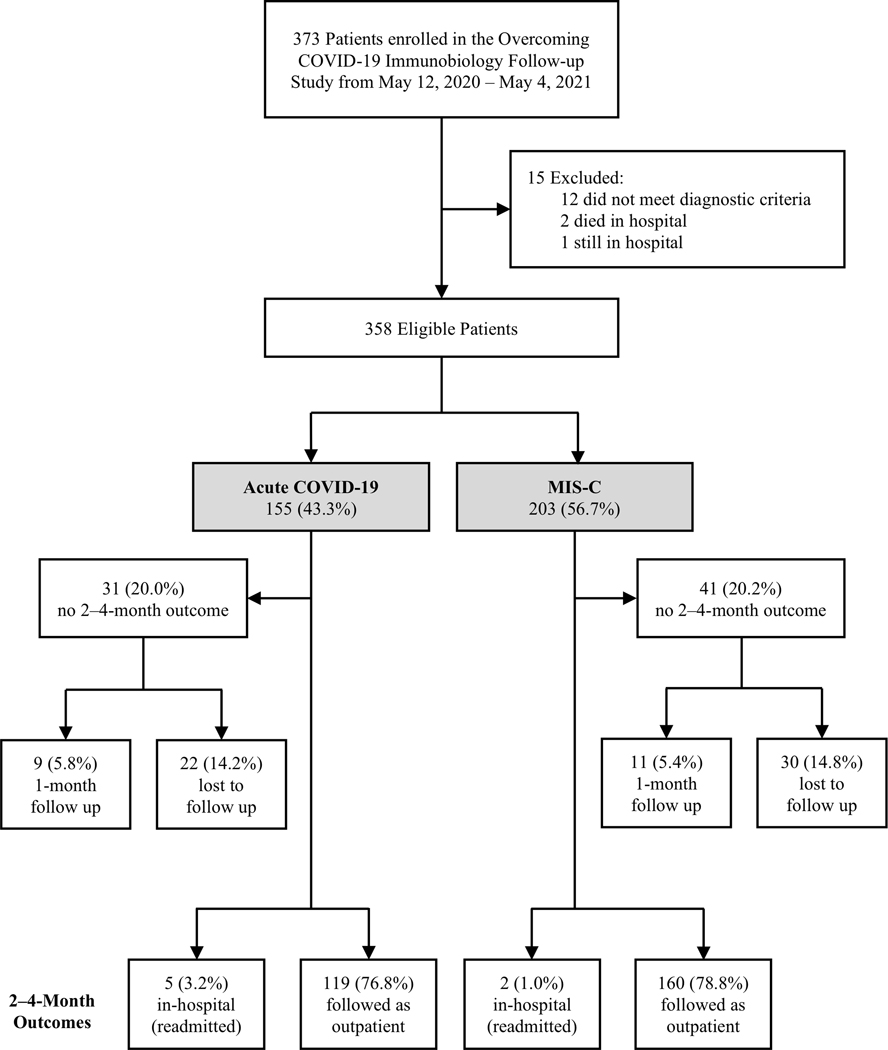
We enrolled 373 children. Fifteen patients were excluded including two in-hospital deaths and one that remained hospitalized (Figure 1). Of 358 patients eligible for follow up, 155 (43.3%) had acute COVID-19 and 203 (56.7%) had MIS-C. At 2–4 month follow-up, 7 patients were readmitted, for whom outpatient survey data were unavailable. We report 2–4 month outcomes for the 279/351 (79.5%) patients who were not readmitted and had 2–4 month survey data available including 119/150 (79.3%) patients with acute COVID-19 and 160/201 (79.6%) with MIS-C. Patient demographics did not differ between patients with and without 2–4 month follow-up data (Supplemental Table 2). Patients with acute COVID-19 and missing 2–4 month survey data were more frequently admitted to the ICU, supported with invasive mechanical ventilation, and had more significant organ dysfunction than those with 2–4 month survey data. For patients with MIS-C requiring mechanical ventilation (n=41), mechanical ventilation duration was shorter in patients without versus with 2–4 month survey data.
Figure 1. Enrollment and follow-up of patients hospitalized for acute COVID-19 or MIS-C across 25 U.S. sites in the Overcoming COVID-19 network.
COVID-19: acute coronavirus 19 disease; MIS-C: multisystem inflammatory syndrome in children.
Patients with Acute COVID-19
Patients with acute COVID-19 and 2–4 month survey data (n=119) demonstrated a bimodal age distribution with more patients in the < 2 and ≥ 13 years old age groups, 59 (49.6%) were male, 79 (66.4%) had pre-existing conditions, and 39 (32.8%) were obese (Table 1). Median duration of hospitalization was 4 days [IQR 2, 10].
Table 1.
Patient and Hospitalization Characteristics of Patients with 2–4 Month Survey Data
| Acute COVID-19 (n=119) | MIS-C (n=160) | P valuea | |
|---|---|---|---|
| Age Group | |||
| < 2 years old | 29 (24.4) | 7 (4.4) | <0.001 |
| ≥ 2 and < 5 years old | 8 (6.7) | 20 (12.5) | |
| ≥ 5 and < 13 years old | 24 (20.2) | 74 (46.2) | |
| ≥ 13 and < 21 years old | 58 (48.7) | 59 (36.9) | |
| Sex, male | 59 (49.6) | 93 (58.1) | 0.18 |
| Race | |||
| White | 64 (53.8) | 68 (42.5) | 0.17 |
| Black | 28 (23.5) | 59 (36.9) | |
| Asian | 3 (2.5) | 2 (1.2) | |
| Other or Unknown | 24 (20.2) | 31 (19.4) | |
| Ethnic Group | |||
| Not Hispanic | 78 (65.5) | 115 (71.9) | 0.12 |
| Hispanic | 38 (31.9) | 36 (22.5) | |
| Unknown | 3 (2.5) | 9 (5.6) | |
| Underlying Conditions | |||
| Previously healthy | 40 (33.6) | 111 (69.4) | <0.001 |
| Pre-existing respiratory conditionb | 36 (30.3) | 19 (11.9) | |
| Other pre-existing conditionc | 43 (36.1) | 30 (18.8) | |
| Obesityd | 39 (32.8) | 50 (31.2) | 0.13 |
| Hospitalization Characteristics | |||
| Admission PELOD-2 score, median (IQR) | 0 (0, 2) | 2 (0, 3) | <0.001 |
| Maximum PELOD-2 score, median (IQR) | 1 (0, 1) | 3 (1, 5) | <0.001 |
| Organ Systems Involved, median (IQR) | 2 (2, 3) | 5 (4, 6) | <0.001 |
| Intensive Care Unit Admission, n (%) | 60 (50.4) | 137 (85.6) | <0.001 |
| Invasive Mechanical Ventilation, n (%) | 21 (17.6) | 33 (20.6) | 0.65 |
| Length of Invasive Mechanical Ventilation, median (IQR)e | 7 (3, 15) | 5 (2, 8) | 0.17 |
| Left Ventricular Ejection Fraction < 55%, n (%) | NA | 86 (53.8) | NA |
| Vasopressor-Dependent Shock, n (%) | 19 (16.0) | 104 (65.0) | <0.001 |
| Extracorporeal Membrane Oxygenation, n (%) | 5 (4.2) | 10 (6.2) | 0.59 |
| Hospitalization Outcomes | |||
| Intensive Care Unit Length of Stay, median (IQR)e | 5 (2, 11) | 4 (2, 5) | 0.07 |
| Hospital Length of Stay, median (IQR) | 4 (2, 10) | 6 (5, 9) | <0.001 |
Compares patients admitted with acute coronavirus disease (COVID-19) to patients admitted with multisystem inflammatory disease in children (MIS-C).
Of the 36 patients with acute COVID-19 and a pre-existing respiratory condition, 15 (42%) had isolated asthma or reactive airways disease whereas 17/19 (89%) patients with MIS-C and a pre-existing respiratory condition had isolated asthma.
Other pre-existing conditions categorized as (not mutually exclusive): Acute COVID-19 (gastrointestinal/hepatic n=31 [26%], neurologic/neuromuscular n=29 [24%], endocrine/metabolic excluding obesity n=24 [20%], cardiovascular n=11 [9%], hematologic n=13 [11%], oncologic/immunosuppressive n=7 [6%], renal/urologic n=6 [5%]) and MIS-C (gastrointestinal/hepatic n=3 [2%], neurologic/neuromuscular n=6 [4%], endocrine/metabolic excluding obesity n=8 [5%], cardiovascular n=3 [2%], hematologic n=2 [1%], oncologic/immunosuppressive n=1 [<1%], renal/urologic n=2 [1%])
Only patients older than 2 years were eligible to be categorized as obese. Patients younger than 2 years old were categorized as not obese.
In patients supported by invasive mechanical ventilation or admitted to the Intensive Care Unit, respectively.
IQR: interquartile range; PELOD-2: pediatric logistic organ dysfunction score version 2.
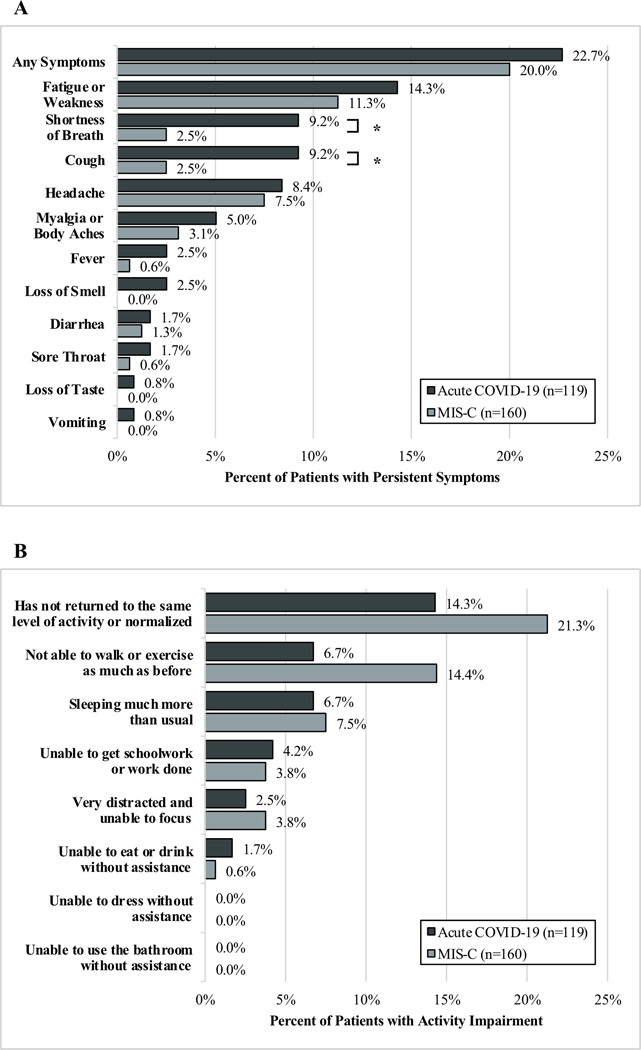
By 2–4 months after hospitalization, 32/119 (26.9%) patients with acute COVID-19 had persistent symptoms or activity impairment; with 27/119 (22.7%) having persistent symptoms (Figure 2A) and 17/119 (14.3%) having activity impairments (Figure 2B). Fever and respiratory symptoms which were common upon admission, had resolved at follow-up, but fatigue or weakness persisted in 14.3% of patients (Supplemental Figure 1). Patients with persistent symptoms or activity impairment most frequently had respiratory, hematologic, or gastrointestinal organ system involvement during hospitalization (Supplemental Figure 2). Patients with impaired activity had longer ICU stays compared with those without impaired activity (11.0 days [IQR 4.0, 26.5] versus 4.0 days [IQR 2.0, 7.0], p=0.01) whereas there was no difference in length of ICU stay among patients with and without persistent symptoms (Supplemental Table 3). Patients reporting persistent symptoms or activity impairments had longer hospitalizations compared with those without persistent symptoms or activity impairments. Of the 31 patients with acute COVID-19 without 2–4 month outcomes, 9 (29%) had survey data available at 1 month and 7/9 (78%) reported symptom resolution and normalized activity.
Figure 2. Outcomes of patients hospitalized for acute COVID-19 or MIS-C with A.) Persistent Symptoms and B.) Ongoing Activity Impairment 2–4 months after hospitalization.
*Denotes significant difference (p<0.05) between patients with acute COVID-19 and MIS-C. COVID-19: acute coronavirus 19 disease; MIS-C: multisystem inflammatory syndrome in children.
Pre-illness and hospital discharge FSS scores were available for 115 of 119 patients with acute COVID-19. New morbidities were present at hospital discharge in 10/115 (9%) patients. The most frequently affected domains were respiratory (n=6; 5%), communication (n=3; 3%), motor (n=3; 3%), and feeding (n=3; 3%). Three of the 10 patients with new morbidities at discharge had persistent morbidities at 2–4 months while 7 patients’ morbidities had resolved. An additional 6 patients had new morbidities that were not present at hospital discharge (data not shown). Thirteen (11.0%) patients with acute COVID-19 were readmitted within 2–4 months (Supplemental Table 4). Of the patients who were readmitted, 1 was previously healthy and was readmitted for epistaxis and the other 12 patients had pre-existing conditions which were commonly associated with their reason for readmission.
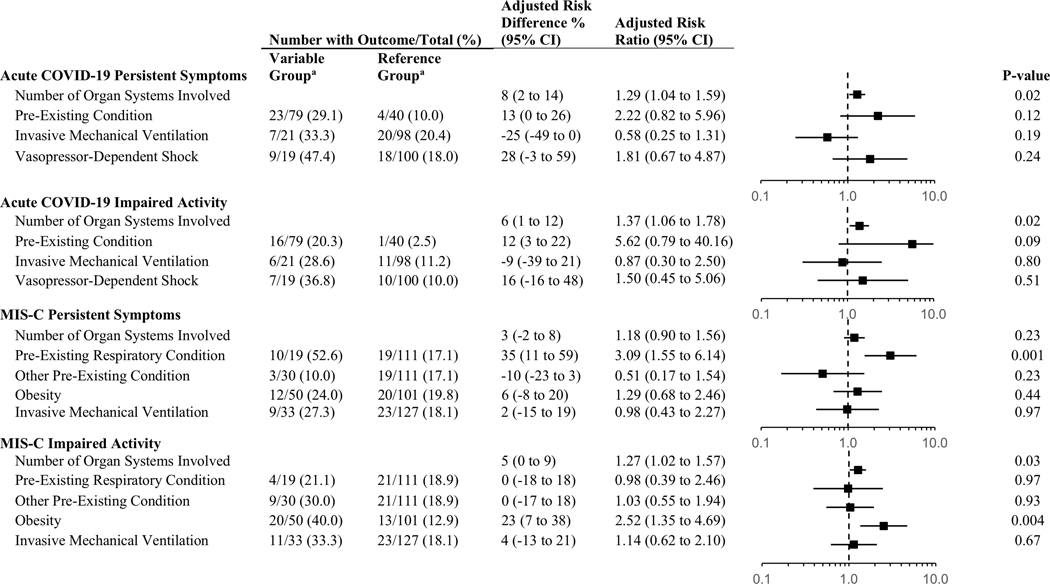
Patient and hospitalization characteristics associated with persistent symptoms and impaired activity in the univariate analyses are included in Supplemental Table 5. In the adjusted model for patients with acute COVID-19, number of organ systems involved was independently associated with persistent symptoms (aRR 1.29[95% CI, 1.04–1.59]) and activity impairment (aRR 1.37[95% CI, 1.06–1.78]) (Figure 3). A sensitivity analysis including 30-day survey data for patients with missing 2–4 month outcomes but resolution of symptoms and without activity impairment at 1 month had similar results (Supplemental Table 6).
Figure 3. Multivariable models evaluating factors associated with persistent symptoms or impaired activity 2–4 months after hospitalization for COVID-19 or MIS-C.
aVariable versus Reference Group corresponds to proportion of subjects with the outcome in “No” versus “Yes” for each variable. Organ system involvement is a continuous variable. COVID-19: acute coronavirus 19 disease; MIS-C: multisystem inflammatory syndrome in children.
Patients with MIS-C
Patients with MIS-C and 2–4 month survey data (n=160) were most frequently 5 years or older, 93 (58.1%) were male, 111 (69.4%) were previously healthy, 19 (11.9%) had a pre-existing respiratory condition which was most frequently asthma (17/19; 89%), and 50 (31.2%) were obese (Table 1). During hospitalization, 153 (95.6%) patients with MIS-C received steroids. Median duration of hospitalization was 6 days [IQR 5, 9].
At 2–4 month follow up, 48 (30.0%) patients with MIS-C had persistent symptoms or activity impairment; 32 (20.0%) had persistent symptoms (Figure 2A) and 34 (21.3%) had activity impairments (Figure 2B). Signs or symptoms common at presentation, including fever and gastrointestinal illness, resolved in most, but fatigue and weakness persisted in 18 (11.3%) patients and headache in 12 (7.5%) (Supplemental Figure 1). Length of ICU stay amongst those admitted to the ICU did not differ in patients with and without persistent symptoms or impaired activity (Supplemental Table 3). Hospital lengths of stay were longer in patients with persistent symptoms or activity impairments compared with those without persistent symptoms or activity impairments. Of the 41 patients with MIS-C without 2–4 month outcomes, 11 (27%) had 1-month survey data available and 5/11 (45%) reported symptom resolution and normalized activity.
Pre-illness baseline and hospital discharge FSS scores were available for 141 of the 160 patients with MIS-C. New morbidities at hospital discharge occurred in 2 (1%) patients with both resolving the morbidity prior to 2–4 month follow-up. At 2–4 month follow-up, 5/136 (4%) patients with MIS-C had a new morbidity with domain-specific morbidities in the mental status (n=4) and motor (n=1) domains (data not shown). By 2–4 month follow-up, 12/160 (7.5%) patients with MIS-C had been readmitted (Supplemental Table 4). Of the 12 readmitted patients, 8 did not have pre-existing conditions, although two met criteria for obesity. Readmissions amongst these 8 patients were for fever or infection, MIS-C flare up, memory loss or psychosis, gastrointestinal bleed, hyperglycemia, catheter site infection, and a non-COVID lower respiratory tract infection. Duration of readmission was determined in 7 of the 8 patients without pre-existing conditions and, excepting the patient readmitted for a gastrointestinal bleed, the durations were 3 days or shorter.
In multivariable analyses, having a pre-existing respiratory condition (17 of 19 had isolated asthma) was associated with persistent symptoms (aRR 3.09[95% CI, 1.55–6.14]) and obesity was associated with ongoing activity impairment (aRR 2.52[95% CI, 1.35–4.69]) (Figure 3). There was also an association between number of organ systems involved and activity impairment (aRR 1.27[95% CI, 1.02–1.57]). A sensitivity analysis including 1-month survey data for patients with missing 2–4 month outcomes but resolution of symptoms and activity impairment at 1 month had similar results (Supplemental Table 6).
Discussion
In this multicenter follow-up study of U.S. children and adolescents hospitalized with acute COVID-19 or MIS-C, more than one in four patients had persistent symptoms or activity impairment after having two to four months to recover from their illness. Fatigue or weakness were the most common symptoms in both children with acute COVID-19 and MIS-C, followed by cough and shortness of breath in the acute COVID-19 group and headache in the MIS-C group. More than one in five patients with MIS-C were not able to walk or exercise at their prior level. Factors associated with persistent symptoms or activity impairment differed between children hospitalized for acute COVID-19 and MIS-C. Most children with acute COVID-19 had underlying conditions, and the number of organ systems affected during the index hospitalization predicted persistent symptoms and activity impairment. In contrast, most patients with MIS-C were previously healthy, but pre-existing respiratory conditions (mostly asthma) were associated with persistent symptoms and obesity was associated with ongoing activity impairment. Few patients with MIS-C reported major functional deficits, but hospital readmissions related to their prior illness did occur within the assessment period. Our study highlights that, while most children recover, many children with both acute COVID-19 and MIS-C have persistent sequelae and further follow-up to determine if these sequelae persist is warranted.
The frequency of persistent symptoms in these U.S. children and adolescents after hospitalization for acute COVID-19 is similar to a prospective follow-up study by Osmanov et al. from Russia describing 518 children hospitalized for acute COVID-19 followed to five months post-discharge where one in four had persistent symptoms.10 These authors identified fatigue and sleep disturbance as common ongoing symptoms, reporting that older children and those with pre-existing allergic conditions were at higher risk of PCC. Despite these similarities, 51% of our patients versus 3% of the patients in the Russian cohort were admitted to the ICU. A smaller Iranian study of 58 children hospitalized for acute COVID-19, including 10 admitted to the ICU, reported prolonged symptoms in 45% of patients at 3 months.9 Similar to our study, fatigue, shortness of breath, and activity intolerance were the most common symptoms and ICU admission was a risk factor for prolonged symptoms. Taken together, these studies suggest that children hospitalized with acute COVID-19, particularly critically ill children, are at an increased risk of PCC relative to non-hospitalized cohorts where rates of PCC are reported as less than 2%.19
We report that nearly one in three patients with MIS-C had persistent symptoms or activity impairment 2–4 months after hospitalization. This frequency is similar to a single center report of 46 pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) patients from London.11 Those investigators conducted comprehensive direct patient assessments 6 weeks and 6 months after hospitalization, reporting that one in three participants had impairments in physical, emotional, or psychosocial domains 6 weeks after illness despite normalization of laboratory data.11 Unlike our study, they followed these children until 6 months where 45% of them did very poorly on the 6-minute walk test. Congruent with their findings, walking and exercise limitations were the most common ongoing impairment in this U.S. cohort with MIS-C.
In our cohort, most patients were admitted to the ICU and, thus, at risk of developing Post-Intensive Care Syndrome (PICS) including functional impairment.20,21 New functional impairment after pediatric critical illness varies across cohorts but is estimated to be 5% at discharge in general PICU populations and 20% six months post-discharge in children with acute respiratory failure.22,23 Although it is prohibitively challenging to separate the relative contributions of the direct effects of SARS-CoV-2 infection from ICU-specific sequelae, ICU interventions were not predictive of ongoing symptoms or activity impairment in the multivariable models. For patients with MIS-C, where a higher proportion required ICU-level support, pre-existing factors such as asthma and obesity were more predictive of future outcomes than critical illness factors. While there is some overlap, the pathobiology driving persistent symptoms in patients with MIS-C likely differs from that in patients with acute COVID-19. The often-prolonged immunomodulatory treatments used for MIS-C including glucocorticoids could contribute to long-term sequelae such as fatigue and weakness.24
This study has important limitations. Most of our patients were admitted to the ICU, and intentionally our outcomes represent those of severely ill hospitalized patients. Some patients (14%) were lost to follow-up, introducing a potential for selection bias as those with symptoms may be more likely to participate. We did not assess social vulnerability. We also did not have a control group of patients with negative SARS-CoV-2 testing for comparison due to enrollment limitations. We relied on caregiver report of symptoms and activity impairments and did not conduct objective assessments of the child’s functional limitations. While the FSS is a validated assessment to identify new morbidities, our questionnaire to delineate symptomatology and activity intolerance was modified from other validated instruments and abbreviated, leading to potential under-assessment of sequelae.16,25 Patients were enrolled prior to the emergence of the B.1.617.2 (Delta) and B.1.1.529 (Omicron) SARS-CoV-2 variants, which may differ in their longer term impacts.2 We did not evaluate the association between practices aimed at preventing morbidities (e.g., early mobility) or treatments for acute COVID-19 or MIS-C and outcomes. Finally, at the time of this study, no patients were vaccinated. Across these sites there is evidence that vaccination effectively prevents severe acute COVID-19 and MIS-C and therefore may also decrease the risk of post-discharge sequelae.26,27
Conclusions
Over one in four children and adolescents in this study developed sequelae after hospitalization for acute COVID-19 or MIS-C. Acute COVID-19, especially when it impacts multiple organ systems, can precipitate ongoing symptoms and activity impairments. These findings highlight the importance of post-discharge follow-up of these severely ill patients. In patients with MIS-C, underlying respiratory conditions, most frequently asthma, were associated with ongoing symptoms and obesity was associated with activity impairment. Additional interventions (e.g., occupational or physical therapy) and clinical follow up of these high-risk cohorts may mitigate long-term sequelae. Most children hospitalized for MIS-C or acute COVID-19 recovered and were back to baseline within two months, which is reassuring.
Supplementary Material
Research in context.
What’s Known on This Subject
Many adults hospitalized with COVID experience post-COVID conditions. Research evaluating post-discharge outcomes of children and adolescents hospitalized for acute COVID-19 or MIS-C are limited, especially for children who became critically ill.
What This Study Adds
Over one in four children and adolescents hospitalized with COVID-19 or MIS-C had sequelae beyond two months post-hospitalization. Patients with COVID-19 and more organ system involvement and patients with MIS-C and underlying respiratory conditions or obesity were at increased risk.
Contributors’ Statement Page
Dr. Maddux participated in acquisition of data, design of statistical analyses, interpretation of data, drafting and revision of the article.
Mr. Young, Ms. Newhams, Ms. FitzGerald, Ms. Chen, and Dr. Kucukak participated in acquisition of data, interpretation of data, development of figures, and critically reviewed the manuscript for important intellectual content.
Dr. Randolph conceptualized and designed the study, coordinated and supervised data collection, participated in interpretation of the data, and critically reviewed the manuscript for important intellectual content.
Drs. Campbell, Feldstein, Zambrano, Patel, and Tenforde participated in interpretation of the data and critically reviewed the manuscript for important intellectual content.
Ms. Berbert, Dr. Weller, Ms. Jie, and Ms. Miller carried out the analyses and critically reviewed the manuscript for important intellectual content.
Drs Halasa, Cvijanovich, Loftis, Walker, Schwartz, Gertz, Tarquinio, Fitzgerald, Kong, Schuster, Mack, Hobbs, Rowan, Staat, Zinter, Irby, Crandall, Flori, Cullimore, Nofziger, Shein, Gaspers, Hume, and Levy participated in acquisition of data and critical review of the manuscript for important intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding:
Centers for Disease Control and Prevention and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Maddux, K23HD096018).
Role of Funder:
This study was funded by the Centers for Disease Control and Prevention who reviewed and approved submission of the study results.
Abbreviations:
- MIS-C
multisystem inflammatory syndrome in children
- aRR
adjusted risk ratios
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- COVID-19
coronavirus disease 2019
- PCC
post-COVID conditions
- CDC
Centers for Disease Control and Prevention
- ICU
Intensive Care Unit
- FSS
Functional Status Scale
- IQR
interquartile range
- aRD
adjusted risk difference
- CI
confidence intervals
- PIMS-TS
pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2
- PICS
Post-Intensive Care Syndrome
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
Article Summary: This multicenter prospective study evaluates risk factors for post-discharge sequelae in children and adolescents after hospital admission for acute COVID-19 or MIS-C.
A complete list of study group members appears in the Online Appendix.
Conflict of Interest Disclosure: The authors have no conflicts of interest relevant to this article to disclose.
References:
- 1.Demographic Trends of COVID-19 cases and deaths in the US reported to CDC. Center for Disease Control and Prevention; 2021. https://covid.cdc.gov/covid-data-tracker/#demographics. Accessed March 28, 2022.
- 2.COVID Data Tracker. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions. Published 2021. Updated March 27, 2022. Accessed March 28, 2022.
- 3.Emergency preparedness and response: HAN00432. CDC Health Alert Network May 14, 2020. [Google Scholar]
- 4.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. 2021;325(11):1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasserie T, Hittle M, Goodman SN. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw Open. 2021;4(5):e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munblit D, Sigfrid L, Warner JO. Setting Priorities to Address Research Gaps in Long-term COVID-19 Outcomes in Children. JAMA Pediatr. 2021;175(11):1095–1096. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Romieu AC, Carton TW, Saydah S, et al. Prevalence of Select New Symptoms and Conditions Among Persons Aged Younger Than 20 Years and 20 Years or Older at 31 to 150 Days After Testing Positive or Negative for SARS-CoV-2. JAMA Netw Open. 2022;5(2):e2147053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asadi-Pooya AA, Nemati H, Shahisavandi M, et al. Long COVID in children and adolescents. World J Pediatr. 2021;17(5):495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur Respir J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penner J, Abdel-Mannan O, Grant K, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health. 2021;5(7):473–482. [DOI] [PubMed] [Google Scholar]
- 12.Kahn R, Berg S, Berntson L, et al. Population-based study of multisystem inflammatory syndrome associated with COVID-19 found that 36% of children had persistent symptoms. Acta Paediatr. 2022;111(2):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defining Childhood Weight Status: BMI for Children and Teens. Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. https://www.cdc.gov/obesity/childhood/defining.html. Published 2021. Updated June 21, 2021. Accessed September 2, 2021.
- 14.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761–1773. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Critical care medicine. 2015;43(8):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatr Crit Care Med. 2018;19(4):298–300. [DOI] [PubMed] [Google Scholar]
- 21.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. [DOI] [PubMed] [Google Scholar]
- 22.Pollack MM, Holubkov R, Funai T, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15(9):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson RS, Asaro LA, Hutchins L, et al. Risk Factors for Functional Decline and Impaired Quality of Life after Pediatric Respiratory Failure. Am J Respir Crit Care Med. 2019;200(7):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son MBF, Murray N, Friedman K, et al. Multisystem Inflammatory Syndrome in Children - Initial Therapy and Outcomes. N Engl J Med. 2021;385(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 26.Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents. N Engl J Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrano LD, Newhams MM, Olson SM, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.