Abstract
Mechano growth factor (MGF), an isoform of insulin-like growth factor 1 (IGF-1), is recognized as a typical mechanically sensitive growth factor and has been shown to play an indispensable role in the skeletal system. In the joint cavity, MGF is highly expressed in chondrocytes, especially in the damaged cartilage tissue caused by trauma or degenerative diseases such as osteoarthritis (OA). Cartilage is an extremely important component of joints because it functions as a shock absorber and load distributer at the weight-bearing interfaces in the joint cavity, but it can hardly be repaired once injured due to its lack of blood vessels, lymphatic vessels, and nerves. MGF has been proven to play an important role in chondrocyte behaviors, including cell proliferation, migration, differentiation, inflammatory reactions and apoptosis, in and around the injury site. Moreover, under the normalized mechanical microenvironment in the joint cavity, MGF can sense and respond to mechanical stimuli, regulate chondrocyte activity, and maintain the homeostasis of cartilage tissue. Recent reports continue to explain its effects on various cell types and sport-related tissues, but its role in cartilage development, homeostasis and disease occurrence is still controversial, and its internal biological mechanism is still elusive. In this review, we summarize recent discoveries on the role of MGF in chondrocytes and cartilage defects, including tissue repair at the macroscopic level and chondrocyte activities at the microcosmic level, and discuss the current state of research and potential gaps in knowledge.
Keywords: mechano growth factor, insulin-like growth factor 1, cartilage defect, chondrocyte
Introduction
Mechano growth factor (MGF), also known as insulin-like growth factor 1 Ec (IGF-1Ec) in humans, is one of the splice variants of the igf gene [1]. MGF has a different C-terminal peptide sequence and exhibits a distinct function that is sensitive to mechanical stimuli compared with other IGF-1 subtypes, including IGF-1Ea and IGF-1Eb [ 2, 3] . MGF has been found in many vertebrate tissues, including the myocardium, skeletal muscle, brain and other tissues [ 4‒ 7] . Among them, the function of MGF in skeletal muscle has most extensively been investigated. MGF, as well as other IGF-1 subtypes, is upregulated during exercise and in injured/damaged skeletal muscles [ 8, 9] , and increased MGF could activate muscle satellite (stem) cells and recruit macrophages to enhance skeletal muscle regeneration [ 10, 11] , which makes it attractive as a potential therapeutic target. Overall, current reports have provided evidence that MGF has potent repair capacity in wound healing, and it also serves as an effector of external mechanical stimulations. Since cartilage is prominently exposed to a series of mechanical stresses [12], we assume that the potential role of MGF in cartilage repair may be related to the remodelling in cartilage sport injury.
In the development of the skeletal system, bone marrow mesenchymal stem cells (BMSCs) and growth plate chondrocytes play a vital role. BMSCs have self-renewal capabilities and can be differentiated into many tissue-specific lineages, including chondroblasts, osteoblasts, adipocytes and other types of adult cells [ 13, 14] . In the joint microenvironment, mechanical stimuli have a critical impact on the differentiation of BMSCs [15]; for example, cyclic uniaxial strain may promote the differentiation of mesenchymal stem cells (MSCs) into smooth muscle cells [16], and cyclic hydrostatic pressure can enhance the chondrogenic phenotype of MSCs [17]. The growth plate is a cartilaginous region located at the end of immature long bones [18] and completes endochondral ossification to promote postnatal bone development. The morphology and gene expression of growth plates are influenced by biomechanics, including the frequency, amplitude and duration of mechanical stress [19]. Recent reports have indicated that MGF is expressed in BMSCs and growth plate chondrocytes and plays a protective role in joint trauma by mediating cell behaviors, including cell proliferation, migration, differentiation and apoptosis, in and around the injury site [20].
Because chondrocytes are exposed to continuous mechanical stress under physiological conditions and there are no blood vessels, nerves or lymph in cartilage tissue [21], they are difficult to repair once damaged. In particular, even small articular cartilage defects, if not treated, may develop into scar tissues mainly composed of early fibrocartilage, eventually leading to osteoarthritis (OA) [22]. OA is one of the most common joint diseases around the world [ 23, 24] and can further result in synovitis, lesions of the meniscus, degeneration of the anterior cruciate ligament and so on [ 25, 26] . Mechanical stress is one of the key factors of cartilaginous damage, and excess mechanical stress not only directly damages chondrocytes and the cartilage extracellular matrix but also activates inflammatory responses ultimately causing OA [ 27‒ 29] . High level of MGF is accompanied by the occurrence, progression and deterioration of OA disease, and recent evidence supports that high MGF expression is correlated with the pathogenesis of OA, indicating its potential as a therapeutic target. However, a general review on the recent discoveries in MGF is still lacking, and it is necessary to summarize existing studies on MGF and discuss future directions in MGF research. Therefore, in this review, we aim to summarize recent discoveries on the expression, functions, and mechanisms of MGF in cartilaginous tissues, and discuss the current research state of potential gaps in knowledge.
Overview of IGF-1
IGF-1, together with IGF-2 and insulin, belongs to the insulin superfamily. Growth hormone (GH) is essential for the synthesis and release of IGF-1 [30], and this GH-mediated IGF-1 axis is well known to promote postnatal individual growth [ 31, 32] , maintain homeostasis of the skeleton system [33], and mediate a variety of cellular biological processes, including cellular proliferation, differentiation, and mitochondrial fission-fusion dynamic equilibrium [34]. IGF-1 is mainly secreted by the liver in an endocrine way [35], and para- and/or autocrine activity is also seen in other tissues, including the lung, kidney, skeletal muscle, heart and white adipose tissue [ 36‒ 39] . Reports have shown that the IGF-1 protein and its isoforms are eventually secreted into the extracellular space and function through several receptors, including IGF-1 type I receptor (IGF-1R), type II (IGF-2R), insulin receptor (INSR) and hybrid receptors (IGF-1R/INSR) [40]. However, the different isoforms of IGF-1 have different destinations. Tan et al. [41] have shown that the IGF-1 isoform that includes exon 5 is concentrated in the nucleolus, which suggests that pro-IGF-1B might be an active intracellular form of IGF-1. Mature IGF-1 protein is different from IGF-1 isoforms because it lacks the E-peptides at the C-terminal [42], and mature IGF-1 production is independent of but influenced by the isoforms [ 37, 43] . Experiments in murine cells suggest that EA and EB have little effect on IGF-1 secretion but can promote the entry of IGF-1 into the cells [44].
Genomic context and alternative splicing of IGF-1
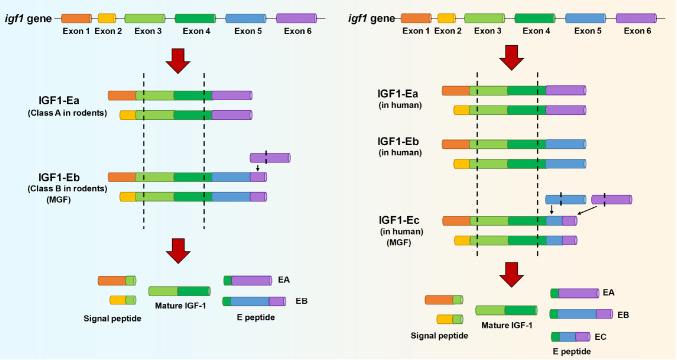
The igf1 gene spans more than 90 kb of DNA [ 2, 45] , and the igf1 gene is first translated into pro-IGF-1 protein, which is the precursor protein of mature IGF-1 and IGF-1 isoforms (IGF-1A, IGF-1B and IGF-1C, named IGF-1Ea, IGF-1Eb and IGF-1Ec, respectively). The gene structure contains 6 exons and 5 introns [9] ( Figure 1). Alternative splicing at the 5′ and 3′ ends of genes can produce several different splicing forms or isoforms, which vary among species [46]. Exons 1 and 2 have distinct promoter sequences and are used interchangeably, giving rise to different IGF-1 isoforms [ 46‒ 48] . Exons 3 and 4 are relatively conserved, encoding the core IGF-1 protein, which is the mature form of the protein found in peripheral blood [ 9, 46] . The alternatively spliced exons 5 and 6 encode the peptide domain E which is present in IGF-1 precursor proteins [49].
Figure 1 .
The structure of the igf1 gene
Alternative splicing of the igf1 gene in rodents and human. The rodent and human genes both have 6 exons, and their translation arises from exon 1 or 2, respectively. In rodents (left), IGF-1Ea excludes exon 5, while IGF-1Eb has a premature termination of exon 6. In humans (right), IGF-1Ea has a similar pattern in humans and rodents; IGF-1Eb in humans excludes exon 6, and IGF-1Ec in humans includes an internal splice site within exon 5 and a premature termination in exon 6. “Pre” indicates the presence of a signal peptide, while “pro” indicates the presence of E-peptides.
In rodents, most IGF-1 transcripts skip exon 5 and splice exon 4 directly to exon 6 and are defined as class A [46]. In the class B IGF-1, exon 5 is included, resulting in the occurrence of a premature stop codon within exon 6 [46]. As in rodents, human class A IGF-1 mRNAs contain exon 4 spliced to exon 6 and is designated as IGF-1Ea [50], whereas human class B IGF-1 contains only exon 5, resulting in a unique E peptide extension that has not been observed in other species [46]. The third isoform detected in humans is named IGF-1Ec or MGF ( Figure 1). It is similar to rodent class B IGF-1 which contains exon 4 spliced to exon 5 and exon 6 [ 51, 52] . Several studies have suggested that this C-terminal peptide (corresponding to the Ec fragment) has physiological function which is distinct from that of IGF-1 [ 1, 2, 53] . Evidence has supported the importance of a serine residue for hMSCs, and the serine residue exists in MGF rather than in other IGF-1 isoforms, explaining the different functions of IGF-1 isoforms [54]. Mature IGF-1 protein has the same structure as IGF-1 isoforms but lacks the E-peptides at the C-terminal [42]. Moreover, three E-peptides are produced in humans: EA (Ea), EB (Eb), and EC (Ec), and two are produced in rodents: EA (Ea) and EB (Eb) ( Figure 1).
The role of IGF-1 isoforms
IGF-1 is found to influence the development of growth plate [ 55‒ 58] . In rodent growth plate, it is found that the expression of IGF-1Ea containing exon 1 (class 1Ea) is the highest in all layers of two- and six-week rodent growth plate, and the peaks appear in the proliferative and hypertrophic zones, respectively [59]. After 4 weeks, class 1Ea is still expressed the most, but the expression is surpassed by IGF-1Eb containing exon 1 in the hypertrophic zone. IGF-1Ea containing exon 2 is expressed at very low levels, and the mRNA expression of IGF-1Eb containing exon 2 is negligible in rodent growth plate [59]. Moreover, the highest expression of IGF-1 is detected in the upper hypertrophic layer in normal rats [60].
IGF1 isoforms are also expressed in resting muscle, and the expression of MGF is significantly lower than that of IGF-1Ea. A significant increase in MGF mRNA is observed after high resistance exercise in the young rather than the elderly subjects, and IGF-1Ea mRNA levels remain stable throughout the experiment [61]. Moreover, it has been observed that after overexpression of IGF-1Ea in skeletal muscle, differentiation into myotubes is enhanced and muscle hypertrophy appears [62]. It has been confirmed that MGF inhibits the differentiation of myoblasts into myotubes and promotes myoblast proliferation [53], but Fornaro et al. [63] showed the opposite result: only full‐length IGF-1Eb rather than Eb peptide (MGF) alone is able to promote anabolic effects on muscle. Overexpression of IGF-1Ea, IGF-1Eb or mature IGF-1 in mice through AVV demonstrates that p-ERK1/2 is increased in both the IGF-1Ea and IGF-1Eb groups, and p-Akt is reduced in the IGF-1Eb group compared with that in the mature IGF-1 group [64].
IGF-1 in skeletal development and repair
IGF-1 is essential in the process of embryonic and postnatal skeletal development [ 53, 65] , and its activities are mostly mediated by IGF-1R [66].
It has been acknowledged that IGF-1 is a regulating factor of cartilage metabolism. In cartilage, proteoglycan is an essential component of the extracellular matrix (ECM). An in vitro study showed that IGF-1 can accelerate the synthesis of proteoglycans [67], and similar results were also obtained in human synovial fluid [68] and periosteal explants during culture [69], revealing the critical role of IGF-1 in cartilage metabolism. Moreover, IGF-1 can promote the differentiation of BMSCs towards chondrocytes, and the effects are additive to and independent of the transforming growth factor beta (TGF-β) superfamily [ 70, 71] . When cartilage is damaged, IGF-1 is rapidly recruited from the synovial fluid or periosteum to the injured site, which induces the chondrogenic differentiation of local MSCs [72]. In in vivo experiments, for example, in mechanically induced cartilage lesions of the patellar groove of the rat femur, cells infected with both adenoviral vectors carrying bone morphogenetic protein 2 ( BMP-2) and IGF-1 produce matrix rich in collagen II (Col2) rather than collagen I (Col1) and restore the smooth articular surface in most lesions, while uninfected cells fail to repair the defects [73]. Moreover, local application of IGF-1 and TGF-β1 accelerates early cellular processes during fracture healing [74], and chronic GH/IGF-1 deficiency aggravates the damage of articular cartilage lesions of OA without bony lesions [75]. By inhibiting IκB-α kinase, IGF-1 can reduce inflammation and alleviate cartilage degradation and chondrocyte apoptosis [76]. Taken together, these results show the importance of IGF-1 in the cartilage healing process.
If IGF-1 is overexpressed in osteoblasts, the osteocyte lacunae occupancy, bone formation rate (BFR), bone volume (BV) and bone mineral density (BMV) are all increased in vivo, with no change in total osteoblasts or osteoclast numbers [ 77‒ 79] .
The signaling pathways of IGF-1
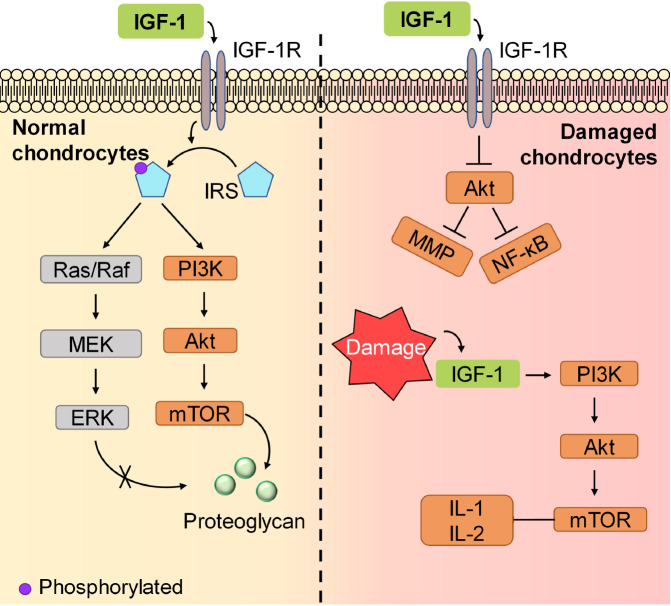
IGF-1 promotes chondrogenic differentiation and chondrocyte proliferation mainly via activating IGF-1R. The major downstream molecules of IGF-1R are sulfated glycosaminoglycan (Shc) and members of the insulin receptor-substrate (IRS) family, including IRS-1 and IRS-2. In chondrocytes, IRS is then phosphorylated and activates downstream signaling pathways, including the phosphoinositide 3-kinase (PI3K) cascade and extracellular-signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) cascade. Zhang et al. [80] revealed that in rat endplate chondrocytes, the PI3K pathway triggered by IGF-1 is mainly responsible for the expression of Col2 in the cartilage matrix, while the ERK pathway mediated by IGF-1 mainly acts on the expression of matrix metalloproteinase (MMP)-13. Moreover, it is the PI3K rather than the ERK/MERK pathway that plays a vital role in proteoglycan synthesis [ 81, 82] , although inhibition of the ERK1/2 pathway can result in a decrease in Shc production [83] ( Figure 2). IGF-1 induces the accumulation of proteoglycans and the chondrogenesis of limb bud mesenchymal cells through the activation of PI3K and Akt, thus activating protein kinase C (PKC)-alpha. IGF-1 also plays a role by upregulating p38 kinase and downregulating ERK1/2 expression [84]. Moreover, hypoxia-inducible factor 1α (HIF-1α), a molecule that is vital for chondrocytes to adapt to low oxygen levels, is upregulated by a posttranscriptional mechanism involving the PI3K/mammalian target of rapamycin (mTOR) and the ERK/MERK pathways [ 85, 86] ( Figure 2).
Figure 2 .
The signaling pathways of IGF-1
IGF-1 functions differently in normal or damaged chondrocytes. In normal chondrocytes (left), IGF-1 phosphorylates IRS, but only the PI3K/Akt signaling pathway plays a vital role in proteoglycan production. In damaged chondrocytes (right), the PI3K/Akt signaling pathway is activated by IGF-1 but produces IL-1 and IL-2, aggravating inflammation.
However, in cartilage defects, some studies showed the opposite results in signaling activation. For example, after IGF-1 treatment, AKT is downregulated in rabbit articular chondrocytes, resulting in the inhibition of MMP expression and nuclear factor kappa B (NF-κB) activation and eventually beneficial to OA relief [ 76, 87] . Moreover, the activation of PI3K/AKT/mTOR signaling induced by IGF-1 could lead to an increase in the expression of inflammatory factors, including IL-1 and IL-2, in mice with lumbar disc herniation [88] as well as in autophagy and apoptosis of articular cartilage in OA mice [89] ( Figure 2). These results indicate that IGF-1 is vital for the maintenance and development of normal cartilage, and it is upregulated after cartilage defects, which further damages the cartilage.
The Expression and Functions of MGF
The expression and functions of MGF in chondrocytes
IGF-1 mRNA is expressed in the growth plate of rats, and the expression pattern of MGF changes from 2 to 6 weeks of age [59] ( Table 1). MGF is slightly expressed in the resting, proliferative and hypertrophic fractions in the costochondral growth plate of two-week-old rats, while relatively high expression of MGF is observed in the hypertrophic fraction in four- to six-week-old rats [59]. Cyclic mechanical stress facilitates MGF mRNA expression in growth plate chondrocytes, which may subsequently regulate growth plate development [90]. MGF is expressed in the growth plate and is not associated with growth plate chondrocyte proliferation [ 9, 90] . Transwell system and wound healing assay in vitro show that the MGF peptide increases the mobility of MSCs [ 20, 91, 92] .
Table 1 The expression and functions of MGF in the musculoskeletal system
|
Cell behavior/tissue type |
Expression |
Migration |
Proliferation |
Differentiation |
||||
|
Growth plate chondrocyte |
MGF is expressed in the costochondral growth plate of two to six weeks old rats [59]. |
MGF is not associated with growth plate chondrocyte proliferation [ 9, 90] . |
– |
|||||
|
hMSC |
– |
MGF may promote cell migration of MSCs and chondrocytes [ 40, 92, 93] . |
MGF peptide fails to enhance hMSC proliferation [ 20, 54, 92] . |
MGF can accelerate the differentiation of BMSCs to chondrocytes in the presence of TGF-β3 [20]. |
||||
|
Cartilage with defect |
The expressions of MGF gene and mRNA are observed to be upregulated when cartilage is injured. |
Synthesized MGF peptide could promote chondrocyte migration in both excessive stress and severe hypoxia [ 29, 90] . |
MGF promotes the proliferation of chondrocytes from OA patients [40]. |
– |
||||
|
Muscle tissue |
MGF expression increases in satellite cells and in proliferating myoblasts during muscle repair [94] and after mechanical stimulation [95]. |
MGF promotes the in vitro migration of human myoblasts [96]. |
MGF facilitates proliferation of the satellite cells when muscle is damaged [ 40, 97, 98] . |
After muscle damage, MGF promotes the development into mononucleated myoblasts of the satellite cells [ 40, 97, 98] . |
In MSCs, MGF has similar expression and functions to those in growth plate chondrocytes ( Table 1). Recent studies have revealed that the MGF peptide fails to enhance hMSC proliferation [ 20, 54, 92] . However, it was reported that mechanical stimulation induces osteoblast proliferation and the expression of MGF mRNA in osteoblasts [99]. Moreover, MGF can accelerate the differentiation of BMSCs to chondrocytes in the presence of TGF-β3 [20], but MGF alone fails to induce chondrogenic differentiation of BMSCs [ 20, 90] . MGF promotes the growth and osteogenic differentiation of rMSCs [100]. In addition, MGF may promote the migration of many cell types, such as tendon cells, MSCs and chondrocytes, providing great potency in tissue repair [ 40, 92, 93] .
The expression and functions of MGF in cartilage defects
The expression of MGF gene and mRNA was observed to be upregulated when cartilage was injured ( Table 1). Through in vitro experiments, it was found that MGF is helpful in alleviating chondrocyte apoptosis and inflammation, either under mechanical overload [90] or in a CoCl 2-stimulated hypoxic microenvironment [29]. Moreover, a low concentration of MGF has better effects on preventing chondrocyte apoptosis under hypoxic circumstances [101]. Synthesized MGF peptide could also promote focal adhesion formation and cytoskeleton reorganization [90] and then further promote growth plate chondrocyte migration in both microenvironments of excessive stress and severe hypoxia [ 29, 90] , while no obvious effect is observed on the viability of chondrocytes under normoxia [29]. In chondrocytes derived from OA patients, MGF has been verified to inhibit chondrocyte apoptosis and promote chondrocyte migration and proliferation and has been demonstrated to attenuate the progression of the physiopathology of OA and increase the stiffness of OA chondrocytes [40].
The expression and functions of MGF in other tissues
MGF is sensitive to mechanical signals in skeletal muscle [97] ( Table 1). In the process of muscle repair, high expression of MGF occurs first in satellite cells and in proliferating myoblasts among the IGF family, while IGF-1a and IGF-2 are found during muscle fibre formation [ 1, 94] . In resting muscle, MGF is rapidly induced by stretching or strenuous physical activity, and the basal expression of the MGF isoform is approximately one order of magnitude lower in females than in males [95]. In active muscle, passive stretching increases MGF mRNA expression, and enhanced MGF expression is observed upon treatment with electric stimuli at 10 Hz [ 1, 2] . Increased expression of MGF has been shown in response to both short- and long-term loading exercise [ 61, 102] , and the MGF mRNA level increases in muscle subjected to electric stimulation [ 2, 97] . After muscle damage, MGF in satellite cells is upregulated, resulting in the activation, proliferation and development of satellite cells into mononucleated myoblasts, thus repairing the muscle tissue [ 40, 97, 98] . However, the ability of skeletal muscle to produce MGF in response to damage or overload diminishes with age in both humans and rats [ 61, 103] . C2C12 cells that overexpress MGF present signs of proliferation but remain in the mononucleated state, suggesting that satellite cells can be activated by MGF [53]. The repair function of MGF in skeletal muscle is also attributed to the migration of myoblasts, and migration is associated with increased matrix metalloproteinase expression, an activated fibrinolytic system [96] and enhanced myoblast stem cell differentiation [104] after MGF treatment. However, the terminal differentiation of C2C12 cells is finally inhibited in the presence of MGF [53].
Emerging evidence has indicated that MGF can be regarded as a cellular repair factor as well as a growth factor [105] and is expressed in different types of tissues [106]. In the myocardium, MGF mRNA level increases after infarction or ischemia, protecting cardiomyocytes from hypertrophy and fibrosis in vivo [ 107, 108] , whereas only IGF-1Ea is present in the resting heart [3]. In osteoblasts, the expression of MGF is increased to a greater degree after cyclic stretching than after static stretching [99]. Moreover, in tendons, MGF and IGF-1Ea are upregulated more significantly after eccentric training than after concentric training [109]. In addition, endogenous MGF is overexpressed in the ischemic brain, especially in areas resistant to damage [ 97, 110] , playing a role in neuroprotection [110]. The expression of MGF can hardly be detected in the hippocampus of the normal brain, and very low expression of MGF can be detected in other areas of the brain by western blot analysis and in situ hybridization [110]. In addition, MGF can facilitate the repair of fibroblast-like synoviocytes in OA [111] and can improve collagen synthesis and cell proliferation of injured human anterior cruciate ligament fibroblasts [112].
The Mechanism of MGF in Chondrocytes and Cartilage Defects
The mechanism of MGF in cell differentiation and proliferation
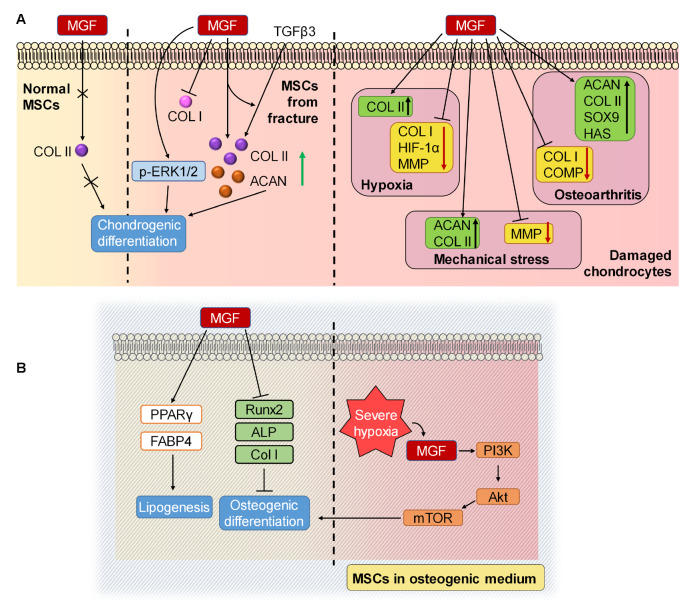
MGF promotes the production of cartilage extracellular matrix and chondrocyte differentiation. MGF receptor is expressed at high levels in rabbit MSCs, indicating that MSCs are sensitive to MGF [100]. Studies have shown that MGF can significantly upregulate the mRNA expression of Col2 and aggrecan induced by TGF-β3 in human MSCs collected from open femur fracture patients but downregulate the expression of Col1, indicating that MGF can promote the differentiation to chondrocytes induced by TGF-β3 [ 20, 113] , and MGF stimulation alone can also significantly upregulate the mRNA expression of Col2, especially from day 2 to day 8 of in vitro culture [113]. Moreover, exogenous administration of MGF upregulates p-ERK1/2 signaling, whose phosphorylation is related to the differentiation of MSCs to chondrocytes [113]. However, MSCs isolated from the long bones of mice showed no difference in the protein expression of Col2 when induced in chondrogenic differentiation media of MGF for 7 days [90], and another study also showed that in normal culture media, MGF alone failed to upregulate the protein expression of Col2 after 15 days of induction, indicating that MGF has no effect on chondrogenic differentiation [20] ( Figure 3A). It appears that MGF can induce the expression of Col2 in early damaged cartilage tissue rather than in healthy tissue and is unable to promote chondrogenic differentiation in both situations.
Figure 3 .
The mechanism of MGF in cell differentiation
Expression of MGF in normal cartilage and damaged cartilage. (A) In MSCs from normal cartilage, MGF fails to upregulate COL II expression and promote chondrogenic differentiation. However, in MSCs derived from fracture marrow, MGF enhances the differentiation of BMSCs induced by TGFβ3 and upregulates the expression of p-ERK1/2, which induces chondrogenic differentiation. In chondrocytes from damaged tissues, MGF influences the expression of chondrocytes and facilitates the repair process. (B) In MSCs cultured in osteogenic medium, MGF can upregulate the expressions of lipogenesis-related genes and downregulate the expressions of osteogenesis-related genes in normal tissue but promote osteogenic differentiation through PI3K/Akt under severe hypoxia. ACAN, aggrecan; Col II, type II collagen; Col I, type I collagen; TGFβ3, transforming growth factor β3; p-ERK1/2, phosphorylated extracellular signal-regulated kinase; HIF-1α, hypoxia-inducible factor 1α; MMP, matrix metalloproteinase; SOX9, sex-determining region Y-type high mobility group box protein 9; HAS, hyaluronan synthase; COMP, cartilage oligomeric matrix protein; ALP, alkaline phosphatase; PPARγ, peroxisome proliferator-activated receptor gamma; FABP4, fatty acid binding protein 4; OCN, osteocalcin; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mechanistic target of rapamycin.
Apart from its effects on normal cartilage, MGF also has a protective role in defective cartilage. MGF fails to promote chondrocyte proliferation in vitro [90], but under severe hypoxia, MGF promotes the proliferation of chondrocytes through the PI3K-Akt and MEK-ERK1/2 pathways [ 29, 114] . Moreover, under severe hypoxia induced by CoCl 2, MGF reduces the expressions of HIF-1α, Col1, and MMP1/13 but facilitates the expression of Col2 in chondrocytes, weakening the adverse effects of cartilage damage [29]. Furthermore, in OA chondrocytes treated with MGF, the mRNA levels of Sox9, Acan, Col2 and hyaluronan synthase (HAS) are upregulated, while the levels of Col1 and cartilage oligomeric matrix protein (Comp) are downregulated, resulting in the promotion of cartilage extracellular matrix production and the prevention of fibrocartilage formation [40]. In agreement with this evidence, exogenous MGF protects cartilage suffering mechanical stress by downregulating the mRNA expression of MMP3 and upregulating the mRNA expressions of Acan and Col2 [90] ( Figure 3A).
In subchondral bone, MGF was found to influence the process of osteogenesis. After treatment with MGF in conditioned osteogenic media, BMSCs were found to downregulate the gene expressions of Runx2, ALP and Col1 and decrease the activity of ALP, indicating an inhibitory role on osteogenic differentiation [92]. It has also been found that the expressions of lipogenesis-related genes, peroxisome proliferator activated receptor gamma ( PPARγ) and fatty acid binding protein 4 ( FABP4) are increased in MGF-treated BMSCs [92]. However, some studies presented the opposite results. For example, Lauzon et al. [115] and Baker et al. [116] claimed that the activation of PI3K/AKT/mTOR signaling can promote osteoblastic differentiation in preosteoblasts and BMSCs, and MGF was proved to promote PI3K/AKT signaling in osteogenic media under severe hypoxia [ 100, 114] ( Figure 3B).
Taken together, these results suggest that MGF has little effect on normal tissue but promotes cell differentiation and proliferation once tissue is injured or damaged.
The mechanism of MGF in inflammatory reactions and apoptosis
Cartilage damage is often associated with the inflammatory response and can induce various types of cell death, including apoptosis [117]. Inflammatory reactions can be caused by intracellular contents spilled by cell necrosis, and inflammatory reactions can induce pyroptosis [118]. Apoptosis is a form of programmed cell death and is characterized by the activation of caspases [119]. Moreover, reports have shown that cell death can change from apoptosis to necrosis [ 117, 120] . Mechanical stress can lead to cartilage inflammatory reactions and apoptosis, although the specific relationship and mechanism have not been clearly described.
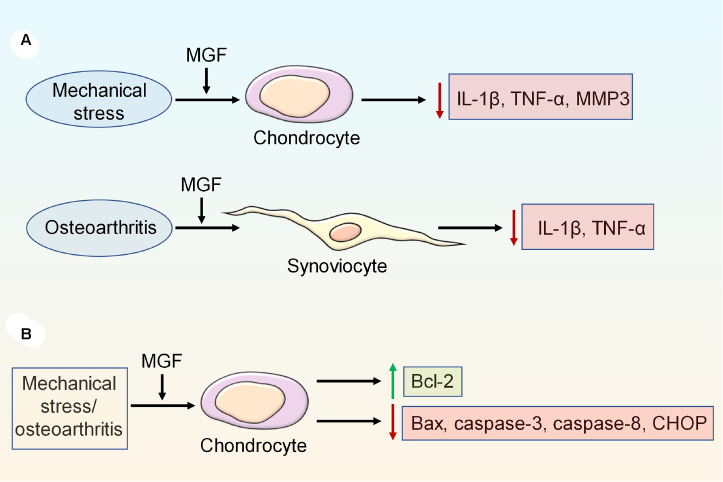
Mechanical stress can induce inflammatory reactions, resulting in the upregulation of IL-1β, tumor necrosis factor (TNF)-α and MMP3 expressions and the downregulation of Acan and Col2 expressions, but the expressions of inflammatory factors can be reversed by exogenous MGF [90]. Moreover, MGF can regulate the inflammatory reaction in fibroblast-like synoviocytes of OA by decreasing the expressions of TNF-α and IL-1β [111] ( Figure 4A).
Figure 4 .
The mechanism of MGF in inflammatory reactions and cell apoptosis
(A) MGF can downregulate the expressions of inflammatory factors, such as IL-1β, TNF-α, and MMP3, in chondrocytes and synoviocytes. (B) Excessive mechanical stress and OA induce cell apoptosis, which can be inhibited by exogenous MGF by regulating the expressions of Bcl-2, Bax, caspase-3, caspase-8 and CHOP.
MGF could inhibit the gene and protein expressions of Bcl-2-associated X protein (Bax) and caspase-3 but upregulate the gene and protein expressions of Bcl-2 in chondrocytes under mechanical stress [ 90, 121] . Moreover, in chondrocytes from OA patients, the expressions of caspase-3, caspase-8 and C/EBP homologous protein (CHOP) are significantly downregulated after MGF treatment, revealing that MGF inhibits chondrocyte apoptosis [40] ( Figure 4B).
The mechanism by which MGF affects cell migration and unfolded protein response
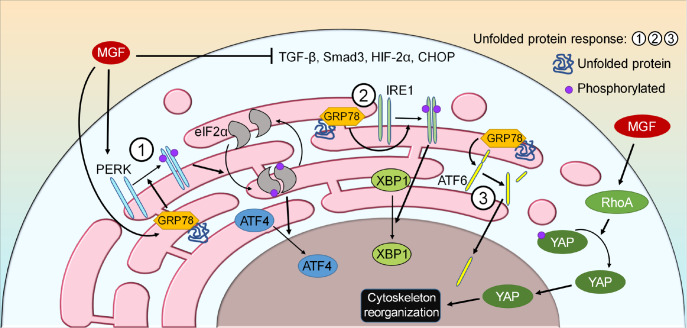
Furthermore, MGF can promote MSC and chondrocyte migration. Transwell assay revealed that MGF can promote OA chondrocyte passage through the Transwell membrane [40], and an in vitro wound healing assay showed that the wound repair rate is increased by both MGF and TGF-β3 [20]. The cell migration promoted by MGF is achieved through the activation of RhoA-Yes associated protein (YAP) signaling [90] ( Figure 5). YAP can be regulated by RhoA, transcriptionally controls focal adhesion (FA) formation and cytoskeleton stability, and determines cell shape, migration and differentiation [122]. RhoA is a Ras-related small GTP-binding protein that is related to the Rho GTPase subfamily, and the activation of RhoA stimulates the formation of the cytoskeleton [ 123, 124] . MGF can directly activate RhoA and its target genes, including YAP, and stimulate the formation of focal adhesion and the cytoskeleton, indicating the role of MGF in cytoskeleton promotion [90]. In our lab, we also confirmed the role of MGF in cytoskeletal reorganization in chondrocytes ( Figure 6).
Figure 5 .
The mechanism by which MGF affects cell migration and the unfolded protein response
MGF induces cell migration via the RhoA/YAP signaling pathway. The unfolded protein response is activated mainly via three branches in response to stress, and MGF can influence one of the branches. GRP78 binds to unfolded or misfolded proteins and activates three ER transmembrane proteins (PERK, IRE1, and ATF6). Exogenous MGF increases the expressions of PERK and GRP78 and decreases the expressions of TGF-β, Smad3, HIF-2α, and CHOP.
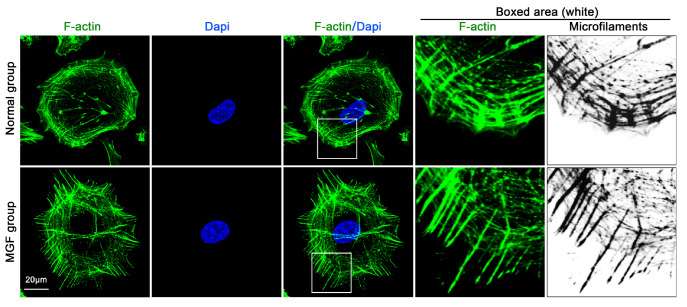
Figure 6 .
IGF enhances the reorganization of the cytoskeleton in chondrocytes
Immunofluorescence staining of F-actin shows the morphological changes in cytoskeleton reorganization between the normal group and MGF group. The boxed area shows that MGF significantly orchestrates cytoskeletal components and thus might facilitate cell synaptic extension. Cytoskeleton (F-actin, FITC), green; nucleus (Dapi), blue. These data from our lab were collected based on at least three independent experiments ( n ≥ 3).
Additionally, OA can destroy the homeostasis of the endoplasmic reticulum (ER), but it can be rebuilt by the unfolded protein response (UPR), which is mainly mediated by protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE-1α) and activating transcription factor 6 (ATF6) ( Figure 5) [ 125‒ 127] . Among these pathways, both PERK and IRE-1α can regulate actin cytoskeleton dynamics through the binding with filamin A, and filamin A regulates the action of actin filaments and cytoskeleton dynamics, facilitating cell migration [128]. MGF was found to increase the expressions of glucose-regulated protein 78 (GRP78) and PERK but decrease the expressions of TGF-β, Smad3, HIF-2α and Chop [40], indicating that MGF can effectively induce UPR. Furthermore, upon treatment with MGF and pERK siRNA, the expressions of TGF-β, Smad3, HIF-2α and Chop were decreased, whereas that of GRP78 was increased [40], suggesting that GRP78 may play a compensating role for pERK.
Conclusions and Perspectives
MGF, as one of the isoforms of IGF-I, functions differently from other IGF-I subtypes. The former functions mainly in injured or damaged cartilage, and the latter is effective in both normal and damaged tissues. In injured or damaged cartilage, MGF shows mechanical sensitivity and has the capacity to induce cell proliferation, differentiation and migration, inhibit cell apoptosis and provoke inflammatory reactions. Although many studies have proven that MGF has an important impact on cells and their living microenvironment, the underlying biological mechanism is still unclear and needs to be further elucidated. Another challenge facing MGF research is the lack of specific antibodies. Although an antibody recognizing a sequence within the pro-IGF-1Eb (rodent) peptide has been generated [ 129, 130] , the precise sequence of synthetic MGF cannot be recognized, and the key protease for producing endogenous MGF has not yet been found [2]. Although some achievements have been made, further studies are required to determine the precise mechanism of MGF in vivo. Many studies have recently been conducted in vitro, while the environment in vivo is much more complicated, and the conclusions in vitro may be inconsistent with those in vivo. Thus, more in vivo research needs to be done. Moreover, research has found an increase in MMPs with low-dose MGF treatment but a decrease with high-dose MGF treatment, implying that MGF may have different mechanisms when the dose changes [40], but the mechanisms remain unknown. Finally, signaling pathways activated by MGF seem to function differently under different situations and their precise roles should be further confirmed. For example, the PI3K/AKT signaling pathway promotes ECM anabolism and chondrocyte proliferation and inhibits chondrocyte apoptosis when activated [ 131, 132] . In cartilage with OA, the PI3K/AKT signaling pathway is downregulated [28] but can be upregulated by MGF [114]. To interpret this contradiction, more research, both in vitro and in vivo, is needed to disclose its regulatory mechanism.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No. 81771047 to J.X.) and the Sichuan Science and Technology Innovation Talent Project (No. 2022JDRC0044 to J.X.).
References
- 1.Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. . 2013;70:4117–4130. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matheny RW. Jr, Nindl BC, Adamo ML. Minireview: mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology. . 2010;151:865–875. doi: 10.1210/en.2009-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G. Expression of insulin growth factor‐1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol. . 1999;516:583–592. doi: 10.1111/j.1469-7793.1999.0583v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stavropoulou A, Halapas A, Sourla A, Philippou A, Papageorgiou E, Papalois A, Koutsilieris M. IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol Med. . 2009;15:127–135. doi: 10.2119/molmed.2009.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDermott MM, Ferrucci L, Gonzalez-Freire M, Kosmac K, Leeuwenburgh C, Peterson CA, Saini S, et al. Skeletal muscle pathology in peripheral artery disease. Arterioscler Thromb Vasc Biol. . 2020;40:2577–2585. doi: 10.1161/ATVBAHA.120.313831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunç BS, Toprak F, Toprak SF, Sozer S. In vitro investigation of growth factors including MGF and IGF-1 in neural stem cell activation, proliferation, and migration . Brain Res. . 2021;1759:147366. doi: 10.1016/j.brainres.2021.147366. [DOI] [PubMed] [Google Scholar]
- 7.Armakolas A, Philippou A, Panteleakou Z, Nezos A, Sourla A, Petraki C, Koutsilieris M. Preferential expression of IGF-1Ec (MGF) transcript in cancerous tissues of human prostate: evidence for a novel and autonomous growth factor activity of MGF E peptide in human prostate cancer cells. Prostate. . 2010;70:1233–1242. doi: 10.1002/pros.21158. [DOI] [PubMed] [Google Scholar]
- 8.Sun KT, Cheung KK, Au SWN, Yeung SS, Yeung EW. Overexpression of mechano-growth factor modulates inflammatory cytokine expression and macrophage resolution in skeletal muscle injury. Front Physiol. . 2018;9:999. doi: 10.3389/fphys.2018.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlegel W, Raimann A, Halbauer D, Scharmer D, Sagmeister S, Wessner B, Helmreich M, et al. Insulin-like growth factor I (IGF-1) Ec/mechano growth factor—a splice variant of IGF-1 within the growth plate. PLoS One. . 2013;8:e76133. doi: 10.1371/journal.pone.0076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anatomy. . 2003;203:89–99. doi: 10.1046/j.1469-7580.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Zeng Z, Zhao L, Chen P, Xiao W. Impaired skeletal muscle regeneration induced by macrophage depletion could be partly ameliorated by MGF injection. Front Physiol. . 2019;10:601. doi: 10.3389/fphys.2019.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan M, Wang Q, Liu Y, Xie J. The role of TGF-β2 in cartilage development and diseases. Bone Joint Res. . 2021;10:474–487. doi: 10.1302/2046-3758.108.BJR-2021-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. . 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Wei J, Li J, Cui Y, Zhou X, Xie J. Lipid metabolism in cartilage and its diseases: a concise review of the research progress. Acta Biochim Biophys Sin. . 2021;53:517–527. doi: 10.1093/abbs/gmab021. [DOI] [PubMed] [Google Scholar]
- 15.Feng Q, Gao H, Wen H, Huang H, Li Q, Liang M, Liu Y, et al. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: insights from a microgel model. Acta Biomater. . 2020;113:393–406. doi: 10.1016/j.actbio.2020.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, Chu JSF, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. . 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 17.Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. . 2003;21:451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C, Cui Y, Yang Y, Guo D, Zhang D, Fan Y, Li X, et al. Runx1 protects against the pathological progression of osteoarthritis. Bone Res. . 2021;9:50. doi: 10.1038/s41413-021-00173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Li Z, Wang C, Bai H, Wang Z, Liu Y, Bao Y, et al. Enlightenment of growth plate regeneration based on cartilage repair theory: a review. Front Bioeng Biotechnol. . 2021;9:654087. doi: 10.3389/fbioe.2021.654087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D′Andrea CR, Alfraihat A, Singh A, Anari JB, Cahill PJ, Schaer T, Snyder BD, et al. Part 1. Review and meta‐analysis of studies on modulation of longitudinal bone growth and growth plate activity: a macro‐scale perspective. J Orthop Res. . 2021;39:907–918. doi: 10.1002/jor.24976. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Zhang D, Lin Y, Yuan Q, Zhou X. Anterior cruciate ligament transection–induced cellular and extracellular events in menisci: implications for osteoarthritis. Am J Sports Med. . 2018;46:1185–1198. doi: 10.1177/0363546518756087. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Chen H, Zhang D, Xie J, Zhou X. The role of stromal cell-derived factor 1 on cartilage development and disease. Osteoarthritis Cartilage. . 2021;29:313–322. doi: 10.1016/j.joca.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Li J, Zhang D, Zhou X, Xie J. Role of the fibroblast growth factor 19 in the skeletal system. Life Sci. . 2021;265:118804. doi: 10.1016/j.lfs.2020.118804. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Cui Y, Zhang D, Xie J, Zhou X. The role of fibroblast growth factor 8 in cartilage development and disease. J Cell Mol Medi. . 2022;26:990–999. doi: 10.1111/jcmm.17174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Zinkle A, Chen L, Mohammadi M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat Rev Rheumatol. . 2020;16:547–564. doi: 10.1038/s41584-020-0469-2. [DOI] [PubMed] [Google Scholar]
- 26.Kan S, Duan M, Liu Y, Wang C, Xie J. Role of mitochondria in physiology of chondrocytes and diseases of osteoarthritis and rheumatoid arthritis. Cartilage. . 2021;13:1102S–1121S. doi: 10.1177/19476035211063858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichler K, Herbert V, Schmidt B, Fischerauer EE, Leithner A, Weinberg AM. Expression of matrix metalloproteinases in human growth plate chondrocytes is enhanced at high levels of mechanical loading: a possible explanation for overuse injuries in children. Bone Joint J. . 2013;95-B:568–573. doi: 10.1302/0301-620X.95B4.30639. [DOI] [PubMed] [Google Scholar]
- 28.Zhou C, Wang C, Xu K, Niu Z, Zou S, Zhang D, Qian Z, et al. Hydrogel platform with tunable stiffness based on magnetic nanoparticles cross-linked GelMA for cartilage regeneration and its intrinsic biomechanism. Bioactive Mater. . 2023;25:615–628. doi: 10.1016/j.bioactmat.2022.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosa SC, Rufino AT, Judas F, Tenreiro C, Lopes MC, Mendes AF. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthritis Cartilage. . 2011;19:719–727. doi: 10.1016/j.joca.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. . 2007;13:663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res Paediatr. . 2009;71:36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- 32.Feigerlova E, Hwa V, Derr MA, Rosenfeld RG. Current issues on molecular diagnosis of GH signaling defects. Endocr Dev. 2013, 24: 118-127. . [DOI] [PubMed]
- 33.Caputo M, Pigni S, Agosti E, Daffara T, Ferrero A, Filigheddu N, Prodam F. Regulation of GH and GH signaling by nutrients. Cells. . 2021;10:1376. doi: 10.3390/cells10061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poudel SB, Dixit M, Neginskaya M, Nagaraj K, Pavlov E, Werner H, Yakar S. Effects of GH/IGF on the aging mitochondria. Cells. . 2020;9:1384. doi: 10.3390/cells9061384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee A, Alzhanov D, Rotwein P. Defining human insulin-like growth factor I gene regulation. Am J Physiol Endocrinol Metab. . 2016;311:E519–E529. doi: 10.1152/ajpendo.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci USA. . 1986;83:77–81. doi: 10.1073/pnas.83.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasprzak A, Szaflarski W. Role of alternatively spliced messenger RNA (mRNA) isoforms of the insulin-like growth factor 1 (IGF1) in selected human tumors. Int J Mol Sci. . 2020;21:6995. doi: 10.3390/ijms21196995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol. . 2010;108:1069–1076. doi: 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocrine Rev. . 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 40.Du X, Cai L, Xie J, Zhou X. The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res. . 2023;11:2. doi: 10.1038/s41413-022-00239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan DS, Cook A, Chew SL. The role of TGF-beta3 in cartilage development and osteoarthritis. BMC Cell Biol. . 2002;3:17. doi: 10.1186/1471-2121-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brisson BK, Barton ER. New modulators for IGF-I activity within IGF-I processing products. Front Endocrinol. . 2013;4:42. doi: 10.3389/fendo.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annibalini G, Contarelli S, De Santi M, Saltarelli R, Di Patria L, Guescini M, Villarini A, et al. The intrinsically disordered E-domains regulate the IGF-1 prohormones stability, subcellular localisation and secretion. Sci Rep. . 2018;8:8919. doi: 10.1038/s41598-018-27233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brisson BK, Barton ER. Insulin-like growth factor-I E-peptide activity is dependent on the IGF-I receptor. PLoS One. . 2012;7:e45588. doi: 10.1371/journal.pone.0045588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philippou A, Maridaki M, Pneumaticos S, Koutsilieris M. The complexity of the IGF1 gene splicing, posttranslational modification and bioactivity. Mol Med. . 2014;20:202–214. doi: 10.2119/molmed.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. . 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 47.Adamo ML, Ben-Hur H, Roberts Jr CT, LeRoith D. Regulation of start site usage in the leader exons of the rat insulin-like growth factor-I gene by development, fasting, and diabetes. Mol Endocrinol. . 1991;5:1677–1686. doi: 10.1210/mend-5-11-1677. [DOI] [PubMed] [Google Scholar]
- 48.Dickson MC, Saunders JC, Gilmour RS. The ovine insulin-like growth factor-I gene: characterization, expression and identification of a putative promoter. J Mol Endocrinol. . 1991;6:17–31. doi: 10.1677/jme.0.0060017. [DOI] [PubMed] [Google Scholar]
- 49.Lowe WL Jr, Lasky SR, LeRoith D, Roberts Jr CT. Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol. . 1988;2:528–535. doi: 10.1210/mend-2-6-528. [DOI] [PubMed] [Google Scholar]
- 50.Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. . 1986;261:4828–4832. doi: 10.1016/S0021-9258(19)89179-2. [DOI] [PubMed] [Google Scholar]
- 51.Bell GI, Stempien MM, Fong NM, Rall LB. Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucl Acids Res. . 1986;14:7873–7882. doi: 10.1093/nar/14.20.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chew SL, Lavender P, Clark AJ, Ross RJ. An alternatively spliced human insulin-like growth factor-I transcript with hepatic tissue expression that diverts away from the mitogenic IBE1 peptide. Endocrinology. . 1995;136:1939–1944. doi: 10.1210/endo.136.5.7720641. [DOI] [PubMed] [Google Scholar]
- 53.Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. . 2002;522:156–160. doi: 10.1016/S0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- 54.Collins JM, Goldspink PH, Russell B. Migration and proliferation of human mesenchymal stem cells is stimulated by different regions of the mechano-growth factor prohormone. J Mol Cell Cardiol. . 2010;49:1042–1045. doi: 10.1016/j.yjmcc.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, He Q, Tsang WP, Garvey WT, Chan WY, Wan C. Insulin exerts direct, IGF-1 independent actions in growth plate chondrocytes. Bone Res. . 2014;2:14012. doi: 10.1038/boneres.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. . 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lui JC, Colbert M, Cheung CSF, Ad M, Lee A, Zhu Z, Barnes KM, et al. Cartilage-targeted IGF-1 treatment to promote longitudinal bone growth. Mol Ther. . 2019;27:673–680. doi: 10.1016/j.ymthe.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Racine HL, Serrat MA. The actions of IGF-1 in the growth plate and Its role in postnatal bone elongation. Curr Osteoporos Rep. . 2020;18:210–227. doi: 10.1007/s11914-020-00570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin WW, Oberbauer AM. Spatiotemporal expression of alternatively spliced IGF-I mRNA in the rat costochondral growth plate. J Endocrinol. . 1999;160:461–467. doi: 10.1677/joe.0.1600461. [DOI] [PubMed] [Google Scholar]
- 60.Reinecke M, Schmid AC, Heyberger-Meyer B, Hunziker EB, Zapf J. Effect of growth hormone and insulin-Like growth factor I (IGF-I) on the expression of IGF-I messenger ribonucleic acid and peptide in rat tibial growth plate and articular chondrocytes in vivo . Endocrinology. . 2000;141:2847–2853. doi: 10.1210/endo.141.8.7624. [DOI] [PubMed] [Google Scholar]
- 61.Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SDR. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. . 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. . 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 63.Fornaro M, Hinken AC, Needle S, Hu E, Trendelenburg AU, Mayer A, Rosenstiel A, et al. Mechano-growth factor peptide, the COOH terminus of unprocessed insulin-like growth factor 1, has no apparent effect on myoblasts or primary muscle stem cells. Am J Physiol Endocrinol Metab. . 2014;306:E150–E156. doi: 10.1152/ajpendo.00408.2013. [DOI] [PubMed] [Google Scholar]
- 64.Brisson BK, Spinazzola J, Park SH, Barton ER. Viral expression of insulin-like growth factor I E-peptides increases skeletal muscle mass but at the expense of strength. Am J Physiol Endocrinol Metab. . 2014;306:E965–E974. doi: 10.1152/ajpendo.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Nishida S, Sakata T, Elalieh HZ, Chang W, Halloran BP, Doty SB, et al. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology. . 2006;147:4753–4761. doi: 10.1210/en.2006-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993, 75: 59–72 . [PubMed]
- 67.McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. . 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schalkwijk J, Joosten LAB, Van Den Berg WB, Van Wyk JJ, Van Putte LAD. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Care Res. . 1989;32:66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- 69.Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O′Driscoll SW. Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro . Osteoarthritis Cartilage. . 2003;11:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 70.Longobardi L, O′Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J Bone Miner Res. . 2006;21:626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 71.Gugjoo MB, Amarpal MB, Abdelbaset-Ismail A, Aithal HP, Kinjavdekar P, Pawde AM, Kumar GS, et al. Mesenchymal stem cells with IGF-1 and TGF-β1 in laminin gel for osteochondral defects in rabbits. Biomed Pharmacother. . 2017;93:1165–1174. doi: 10.1016/j.biopha.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 72.Wen C, Xu L, Xu X, Wang D, Liang Y, Duan L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res Ther. . 2021;23:277. doi: 10.1186/s13075-021-02662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheumatism. . 2003;48:430–441. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- 74.Wildemann B, Schmidmaier G, Ordel S, Stange R, Haas NP, Raschke M. Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-β1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl BioMater. . 2003;65B:150–156. doi: 10.1002/jbm.b.10512. [DOI] [PubMed] [Google Scholar]
- 75.Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS. Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum. . 2006;54:3850–3858. doi: 10.1002/art.22254. [DOI] [PubMed] [Google Scholar]
- 76.Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One. . 2011;6:e28663. doi: 10.1371/journal.pone.0028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. . 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. . 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 79.Mohan S, Baylink DJ. Impaired skeletal growth in mice with haploinsufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol. . 2005;185:415–420. doi: 10.1677/joe.1.06141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD, Tang D, Sheu TJ, et al. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthritis Cartilage. . 2009;17:100–106. doi: 10.1016/j.joca.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Starkman BG, Cravero JD, Delcarlo Jr M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. . 2005;389:723–729. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Zhang R, Zhang Q, Xu Z, Xu F, Li D, Li Y. Microtia patients: auricular chondrocyte ECM is promoted by CGF through IGF‐1 activation of the IGF‐1R/PI3K/AKT pathway. J Cell Physiol. . 2019;234:21817–21824. doi: 10.1002/jcp.27316. [DOI] [PubMed] [Google Scholar]
- 83.McMahon LA, Prendergast PJ, Campbell VA. A comparison of the involvement of p38, ERK1/2 and PI3K in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. . 2008;368:990–995. doi: 10.1016/j.bbrc.2008.01.160. [DOI] [PubMed] [Google Scholar]
- 84.Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem. . 2003;278:36563–36571. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- 85.Sartori-Cintra AR, de Mara CS, Argolo DL, Coimbra IB. Regulation of hypoxia-inducible factor-1α (HIF-1α) expression by interleukin-1β (IL-1β), insulin-like growth factors I (IGF-I) and II (IGF-II) in human osteoarthritic chondrocytes. Clinics. . 2012;67:35–40. doi: 10.6061/clinics/2012(01)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gelse K, Mühle C, Knaup K, Swoboda B, Wiesener M, Hennig F, Olk A, et al. Chondrogenic differentiation of growth factor-stimulated precursor cells in cartilage repair tissue is associated with increased HIF-1α activity. Osteoarthritis Cartilage. . 2008;16:1457–1465. doi: 10.1016/j.joca.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Deng Z, Lin Z, Zhong Q, Lu M, Fang H, Liu J, Duan L, et al. Interleukin 1 beta-induced chloride currents are important in osteoarthritis onset: an in vitro study . Acta Biochim Biophys Sin. . 2021;53:400–409. doi: 10.1093/abbs/gmab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raj AT, Kheur S, Bhonde R, Gupta AA, Patil S. Assessing the effect of human mesenchymal stem cell-derived conditioned media on human cancer cell lines: a systematic review. Tissue Cell. . 2021;71:101505. doi: 10.1016/j.tice.2021.101505. [DOI] [PubMed] [Google Scholar]
- 89.Yu Q, Zhao B, He Q, Zhang Y, Peng X. microRNA‐206 is required for osteoarthritis development through its effect on apoptosis and autophagy of articular chondrocytes via modulating the phosphoinositide 3‐kinase/protein kinase B‐mTOR pathway by targeting insulin‐like growth factor‐1. J Cell Biochem. . 2019;120:5287–5303. doi: 10.1002/jcb.27803. [DOI] [PubMed] [Google Scholar]
- 90.Jing X YY, Bao Y, Zhang J, Huang J, Wang R, Guo J, Guo F. Mechano-growth factor protects against mechanical overload induced damage and promotes migration of growth plate chondrocytes through RhoA/YAP pathway. Exp Cell Res. 2018, 388: 81-91. . [DOI] [PubMed]
- 91.Li J, Fu X, Zhang D, Guo D, Xu S, Wei J, Xie J, et al. Co-culture with osteoblasts up-regulates glycolysis of chondrocytes through MAPK/HIF-1 pathway. Tissue Cell. . 2022;78:101892. doi: 10.1016/j.tice.2022.101892. [DOI] [PubMed] [Google Scholar]
- 92.Cui H, Yi Q, Feng J, Yang L, Tang L. Mechano growth factor E peptide regulates migration and differentiation of bone marrow mesenchymal stem cells. J Mol Endocrinol. . 2014;52:111–120. doi: 10.1530/JME-13-0157. [DOI] [PubMed] [Google Scholar]
- 93.Zhang B, Luo Q, Sun J, Xu B, Ju Y, Yang L, Song G. MGF enhances tenocyte invasion through MMP-2 activity via the FAK-ERK1/2 pathway. Wound Repair Regeneration. . 2015;23:394–402. doi: 10.1111/wrr.12293. [DOI] [PubMed] [Google Scholar]
- 94.Kandalla PK, Goldspink G, Butler-Browne G, Mouly V. Mechano growth factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech Ageing Dev. . 2011;132:154–162. doi: 10.1016/j.mad.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 95.Greig CA, Hameed M, Young A, Goldspink G, Noble B. Skeletal muscle IGF-I isoform expression in healthy women after isometric exercise. Growth Hormone IGF Res. . 2006;16:373–376. doi: 10.1016/j.ghir.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Mills P, Lafrenière JF, Benabdallah BF, El Fahime EM, Tremblay JP. A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res. . 2007;313:527–537. doi: 10.1016/j.yexcr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 97.Dai Z, Wu F, Yeung EW, Li Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Hormone IGF Res. . 2010;20:275–281. doi: 10.1016/j.ghir.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 98.Schönenberger C, Schütz A, Franco-Obregón A, Zenobi-Wong M. Efficient electroporation of peptides into adherent cells: investigation of the role of mechano-growth factor in chondrocyte culture. Biotechnol Lett. . 2011;33:883–888. doi: 10.1007/s10529-010-0508-1. [DOI] [PubMed] [Google Scholar]
- 99.Tang LL, Xian CY, Wang YL. The MGF expression of osteoblasts in response to mechanical overload. Arch Oral Biol. . 2006;51:1080–1085. doi: 10.1016/j.archoralbio.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 100.Tong Y, Feng W, Wu Y, Lv H, Jia Y, Jiang D. Mechano-growth factor accelerates the proliferation and osteogenic differentiation of rabbit mesenchymal stem cells through the PI3K/AKT pathway. BMC Biochem. . 2015;16:1. doi: 10.1186/s12858-015-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sha Y, Yang L, Lv Y. MGF E peptide improves anterior cruciate ligament repair by inhibiting hypoxia‐induced cell apoptosis and accelerating angiogenesis. J Cell Physiol. . 2019;234:8846–8861. doi: 10.1002/jcp.27546. [DOI] [PubMed] [Google Scholar]
- 102.Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty Jr CL, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. . 2001;280:E383–E390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- 103.Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. . 2001;505:259–263. doi: 10.1016/S0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- 104.Ates K, Yang SY, Orrell RW, Sinanan ACM, Simons P, Solomon A, Beech S, et al. The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Lett. . 2007;581:2727–2732. doi: 10.1016/j.febslet.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 105.Goldspink G. Impairment of IGF-I gene splicing and MGF expression associated with muscle wasting. Int J Biochem Cell Biol. . 2006;38:481–489. doi: 10.1016/j.biocel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 106.Vassilakos G, Philippou A, Koutsilieris M. Identification of the IGF-1 processing product human Ec/rodent Eb peptide in various tissues: evidence for its differential regulation after exercise-induced muscle damage in humans. Growth Hormone IGF Res. . 2017;32:22–28. doi: 10.1016/j.ghir.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Carpenter V, Matthews K, Devlin G, Stuart S, Jensen J, Conaglen J, Jeanplong F, et al. Mechano-growth factor reduces loss of cardiac function in acute myocardial infarction. Heart Lung Circ. . 2008;17:33–39. doi: 10.1016/j.hlc.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 108.Cai M, Wang Q, Liu Z, Jia D, Feng R, Tian Z. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. . 2018;213:40–49. doi: 10.1016/j.lfs.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 109.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. . 1985;102:573–581. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- 110.Dluzniewska J, Sarnowska A, Beręsewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, et al. A strong neuroprotective effect of the autonomous C‐terminal peptide of IGF‐1 Ec (MGF) in brain ischemia. FASEB J. . 2005;19:1896–1898. doi: 10.1096/fj.05-3786fje. [DOI] [PubMed] [Google Scholar]
- 111.Li H, Lei M, Yu C, Lv Y, Song Y, Yang L. Mechano growth factor-E regulates apoptosis and inflammatory responses in fibroblast-like synoviocytes of knee osteoarthritis. Int Orthopaedics (SICOT) . 2015;39:2503–2509. doi: 10.1007/s00264-015-2974-5. [DOI] [PubMed] [Google Scholar]
- 112.Sha Y, Afandi R, Zhang B, Yang L, Lv Y. MGF E peptide pretreatment improves collagen synthesis and cell proliferation of injured human ACL fibroblasts via MEK-ERK1/2 signaling pathway. Growth Factors. . 2017;35:29–38. doi: 10.1080/08977194.2017.1327856. [DOI] [PubMed] [Google Scholar]
- 113.Armakolas N, Dimakakos A, Armakolas A, Antonopoulos A, Koutsilieris M. Possible role of the Ec peptide of IGF-1Ec in cartilage repair. Mol Med Rep. . 2016;14:3066–3072. doi: 10.3892/mmr.2016.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sha Y, Lv Y, Xu Z, Yang L, Hao X, Afandi R. MGF E peptide pretreatment improves the proliferation and osteogenic differentiation of BMSCs via MEK-ERK1/2 and PI3K-Akt pathway under severe hypoxia. Life Sci. . 2017;189:52–62. doi: 10.1016/j.lfs.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 115.Lauzon MA, Drevelle O, Daviau A, Faucheux N. Effects of BMP-9 and BMP-2 on the PI3K/Akt pathway in MC3T3-E1 Preosteoblasts. Tissue Eng Part A. . 2016;22:1075–1085. doi: 10.1089/ten.tea.2016.0151. [DOI] [PubMed] [Google Scholar]
- 116.Baker N, Sohn J, Tuan RS. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res Ther. . 2015;6:238. doi: 10.1186/s13287-015-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kühn K, D′Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. . 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 118.Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol. . 2016;26:R568–R572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 119.Hsu SK, Li CY, Lin IL, Syue WJ, Chen YF, Cheng KC, Teng YN, et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics. . 2021;11:8813–8835. doi: 10.7150/thno.62521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. . 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Q, Fang H, Zhao L, Zhang C, Zhang L, Tian B. Mechano growth factor attenuates mechanical overload-induced nucleus pulposus cell apoptosis through inhibiting the p38 MAPK pathway. Biosci Rep. . 2019;39:BSR20182462. doi: 10.1042/BSR20182462. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skládal P, Pešl M, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. . 2017;8:15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim J, Islam R, Cho JY, Jeong H, Cap K, Park Y, Hossain AJ, et al. Regulation of RhoA GTPase and various transcription factors in the RhoA pathway. J Cell Physiol. . 2018;233:6381–6392. doi: 10.1002/jcp.26487. [DOI] [PubMed] [Google Scholar]
- 124.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. . 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 125.Yu M, Lun J, Zhang H, Wang L, Zhang G, Zhang H, Fang J. Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy. Acta Biochim Biophys Sin. . 2021;53:1417–1427. doi: 10.1093/abbs/gmab131. [DOI] [PubMed] [Google Scholar]
- 126.Ramirez MU, Hernandez SR, Soto-Pantoja DR, Cook KL. Endoplasmic reticulum stress pathway, the unfolded protein response, modulates immune function in the tumor microenvironment to impact tumor progression and therapeutic response. Int J Mol Sci. . 2019;21:169. doi: 10.3390/ijms21010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Long D, Chen K, Yang Y, Tian X. Unfolded protein response activated by endoplasmic reticulum stress in pancreatic cancer: potential therapeutical target. Front Biosci (Landmark Ed) . 2021;26:1689–1696. doi: 10.52586/5061. [DOI] [PubMed] [Google Scholar]
- 128.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. . 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kravchenko IV, Furalyov VA, Khotchenkov VP, Popov VO. Monoclonal antibodies to mechano-growth factor. Hybridoma. . 2006;25:300–305. doi: 10.1089/hyb.2006.25.300. [DOI] [PubMed] [Google Scholar]
- 130.Philippou A, Stavropoulou A, Sourla A, Pissimissis N, Halapas A, Maridaki M, Koutsilieris M. Characterization of a rabbit antihuman mechano growth factor (MGF) polyclonal antibody against the last 24 amino acids of the E domain. In Vivo. 2008, 22: 27–35 . [PubMed]
- 131.Stanic I, Facchini A, Borzì RM, Vitellozzi R, Stefanelli C, Goldring MB, Guarnieri C, et al. Polyamine depletion inhibits apoptosis following blocking of survival pathways in human chondrocytes stimulated by tumor necrosis factor-α. J Cell Physiol. . 2006;206:138–146. doi: 10.1002/jcp.20446. [DOI] [PubMed] [Google Scholar]
- 132.Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. . 2020;28:400–409. doi: 10.1016/j.joca.2020.02.027. [DOI] [PubMed] [Google Scholar]