Abstract
Introduction
Although cases of acute cholecystitis, acute pancreatitis, and acute appendicitis following dengue virus infections have been documented, very few large-scale studies have investigated the postdengue risk of these acute abdominal conditions.
Methods
This retrospective population-based cohort study included all patients with laboratory-confirmed dengue from 2002 to 2015 in Taiwan and 1:4 nondengue individuals matched by age, sex, area of residence, and symptom onset time. Multivariate Cox proportional hazards regression models were used to investigate the short-term (≤ 30 days), medium-term (31–365 days), and long-term (> 1 year) risks of acute cholecystitis, pancreatitis, and appendicitis after dengue infection, adjusted for age, sex, area of residence, urbanization level, monthly income level, and comorbidities. Bonferroni correction was used for multiple testing; E-values were used to assess the robustness of the results to unmeasured confounding.
Results
This study included 65,694 individuals with dengue and 262,776 individuals without dengue. Patients with dengue had a significantly increased risk of acute cholecystitis (adjusted hazard ratio (aHR) 60.21; 95% CI 29.11–124.54; P < 0.0001, E-value = 119.92) and acute pancreatitis (aHR 17.13; 95% CI 7.66–38.29; P < 0.0001, E-value = 33.75) within the first 30 days postinfection compared to those without dengue, but this increased risk was not present after that. The incidence rates of acute cholecystitis and pancreatitis in the first 30 days were 18.79 and 5.27 per 10,000, respectively. No increased risk of acute appendicitis was observed among patients with acute dengue infection.
Conclusion
This study was the first large epidemiological study to show a significantly increased risk of acute cholecystitis and pancreatitis among patients with dengue during the acute phase of dengue infection, while no such association was observed for acute appendicitis. Early identification of acute cholecystitis and pancreatitis in patients with dengue is crucial for preventing fatal complications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00821-1.
Keywords: Acute abdomen, Dengue virus, Dengue, Acute cholecystitis, Acute pancreatitis, Acute appendicitis, Cohort study, Taiwan
Key Summary Points
| Why carry out the study? |
| This population-based cohort study included 65,694 individuals with laboratory-confirmed dengue and 1:4 matched nondengue individuals to investigate the risks of acute cholecystitis, acute pancreatitis, and acute appendicitis after dengue infection. |
| What was learned from the study? |
| Patients with dengue had a significantly increased risk of acute cholecystitis (aHR 60.21; 95% CI 29.11–124.54) and acute pancreatitis (aHR 17.13; 95% CI 7.66–38.29) within the first 30 days postinfection compared to those without dengue. |
| No increased risk of acute appendicitis was observed among patients with acute dengue infection. |
| Early identification of acute cholecystitis and pancreatitis in patients with dengue is crucial for preventing fatal complications. |
Introduction
For decades, arboviral diseases were thought to have minimal impact on global mortality and disability. However, the unprecedented emergence and resurgence of arboviral diseases over the past five decades, including dengue, Zika virus disease, chikungunya, and yellow fever, have changed perspectives on their influence on global mortality and disability [1]. The emergence of arboviral diseases is likely driven by the triad of the modern world: urbanization, globalization, and increased international mobility [1]. Additionally, increased urbanization facilitates the expansion and persistence of Aedes mosquitoes in endemic regions, leading to intensified endemic transmission of arboviruses [2]. Dengue is one of the most important mosquito-borne diseases in humans and is caused by four distinct but closely related dengue virus (DENV) serotypes. During the past five decades, the incidence of dengue has risen 30-fold [3]. Some 100–400 million DENV infections occur annually in 128 countries, with an estimated population at risk of up to 3.97 billion people [4, 5].
The broad spectrum of manifestations in dengue ranges from subclinical disease, classic dengue fever, to severe dengue, previously known as dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) [6]. The initial manifestations of dengue include high fever, skin rash, headache, arthralgia, myalgia, nausea, and vomiting. Some symptoms have been identified as warning signs, which are associated with an increased risk of progression to severe dengue. These warning signs include abdominal pain, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy, restlessness, and hepatomegaly. Patients with warning signs require further intensive medical care [6].
Abdominal pain is a common symptom experienced by approximately 11.5–57% of patients with dengue and serves as a well-established warning sign for severe dengue [7–9]. The most frequent causes of abdominal pain in patients with dengue are acute hepatitis and nonspecific pain [10–12]. Acute abdomen, defined as the rapid onset of abdominal pain, fever, and peritoneal signs, is much less common in patients with dengue but has been documented in several case reports and case series involving individuals with dengue [13, 14]. A recent review of 9365 patients with dengue from 22 studies revealed that 1501 (16%) of patients presented with acute abdomen; the most prevalent causes were acute cholecystitis (45.4%), acute pancreatitis (7.7%), and acute appendicitis (2.7%) [13]. However, most patients included in this review were hospitalized in tertiary care hospitals, which may significantly overestimate the incidence of acute abdomen in DENV infection as a result of the selection bias of more severe cases. Another limitation of these previous studies is the lack of comparison groups to thoroughly investigate the association between dengue and acute abdomen.
Therefore, this population-based cohort study aimed to investigate the risk of acute cholecystitis, acute pancreatitis, and acute appendicitis following DENV infections by including all laboratory-confirmed patients with dengue, both inpatients and outpatients, from 2002 to 2015 in Taiwan, and comparing them to matched nondengue individuals. Failure to promptly identify these conditions in patients with dengue may result in life-threatening complications, such as gallbladder rupture and pancreatic necrosis. Understanding the risk of these three conditions among patients with dengue can increase knowledge of potential dengue complications and assist healthcare professionals in early recognition and proper management [12].
Methods
A single-payer national health insurance program was launched in Taiwan in 1995. This nationwide population-based cohort study was conducted using data from the National Health Insurance Research Database (NHIRD) in Taiwan, which comprises health insurance claims data for more than 99% of the over 23 million Taiwanese population as well as foreign nationals who possess a valid residence permit. It has become a valuable resource for health-related studies in recent years [15]. Comprehensive healthcare information for insured people, including demographic data (age, sex, area of residency, and income) and clinical data (medical diagnoses, visit dates, drugs and procedures from outpatient, inpatient, and emergency departments) is available in the NHIRD. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) was used in the NHIRD between 2000 and 2015, and the ICD-10-CM was introduced in 2016. To protect privacy, personal identifiers in the database were encrypted. The NHIRD can be linked to multiple national databases, including registries of birth, deaths, cancers, and reportable infectious diseases.
This study was commenced after obtaining approval from the Institutional Review Board of National Cheng Kung University Hospital (B-ER-106-184). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Because the data were deidentified and analyzed for research purposes, the need for informed consent was waived.
Study Population
All cases of laboratory-confirmed dengue in Taiwan between 2002 and 2015, including hospitalized and outpatient cases, were identified from the Notifiable Disease Dataset of Confirmed Cases (NDDCC), released by the Taiwan Center for Disease Control. The criteria for laboratory confirmation included isolation of DENV, positive real-time reverse transcription polymerase chain reaction, a fourfold rise in the IgG titer in paired acute- and convalescent-phase samples, positive nonstructural protein 1 (NS1) testing, and detection of dengue-specific IgM and IgG antibodies in a single serum sample (before 2009) [16–18]. The date of symptom onset recorded in the NDDCC was set as the index date for each patient with dengue. Patients with missing or invalid data or not enrolled in the NHIRD were excluded. For each case with dengue, four nondengue controls were randomly selected from all beneficiaries of Taiwan’s National Health Insurance Program by matching on age, sex, area of residence, and the calendar year and month of the index date. The matched controls had the same index date as those for their corresponding case with dengue. Those in both the dengue and nondengue groups who had a diagnosis of cholelithiasis (ICD-9-CM code 574.x), other disorders of the gallbladder (ICD-9-CM code 575.x), diseases of the pancreas (ICD-9-CM code 577.x), and appendicitis (ICD-9-CM code 540-542) before the index date were excluded.
Study Outcome and Follow-up
All the study participants’ medical claim data were obtained from the NHIRD; their mortality records were retrieved from the Cause of Death Database. The outcomes of this study included acute cholecystitis, acute pancreatitis, and acute appendicitis, which were defined by one hospital admission with relevant ICD-9-CM codes or ICD-10-CM codes (Supplemental Table 1). We followed all the subjects from the index date to the first date of hospital admission due to the above outcomes, death, or the end of 2018, whichever occurred first.
Covariates
In addition to age and sex, area of residence was also used as a matching variable because dengue epidemics mainly occurred in southern Taiwan, including Tainan, Kaohsiung, and Pingtung, while other places had only sporadic cases. Urbanization level and monthly income level were also considered sociodemographic variables in this study, as previously described [17, 18]. We also considered the following comorbidities as potential confounders: alcohol-related diseases, chronic obstructive pulmonary disease (COPD), dyslipidemia, diabetes mellitus (DM), and hypertension, which were defined by at least three outpatient visits or one hospital admission before the index date with ICD-9-CM codes listed in Supplementary Material Table 1.
Statistical Analysis
We used the standardized mean difference (SMD) to compare the baseline characteristics between the dengue group and the nondengue group, and an SMD > 0.1 was considered a meaningful difference [19]. The incidence rates of acute cholecystitis, acute pancreatitis, and acute appendicitis were calculated as the number of events during the follow-up period divided by the total follow-up time in person-months. We constructed multivariate Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the abovementioned outcomes in cases with dengue compared to nondengue subjects, controlling for age, sex, area of residence, urbanization level, monthly income level, and comorbidities. Proportional hazard assumptions were examined by using log–log survival plots. Analyses stratified by the follow-up time were also performed to calculate the HRs of three outcomes at different periods (≤ 30 days, 31–365 days, and > 365 days after the index date) to investigate the short-term, medium-term, and long-term risks of acute cholecystitis, acute pancreatitis, and acute appendicitis after DENV infection. To further investigate whether the risk of acute abdomen differed between the most acute stage and the convalescent stage, we further stratified the first 30 days into ≤ 10 days and 11–30 days. To account for multiple comparisons for three different outcomes and analyses stratified by sex, age, and follow-up time, a post hoc Bonferroni correction was performed to derive an adjusted threshold for p values. Sensitivity analyses using subdistribution hazard models proposed by Fine and Gray to account for the competing risk of death were also performed [20]. In addition, we calculated E-values to evaluate how strong the unmeasured confounding would have to be to negate the observed association between DENV infection and three outcomes [21–23].
Results
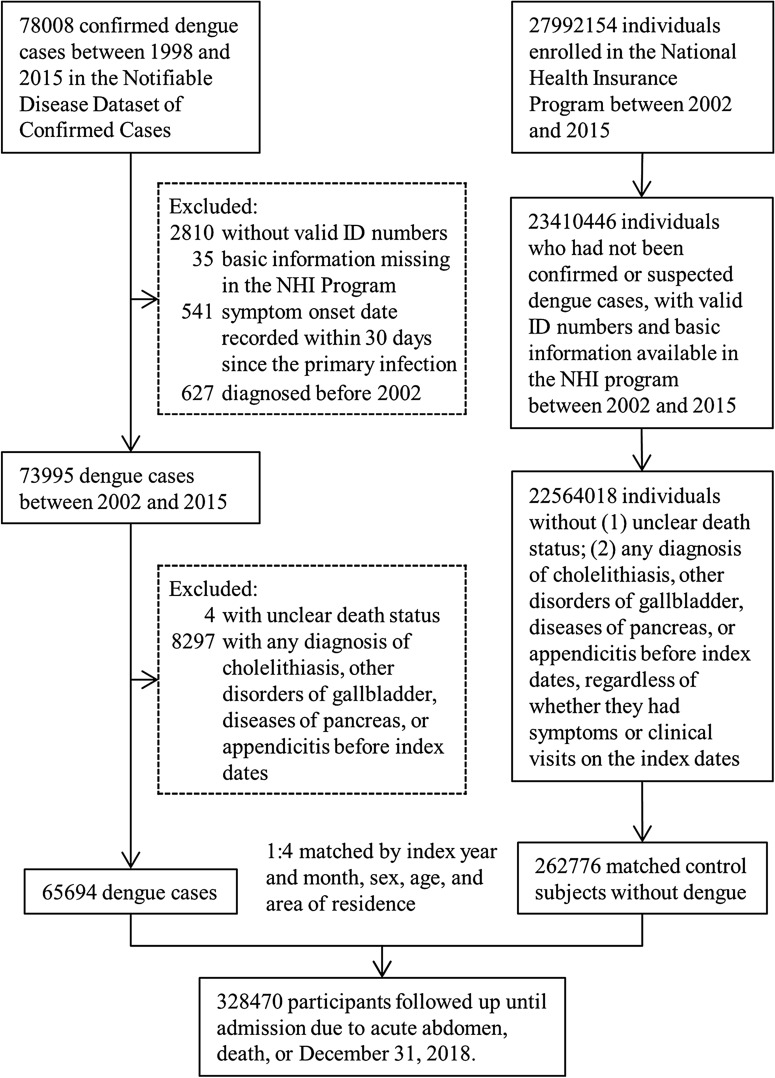
A total of 65,694 individuals with laboratory-confirmed dengue and 262,776 matched individuals without dengue were included in this study. The flow diagram to identify and select study subjects is depicted in Fig. 1. The mean follow-up period was 4.95 ± 3.55 years for the dengue group and 4.95 ± 3.32 years for the nondengue group. We found that 0.53% of patients with dengue and 0.06% of individuals without dengue died in the first month, while 4.23% of patients with dengue and 4.86% of nondengue individuals died during the whole follow-up period. The baseline demographic and clinical characteristics of the two groups are tabulated in Table 1. Patients with dengue seemed to reside in more urbanized areas than nondengue subjects. Other baseline characteristics were not significantly different between the two groups.
Fig. 1.
Flow diagram of the selection of the study population
Table 1.
Demographic and clinical characteristics in dengue and nondengue groups
| Dengue cohort (n = 65,694) | Nondengue cohort (n = 262,776) | SMD | |
|---|---|---|---|
| Sex | |||
| Male | 33,215 (50.6) | 132,860 (50.6) | – |
| Female | 32,479 (49.4) | 129,916 (49.4) | – |
| Age (years) | 43.9 (20.3) | 43.9 (20.3) | – |
| 0–17 | 7775 (11.8) | 31,100 (11.8) | – |
| 18–35 | 16,042 (24.4) | 61,468 (24.4) | – |
| 36–50 | 14,416 (21.9) | 57,664 (21.9) | – |
| 51–64 | 16,431 (25.0) | 65,724 (25.0) | – |
| ≥ 65 | 11,030 (16.8) | 44,120 (16.8) | – |
| Area of residence | |||
| Tainan | 23,011 (35.0) | 92,044 (35.0) | – |
| Kaohsiung | 38,979 (59.3) | 159,916 (59.3) | – |
| Pingtung | 1645 (2.5) | 6580 (2.5) | – |
| Others | 2059 (3.1) | 8236 (3.1) | – |
| Urbanization | |||
| 1 | 23,623 (36.0) | 61,616 (23.5) | 0.284 |
| 2 | 26,380 (40.2) | 85,442 (32.5) | 0.161 |
| 3 | 13,075 (19.9) | 70,804 (26.9) | 0.161 |
| 4–7 | 2616 (4.0) | 44,914 (17.1) | 0.355 |
| Income | |||
| Not-employed | 12,094 (18.4) | 45,502 (17.3) | 0.029 |
| Low | 26,660 (40.6) | 115,695 (44.0) | 0.070 |
| High | 26,940 (41.0) | 101,579 (38.7) | 0.048 |
| Comorbidity | |||
| Alcohol-related disease | 226 (0.3) | 1065 (0.4) | 0.010 |
| COPD | 10,495 (16.0) | 36,889 (14.0) | 0.055 |
| Diabetes mellitus | 7694 (11.7) | 28,491 (10.8) | 0.028 |
| Dyslipidemia | 12,734 (19.3) | 43,737 (16.6) | 0.073 |
| Hypertension | 15,523 (23.6) | 57,338 (21.8) | 0.044 |
Data are number (%) or mean (standard deviation), unless otherwise stated
SMD standardized mean difference, COPD chronic obstructive pulmonary disease
The overall incidence rates of acute cholecystitis, acute pancreatitis, and acute appendicitis were 1.44, 0.63, and 0.88 per 10,000 person-months in the dengue group and 1.06, 0.50, and 0.70 per 10,000 person-months in the nondengue group, respectively. Multivariate Cox proportional hazards regression models revealed that patients with dengue had significantly higher risks of developing acute cholecystitis (adjusted HR (aHR) 1.33; 95% CI 1.20–1.46; P < 0.0001, E-value = 1.99), acute pancreatitis (aHR 1.28; 95% CI 1.11–1.48; P = 0.0009, E-value = 1.88), and acute appendicitis (aHR 1.27; 95% CI 1.12–1.43; P = 0.0002, E-value = 1.86) than nondengue subjects, after adjusting for age, sex, area of residence, urbanization level, monthly income level, and comorbidities.
To adjust for the multiple comparison problem, the Bonferroni post hoc method required a significance level of P < 0.0013 (three different outcomes and analyses stratified by sex (two groups), age (five groups), and follow-up time (five groups); total 39 tests in Tables 2 and 3). Analyses stratified by sex and age revealed that the risk of acute cholecystitis among patients with dengue remained significantly higher than that among subjects without dengue for male participants (aHR 1.25; 95% CI 1.17–1.55; P < 0.0001, E-value = 1.81), female participants (aHR 1.30; 95% CI 1.13–1.49; P = 0.0003, E-value = 1.92), and those aged 0–17 years (aHR 9.22; 95% CI 2.95–28.80; P = 0.0001, E-value = 17.93) and ≥ 65 years (aHR 1.42; 95% CI 1.21–1.67; P < 0.0001, E-value = 2.19) (Table 2). Subdistribution hazard models were used in sensitivity analyses to account for competing risks, and the results were similar to those obtained from Cox regression models (Tables 2 and 3).
Table 2.
Comparison of incidence and hazard ratio of acute abdomen between dengue and nondengue cohorts stratified by sex and age
| Acute abdomen | Dengue cohort | Nondengue cohort | Adjusted HRb (95% CId) |
P valuee | Adjusted SHRc (95% CId) |
P valuee | ||
|---|---|---|---|---|---|---|---|---|
| No. of events | IRa | No. of events | IRa | |||||
| Acute cholecystitis | 564 | 1.44 | 1657 | 1.06 | 1.33 (1.20–1.46) | < 0.0001* | 1.34 (1.21–1.48) | < 0.0001* |
| Sex | ||||||||
| Male | 291 | 1.48 | 818 | 1.05 | 1.25 (1.17–1.55) | < 0.0001* | 1.37 (1.19–1.57) | < 0.0001* |
| Female | 273 | 1.39 | 839 | 1.07 | 1.30 (1.13–1.49) | 0.0003* | 1.31 (1.14–1.51) | 0.0002* |
| Age (years) | ||||||||
| 0–17 | 9 | 0.20 | 6 | 0.03 | 9.22 (2.95–28.80) | 0.0001* | 9.23 (3.42–24.91) | < 0.0001* |
| 18–35 | 45 | 0.47 | 175 | 0.45 | 1.02 (0.74–1.43) | 0.8873 | 1.03 (0.74–1.43) | 0.8879 |
| 36–50 | 101 | 1.08 | 296 | 0.79 | 1.37 (1.09–1.73) | 0.0069 | 1.38 (1.09–1.74) | 0.0073 |
| 51–64 | 188 | 1.85 | 584 | 1.44 | 1.23 (1.04–1.45) | 0.0180 | 1.24 (1.05–1.48) | 0.0128 |
| ≥ 65 | 221 | 3.91 | 596 | 2.64 | 1.42 (1.21–1.67) | < 0.0001* | 1.45 (1.23–1.71) | < 0.0001* |
| Acute pancreatitis | 250 | 0.63 | 793 | 0.50 | 1.28 (1.11–1.48) | 0.0009* | 1.30 (1.12–1.50) | 0.0005* |
| Sex | ||||||||
| Male | 131 | 0.67 | 450 | 0.57 | 1.18 (0.96–1.44) | 0.1087 | 1.19 (0.98–1.46) | 0.0852 |
| Female | 119 | 0.60 | 343 | 0.43 | 1.40 (1.13–1.74) | 0.0023 | 1.42 (1.14–1.76) | 0.0016 |
| Age (years) | ||||||||
| 0–17 | 3 | 0.07 | 7 | 0.04 | 1.99 (0.47–8.49) | 0.3505 | 2.00 (0.51–7.78) | 0.3200 |
| 18–35 | 26 | 0.27 | 91 | 0.24 | 1.14 (0.73–1.77) | 0.5738 | 1.14 (0.73–1.78) | 0.5751 |
| 36–50 | 50 | 0.53 | 190 | 0.51 | 1.12 (0.82–1.54) | 0.4770 | 1.13 (0.82–1.54) | 0.4537 |
| 51–64 | 83 | 0.81 | 244 | 0.60 | 1.31 (1.02–1.70) | 0.0373 | 1.34 (1.03–1.73) | 0.0280 |
| ≥ 65 | 88 | 1.54 | 261 | 1.15 | 1.36 (1.05–1.76) | 0.0191 | 1.38 (1.07–1.79) | 0.0134 |
| Acute appendicitis | 346 | 0.88 | 1113 | 0.70 | 1.27 (1.12–1.43) | 0.0002* | 1.27 (1.12–1.44) | 0.0002* |
| Sex | ||||||||
| Male | 188 | 0.96 | 604 | 0.77 | 1.30 (1.10–0.1)54 | 0.0020 | 1.31 (1.11–1.55) | 0.0018 |
| Female | 158 | 0.80 | 509 | 0.65 | 1.23 (1.03–1.48) | 0.0250 | 1.24 (1.03–1.49) | 0.0235 |
| Age (years) | ||||||||
| 0–17 | 52 | 1.17 | 134 | 0.75 | 1.63 (1.18–2.27) | 0.0035 | 1.63 (1.18–2.26) | 0.0032 |
| 18–35 | 96 | 1.00 | 312 | 0.81 | 1.25 (0.99–1.58) | 0.0607 | 1.25 (0.99–1.58) | 0.0589 |
| 36–50 | 75 | 0.80 | 263 | 0.70 | 1.15 (0.89–1.50) | 0.2820 | 1.16 (0.89–1.50) | 0.2764 |
| 51–64 | 73 | 0.71 | 248 | 0.61 | 1.14 (0.87–1.48) | 0.3496 | 1.14 (0.88–1.49) | 0.3256 |
| ≥ 65 | 50 | 0.87 | 156 | 0.69 | 1.40 (0.99–1.96) | 0.0543 | 1.42 (1.00–2.00) | 0.0480 |
aIR = incidence rate per 10,000 person-months
bHazard ratio adjusted for age, sex, area of residence, urbanization level, monthly income level, and comorbidities
cSubdistribution hazard ratio adjusted for age, sex, area of residence, urbanization level, monthly income level, and comorbidities
d95% CIs were not adjusted for multiple comparisons and thus cannot be directly used for hypothesis testing or inference
ePost hoc adjustment for multiple comparisons by the Bonferroni method required a significance level of P < 0.0013
*Achieved statistical significance after Bonferroni correction for multiple comparisons (P < 0.0013)
Table 3.
Comparison of incidence and hazard ratio of acute abdomen between dengue and nondengue cohorts stratified by follow-up period
| Acute abdomen | Dengue cohort | Non-dengue cohort | Adjusted HRb (95% CI)d |
P valuee | Adjusted SHRc (95% CI)d |
P valuee | ||
|---|---|---|---|---|---|---|---|---|
| No. of events | IRa | No. of events | IRa | |||||
| Acute cholecystitis | ||||||||
| ≤ 30 days | 121 | 18.79 | 8 | 0.31 | 60.21 (29.11–124.54) | < 0.0001* | 60.01 (28.24–127.53) | < 0.0001* |
| ≤ 10 days | 110 | 51.13 | 4 | 0.46 | 111.67 (40.80–305.65) | < 0.0001* | 111.44 (39.76–312.31) | < 0.0001* |
| 11–30 days | 11 | 2.56 | 4 | 0.23 | 9.25 (2.79–30.65) | 0.0003* | 9.23 (2.57–33.18) | 0.0007* |
| 31–365 days | 68 | 0.95 | 212 | 0.74 | 1.32 (0.99–1.74) | 0.0579 | 1.32 (0.99–1.75) | 0.0576 |
| > 365 days | 375 | 1.19 | 1437 | 1.14 | 1.01 (0.90–1.13) | 0.9293 | 1.03 (0.91–1.16) | 0.6488 |
| Acute pancreatitis | ||||||||
| ≤ 30 days | 34 | 5.27 | 8 | 0.31 | 17.13 (7.66–38.29) | < 0.0001* | 17.02 (7.95–36.46) | < 0.0001* |
| ≤ 10 days | e | e | e | e | 45.65 (10.64–195.93) | < 0.0001* | 45.51 (11.77–176.01) | < 0.0001* |
| 11–30 days | e | e | e | e | 5.54 (1.66–18.44) | 0.0053 | 5.53 (1.54–19.90) | 0.0088 |
| 31–365 days | 33 | 0.46 | 119 | 0.41 | 1.15 (0.77–1.71) | 0.4986 | 1.15 (0.78–1.71) | 0.4885 |
| > 365 days | 183 | 0.58 | 666 | 0.53 | 1.11 (0.94–1.32) | 0.2127 | 1.14 (0.96–1.35) | 0.1374 |
| Acute appendicitis | ||||||||
| ≤ 30 days | 10 | 1.55 | 23 | 0.89 | 1.65 (0.78–3.49) | 0.1952 | 1.64 (0.76–3.55) | 0.2086 |
| ≤ 10 days | e | e | e | e | 3.74 (1.39–10.09) | 0.0091 | 3.74 (1.29–10.83) | 0.0150 |
| 11–30 days | e | e | e | e | 0.51 (0.11–2.25) | 0.3720 | 0.51 (0.12–2.15) | 0.3560 |
| 31–365 days | 51 | 0.71 | 208 | 0.72 | 1.01 (0.74–1.38) | 0.9591 | 1.01 (0.74–1.38) | 0.9558 |
| > 365 days | 285 | 0.90 | 882 | 0.70 | 1.32 (1.15–1.51) | < 0.0001* | 1.33 (1.12–1.52) | < 0.0001* |
aIR = incidence rate per 10,000 person-months
bHazard ratio adjusted for age, sex, area of residence, urbanization level, monthly income level, and comorbidities
cSubdistribution hazard ratio adjusted for age, sex, area of residence, urbanization level, monthly income level, and comorbidities
d95% CIs were not adjusted for multiple comparisons and thus cannot be directly used for hypothesis testing or inference
eThe cells left blank indicated that the numbers of events in some cells was too small and were therefore not allowed to be exported under the regulations of the Health and Welfare Data Science Center of Taiwan to prevent re-identification
fPost hoc adjustment for multiple comparisons by the Bonferroni method required a significance level of P < 0.0013
*Achieved statistical significance after Bonferroni correction for multiple comparisons (P < 0.0013)
We further analyzed the time trend for the risks of the three outcomes after DENV infection (Table 3). Patients with dengue had a significantly increased risk of developing acute cholecystitis in the first 30 days after symptom onset (incidence rate 18.79 per 10,000 person-months; aHR 60.21; 95% CI 29.11–124.54; P < 0.0001, E-value = 119.92), but no increased risk was observed after 30 days. A significantly higher risk of developing acute pancreatitis was also observed in the first 30 days (incidence rate 5.27 per 10,000 person-months; aHR 17.13; 95% CI 7.66–38.29; P < 0.0001, E-value = 33.75) but not thereafter. For acute appendicitis, the results showed no increased risk in the first year but an increased risk after 1 year of DENV infection (aHR 1.32; 95% CI 1.15–1.51; P < 0.0001, E-value = 1.97).
To further investigate the differences in acute abdomen risk between the most acute phase and the convalescent phase, we subdivided the initial 30 days into two periods: the first 10 days and the subsequent 11–30 days (Table 3). In accordance with the regulations of the Health and Welfare Data Science Center of Taiwan, the number of events was too small to allow data to be reported in certain instances in order to prevent re-identification; therefore, the case numbers and incidence rates of acute pancreatitis and appendicitis were not shown. The results showed that the risk of acute cholecystitis was highest during the first 10 days after symptom onset in patients with dengue (aHR 111.67; 95% CI 40.80–305.65; P < 0.0001, E-value = 222.84), and the risk dropped but remained significantly higher compared to nondengue subjects between 11 and 30 days after infection (aHR 9.25; 95% CI 2.79–30.65; P = 0.0003, E-value = 17.99). The risk of acute pancreatitis among patients with dengue was also highest during the first 10 days (aHR 45.65; 95% CI 10.64–195.93; P < 0.0001, E-value = 90.8), but the aHR became statistically non-significant in the convalescent stage after considering multiple comparisons (aHR 5.54; 95% CI (1.66–18.44); P = 0.0053).
Discussion
This study demonstrated that patients with dengue had a significantly higher risk of acute cholecystitis and pancreatitis compared to nondengue subjects, specifically in the first month after symptom onset. The aHRs (60.21 and 17.13, respectively) and corresponding E-values (119.92 and 33.75, respectively) were very large, suggesting that the results were robust and unlikely to be caused by unmeasured confounding. The risk of acute cholecystitis and pancreatitis was highest during the most acute stage (first 10 days), which is consistent with existing literature that indicates most case reports of acute cholecystitis and pancreatitis in patients with dengue occur during the first week [24–28]. However, we reported a novel finding that patients with dengue still had an increased risk of acute cholecystitis between 11 and 30 days after symptom onset. This might be because some patients with more severe diseases had a prolonged clinical course and thus still had an increased risk of acute cholecystitis after 10 days.
Despite observing high aHRs for the correlation between dengue and acute cholecystitis and pancreatitis, we found that the incidence rates of acute cholecystitis, acute pancreatitis, and acute appendicitis in the first month of DENV infection were only 18.79, 5.27, and 1.55 per 10,000, respectively. This is consistent with a large multicenter prospective study of 1165 hospitalized patients, which found that 11.5% of patients with dengue fever and 21.9% of patients with DHF had abdominal pain; however, acute cholecystitis, acute pancreatitis, and acute appendicitis were not identified in this study [9]. Therefore, although abdominal pain is prevalent in patients with dengue and is considered an important warning sign of progression to severe dengue [29], cases presenting with acute abdomen are rare. However, the reported risks of acute cholecystitis and acute pancreatitis during acute DENV infection in other literature vary widely, with ranges of 0.15–57.0% and 0.03–15.0%, respectively [13, 30–49]. This wide range of reported risks probably results from different selection criteria in previous studies. For example, although most of the studies included hospitalized patients, some selected only cases with DHF [50], cases with abdominal pain [31], or cases admitted to surgical units [14]. In addition, previous studies did not include nondengue subjects for comparison to investigate the association between dengue and acute abdomen. Our study was a population-based study including all cases of laboratory-confirmed dengue in Taiwan from 2002 to 2015, providing an accurate estimated risk of the three diseases after DENV infection and unraveling the association between DENV infection and three acute abdominal conditions.
Acute acalculous cholecystitis and pancreatitis were previously reported after DENV infection, especially in patients with DHF [24, 25, 27, 51–54]. Detailed mechanisms of acute cholecystitis and pancreatitis remain unknown in the acute phase of DENV infection. One proposed hypothesis is that direct virus invasion triggers a cascade of events, including local inflammation, tissue swelling, and destruction of gall bladder and pancreatic acinar cells, leading to biliary stasis and obstruction of pancreatic fluid outflow [12, 54]. Other possible contributing factors for developing acute cholecystitis include systemic inflammatory responses, endotoxemia, secondary bacterial translocation, spasms of the ampulla of Vater, microangiopathic changes, and ischemia–reperfusion injury [13, 30]. An autoimmune response to pancreatic islet cells triggered by DENV infection may play a role in the development of acute pancreatitis [12]. Gallbladder wall thickening and ascites usually resolve entirely with the recovery of acute dengue. Surgical treatment is generally not recommended in dengue-associated acute cholecystitis in the absence of complications such as gallbladder perforation [13, 54]. Identifying acute cholecystitis and pancreatitis in patients with dengue, particularly in critical cases, can be challenging. However, it is crucial to detect these conditions early in patients with dengue and closely monitor potentially fatal complications, such as gallbladder rupture and pancreatic necrosis, to reduce mortality. Deteriorating biochemical indicators and clinical decline with abdominal guarding and stiffness can aid in diagnosis [13].
Our study investigated the risk of acute abdomen among patients diagnosed with dengue. On the other hand, there have been reports of cases initially presenting with acute abdomen in surgical units but later diagnosed with dengue [14]. In a separate instance, a patient in Germany who had contracted dengue abroad developed acute acalculous cholecystitis, underwent cholecystectomy, and unfortunately died from massive bleeding due to the initial failure to diagnose dengue [55]. Given the strong association between dengue and acute cholecystitis and pancreatitis found in our study, healthcare providers should exercise caution and consider dengue as a potential diagnosis when evaluating patients with acute abdomen during dengue epidemics or when encountering patients with relevant travel histories. Misdiagnosis and unnecessary surgical intervention can lead to poor outcomes, as DENV infection can cause microvascular changes and coagulopathy, making surgical intervention highly risky [14].
Although case reports of acute appendicitis during acute DENV infection have been documented [13, 39, 56, 57], our study found no significant increase in the risk of acute appendicitis in the first month or first year following DENV infection. However, we found a slightly increased risk of acute appendicitis after 1 year of DENV infection (aHR 1.32; P < 0.0001); the results remained statistically significant after adjusting for multiple comparisons, suggesting that the results were unlikely due to type 1 error. It is difficult to explain why patients with dengue had a long-term increased risk of developing appendicitis in our study. Our previous study found a significantly higher risk of leukemia after DENV infection [17]. The appendix contains abundant lymphoid tissue, and recent evidence suggests that it may be an essential part of the immune system [58]. Therefore, it is possible that DENV infection has a long-term effect on the hematolymphoid system through certain currently unknown mechanisms. However, the E-value in our study was only 1.97, suggesting that unmeasured and uncontrolled confounding might explain away this observed association. Acute appendicitis has also been reported after coxsackievirus, measles virus, and cytomegalovirus infection [59–63]. A previous study suggests that viral infections are unlikely to be the immediate cause of appendicitis; however, there may be common etiological determinants, pathogenic mechanisms, or environmental factors that impact the incidence of both viral infections and appendicitis [59].
The strengths of our study included the following. First, this study has a large number of patients diagnosed with dengue in Taiwan, comprising both hospitalized and outpatient patients, which provides better generalizability compared to many studies that only included hospitalized patients. In addition, the outcomes were retrieved from the national health insurance database with a coverage rate of greater than 99%. Therefore, selection bias due to different enrollment criteria or loss to follow-up was unlikely. Second, corrections for multiple testing by the Bonferroni method were performed, reducing the chance of false-positive findings. Third, sensitivity analyses using the Fine and Gray methods to adjust for competing risk of death showed similar results to the original analyses. Finally, we calculated E-values to evaluate the robustness of study findings with respect to potential uncontrolled and unmeasured confounding.
This study also has some limitations. First, the NHIRD did not contain information on some potential confounders, such as family history, body weight, diet, and self-paid hormone use, such as oral contraceptives. Second, although all the patients were laboratory-confirmed, some nondengue subjects might have been misclassified because DENV infection might be subclinical and underreported. Although this misclassification may occur, it is not expected to significantly impact our results because dengue is currently not endemic in Taiwan, with a relatively low overall incidence and seroprevalence [64, 65]. Third, the criteria for laboratory confirmation of dengue before 2009 involved detecting DENV IgM or IgG in a single serum sample, which might have led to some misclassification due to cross-reactivity with other similar flaviviruses. However, since dengue is not endemic in Taiwan and the seroprevalence was low before 2009, the misclassification bias should not pose a significant issue. Fourth, the NHIRD used in this study is a claims database used for insurance reimbursements, which includes records of diagnoses, drugs, and procedures received by all beneficiaries of Taiwan’s National Health Insurance. However, detailed medical records, such as test results and clinical symptoms, are not available. Therefore, we could not classify the severity of dengue on the basis of the 1997 or 2009 World Health Organization (WHO) classification criteria. In addition, for patients with acute cholecystitis, acute pancreatitis, or acute appendicitis, we only know that they had been hospitalized during the specified period with a discharge diagnosis of these three diseases; the exact dates of the occurrence of the diseases were unknown. Finally, further analyses stratified by both follow-up and age groups were not performed because the results of fewer than three persons could not be reported to prevent re-identification under the privacy regulation of the Health and Welfare Data Science Center in Taiwan.
Conclusion
This study revealed that patients with dengue had a significantly increased risk of developing acute cholecystitis and acute pancreatitis during the initial month compared to those without dengue but no increased risk of acute appendicitis. Early detection of acute cholecystitis and pancreatitis in patients with dengue is crucial to prevent fatal complications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was partially supported by grants from the Ministry of Science and Technology, Taiwan (MOST-111-2625-M-006-016-[HIS], National Health Research Institute (MR-110-GP-03 [CYC] and MR-111-GP-05 [CYC]), and National Cheng Kung University Hospital (NCKUH-11203040[HIS]). The Journal’s Rapid Service Fee was funded by the authors.
Medical Writing/Editorial Assistance
The authors would like to acknowledge the use of ChatGPT, an AI language model developed by OpenAI, for providing editorial assistance in improving the English language of this manuscript. It is important to note that the assistance provided by ChatGPT was limited to language editing, while all original content, ideas, data analysis, results, and discussions were solely generated by the authors. After using this tool, the authors reviewed and edited the content as needed and will take full responsibility for the content of the publication.
Author Contributions
Hsin-I Shih: Conceptualization, Methodology, Investigation, Original Draft Preparation, Writing – Review & Editing. Chia-Yu Chi: Funding Acquisition, Writing – Review & Editing. Yu-Ping Wang: Data Curation, Investigation, Software, Yu-Wen Chien: Conceptualization, Formal Analysis, Funding Acquisition, Methodology, Investigation, Original Draft Preparation, Writing – Review & Editing.
Disclosures
Hsin-I Shih, Chia-Yu Chi, Yu-Ping Wang, and Yu-Wen Chien all confirm that they have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
This study was commenced after obtaining approval from the Institutional Review Board of National Cheng Kung University Hospital (B-ER-106–184). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Because the data were deidentified and analyzed for research purposes, the need for informed consent was waived.
Data Availability
This study used national databases obtained from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare in Taiwan (https://dep.mohw.gov.tw/dos/cp-5119-59201-113.html). All data obtained were anonymized and deidentified by the HWDC. The data used in this study must be accessed and analyzed in the HWDC after filling out an application according to the relevant regulations and thus cannot be shared. Permission was required to use the data in NHIRD, and our study group obtained the necessary permission. Contact information for data application, analysis and inquiry (https://dep.mohw.gov.tw/dos/cp-2516-59203-113.html).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hsin-I. Shih and Chia-Yu Chi contributed equally to this manuscript.
References
- 1.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17(3):e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer MUG, Reiner RC, Jr, Brady OJ, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4(5):854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control: new edition. WHO, Geneva. 2009. https://www.ncbi.nlm.nih.gov/books/NBK143157/. [PubMed]
- 4.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Ooi EE, Horstick O, Wills B. Dengue. Lancet. 2019;393(10169):350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- 7.Mendez A, Gonzalez G. [Dengue haemorrhagic fever in children: ten years of clinical experience]. Biomedica. 2003;23(2):180–93. https://www.ncbi.nlm.nih.gov/pubmed/12872557. [PubMed]
- 8.Wang JY, Tseng CC, Lee CS, Cheng KP. Clinical and upper gastroendoscopic features of patients with dengue virus infection. J Gastroenterol Hepatol. 1990;5(6):664–668. doi: 10.1111/j.1440-1746.1990.tb01122.x. [DOI] [PubMed] [Google Scholar]
- 9.Jayarajah U, de Silva PK, Jayawardana P, et al. Pattern of dengue virus infections in adult patients from Sri Lanka. Trans R Soc Trop Med Hyg. 2018;112(3):144–153. doi: 10.1093/trstmh/try034. [DOI] [PubMed] [Google Scholar]
- 10.Shabbir B, Qadir H, Mahboob F. Acute abdominal pain in dengue fever. Pak J Med Health Sci. 2012;6:155–158. [Google Scholar]
- 11.Khanna S, Vij JC, Singal D, Kumar, Tandon R (2005) Etiology of abdominal pain in dengue fever. WHO Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/164137.
- 12.Gupta B, Nehara H, Parmar S, Meena S, Gajraj S, Gupta J. Acute abdomen presentation in dengue fever during recent outbreak. J Acute Dis. 2017;6(5):198–204. doi: 10.4103/2221-6189.219612. [DOI] [Google Scholar]
- 13.Jayarajah U, Lahiru M, De Zoysa I, Seneviratne SL. Dengue infections and the surgical patient. Am J Trop Med Hyg. 2021;104(1):52–59. doi: 10.4269/ajtmh.20-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasundara B, Perera L, de Silva A. Dengue fever may mislead the surgeons when it presents as an acute abdomen. Asian Pac J Trop Med. 2017;10(1):15–19. doi: 10.1016/j.apjtm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD) Epidemiol Health. 2018;40:e2018062. doi: 10.4178/epih.e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang K, Lu PL, Ko WC, et al. Dengue fever scoring system: new strategy for the early detection of acute dengue virus infection in Taiwan. J Formos Med Assoc. 2009;108(11):879–885. doi: 10.1016/s0929-6646(09)60420-4. [DOI] [PubMed] [Google Scholar]
- 17.Chien YW, Wang CC, Wang YP, Lee CY, Perng GC. Risk of leukemia after dengue virus infection: a population-based cohort study. Cancer Epidemiol Biomark Prev. 2020;29(3):558–564. doi: 10.1158/1055-9965.epi-19-1214. [DOI] [PubMed] [Google Scholar]
- 18.Chien YW, Chuang HN, Wang YP, Perng GC, Chi CY, Shih HI. Short-term, medium-term, and long-term risks of nonvariceal upper gastrointestinal bleeding after dengue virus infection. PLoS Negl Trop Dis. 2022;16(1):e0010039. doi: 10.1371/journal.pntd.0010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330(7497):960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/circulationaha.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 22.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 23.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29(5):e45–e47. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurung S, Karki S, Khadka M, Gurung S, Dhakal S. Acute acalculous cholecystitis in a patient with dengue fever: a case report. Ann Med Surg (Lond) 2022;84:104960. doi: 10.1016/j.amsu.2022.104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setyawati AN, Tjahjono DK, Chionardes MA, Arkhaesi N. Acute acalculous cholecystitis in a pediatric dengue hemorrhagic fever patient: a case report, lesson learned from limited resource setting. Ann Med Surg (Lond) 2022;81:104437. doi: 10.1016/j.amsu.2022.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasim A. Dengue fever presenting as acute acalculous cholecystitis. J Coll Physicians Surg Pak. 2009;19(8):531–3. https://www.ncbi.nlm.nih.gov/pubmed/19651022. [PubMed]
- 27.Jain V, Gupta O, Rao T, Rao S. Acute pancreatitis complicating severe dengue. J Glob Infect Dis. 2014;6(2):76–78. doi: 10.4103/0974-777X.132050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijekoon CN, Wijekoon PW. Dengue hemorrhagic fever presenting with acute pancreatitis. Southeast Asian J Trop Med Public Health. 2010;41(4):864–6. https://www.ncbi.nlm.nih.gov/pubmed/21073060. [PubMed]
- 29.Weerakoon KGAD, Chandrasekaramb, S, Jayabahu, JPSNK, Gunasena S, Kularatne SAM. Acute abdominal pain in dengue haemorrhagic fever: a study in Sri Lanka. 2009. https://apps.who.int/iris/handle/10665/170944.
- 30.Wu KL, Changchien CS, Kuo CM, et al. Dengue fever with acute acalculous cholecystitis. Am J Trop Med Hyg. 2003;68(6):657–60. https://www.ncbi.nlm.nih.gov/pubmed/12887023. [PubMed]
- 31.Khanna S, Vij JC, Kumar A, Singal D, Tandon R. Dengue fever is a differential diagnosis in patients with fever and abdominal pain in an endemic area. Ann Trop Med Parasitol. 2004;98(7):757–760. doi: 10.1179/000349804X3153. [DOI] [PubMed] [Google Scholar]
- 32.Sharma N, Mahi S, Bhalla A, Singh V, Varma S, Ratho R. Dengue fever related acalculous cholecystitis in a North Indian tertiary care hospital. J Gastroenterol Hepatol. 2006;21:664–667. doi: 10.1111/j.1440-1746.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee I, Khor B, Kee K, Yang K, Liu J. Hyperlipasemia/pancreatitis in adults with dengue hemorrhagic fever. Pancreas. 2007;35:381–382. doi: 10.1097/01.mpa.0000297828.05678.7a. [DOI] [PubMed] [Google Scholar]
- 34.Premaratna R, Bailey M, Ratnasena B, De Silva H. Dengue fever mimicking acute appendicitis. Trans R Soc Trop Med Hyg. 2007;101:683–685. doi: 10.1016/j.trstmh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Bhatty S, Shaikh N, Fatima M, Sumbhuani A. Acute acalculous cholecystitis in dengue fever. J Pak Med Assoc. 2009;59:519–521. [PubMed] [Google Scholar]
- 36.Weerakoon K, Chandrasekaramb S, Jayabahu J, Gunasena S, Kularatne S. Acute abdominal pain in dengue haemorrhagic fever: a study in Sri Lanka. Dengue Bull. 2009;33:70–74. [Google Scholar]
- 37.Laul A, Laul P, Merugumala V, Pathak R, Miglani U, Saxena P. Clinical profiles of dengue infection during an outbreak in northern India. J Trop Med. 2016;2016:5917934. doi: 10.1155/2016/5917934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhamb R, Kumar A, Ranga G, Rathi N. Unusual manifestations in dengue outbreak 2009, Delhi, India. J Commun Dis. 2010;42:255–261. [PubMed] [Google Scholar]
- 39.Shamim M. Frequency, pattern and management of acute abdomen in dengue fever in Karachi, Pakistan. Asian J Surg. 2010;33(3):107–113. doi: 10.1016/S1015-9584(10)60019-X. [DOI] [PubMed] [Google Scholar]
- 40.Chakravarti A, Suresh K, Neha S, Malik S. Dengue outbreak in Delhi in 2009: study of laboratory and clinical parameters. J Commun Dis. 2009;44:163–168. [PubMed] [Google Scholar]
- 41.Majumdar R, Jana C, Ghosh S, Biswas U. Clinical spectrum of dengue fever in a tertiary care centre with particular reference to atypical presentation in the 2012 outbreak in Kolkata. J Indian Med Assoc. 2012;110:904–906. [PubMed] [Google Scholar]
- 42.Ahmad F, Nadeem A, Faisal M, Shaukat M, Siddique K. Management experience of surgical complications of dengue fever patients at hameed Latif hospital, Lahore. Ann King Edward Med Univ. 2013;19:49. [Google Scholar]
- 43.Chatterjee N, Mukhopadhyay M, Ghosh S, Mondol M, Das C, Patar K. An observational study of dengue fever in a tertiary care hospital of eastern India. J Assoc Physicians India. 2014;62(3):224–7. https://www.ncbi.nlm.nih.gov/pubmed/25327063. [PubMed]
- 44.Sreeramulu P, Shashirekha C, Katti P. Incidence and management of acalculus cholecystitis in dengue fever—a retrospective study. Int J Biomed Adv Res. 2014;5:422–424. doi: 10.7439/ijbar.v5i9.855. [DOI] [Google Scholar]
- 45.Pothapregada S, Kamalakannan B, Thulasingam M. Clinical profile of atypical manifestations of dengue fever. Indian J Pediatr. 2016;83:493–499. doi: 10.1007/s12098-015-1942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayasundara B, Perera L, de Silva A. Dengue fever may mislead the surgeons when it presents as an acute abdomen. Asian Pac J Trop Med. 2016;10:15–19. doi: 10.1016/j.apjtm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Shashirekha C, Sreeramulu P, Ravikiran H. Surgical presentations with abdominal pain in dengue fever. Int Surg J. 2016;3:754–756. [Google Scholar]
- 48.Chandey M, Kaur H, Kaur S. Acute acalculous cholecystitis in dengue fever patients. Int J Adv Med. 2017;4:375–377. doi: 10.18203/2349-3933.ijam20170923. [DOI] [Google Scholar]
- 49.Gupta B, Nehara H, Parmar S, Meena S, Gajraj S, Gupta J. Acute abdomen presentation in dengue fever during recent outbreak. J Acute Dis. 2017;6:198. doi: 10.4103/2221-6189.219612. [DOI] [Google Scholar]
- 50.Khor BS, Liu JW, Lee IK, Yang KD. Dengue hemorrhagic fever patients with acute abdomen: clinical experience of 14 cases. Am J Trop Med Hyg. 2006;74(5):901–904. doi: 10.4269/ajtmh.2006.74.901. [DOI] [PubMed] [Google Scholar]
- 51.Naik S, Mahajan S, Talwar D, Jagtap G. Acute pancreatitis complicating a case of dengue fever: double trouble. Cureus. 2021;13(11):e19523. doi: 10.7759/cureus.19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen TC, Perng DS, Tsai JJ, Lu PL, Chen TP. Dengue hemorrhagic fever complicated with acute pancreatitis and seizure. J Formos Med Assoc. 2004;103(11):865–8. https://www.ncbi.nlm.nih.gov/pubmed/15549156. [PubMed]
- 53.Seetharam P, Rodrigues G. Dengue Fever presenting as acute pancreatitis. Eurasian J Med. 2010;42(3):151–152. doi: 10.5152/eajm.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai YT, Kalimuddin S, Ng HJH, Tay GCA. Acute acalculous cholecystitis in dengue fever: a case series. Singapore Med J. 2021. 10.11622/smedj.2021168. [DOI] [PMC free article] [PubMed]
- 55.Schmidt-Chanasit J, Tenner-Racz K, Poppert D, et al. Fatal dengue hemorrhagic fever imported into Germany. Infection. 2012;40(4):441–443. doi: 10.1007/s15010-011-0208-3. [DOI] [PubMed] [Google Scholar]
- 56.Thadchanamoorthy V, Ganeshrajah A, Dayasiri K, Jayasekara NP. Acute appendicitis during the recovery phase of dengue hemorrhagic fever: two case reports. J Med Case Rep. 2022;16(1):219. doi: 10.1186/s13256-022-03443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Premaratna R, Bailey MS, Ratnasena BG, de Silva HJ. Dengue fever mimicking acute appendicitis. Trans R Soc Trop Med Hyg. 2007;101(7):683–685. doi: 10.1016/j.trstmh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Kooij IA, Sahami S, Meijer SL, Buskens CJ, Te Velde AA. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol. 2016;186(1):1–9. doi: 10.1111/cei.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alder AC, Fomby TB, Woodward WA, Haley RW, Sarosi G, Livingston EH. Association of viral infection and appendicitis. Arch Surg. 2010;145(1):63–71. doi: 10.1001/archsurg.2009.250. [DOI] [PubMed] [Google Scholar]
- 60.Tobe T, Horikoshi Y, Hamada C, Hamashima Y. Virus infection as a trigger of appendicitis: experimental investigation of Coxsackie B5 virus infection in monkey intestine. Surgery. 1967;62(5):927–34. https://www.ncbi.nlm.nih.gov/pubmed/4964128. [PubMed]
- 61.Andersson R, Hugander A, Thulin A, Nystrom PO, Olaison G. Clusters of acute appendicitis: further evidence for an infectious aetiology. Int J Epidemiol. 1995;24(4):829–833. doi: 10.1093/ije/24.4.829. [DOI] [PubMed] [Google Scholar]
- 62.Searle A, Owen WJ. Measles appendicitis. Br J Clin Pract. 1990;44(12):749. https://www.ncbi.nlm.nih.gov/pubmed/2102225. [PubMed]
- 63.Lin J, Bleiweiss IJ, Mendelson MH, Szabo S, Schwartz IS. Cytomegalovirus-associated appendicitis in a patient with the acquired immunodeficiency syndrome. Am J Med. 1990;89(3):377–379. doi: 10.1016/0002-9343(90)90353-f. [DOI] [PubMed] [Google Scholar]
- 64.Chien YW, Huang HM, Ho TC, et al. Seroepidemiology of dengue virus infection among adults during the ending phase of a severe dengue epidemic in southern Taiwan, 2015. BMC Infect Dis. 2019;19(1):338. doi: 10.1186/s12879-019-3946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YH, Hsieh YC, Chen CJ, Lin TY, Huang YC. Retrospective seroepidemiology study of dengue virus infection in Taiwan. BMC Infect Dis. 2021;21(1):96. doi: 10.1186/s12879-021-05809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used national databases obtained from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare in Taiwan (https://dep.mohw.gov.tw/dos/cp-5119-59201-113.html). All data obtained were anonymized and deidentified by the HWDC. The data used in this study must be accessed and analyzed in the HWDC after filling out an application according to the relevant regulations and thus cannot be shared. Permission was required to use the data in NHIRD, and our study group obtained the necessary permission. Contact information for data application, analysis and inquiry (https://dep.mohw.gov.tw/dos/cp-2516-59203-113.html).