Abstract
Bacteria from the Turicibacter genus are prominent members of the mammalian gut microbiota and correlate with alterations in dietary fat and body weight, but the specific connections between these symbionts and host physiology are poorly understood. To address this knowledge gap, we characterize a diverse set of mouse- and human-derived Turicibacter isolates, and find they group into clades that differ in their transformations of specific bile acids. We identify Turicibacter bile salt hydrolases that confer strain-specific differences in bile deconjugation. Using male and female gnotobiotic mice, we find colonization with individual Turicibacter strains leads to changes in host bile acid profiles, generally aligning with those produced in vitro. Further, colonizing mice with another bacterium exogenously expressing bile-modifying genes from Turicibacter strains decreases serum cholesterol, triglycerides, and adipose tissue mass. This identifies genes that enable Turicibacter strains to modify host bile acids and lipid metabolism, and positions Turicibacter bacteria as modulators of host fat biology.
Subject terms: Microbiome, Fat metabolism, Bacteriology
Mechanisms by which the gut microbiota affects its host are a main research focus. Here, Lynch et al. characterize bile acid modifications performed by a prevalent bacterial taxon from the gut, the genus Turicibacter, and found they broadly altered host lipids, connecting Turicibacter functions and host physiology.
Introduction
The gut microbiota forms complex relationships with its host organism, modulating broad aspects of host physiology including metabolism1,2 and neurobiology3,4. Often, the connections between the gut microbiota and host physiology are easiest to decipher through presence/absence of large sectors of the microbial community (examples in refs. 5–7), but in some cases, specific microbial features and/or taxa serve important roles in host biology8–10.
The bacterium Turicibacter sanguinis is the best-studied species of the Turicibacter genus, which is prevalent in the gut microbiota of several animals, including humans11, mice7, cows12, pigs13, and chickens14. Turicibacter can reach relative abundances of >20% in the rodent small intestine15 and 0.5% in the human fecal microbiota16,17. A study of 1126 sets of twins found Turicibacter was a highly heritable taxon in the human fecal microbiota18, supporting associations between Turicibacter abundance and host genetics found in Diversity Outbred mouse collections19–21. Long-standing co-evolutionary connections between this taxon and humans are suggested by the high abundance of Turicibacter sequences that have been measured in ancient and non-industrial human gut microbiotas17,22–24.
The mammalian gut microbiota has long been associated with obesity25,26, but studies often provide strong correlations rather than mechanistic determinants of these relationships, indicating a further need for fundamental interrogation into connections between the microbiota and host fat27. Numerous microbiota community profiling studies reveal correlations between Turicibacter and features of host fat metabolism, such as adiposity and dietary lipids28–33, but the nature of these correlations varies34,35. We recently observed that the type strain of T. sanguinis, MOL36136,37, broadly alters the host serum lipidome while decreasing serum triglycerides in mice38. This same strain was also reported to modify bile species through deconjugation and dehydrogenation in vitro21, suggesting at least one potential means by which Turicibacter can influence host lipid status. Based on these findings, we hypothesized that there may be variations in the functional activity of Turicibacter strains that account for differences in host bile and lipid biology, providing a mechanism to connect this taxon to aspects of host physiology.
In this work, we show that diverse Turicibacter isolates influence host metabolites, including lipids and bile acids. These isolates each perform bile transformations in vitro, albeit with differing chemical specificity. We identify and describe bile modifying genes from these isolates and measure their effects on host physiology. We find that these genes are sufficient to broadly alter host lipid and cholesterol states, presenting a means by which bacteria from the Turicibacter genus may modulate biology of their diverse hosts.
Results
Turicibacter isolates separate into genetically distinguishable strains
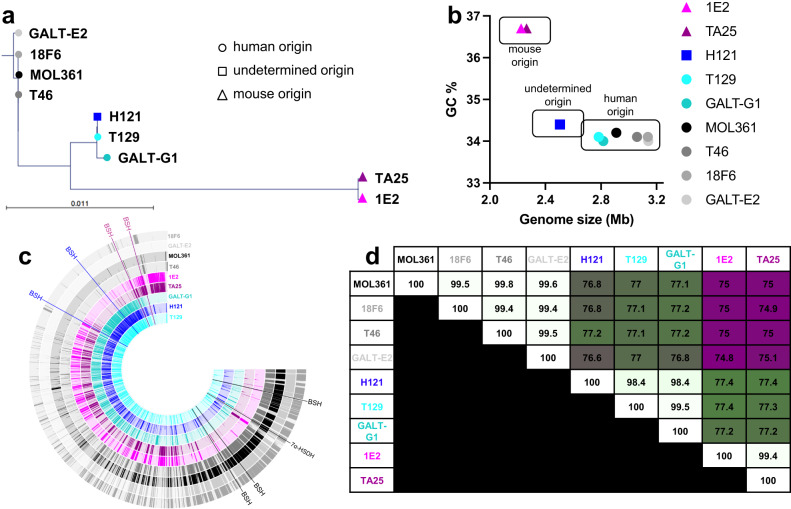
To better understand the diversity within the Turicibacter genus, we gathered nine isolates from the fecal microbiotas of mice and humans that had been identified as T. sanguinis based on their 16S rRNA gene sequence (97% full length 16S rRNA gene sequence similarity cutoff, Supplementary Data 4). Two of these isolates had been previously identified (human-derived type strain MOL361 and H121, which was derived from contaminated germ-free mice36,39); five had been isolated but not published (human isolates 18F6, T46, and T129, and mouse isolates 1E2 and TA25); and two were isolated from a human fecal sample specifically for this study (GALT-E2 and GALT-G1) using an array-based isolation and cultivation platform (see Methods). We performed shotgun short read sequencing and created draft assemblies of each isolate genome. Comparisons of the 16S rRNA gene phylogeny (Fig. 1a), general genome characteristics (Fig. 1b), or specific genome sequence (Fig. 1c, d) revealed that even with this fairly small sample of the 16S rRNA gene-based “T. sanguinis” species designation, there were at least three distinct subgroups: two from humans (exemplar isolates MOL361 and H121, with 99.3% full length 16S rRNA gene similarity), and one from mice (exemplar strain 1E2, 97.5% and 97.8% 16S rRNA gene similarity with MOL361 and H121, respectively). Genomic alignments indicated a substantial amount of shared DNA sequences within members of the same subgroups (all within group average nucleotide identity [ANI] >98.3%) with the remaining amount of genetic variation indicating smaller genetic differences between related isolates. These within-subgroup shared sequences were distinct from members of the other two subgroups (intergroup ANI: MOL361-H121 = 76.80%, MOL361-1E2 = 74.95%, H121-1E2 = 77.43%). It is important to note that the H121-group genomically resembles the newly described species Turicibacter bilis13 (98.8% ANI), currently the only other named species from this genus. Overall, these genomic differences suggest distinct evolutionary histories that correspond at least partially with host origin.
Fig. 1. Genomic comparisons reveal distinct subgroups of Turicibacter.
a Phylogenetic tree comparing full-length 16S rRNA gene sequences from noted Turicibacter isolates. Circles indicate human-derived isolates, triangles indicate mouse-derived isolates, and square indicates a mouse-derived contaminating isolate. b Association between guanine-cytosine % (GC%) and calculated genome size in megabases (Mb) for shotgun-assembled genomes of Turicibacter isolates from a. c Full genome sequence comparisons across Turicibacter strains. Position of predicted bile-modification gene homologs are noted outside of rings, with the color of the gene name denoting the genome family that gene is found in. Each ring represents sequence blocks in one genome. d Average nucleotide identity (ANI) between noted Turicibacter genomes. Number denotes ANI, white-green-maroon scale represents 100%-75% ANI scale.
Turicibacter isolates differ in their impact on host fat biology and circulating metabolome
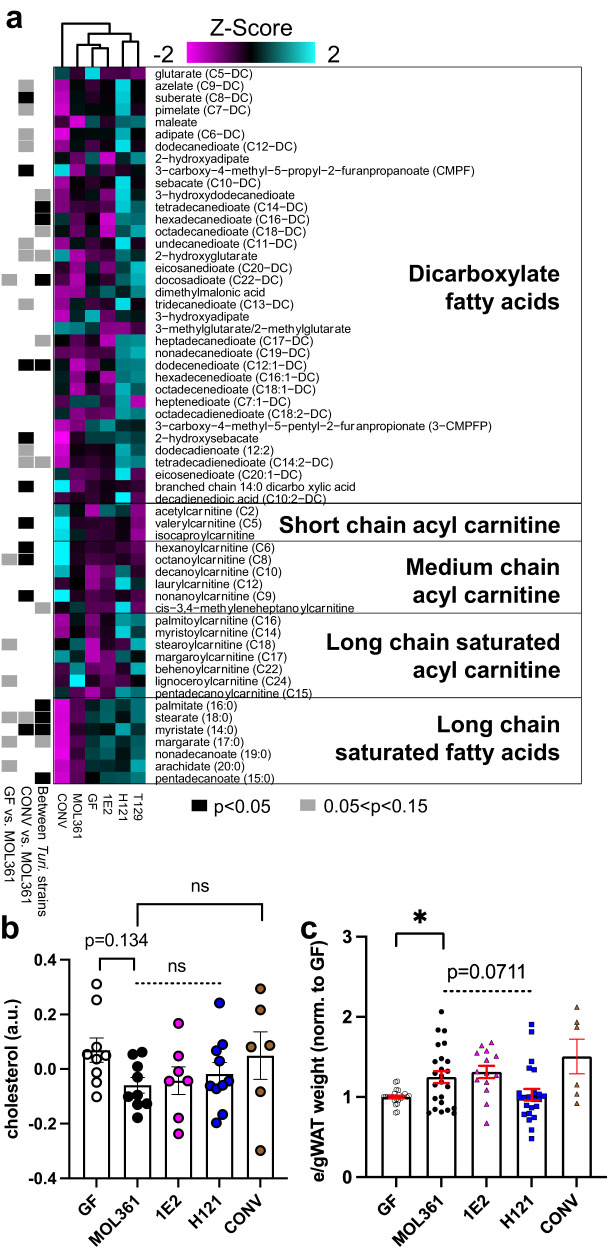
Previous findings revealed that monocolonizing mice with T. sanguinis MOL361 altered host fat tissue and circulating lipids38. Due to the large genomic variation between our Turicibacter strains, we predicted that they would vary in their effects on host lipid biology. We chose representative isolates from each of the distinct phylogenetic subgroups (MOL361, H121 and T129, and 1E2) and measured their effects on circulating metabolites and adipose tissue in monocolonized mice relative to germ-free (GF) and conventionalized controls (i.e., gavaged with complete microbiota, CONV). Compared to GF mice, CONV mice had decreased levels of several dicarboxylate fatty acids, long-chain fatty acids, and long-chain acyl carnitines, with a broad increase in short- and medium-chain acyl carnitines (Fig. 2a). Consistent with a previous report38, colonization with individual Turicibacter strains also induced widespread alterations in host serum lipids, with several strain-level differences in host lipid alterations (Fig. 2a, Supplementary Data 5). Compared to GF controls, MOL361 increased a subset of long-chain acyl carnitines, and decreased many long-chain saturated fatty acids. In addition, colonization with MOL361 elicited trending decreases in host cholesterol to levels below those seen in GF (Fig. 2b). Compared to GF, H121 colonization significantly increased serum levels of several medium-chain fatty acids, dicarboxylic acids, and short-, medium- and long-chain acyl carnitines. 1E2 colonization had a smaller overall effect on host lipids, but led to a decrease in several dicarboxlyate fatty acids (Fig. 2a). In addition to differences between GF or CONV mice and Turicibacter-monocolonized animals, there were also alterations in host lipids that showed some strain-dependency, with trending discrepancies in dicarboxylate fatty acids and significant differences in long chain saturated fatty acids (Fig. 2a) At the tissue level, two of the four Turicibacter strains stimulated statistically significant increases in epididymal/gonadal white adipose tissue (e/gWAT) mass in comparison to GF controls, and a third strain elicited similar increases that were not statistically significant (T129). In contrast, there was no noticeable effect of H121 on e/gWAT mass (Fig. 2c, Supplementary Data 6), distinguishing it from the other Turicibacter strains. Consistent with this, H121 showed the smallest e/gWAT adipocyte size within fat pads (Supplementary Fig. 1a–f). This may be due to lower colonization of H121 in both the small intestine and the colon (Supplementary Fig. 1g, h) The effects of particular Turicibacter strains on serum lipids, cholesterol, and fat mass directed us to investigate molecules that could potentially connect these biomolecules: bile acids.
Fig. 2. Colonization with different Turicibacter strains alters host lipids.
a Heatmap displaying relative abundance of serum lipids from gnotobiotic mice monocolonized with noted Turicibacter strains. Heatmap values (Z-score) represent mean abundance of each detected lipid species from labeled lipid categories scaled across all the means of that individual lipid species. Black (p < 0.05) and gray (0.05 < p < 0.15) rectangles indicate statistically significant differences of that metabolite between: left, GF and MOL361 monocolonized mice; middle, CONV and MOL361 monocolonized mice; and right, between MOL361, 1E2, and H121 monocolonized mice. b Serum cholesterol abundances of mice colonized by noted Turicibacter strains. c Sex and litter-matched relative epidydimal/gonadal white adipose tissue (e/g WAT) mass of mice monocolonized with noted Turicibacter strains. Shapes indicate value for individual mouse, dotted bar represents ANOVA statistic for groups below the line, a.u. = arbitrary units. WAT analysis n: GF = 26, MOL361 = 24, 1E2 = 14, H121 = 23, T129 = 10, CONV = 6. All other analysis n: GF = 9, MOL361 = 9, 1E2 = 7, H121 = 10, T129 = 7, CONV = 6. In all panels Mann–Whitney test for MOL361-GF and MOL361-CONV comparisons, Kruskal–Wallis for intra-Turicibacter comparison, Šidák correction for multiple comparisons86. Errors bars are mean +/− SEM, *p < 0.05, GF-MOL361 in 2c: p = 0.0108. Data are provided as source data file.
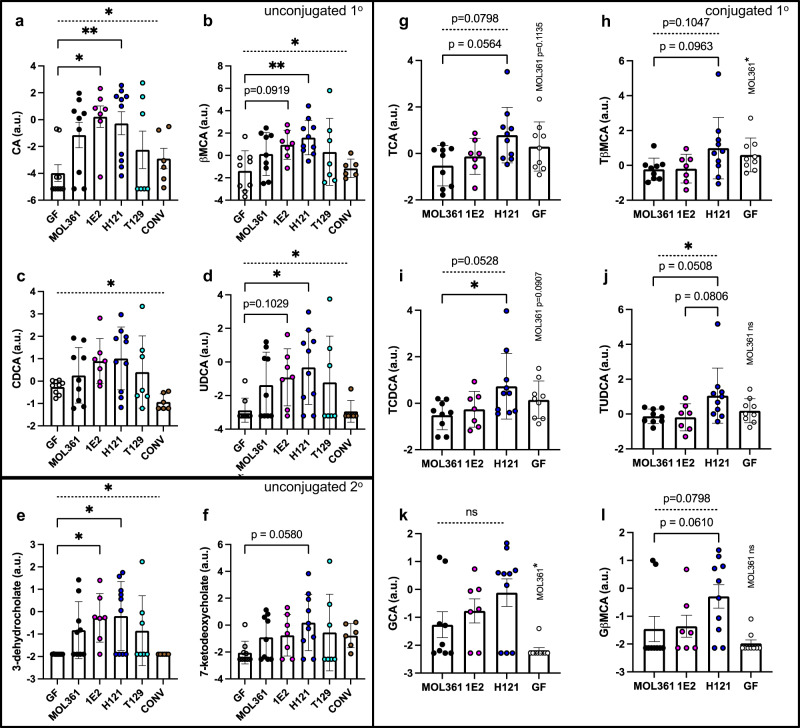
Bile acids can affect circulating host lipids by altering fat digestion and systemic hormonal signaling40. They are produced by the host and released into the small intestine, where they promote digestion of fats by facilitating micelle formation, and can also act through receptors like the farnesoid X receptor (FXR)40 and GPBAR1/TGR541. Gut bacteria can modify bile acids, primarily through transformations like deconjugation. Previous reports found that MOL361 can broadly modify bile acids in vitro21, so to determine if these abilities allowed MOL361 and other Turicibacter strains to modify host bile acids in vivo, we profiled serum and cecal bile acids in Turicibacter-monocolonized mice. Though each strain had some unique impacts on host serum metabolites, we noted consistent patterns in bile acids across monocolonized mice in comparison to either their GF or CONV controls. (Note: bile species with amino acid conjugants are typically referred to as “bile salts,” but for simplicity, we will herein refer to both conjugated and unconjugated bile species as “bile acids”). Colonization with all of the Turicibacter strains led to a general increase in serum levels of unconjugated primary bile acids like cholate (CA), chenodeoxycholate (CDCA), and β-muricholate (βMCA) (Fig. 3a–d), and a similar rise in unconjugated secondary bile acids 3-dehydrocholate and 7-ketodeoxycholate (Fig. 3e, f). These responses were highly variable in the case of T129 colonization, leading us to de-emphasize this strain for subsequent experiments. In response to colonization with MOL361, 1E2, or H121, we also found a similar increase in several unconjugated bile acids in the cecum (Supplementary Fig. S2). In all, these results suggest that these Turicibacter strains are able to impact host bile acids, potentially by deconjugating them in the gut.
Fig. 3. Turicibacter colonization alters circulating host bile species in a strain-specific manner.
Serum abundances of a–d, primary unconjugated bile acids; e, f secondary unconjugated bile acids; or g–l primary conjugated bile acids. Points indicate log-transformed value for individual mouse with shapes and colors matching Fig. 1, error bars represent mean +/− SEM, a.u.=arbitrary units. Kruskal–Wallis test across all noted colonizations with Dunn’s multiple comparisons to GF for a–f. Kruskal–Wallis test between noted Turicibacter strains and multiple comparisons to H121 for g–l. Mann–Whitney test used to compare GF and MOL361 in g–l, and p-values are noted above GF data points. n for each group: GF = 9, MOL361 = 9, 1E2 = 7, H121 = 10, T129 = 7, CONV = 6. Dotted bar represents ANOVA statistic for groups below the line. Error bars are mean +/− SEM, *p < 0.05, **p < 0.005. Corrected p-values (ANOVA/GF-MOL361/GF-1E2/GF-H121/GF-T129/GF-CONV): 2a = (0.0204/0.1785/0.0141/0.0049/0.999/0.999); 2b = (0.0116/0.5123/0.0919/0.0059/0.9256/0.999); 2c = (0.0208/0.999/0.5425/0.5306/0.999/0.4158); 2d = (0.0216/0.4850/0.1029/0.0201/0.8856/0.999); 2e = (0.0121/0.3585/0.0299/0.0405/0.7612/0.999); 2f = (0.2203/0.6556/0.6645/0.0580/0.999/0.4012). Corrected p-values (ANOVA/H121-MOL361/H121-1E2/GF-MOL361): 2g = (0.0798/0.0564/0.3171/0.1135); 2h = (0.1047/0.0963/0.2315/0.400); 2i = (0.0528/0.0351/0.2726/0.0907); 2j = (0.0407/0.0508/0.0806/0.2973); 2k = (0.3988/0.3757/0.7675/0.0226); 2l = (0.0769/0.0610/0.2393/0.7176).
When we investigated potential explanations for increases in unconjugated bile species, we noticed that the serum levels of conjugated bile acids differed between animals colonized by different Turicibacter strains, with the clearest delineation separating H121 from MOL361 and 1E2. MOL361- and 1E2-colonized animals generally had lower levels of taurine-conjugated primary bile acids in comparison to H121-colonized animals (Fig. 3g–j), whereas H121 colonization led to an increase in glyco-beta-muricholic acid (GβMCA) (Fig. 3k). Female mice displayed the most significant Turicibacter-associated changes in circulating bile species, lipids, and cholesterol, indicating sex-dependent responses to Turicibacter colonization (Supplementary Fig. 3). This sex difference has also been reported in C57BL/6 mice monocolonized with T. sanguinis MOL361, albeit with a directionally different change in adipocyte size38. Notably, in the cecum, there was less strain-specific distinction in conjugated bile acids, with taurine-conjugated bile acids decreased by colonization with any of the three Turicibacter strains we surveyed (Supplementary Fig. S2). The one cecal bile acid that did display strain dependency was the secondary bile acid lithocholic acid (Supplementary Fig. S2, bottom). Also, the low levels of detected glycine-conjugated bile acids (specifically glycocholic acid) in both serum and cecal contents was slightly increased by Turicibacter colonization (Fig. 3k, l; Supplementary Fig. S2c) Overall, these data demonstrate that colonization with Turicibacter alters serum bile acids, lipids, and cholesterol, as well as host fat mass. Further, while some changes like increased unconjugated bile acids were conserved across Turicibacter colonizations, levels of specific conjugated bile acids differed between Turicibacter strains, with MOL361 and 1E2 leading to lower levels of taurine-conjugated bile acids than H121 colonization.
Turicibacter strains differ in their ability to modify host bile acids
Based on the large genetic variation between the Turicibacter strains (Fig. 1) and the differences in aspects of serum lipid and bile acid profiles seen in response to colonization with different isolates (Fig. 3), we posited that Turicibacter strains differ in their ability to modify bile acids. To test this, we grew each of our nine isolates to stationary phase in rich medium supplemented with a sub-inhibitory concentration of five bile species21: cholic acid, chenodeoxycholic acid, deoxycholic acid (DCA), taurocholic acid (TCA) and glycochenodeoxycholic acid (GCDCA). We then used liquid chromatography-mass spectrometry (LC-MS) to characterize the resulting bile transformations performed by each isolate (Fig. 4a). We discovered that the strains not only differed in their ability to modify this combination of bile species, but also that these capabilities generally mirrored the groupings identified in genomic comparisons (Fig. 4b). MOL361, 18F6, and GALT-E2 deconjugated both tauro- and glyco-bile acids, and also dehydrogenated CA and CDCA (Fig. 4b). 1E2 and TA25 deconjugated tauro-bile acids, but did not detectably deconjugate glyco-bile acids or perform dehydrogenation (Fig. 4b). H121 and T129 deconjugated glyco-bile acids, but did not readily deconjugate tauro-bile acids nor perform detectable dehydrogenation (Fig. 4b). T46 and GALT-G1 did not have bile-modifying capacity that mirrored their genetic phylogeny; T46 genomically resembled the MOL361 group but performed modifications similar to the H121 group (i.e. glyco- but not tauro- deconjugation, minimal dehydrogenation), whereas GALT-G1, which genomically resembled the H121 group, performed more MOL361-like transformations (i.e., glyco- and tauro-deconjugation, dehydrogenation of CDCA) (Figs. 1, 4b, Supplementary Data. 1). Overall, each strain performed at least one of three bile transformations, with some showing capacity for all three (Fig. 4b, Supplementary Data 1).
Fig. 4. Turicibacter isolates differ in their bile-modifying abilities.
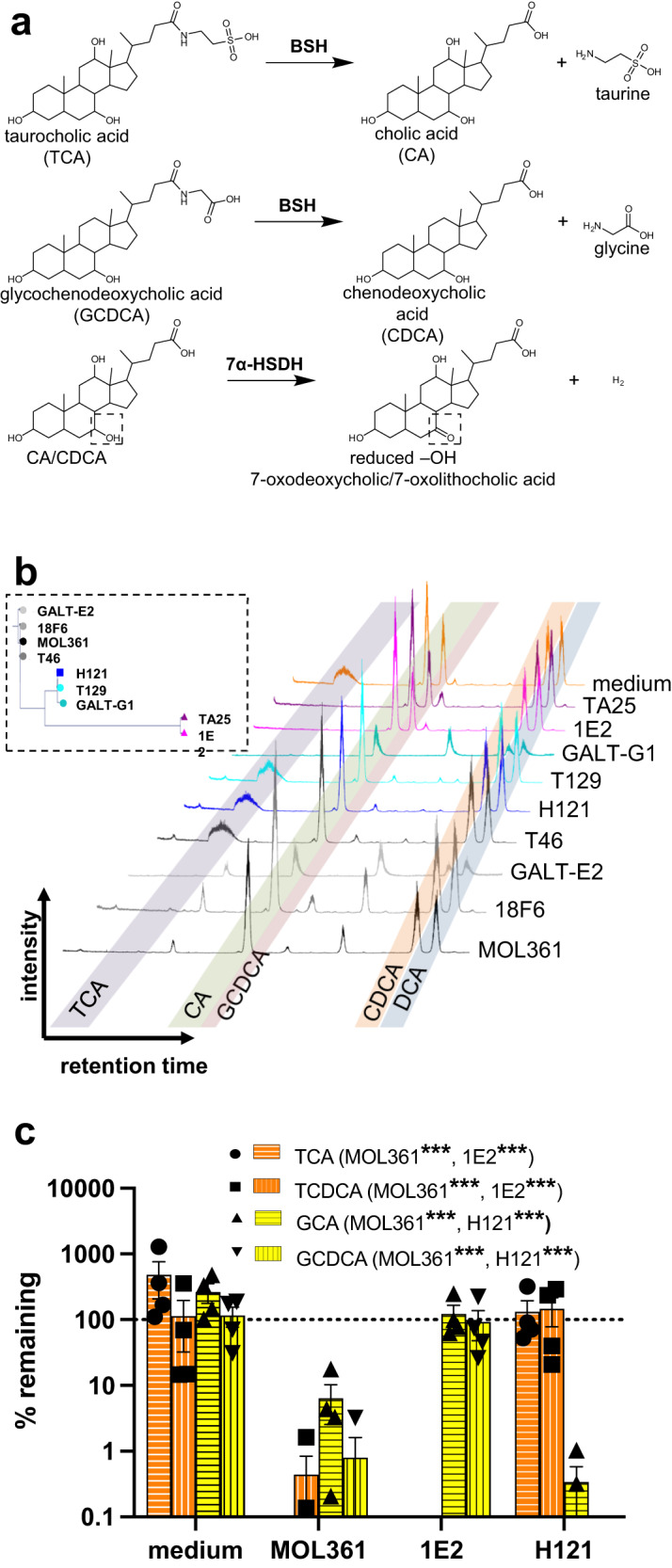
a Schematic for types of bile transformations found to be performed by Turicibacter isolates. b inset: 16S rRNA gene-based phylogenic tree from Fig. 1a. Liquid chromatograms of individual Turicibacter isolates grown for 24 h in media with sub-inhibitory concentrations of five bile acids: taurocholic acid (TCA), cholic acid (CA), glycochenodeoxycholic acid (GCDCA), chenodeoxycholic acid (CDCA), and deoxycholic acid (DCA). Shaded regions indicate expected retention time of each bile species. c Percent remaining (compared to cultures at time = 0) of conjugated bile acids (TCA, taurochenodeoxycholic acid [TCDCA], glycocholic acid [GCA], GCDCA) after 24 growth with noted Turicibacter isolate. Yellow = glycine-conjugated bile acids, orange=taurine-conjugated bile acids. n = 4 independent cultures. Values not shown were below 0.1% remaining. Statistical analysis performed by one sample t-test, annotations of legend denotes strains with significant difference from 100% remaining for each bile acid. p-values for each bile acid (MOL361/1E2/H121): TCA = (< 0.0001/ < 0.0001/0.6327); TCDCA = (< 0.0001/ < 0.0001/0.5410); GCA = (0.0002/0.6348/ < 0.0001); GCDCA = (< 0.0001/0.8803/ < 0.0001). Data are provided as source data file.
To confirm these intragroup distinctions, we chose one isolate from each of the subgroups (MOL361, H121, 1E2), and grew them in the presence of four primary conjugated bile acids: TCA, taurochenodeoxycholic acid (TCDCA), glycocholic acid (GCA), and GCDCA. This supported the same pattern seen above; MOL361 deconjugated both groups of bile acids, 1E2 preferentially deconjugated tauro-conjugates, and H121 preferentially deconjugated glyco-conjugates (Fig. 4c). MOL361 and 1E2 displayed broad deconjugation of tauro-conjugates and were able to process at least six taurine-conjugated bile acids (Supplementary Fig. 4). These data reveal that while all tested strains are efficient modifiers of bile species, their specific transformations differ in a strain-dependent manner, potentially reflecting functions that influence their differential effects on host lipid biology.
Turicibacter genomes have different repertoires of bile salt hydrolases
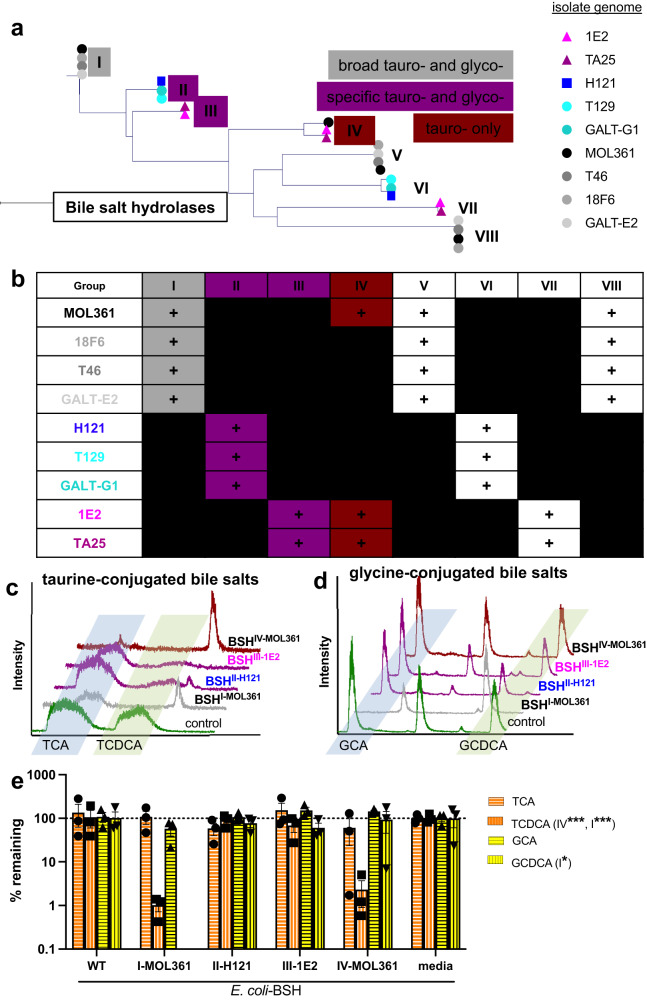
The different bile modification abilities across strain subgroups suggested that each contained unique repertoires of bile-modifying genes. Certain bacteria from the gut microbiota dehydrogenate hydroxyl groups from the steroid core of bile acids42, increasing their polarity and modulating their affinity for host bile acid receptors43. We searched the Turicibacter genomes for homologs of the characterized 7α-hydroxysteroid dehydrogenase (7α-HSDH21) from Clostridium absolum44. This revealed genes with 57% amino acid identity in MOL361, 18F6, T46, and GALT-E2, and homologs with 59% amino acid identity in H121 and T129 (Supplementary Fig. 5a). Though the H121-derived putative homolog had higher overall sequence identity than the MOL361-derived homolog, it lacked certain features predicted to be critical for dehydrogenase activity, such as the analogous Asp38 that is catalytically critical for this reaction44. Because in vitro experiments showed only isolates from the MOL361 group performed bile dehydrogenation, we cloned the putative 7α-HSDH homolog from MOL361 into E. coli C41-pLys and then grew these cells in individual unconjugated bile acids that can be dehydrogenated: CA, CDCA, and DCA. The protein encoded by the gene removed the mass equivalent of two hydrogens from CA (Supplementary Fig. 5b, c). Background transformation by E. coli prevented clear evidence of CDCA dehydrogenation by this putative 7α-HSDH homolog (Supplementary Fig. 5d), but this homolog did not act on DCA (Supplementary Fig. 5e), supporting its annotation as a 7α-HSDH.
Conjugation increases bile acid solubility and emulsification ability45, while deconjugation reverses these effects, leading to decreased dietary lipid absorption. To identify Turicibacter bile salt hydrolase (bsh) genes responsible for the strain-specific differences in bile deconjugation, we first searched our assembled genomes for annotations of “choloylglycine hydrolase,” the broad category that includes these genes. Of these annotated genes, we identified eight groupings of homologous sequences, and found that each Turicibacter strain encodes putative choloylglycine hydrolases from at least two of the eight groups (Fig. 5a, b). Isolates within the same phylogenetic and phenotypic subgroups largely shared similar sequences (Fig. 5a, b). To assay the function of the strain-specific putative bsh genes, we cloned and individually expressed one representative sequence from each of the eight putative choloylglycine hydrolase groups in E. coli C41-pLys and measured the ability of these engineered bacteria to perform the deconjugations we observed in our native Turicibacter. We cultured the individual E. coli strains to stationary phase in the presence of two tauro- (TCA, TCDCA) or glyco- (GCA, GCDCA) bile acids, then measured their ability to deconjugate these bile acid pools. We found that E. coli expressing four of the eight putative bsh gene groups showed deconjugation activity against at least one of the bile acids (Fig. 5c–e). From MOL361, one BSH (group IV) is tauro-specific, and one (group I) deconjugates both glyco- and tauro-conjugates (Fig. 5c, d). 1E2 shares a tauro-specific BSH with MOL361 (group IV), and has another BSH (group III) with moderate activity on TCDCA (Fig. 5c, d). H121 has a BSH (group II) with activity on TCDCA and GCDCA (Fig. 5c, d), although this was blunted when presented with the combined four bile acids (Fig. 5e), potentially because of competitive inhibition. Collectively, these findings reveal that Turicibacter strains contain a range of bile salt hydrolases with deconjugation preferences for different bile acids.
Fig. 5. Turicibacter isolates differ in their genetic capacity to modify bile species.
a Phylogenetic tree of amino acid sequences for each predicted bile salt hydrolase (BSH) sequence from Turicibacter isolates, with observed bile species specificity noted in boxes. We did not detect bile salt hydrolase activity in sequences without boxes, representing groups V-VIII. b Presence (+) or absence of sequence homologs with potential BSH activity in Turicibacter isolates. c Liquid chromatograms of media after 24 h cultures of E. coli expressing individual predicted bsh genes from each sequence grouping and grown with TCA and TCDCA. Control is E. coli with same expression vector but expressing non-bile-modifying gene. d same as c but with GCA and GCDCA instead of tauro-bile acids. e Quantification of percent remaining (compared to media controls) of conjugated bile acid (TCA, TCDCA, GCA, GCDCA) after 24 h growth with E. coli expressing the noted Turicibacter bsh gene. n = 3 independent cultures, annotations in legend indicate *p < 0.05, **p < 0.005, ***p < 0.0005 using one sample t-test comparison with 100% remaining. p-values for each bile acid expressed in E. coli (WT/BSHI-MOL361/BSHII-H121/BSHIII-1E2/BSHIV-MOL361): TCA = (0.6778/0.8401/0.1597/0.5261/0.4015); TCDCA = (0.9252/ < 0.0001/0.6322/0.2703/0.0002); GCA = (0.7814/0.1447/0.6873/0.1938/0.0474); GCDCA = (0.9706/ < 0.0001/0.2851/0.1405/0.9125). BSH nomenclature indicates homolog group (e.g. III) and isolate of origin (e.g. MOL361). Data are provided as source data file.
Strain- and substrate-specific bile salt hydrolases from Turicibacter differentially alter host lipid composition
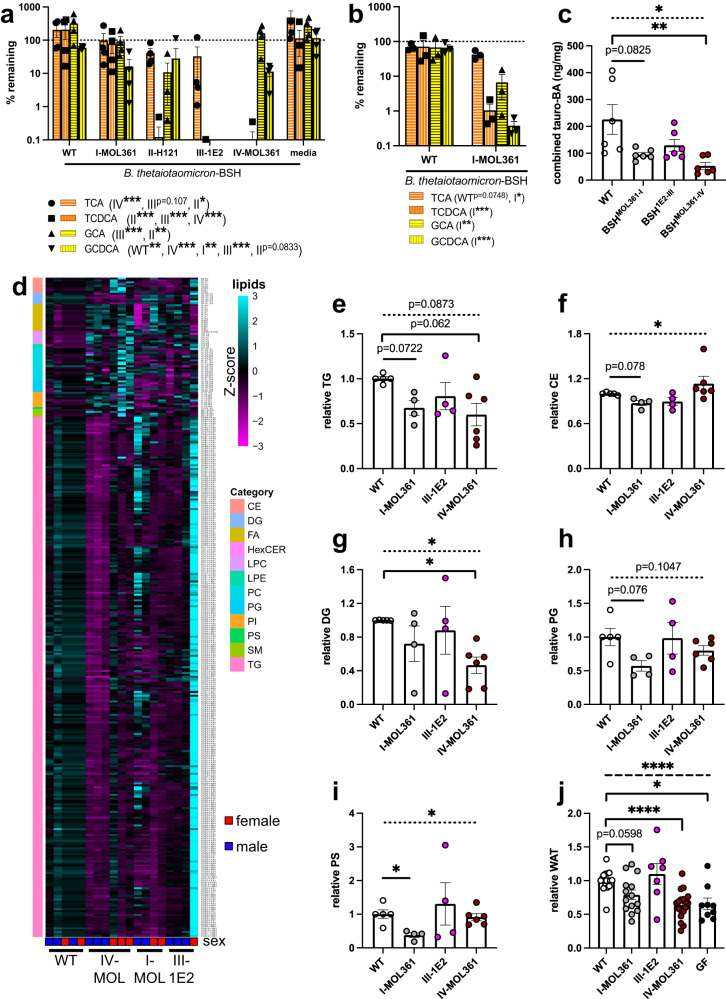
Given that Turicibacter colonization broadly modified host lipid and bile pools (Fig. 2), and that bile transformations have been previously shown to alter host lipids46, 47, we predicted that expressing Turicibacter bile-modifying genes outside the context of Turicibacter colonization would be sufficient to impact host lipid biology. To measure the individual effects of these bile acid transformations, we expressed Turicibacter bsh genes off of a genomically-integrated high expression vector48 in the common gut bacterium Bacteroides thetaiotaomicron. This bacterium was chosen because it stably colonizes the murine gut and, unlike E. coli C41-pLys, contains a homolog of a characterized 7α-HSDH similar to that of T. sanguinis MOL36149,50, allowing the engineered bacteria to more completely mimic Turicibacter bile transformations. As this B. thetaiotaomicron strain has previously been described to have some BSH activity46, we decided to employ the parental strain expressing the non-bile modifying green fluorescent protein (GFP) as a comparison baseline for these experiments (named Bt-WT). These engineered B. thetaiotaomicron strains generally transformed tauro- and glyco- conjugated bile acids as predicted based on the BSH characterization in E. coli, including preferential TCDCA transformation, indicating they were capable of performing Turicibacter-like bile transformations (Fig. 6a, Supplementary Data 2). However, the B. thetaiotaomicron strain that expressed bsh (group III) from strain 1E2 more completely transformed glyco-conjugated bile acids than the strain expressing bsh (group II) from H121, counter to the E. coli findings. This observation led us to not use the bsh (group II)-expressing strain further. Additionally, although we did not notice a significant in vitro growth defect in the engineered B. thetaiotaomicron strains (Supplementary Fig. 6), we observed a bile transformation delay in B. thetaiotaomicron expressing bsh (group I) from MOL361 that we could compensate for by extending the growth period (Fig. 6b).
Fig. 6. Turicibacter bsh expression is sufficient to alter host lipidome and health-associated lipid markers.
a Percent remaining of noted bile acids after 24 h growth with Bacteroides thetaiotaomicron expressing noted bsh genes. n = 4 cultures per strain. b Same as a, but with 48 h growth with noted B. thetaiotaomicron strains. n = 3 cultures per strain. For a and b, points represent individual comparison with media control, legend annotations denote strains with statistical significance for each bile acid using one sample t-test comparison with 100% remaining. c Quantification of combined taurine-conjugated cecal bile acids (BA) from mice colonized with bsh-expressing B. thetaiotaomicron. Statistical analysis was performed with Kruskal–Wallis test with Dunn’s multiple comparisons test. n = 6 animals per colonization. d Heatmap of circulating lipid species significantly altered by expression of at least one Turicibacter bsh in B. thetaiotaomicron. Heatmap Z-score values represent mean abundance of each detected lipid species from labeled lipid categories scaled across all the means of that individual lipid species. Colors on left correspond to lipid class, each column represents one animal. e–i Relative combined circulating concentrations of (e) triglycerides (TG), f cholesterol esters (CE), g diacylglycerides (DG), h phosphotidylglycines (PG), or i phosphotidylserines (PS) of mice monocolonized with bsh-expressing B. thetatiotaomicron. j Relative white adipose tissue weight of mice monocolonized with bsh-expressing B. thetatiotaomicron. n for each condition in d–i: WT = 5(3 M, 2 F); BSHI-MOL361 = 4(2 M, 2 F); BSHIII-1E2 = 4(3 M, 1 F); BSHIV-MOL361 = 6(3 M, 3 F). n for j: WT = 17; BSHI-MOL361 = 14; BSHIII-1E2 = 7; BSHIV-MOL361 = 18. In (e–j), all values normalized to sex-matched littermates, statistical analysis performed by Welch’s ANOVA with Dunnett’s multiple comparisons to Bt-WT, dotted bar in c, e–j represents ANOVA statistic for groups below the line. n for (e–i): Bt-WT = 5, Bt-BSH-MOL361-I = 4, Bt-BSH-1E2-III = 4, Bt-BSH-MOL361-IV = 6. n for (j): Bt-WT = 17, Bt-BSH-MOL361-I = 14, Bt-BSH-1E2-III = 7, Bt-BSH-MOL361-IV = 18. Error bars are mean +/− SEM, *p < 0.05, **p < 0.005, ***p < 0.0005. Figure 6ap-value for B. thetaiotaomicron expressing BSH in each bile acid (WT/BSHI-MOL361/BSHII-H121/BSHIII-1E2/BSHIV-MOL361): TCA = (0.3020/0.9811/0.0286/0.1074/ < 0.0001); TCDCA = (0.3686/0.6211/ < 0.0001/ < 0.0001/ < 0.0001); GCA = (0.1703/0.8672/0.0028/ < 0.0001/0.3291); GCDCA = (0.0007/0.0035/0.0833/ < 0.0001/ < 0.0001). Figure 6bp-values (WT/BSHIV-MOL361): TCA = (0.0748/0.0148); TCDCA = (0.5295/ < 0.0001); GCA = (0.4168/0.0023); GCDCA = (0.1323/ < 0.0001). Figure 6c, e–jp-values (ANOVA/BSHI-MOL361/BSHIII-1E2/BSHIV-MOL361): 6c = (0.0051/0.0825/0.5337/0.0016); 6e = (0.0222/0.0722/0.5767/0.0669); 6f = (0.0377/0.0780/0.2933/0.4864); 6g = (0.0142/0.5490/0.9599/0.0072); 6h = (0.1047/0.0760/0.999/0.4879); 6i = (0.0076/0.0147/0.9449/0.9344); 6j = (0.0042/0.0457/0.8987/ < 0.0001). Data are provided as source data file.
We monocolonized mice with the bsh-expressing B. thetaiotaomicron strains and assessed their circulating lipid profiles and abdominal WAT mass. We found that the engineered strains colonized the gnotobiotic mouse gut at least as well as the control strain, with one strain (BSH-III-1E2) colonizing the cecum slightly better (Supplementary Fig. 7), and that expressing individual Turicibacter bsh genes in the B. thetaiotaomicron background was sufficient to significantly alter host cecal bile levels (Fig. 6c, Supplementary Fig. S8) and the absolute abundance of hundreds of circulating lipid species (Fig. 6d, Supplementary Data 7). In particular, expression of either the group I or group IV bsh led to a decrease in triglycerides (Fig. 6d, e). Expressing the tauro-specific bsh (group IV) from MOL361 also decreased diacylglycerides (Fig. 6g). Expressing the broader specificity bsh (group I) from MOL361 also led to a decrease of phosphatidylglycines, phosphatidylserines, and cholesterol (Fig. 6d–f, h, i). Despite having broad capacity for transformation, B. thetaiotaomicron expressing bsh (group III) from 1E2 did not alter host lipid profiles as much as the other strains (Fig. 6d–i). On a tissue-level, bsh-expression also altered WAT storage in the colonized mice, with the broad tauro-deconjugating BSHs (group I and IV) significantly reducing WAT mass (Fig. 6j). Similar to our findings with Turicibacter monocolonization, we also observed a sex difference in BSH responses, with male mice showing more consistent decrease in triglycerides in response to the tauro-specific BSH from MOL361, and females showing more consistent triglyceride responses to the broadly deconjugating BSH (Supplementary Fig. 10). Overall, these BSH-associated lipid and adipose tissues results were consistent with previous findings on MOL361 colonization in the C57Bl/6 mouse model38, but differed from MOL361 colonization in our Swiss Webster mice (Fig. 2c, Supplementary Fig. S11).
To further explore potential factors that may drive the cholesterol and WAT alterations in response to Turicibacter colonization and bsh expression, we measured liver transcript levels of farnesoid X receptor, (Fxr), a key nuclear receptor for bile acids; cytochrome P450 Family A Subfamily A Member 1/Cholesterol 7α Hydroxylase (Cyp7a1), the rate-limiting enzyme for conversion of cholesterol into bile acids; and glucose-6-phosphatase (G6pase), a key enzyme for gluconeogenesis. There were no differences in Fxr transcript levels across any of the native and engineered bacterial colonization conditions (Supplementary Fig. 12a). However, we found similar increases in Cyp7a1 expression (Supplementary Fig. 12b) and decreases in G6pase expression (Supplementary Fig. 12c) between the Turicibacter and bsh colonizations. These findings suggest that the bile-modifying activities of Turicibacter lead to downstream alterations in liver expression of bile signaling and response elements. In all, these results demonstrate that expressing strain-specific bsh genes from Turicibacter, especially those able to process the abundant taurine-conjugated bile acids present in the murine intestine, is sufficient to alter host cholesterol, bile, and lipid biology.
Discussion
Results from this study show that several strains of Turicibacter bacteria from the mammalian gut microbiota modulate host bile and lipid compositions. We identified and characterized five novel Turicibacter genes capable of performing bile transformations (four bsh, one 7α-HSDH), and revealed that expression of individual bsh genes is sufficient to broadly and differentially alter host lipid profiles. Further, we found that while bile-transforming genes are present in all our surveyed Turicibacter strains, the specific transformation capacity of BSH variants differed by strain in a manner consistent with host environment co-evolution: bile acids in the human gut are a mix of taurine- and glycine-conjugants, whereas murine bile acids are predominantly taurine-conjugants51, 52, providing different bile environments that are preferentially processed by Turicibacter strains isolated from their respective gastrointestinal tracts. This close connection between host-specific bile composition and bacterial modifications may be due in part to the bile sensitivity previously exhibited by MOL361 and/or the high abundance of Turicibacter in the small intestine, causing these bacteria to more strongly associate with host genes for bile reabsorption and lumenal bile levels than other bile-modifying gut bacteria21,53–57.
This work displays metabolic consequences of colonization by specific gut bacteria and improves the resolution of our understanding connecting specific taxa—in this case, at the strain level—with host physiology. In rodent and human studies, Turicibacter relative abundance often negatively correlates with dietary fats29,31,58–62 and host adiposity28,33,34, but some studies have shown opposite relationships30,63,64. This could be a result of the phenotypic diversity identified here among Turicibacter isolates wherein the host may experience different lipid outcomes depending on their own specific Turicibacter strains, but could also vary with other features such as host genetics and sex21,38 (Supplementary Figs. 3, 9, 10), leading to discrepancies in lipid responses between hosts despite similar bile modifications performed by similar taxa in the gut microbiota. Importantly, effects of Turicibacter colonization could also be influenced by biogeographic organization of an individual’s microbiota; in addition to the specific taxonomic membership, Turicibacter positioning in the small and large intestine may affect host consequences from their respective bile modifications by transforming bile pools in either section of the gut tract. Despite genomic and localization differences between them, we found that MOL361 and H121 induced lipid metabolite changes that indicate increases in fatty acid oxidation, suggesting that these strains at least share some features that alter host fatty acid metabolism. While we identified broad groups of host lipids that were differentially modulated by different Turicibacter strains, host complexities likely reduced our power to further resolve these differences, pointing to future research that may still reveal more strain-specific effects of Turicibacter bacteria.
Further research into bsh gene regulation in the Turicibacter genus, the relationship between bile and Turicibacter colonization and transmission behaviors, as well as the native functionality of the putative BSH and 7α-HSDH homologs we tested, will further explain how these bacteria wield their bile modifications in the intestine. In addition, exploring the discrepencies we observed between bacterially-induced serum and cecal bile profiles could describe other complexities in these effects, such as differential host reabsorption of modified bile acids. Several in vitro bile modulations, such as increases in unconjugated bile acids, were consistent between our in vitro characterizations and Turicibacter colonization, but concurrent in vivo increases in expression of bile synthesis genes and levels of glyco-conjugated bile acids seen during Turicibacter colonization and expression of individual Turicibacter BSH indicate the possibility of more complex interactions between host bile production and Turicibacter activity. The fact that the specificity and activity of the individual BSH homologs differed when expressed in different bacterial backgrounds indicates that other unknown cellular or environmental factors influence the way individual BSH act in vivo. This may also include mechanisms that modify the functionality of the other putative BSH homologs we identified from Turicibacter that did not deconjugate the specific conjugated bile acids used in our experiments. These findings may influence the ways that bile-modifying genes can be employed to shape host lipid profiles through microbiota engineering, positioning certain microbial species as more appropriate vectors to impart specific host effects. It will also be informative to determine what other activities performed by Turicibacter lead to WAT gain in colonized animals, which contrasted findings from colonizing mice with specific bsh-overexpressing strains of B. thetaiotaomicron. Given this finding, it is likely that Turicibacter also influences host lipids through other mechanisms in addition to the bile transformations we characterized.
Our work also connects specific Turicibacter members and BSH activity with specific host outcomes. Though some host responses such as broadly decreased triglycerides were consistent across our BSH-recipient mice, the exact lipid and cholesterol responses differed, indicating that the type of deconjugations might have differing connections with host physiology. Further work will continue strengthening the prospect of utilizing Turicibacter and/or its bile modifications to intentionally alter host lipid biology to improve host metabolic and lipid-associated health65,66, as has been proposed with other bacteria54,67. Beyond lipid biology, Turicibacter abundance has been positively correlated with diseases such as Parkinson’s disease68 and depression69, and selective serotonin reuptake inhibitors (SSRIs) have been found to negatively affect Turicibacter growth and colonization70, potentially because they inhibit activity of its unique serotonin transporter38. SSRI use is frequently associated with metabolic side effects like weight gain71,72, and our findings suggests a hypothesis that connects SSRI use and these side effects: SSRI use could diminish gut colonization of bacteria like Turicibacter, thus unintentionally altering their impact on host physiology. Future work may develop strategies to reduce interactions between SSRIs and activity of the microbiota, and minimizing the side effects of these drugs and improving host outcomes. In all, these associations further emphasize the importance of understanding mechanisms connecting members of the diverse Turicibacter genus to host physiology.
Methods
Mouse husbandry
All mouse experiment protocols were approved by the UCLA Institutional Animal Care and Use Committee. Adult (6–8 week old) germ-free Swiss Webster mice were used for all animal experiments (see details in Supplementary Data S3). Mice were reared in flexible gnotobiotic isolators on a 12 h:12 h light dark schedule on standard chow (Labdiet 5K52, 22.1%: 16.6%: 61.3% protein: fat: carbohydrate by calories), then were exited to autoclaved filter top cages with autoclaved chow (Labdiet 5010, 28.7%: 13.1%: 58.2% protein: fat: carbohydrate by calories) and water. For entirety of experiments, mice were kept in 12 h:12 h light:dark cycle under temperature (22o–25 oC) and humidity control. After 1 day of cage acclimation, the noted Turicibacter or Bacteroides thetaiotaomicron strain was grown in YCFA medium (see below) overnight, pelleted by centrifugation, and resuspended in 1X PBS. Mice were colonized by a 200 μL gavage containing ~106 colony-forming units (CFU) of Turicibacter or ~108 CFU of B. thetaiotaomicron. Alternatively, mice were gavaged with the same volume of PBS alone (referred to as germ-free [GF]) or PBS-suspended fecal slurry from a specific pathogen-free adult mouse (referred to as conventionalized [CONV]). Colonization was measured using strain-specific TuriSERT primers (Supplementary Data 8) and quantitative PCR (qPCR) from weight-normalized contents from the distal small intestine and proximal colon after addition of Low Abundance Microbiota Standard (Zymo) and extraction using the ZymoBIOMICS DNA Miniprep kit (Zymo), referenced to a standard curve of CFU derived from serial dilution plating of cultures of that strain.
Bacterial culturing
Turicibacter isolates and Bacteroides thetaiotaomicron strains (Supplementary Data 4) were cultured in a flexible vinyl chamber (Coy) in an anaerobic 85%/10%/5% nitrogen/carbon dioxide/hydrogen mixture (Airgas). Turicibacter was grown on Schaedler’s agar (BD Biosciences) or modified YCFA73 (pH 7.4, per liter: 100 mM MOPS, 10 g casitone, 2.5 g yeast extract, 2 g glucose, 2 g maltose monohydrate, 2 g cellobiose, 44 mg MgSO4, 68 mg CaCl2, 0.9 g NaCl, 10 mg hemin, 0.45 g K2HPO4, 0.45 g KH2PO4, 4 g NaHCO3, 1 g cysteine, 1 mg resazurin, 1.9 mL glacial acetic acid, 0.7 mL propionic acid, 90 μL isobutyric acid, 100 uL isovaleric acid, 100uL valeric acid, 10 mL ATCC vitamin mixture, 0.2% Tween-80) at 37 oC. Cells were normally grown without shaking, but when appropriate, Turicibacter cultures were anaerobically transferred to sealed Hungate tubes or 1.7 mL microcentrifuge tubes and shaken at 225 RPM at 37oC.
For B. thetaiotaomicron growth curves, overnight cultures were grown anaerobically in BHI-S for ~48 h at 37 °C to ensure culture saturation, then were subcultured 1:50 for 6 h at 37 °C (final OD600 = 0.41–0.51). Subcultures were all then diluted to OD600 = 0.1, and then six replicates were further diluted 1:10 in 100 μL BHI-S in a 96 well plate. Plates were anaerobically sealed with parafilm and incubated at 37 °C. OD600 readings were taken every 15 min in a Biotek Synergy H1 microplate reader (Agilent).
Escherichia coli C41-pLys (Lucigen) was used for characterizing putative bile modification genes, which were expressed off the pET21+ plasmid. E. coli was grown aerobically shaking at 37 °C in Luria Broth (LB, 1% NaCl, 1% tryptone, 0.5% yeast extract) supplemented with 100 μg mL−1 ampicillin. Expression of genes was induced by addition of 100 μM IPTG.
Bacterial isolation and identification
A frozen stool sample from an adult human was thawed on ice and diluted 1:10 with PRAS anaerobic dilution blank medium (Anaerobe Systems). 100 μL of the diluted stool was further diluted to 1:1000 with modified YCFA media containing 0.05% bovine bile, 0.2% Tween-80, and 50 mM resorufin and loaded on Prospector® system arrays (Isolation Bio, San Carlos, CA, USA) following manufacturer’s instructions. The fluorescent green signal of the arrays at time 0 was read on the Prospector® instrument in a Coy anaerobic chamber and the arrays incubated at 37 °C in an Anaerobic Systems AS-580 anaerobic chamber (Anaerobe Systems). At 17 and 41 h of incubation the arrays were scanned again and the decrease in green fluorescence from time 0 was used as an indicator for bacterial growth in the array nanowells. Bacteria from the array were transferred to 96-well transfer plates containing 200 μL per well of modified YCFA media, without the addition of 50 mM resorufin. The transfer plates were sealed with a gas permeable film and incubated at 37 °C in a Mitsubishi AnaeroPack jar with a gas-generating sachet (Remel) for 7 days. After incubation, the contents of 538 wells from the transfer plates with visible turbidity were consolidated into secondary 96-well plates, preserved with reduced glycerol, and stored at −80 °C until needed. Unless stated otherwise, all stool and isolate manipulations were conducted anaerobically with a 5% CO2/5% H2/90% N2 atmosphere.
Genomic DNA was extracted in a 96-well format from the consolidated Prospector® culture plates using the Extract All Kit (Applied Biosystems). 20 μL of culture was combined with 20 μL of Lysis Solution and incubated for 10 min at 95 °C, followed by 3 min at room temperature. The DNA was stabilized with the addition of 20 μL of DNA Stabilizing Solution and the resulting DNA lysate stored at −20 °C until needed.
qPCR screening of novel Turicibacter isolates
Genomic DNA from 538 isolates was screened for Turicibacter 16S and the Turicibacter TuriSERT38 gene using a multiplexed primer set (Supplementary Data 8). Each 25 μL qPCR reaction mixture had 1 μL Extract All lysate, 10 μL SYBR Power master mix (Applied Biosystems), 0.5 μL of each of the 10 μM primers, and 12 μL molecular grade water. The reactions were run in a QuantStudio 6 Flex (Thermo Fisher) with a 95 °C hold followed by 40 cycles of 95 °C for 15 s, 50 °C for 30 s, 72 °C for 30 s. Turicibacter sanguinis MOL361 gDNA and water were used as positive and negative controls, respectively.
Molecular cloning
Turicibacter genes were amplified from template culture lysates with Phusion or Q5 DNA polymerase (NEB) and primers designed to amplify denoted Turicibacter genes. pET21- or pWW383748-derived expression plasmids were assembled using Gibson assembly (see Supplementary Data 8 for oligos) for expression in E. coli or Bacteroides thetaiotaomicron, respectively. Cloned constructs were confirmed through Sanger sequencing prior to functional characterization. pWW3837-derived constructs were cloned into B. thetaiotaomicron VPI-5482 as previously described48,74. bsh-expressing B. thetaiotaomicron was compared to B. thetaiotaomicron containing the original pWW3837 construct (referred to as wild-type B. thetaiotaomicron).
Genome assemblies
Each strain was streaked on Schaedler agar plates and incubated anaerobically, then an individual colony from each isolate was picked into YCFA medium and grown overnight at 37 °C. DNA was extracted using the ZymoBIOMICS DNA Miniprep kit (Zymo), with bead beating used to lyse cells. Purified genomic DNA was sequenced by MiGS (migscenter.org), and 151 bp paired-end sequences were assembled using CLC Genomics Workbench (Qiagen). Genome assemblies have been deposited at NCBI at BioProject PRJNA846348.
Whole genome and gene comparisons
anvi’o75 was used to profile and visualize the different Turicibacter strain DNA sequences to locate putative bile salt hydrolase and 7α-HSDH homologs in contig groups, generate variability profiles, and measure gene coverage and detection statistics. Average nucleotide identity (ANI) was calculated using OrthoANIu76 (available https://www.ezbiocloud.net/tools/ani).
Sequences comparisons between 16S rRNA and bsh genes/BSH amino acid sequences were performed in CLC Genomics Workbench (Qiagen). 7α-HSDH sequence comparisons were performed using tblastn (v.20.12.0)77 using the translated amino acid sequence from Clostridium absonum44.
Assessment of bile transformations
In vitro characterization of bile transformations by engineered E. coli or B. thetaiotaomicron strains were performed by growing cells in respective media conditions described above supplemented with 0.5 mM (total combined concentration) of the noted bile species. Cells were grown to stationary phase (shaking at 37 °C), then frozen at −80 °C until further processing. Cells were then thawed, pelleted (5 min at 16,000 × g), and the supernatant was removed to a new microcentrifuge tube. Three volumes of methanol was added, then the mixture was vigorously mixed for 30–60 s and incubated (room temperature, 15 min). Mixtures were centrifuged (5 min, 16,000 × g), the supernatant removed to a clean microcentrifuge tube and dried in a vacuum concentrator. The dried residue was treated with methanol/water/formic acid (50/50/0.1, all by volume) then vigorously mixed and centrifuged as described above. Supernatants were transferred to polypropylene HPLC vials, capped, and maintained at 4 °C while aliquots (typically 5 μL) were injected onto a reversed phase HPLC column (Cadenza CD-C18, 3.0 μm, 250 × 2 mm, Imtakt) equilibrated in solution A (water/formic acid, 100/0.1, vol./vol.) and eluted (0.2 mL minute−1) with an increasing concentration of solution B (acetonitrile/formic acid, 100/0.1, vol./vol.); minute/% B: 0/30, 45/70, 48/100, 50/30, 67/30). The effluent from the column was passed through an electrospray ion source (capillary voltage 42 V, capillary temperature 275 °C, sheath gas flow 15 L min−1, spray voltage 5 kV, and −15 kV conversion dynode with −1.2 kV multipliers) connected to a linear ion trap mass spectrometer (Thermo LTQ) scanning from m/z 95-1000 in the positive ion mode. Spectra were recorded and analyzed with instrument manufacturer supplied software. Confirmation of proposed elemental compositions was achieved using the same chromatography and ion source configuration with the spectra recorded by scanning on an orbitrap mass spectrometer (Thermo LTQ XL).
For bile species quantification, an internal spike-in standard of 100 mM chenodeoxycholic acid-D4 (CDCA-D4, Sigma) was added to initial culture supernatants as a normalization reference. Area under the curve from reconstructed ion chromatograms was used to quantify the abundances of each species.
Serum metabolite analysis
Mice were euthanized with isoflurane and whole blood was collected via cardiac puncture. Blood was allowed to clot in SST Vacutainer tubes (BD) on ice, then centrifuged (4 °C, 1 min, 1500 × g). The supernatant was removed and snap frozen in liquid nitrogen. Serum metabolites were analyzed using global metabolomics platform by Metabolon (Morrisville, NC, USA). Unless otherwise noted, values presented are in arbitrary units (a.u.) for that particular metabolite, determined by the log-transform of the volume-corrected quantification.
Circulating lipid analysis
Mice were fasted for 4–6 h, then euthanized as described above. Blood was collected via cardiac puncture and deposited into anticoagulatory K2EDTA Vacutainer tubes (BD) on ice. Blood was centrifuged (4 °C, 15 min, 2000 × g), then plasma was collected from supernatant and snap frozen in liquid nitrogen. Shotgun lipidomics was performed by the UCLA Lipidomics Core (Los Angeles, CA, USA) with the following protocol. Thawed plasma was pipetted into glass tubes, a mixture of 70 internal standard lipids (Sciex and Avanti) was added, and lipids were extracted using a modified Bligh and Dyer extraction78. The pooled organic layers from two extractions were dried in a vacuum concentrator and resuspended in 50/50 (vol./vol.) methanol/dichloromethane plus 10 mM ammonium acetate. After transfer to robovials, samples were analyzed with a Sciex 5500 with DMS Device (Lipidyzer Platform) using a targeted acquisition list of 1450 lipid species. The Lipidyzer Differential Mobility device was tuned using the EquiSPLASH LIPIDOMIX standard mixture (Avanti). Data were analyzed using an in-house platform using previously described parameters79, and quantitative values were normalized to input volume. Statistical significance identification for species to include in heatmap for Fig. 6d was performed with uncorrected two-tailed Welch’s t-test comparison to Bt-WT (p-value cutoff <0.05). Both raw and corrected p-values are included in Supplementary Data 7.
Total cecal bile analysis
Mice were colonized as described above and contents from the blind end of the cecum were snap frozen upon sacrifice. Samples were thawed on ice and sent to Metabolon for analysis with total bile analysis pipeline. Values shown at “0” were non-quantified and below limit of detection.
Adipocyte area calculation
After sacrifice, mice epidydimal or gonadal white adipose tissue (e/g WAT) pads were weighed and placed into 4% paraformaldehyde in 1X PBS for 48 h at 4 °C. Fat pads were washed twice in 70% ethanol, then submitted to the UCLA Translational Pathology Core Laboratory (Los Angeles, CA, USA) for paraffin embedding, sectioning, and H&E staining. Ten adipocyte images from each animal (five from each fat pad) were visualized with a 20X objective on an EVOS microscope (Thermo). Adipocyte area for all cells contained entirely within the field of view was automatically measured using the Fiji80 Adiposoft81 plug-in (version 1.1.16).
qRT-PCR measurement of liver transcripts
Gnotobiotic mice were colonized as described above, and upon sacrifice, the median lobe of the liver was dissected and either directly snap frozen in liquid nitrogen (all Turicibacter colonized animals) or placed in Trizol, bead beat for 1 min, then frozen in liquid nitrogen (all B. thetaiotaomicron colonized animals). All livers were then transferred to −80 °C until further processing. Directly snap frozen livers were thawed overnight at −20 °C in RNALater-ICE (ThermoFisher), then bead beat in Trizol for 1 min, after which all samples were processed in the same manner. RNA was extracted from thawed Trizol samples using the Direct-Zol RNA Miniprep Kit (Zymo), then cDNA was generated using the qScript cDNA Synthesis Kit (Quantabio). qPCR was performed using the PowerUp SYBR Green Master Mix (ThermoFisher) on a QuantStudio5 Real-Time PCR System (ThermoFisher) (primers82,83 available in Supplementary Data 8) (cycling conditions: 50 °C for 2 min, 95 °C for 2 min, 50 cycles of 95 °C for 15 s, 55 °C for 15 s, 72 °C for 1 min, followed by melt curve). Fold changes in comparison with sex-matched controls (GF for Turicibacter colonizations, Bt-WT for B. thetaiotaomicron colonizations) were calculated using the ΔΔCt method with auto-thresholded Ct values with ppia as the housekeeping gene.
Statistical analysis and reproducibility
Samples sizes were determined using comparison to prior experience, but no statistical method was used to determine sample size. Statistical calculations were performed in in Prism 9.3.1 (Graphpad) or Microsoft Excel 14.7.1. For cecal bile quantifications after Turicibacter colonization, outliers were removed using the ROUT method with Q = 1% and removed from analysis, but original data are included in source data files. No other data in this work were excluded from analysis. Dotted lines denote statistical comparison between groups directly below line, solid lines denote pairwise comparison. Unless otherwise noted, all tests were two-sided, ***p < 0.0005, **p < 0.005, *p < 0.05, written p = 0.05 < 0.15. Heatmaps were created using the pheatmap84 package in R (version 3.6.3)85.
All data are representative of and/or include biological replicates performed across at least two separate experiments performed on different days. All mouse experiments include samples from both sexes of multiple litters except CONV which included both sexes from one litter. Mice were randomly divided into conditions and tagged by a blinded researcher. Adipocyte image analysis was performed by two blinded researchers. All mass spectrometry and chromatograms are representative of at least two experiments from different days.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Turicibacter isolates H121, TA25, T46, and T129 were a gift from Thomas Auchtung. 18F6 and 1E2 were a gift from Kenya Honda. pWW3837 and B. thetaiotaomicron VPI-5482 were gifts from Fatima Enam and Justin Sonnenburg. We would like to thank Alexander Yoon, Gazmend Elezi, and Lauren Seaman for technical assistance with the LC-MS operation and protocols, and Nathan Hwangbo for statistical guidance. This work was supported by funding from the Packard Foundation (E.Y.H.) and Ford Foundation (J.B.L.). E.Y.H. is a New York Stem Cell Foundation—Robertson Investigator. This research was supported in part by The New York Stem Cell Foundation.
Source data
Author contributions
J.B.L., E.L.G, K.C., K.B.Y., J.P., and E.Y.H. conceptualized and planned bacterial and mouse experiments. J.B.L. and K.F.F. designed and performed LC-MS experiments. J.B.L., T.J., A.A., and J.L. performed isolation and characterization of novel Turicibacter isolates. J.B.L. and E.Y.H. wrote manuscript. All authors read and edited final manuscript.
Peer review
Peer review information
Nature Communications thanks Ruth Ley, and the other, anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The metabolomics and lipidomics data generated in this study are attached to this manuscript as supplementary data tables. The mass spectrometry datasets are deposited to Metabolights (www.ebi.ac.uk/metabolights/MTBLS7921/) as Project MTBLS7921. Raw sequencing reads and genome assemblies for Turicibacter strains are deposited at NCBI under BioProject ID PRJNA846348. The data used to generate the figures are included in source files or supplementary data tables unless provided in the sources mentioned above. Source data are provided with this paper.
Materials availability
Bacterial strains from this work may be obtained through reasonable request to the corresponding author. Certain materials may be restricted by MTA requirements.
Competing interests
Findings regarding the host effects of Turicibacter reported in the manuscript are the subject of provisional patent application 63/288980, owned by UCLA, on which J.B.L., E.L.G., K.C., and E.Y.H. are inventors. J.B.L. and E.Y.H. are scientific advisors for PurposeBio, which is licensing this patent but which had no role in study design, data collection, data analysis, or interpretation of this work. No other authors have competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39403-7.
References
- 1.Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019;20:461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahlström A, Sayin SI, Marschall H-U, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Sharon, G., Sampson, T. R., Geschwind, D. H. & Mazmanian, S. K. The central nervous system and the gut microbiome. Cell10.1016/j.cell.2016.10.027 (2016). [DOI] [PMC free article] [PubMed]
- 4.Fung, T. C., Olson, C. A. & Hsiao, E. Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci.10.1038/nn.4476 (2017). [DOI] [PMC free article] [PubMed]
- 5.Rabot S, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 6.Wikoff WR, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl Acad. Sci. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell10.1016/j.cell.2015.02.047 (2015). [DOI] [PMC free article] [PubMed]
- 8.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storelli G, et al. Drosophila perpetuates nutritional mutualism by promoting the fitness of its intestinal symbiont lactobacillus plantarum. Cell Metab. 2018;27:362–377.e8. doi: 10.1016/j.cmet.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CY, et al. Human reference gut microbiome catalog including newly assembled genomes from under-represented Asian metagenomes. Genome Med. 2021;13:134. doi: 10.1186/s13073-021-00950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, et al. The day-to-day stability of the ruminal and fecal microbiota in lactating dairy cows. Microbiologyopen. 2020;9:e990. doi: 10.1002/mbo3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki JJ, Looft T. Turicibacter bilis sp. nov., a novel bacterium isolated from the chicken eggshell and swine ileum. Int. J. Syst. Evol. Microbiol. 2022;72:005153. doi: 10.1099/ijsem.0.005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, et al. Metagenome-assembled genomes and gene catalog from the chicken gut microbiome aid in deciphering antibiotic resistomes. Commun. Biol. 2021;4:1305. doi: 10.1038/s42003-021-02827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo J, et al. Structural and quantitative alterations of gut microbiota in experimental small bowel obstruction. PLoS One. 2021;16:e0255651. doi: 10.1371/journal.pone.0255651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browne, H. P. et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature10.1038/nature17645 (2016). [DOI] [PMC free article] [PubMed]
- 17.Martínez I, et al. The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 18.Goodrich JK, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19:731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson AK, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl Acad. Sci. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Org, E. et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 10.1101/gr.194118.115 (2015). [DOI] [PMC free article] [PubMed]
- 21.Kemis, J. H. et al. Genetic determinants of gut microbiota composition and bile acid profiles in mice. PLoS Genet. 10.1371/journal.pgen.1008073 (2019). [DOI] [PMC free article] [PubMed]
- 22.Santiago-Rodriguez TM, et al. Gut microbiome of an 11th century A.D. Pre-Columbian Andean Mummy. PLoS One. 2015;10:e0138135. doi: 10.1371/journal.pone.0138135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wibowo MC, et al. Reconstruction of ancient microbial genomes from the human gut. Nature. 2021;594:234–239. doi: 10.1038/s41586-021-03532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature10.1038/nature05414 (2006). [DOI] [PubMed]
- 26.Thaiss, C. A. et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature10.1038/nature20796 (2016). [DOI] [PubMed]
- 27.Chadaideh KS, Carmody RN. Host-microbial interactions in the metabolism of different dietary fats. Cell Metab. 2021;33:857–872. doi: 10.1016/j.cmet.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Dhakal S, McCormack L, Dey M. Association of the gut microbiota with weight-loss response within a retail weight-management program. Microorganisms. 2020;8:1246. doi: 10.3390/microorganisms8081246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, et al. Diet- and genetically-induced obesity produces alterations in the microbiome, inflammation and Wnt pathway in the intestine of Apc +/1638N mice: comparisons and contrasts. J. Cancer. 2016;7:1780–1790. doi: 10.7150/jca.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T-T, et al. Polyunsaturated fatty acids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem. Toxicol. 2019;131:110558. doi: 10.1016/j.fct.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Velázquez KT, et al. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019;11:619–637. doi: 10.4254/wjh.v11.i8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao N, et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol. Genom. 2018;50:244–254. doi: 10.1152/physiolgenomics.00114.2017. [DOI] [PubMed] [Google Scholar]
- 33.Petersen C, et al. T cell–mediated regulation of the microbiota protects against obesity. Science. 2019;365:eaat9351. doi: 10.1126/science.aat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golloso-Gubat MJ, et al. Gut microbiota and dietary intake of normal-weight and overweight Filipino children. Microorganisms. 2020;8:1015. doi: 10.3390/microorganisms8071015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, et al. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;8:e8317. doi: 10.7717/peerj.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosshard, P. P., Zbinden, R. & Altwegg, M. Turicibacter sanguinis gen. nov., sp. nov., a novel anaerobic, Gram-positive bacterium. Int. J. Syst. Evol. Microbiol. 10.1099/ijs.0.02056-0 (2002). [DOI] [PubMed]
- 37.Maki JJ, Nielsen DW, Looft T. Complete genome sequence and annotation for turicibacter sanguinis MOL361 T (DSM 14220) Microbiol. Resour. Announc. 2020;9:e00475–20. doi: 10.1128/MRA.00475-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fung, T. C. et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol.10.1038/s41564-019-0540-4 (2019). [DOI] [PMC free article] [PubMed]
- 39.Auchtung, T. A. et al. Complete genome sequence of Turicibacter sp. strain H121, isolated from the feces of a contaminated germ-free mouse. Genome Announc. 10.1128/genomeA.00114-16 (2016). [DOI] [PMC free article] [PubMed]
- 40.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 41.Guo C, Chen W-D, Wang Y-D. TGR5, not only a metabolic regulator. Front. Physiol. 2016;7:646. doi: 10.3389/fphys.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Makishima M, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 44.Lou D, et al. The three-dimensional structure of Clostridium absonum 7α-hydroxysteroid dehydrogenase: new insights into the conserved arginines for NADP(H) recognition. Sci. Rep. 2016;6:22885. doi: 10.1038/srep22885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaver FA, van der Meer R. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl. Environ. Microbiol. 1993;59:1120–1124. doi: 10.1128/aem.59.4.1120-1124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao, L. et al. A selective gut bacterial bile salt hydrolase alters host metabolism. Elife10.7554/elife.37182 (2018). [DOI] [PMC free article] [PubMed]
- 47.Joyce SA, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl Acad. Sci. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitaker WR, Shepherd ES, Sonnenburg JL. Tunable expression tools enable single-cell strain distinction in the gut microbiome. Cell. 2017;169:538–546.e12. doi: 10.1016/j.cell.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hylemon PB, Sherrod JA. Multiple forms of 7-alpha-hydroxysteroid dehydrogenase in selected strains of Bacteroides fragilis. J. Bacteriol. 1975;122:418–424. doi: 10.1128/jb.122.2.418-424.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherrod JA, Hylemon PB. Partial purification and characterization of NAD-dependent 7α-hydroxysteroid dehydrogenase from Bacteroides thetaiotaomicron. Biochim. Biophys. Acta—Lipids Lipid Metab. 1977;486:351–358. doi: 10.1016/0005-2760(77)90031-5. [DOI] [PubMed] [Google Scholar]
- 51.Marion S, et al. Biogeography of microbial bile acid transformations along the murine gut. J. Lipid Res. 2020;61:1450–1463. doi: 10.1194/jlr.RA120001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 2010;51:226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones BV, Begley M, Hill C, Gahan CGM, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl Acad. Sci. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begley M, Hill C, Gahan CGM. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prete R, et al. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020;10:1165. doi: 10.1038/s41598-020-58069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka H, Hashiba H, Kok J, Mierau I. Bile salt hydrolase of bifidobacterium longum -biochemical and genetic characterization. Appl. Environ. Microbiol. 2000;66:2502–2512. doi: 10.1128/AEM.66.6.2502-2512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim G-B, Miyamoto CM, Meighen EA, Lee BH. Cloning and characterization of the bile salt hydrolase genes (bsh) from bifidobacterium bifidum strains. Appl. Environ. Microbiol. 2004;70:5603–5612. doi: 10.1128/AEM.70.9.5603-5612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma D, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 2018;8:6670. doi: 10.1038/s41598-018-25190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao X, et al. Correlations between α-Linolenic acid-improved multitissue homeostasis and gut microbiota in mice fed a high-fat diet. mSystems. 2020;5:e00391–20. doi: 10.1128/mSystems.00391-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Everard A, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8:2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jung M-J, et al. Chronic repression of mTOR Complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 2016;6:30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang J, et al. Supplementation of Bacillus sp. DU-106 reduces hypercholesterolemia and ameliorates gut dysbiosis in high-fat diet rats. Appl. Microbiol. Biotechnol. 2021;105:287–299. doi: 10.1007/s00253-020-10977-2. [DOI] [PubMed] [Google Scholar]
- 63.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22:658–668. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez-Guryn K, et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe. 2018;23:458–469.e5. doi: 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur. J. Clin. Nutr. 2012;66:1234–1241. doi: 10.1038/ejcn.2012.126. [DOI] [PubMed] [Google Scholar]
- 66.Song Z, et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019;7:9. doi: 10.1186/s40168-019-0628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell, B. J. et al. Intestinal Transgene Delivery with Native <em>E. coli</em> Chassis Allows Persistent Physiological Changes. bioRxiv 2021.11.11.468006 10.1101/2021.11.11.468006 (2021).
- 68.Jin M, et al. Analysis of the gut microflora in patients with Parkinson’s disease. Front. Neurosci. 2019;13:1184. doi: 10.3389/fnins.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS. Altered composition of gut microbiota in depression: a systematic review. Front. Psychiatry. 2020;11:541. doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson, M. A. et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 10.1038/s41467-018-05184-7 (2018). [DOI] [PMC free article] [PubMed]
- 71.Ferguson JM. SSRI antidepressant medications. Prim. Care Companion J. Clin. Psychiatry. 2001;03:22–27. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry. 2009;6:16–18. [PMC free article] [PubMed] [Google Scholar]
- 73.Duncan SH. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- 74.Lynch, J. B. & Sonnenburg, J. L. Prioritization of a plant polysaccharide over a mucus carbohydrate is enforced by a Bacteroides hybrid two-component system. Mol. Microbiol. 10.1111/j.1365-2958.2012.08123.x (2012). [DOI] [PMC free article] [PubMed]
- 75.Eren AM, et al. Community-led, integrated, reproducible multi-omics with anvi’o. Nat. Microbiol. 2021;6:3–6. doi: 10.1038/s41564-020-00834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoon S-H, Ha S, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van. Leeuwenhoek. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 77.Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res.10.1093/nar/25.17.3389 (1997). [DOI] [PMC free article] [PubMed]
- 78.Hsieh W-Y, Williams KJ, Su B, Bensinger SJ. Profiling of mouse macrophage lipidome using direct infusion shotgun mass spectrometry. STAR Protoc. 2021;2:100235. doi: 10.1016/j.xpro.2020.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su B, et al. A DMS shotgun lipidomics workflow application to facilitate high-throughput, comprehensive lipidomics. J. Am. Soc. Mass Spectrom. 2021;32:2655–2663. doi: 10.1021/jasms.1c00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galarraga M, et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012;53:2791–2796. doi: 10.1194/jlr.D023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sayin SI, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of Tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi S, et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res. 2016;57:2130–2137. doi: 10.1194/jlr.M071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kolde, R. pheatmap: Pretty Heatmaps. R Package Version 1.10.12. https://cran.r-project.org/web/packages/pheatmap/ (2019).
- 85.Team, R. C. R: A language and environment for statistical computing. R Foundation for Statistical Computinghttps://www.R-project.org/ (Vienna, Austria, 2020).
- 86.Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967;62:626–633. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metabolomics and lipidomics data generated in this study are attached to this manuscript as supplementary data tables. The mass spectrometry datasets are deposited to Metabolights (www.ebi.ac.uk/metabolights/MTBLS7921/) as Project MTBLS7921. Raw sequencing reads and genome assemblies for Turicibacter strains are deposited at NCBI under BioProject ID PRJNA846348. The data used to generate the figures are included in source files or supplementary data tables unless provided in the sources mentioned above. Source data are provided with this paper.
Bacterial strains from this work may be obtained through reasonable request to the corresponding author. Certain materials may be restricted by MTA requirements.