Abstract
Background
Remote monitoring of cardiac implantable electric devices improves patient outcomes and experiences. Alert-based systems notify physicians of clinical or device issues in near real-time, but their effectiveness is contingent upon device connectivity.
Objective
To assess patient connectivity by analyzing alert transmission times from patient transceivers to the CareLink network.
Methods
Alert transmissions were retrospectively gathered from a query of the United States de-identified Medtronic CareLink database. Alert transmission time was defined as the duration from alert occurrence to arrival at the CareLink network and was analyzed by device type, alert event, and alert type. Using data from previous studies, we computed the benefit of daily connectivity checks.
Results
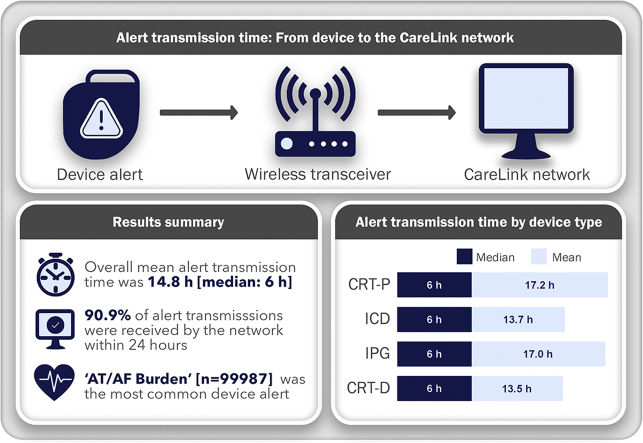
The mean alert transmission time was 14.8 hours (median = 6 hours), with 90.9% of alert transmissions received within 24 hours. Implantable pulse generators (17.0 ± 40.2 hours) and cardiac resynchronization therapy-pacemakers (17.2 ± 42.5 hours) had longer alert transmission times than implantable cardioverter-defibrillators (13.7 ± 29.5 hours) and cardiac resynchronization therapy-defibrillators (13.5 ± 30.2 hours), but the median time was 6 hours for all 4 device types. There were differences in alert times between specific alert events. Based on our data and previous studies, daily connectivity checks could improve daily alert transmission success by 8.5% but would require up to nearly 800 additional hours of staff time on any given day.
Conclusion
Alert transmission performance from Medtronic devices was satisfactory, with some delays likely underscored by patient connectivity issues. Daily connectivity checks could provide some improvement in transmission success at the expense of increased clinic burden.
Keywords: Remote monitoring, Cardiac implantable electronic device (CIED), Alert transmission, Connectivity, CareLink
Graphical abstract
Central Figure_Final
Key Findings.
-
•
The most common cardiac implantable electronic device (CIED) alert received by the CareLink (Medtronic) network was “AT/AF Burden” (n = 99,987).
-
•
Defined as the time from alert detection to receipt by the CareLink network, the mean alert transmission time across all CIED types was 14.8 hours, with a median of 6 hours.
-
•
Our results show robust patient connectivity, with 90.9% of all alert transmissions being received within 24 hours.
Introduction
Cardiac implantable electronic device (CIED) systems traditionally required regular in-clinic follow-ups to monitor CIED performance and ensure diagnostic and therapeutic efficacy for the patient. Devices have since been modified to allow for remote follow-up (remote interrogation), which is more convenient, increases compliance to follow-up appointments, and improves patient outcomes.1,2 Despite these advancements, remote follow-up still follows a calendar-based schedule, imparting significant review burden on staff and resulting in few meaningful actions.3,4 To further improve patient outcomes, many CIED systems now provide exception-based remote monitoring approaches, using alerts to notify physicians of significant clinical changes sensed by the device or of CIED performance issues.5 However, device connectivity is critical for the operation of the alert-based system and thus remains as a substantial barrier to unlocking its full effectiveness.3
The most common way to maintain device connectivity is by proactively identifying disconnected patient transceivers that have not communicated with the remote monitoring system in some time. Maintenance of device connectivity varies by manufacturer. Medtronic legacy (non-Bluetooth) transceivers are checked for connectivity every 15 days and the clinic is notified if a connection has not been established for 14 days. The primary concern with this approach is that an alert from a disconnected device may not be received by the remote monitoring system for up to 15 days. To circumvent this, the Medtronic system uses a built-in retry mechanism, and if an alert or a scheduled transmission fails, the patient is alerted via an audible device tone. The transmission will retry at set intervals until successful, depending on whether the failure was from the CIED to the transceiver or from the transceiver to the network.
Several solutions have been proposed to address the lag time in receiving alerts from disconnected transceivers, including daily transmissions and daily connectivity checks.6,7 Before implementing and assessing new solutions, it is important to fully understand the current system. Previous studies have focused on the clinical benefit of remote monitoring systems or analyzed scheduled transmissions in a controlled trial, but a technical assessment of unscheduled alert transmissions has not been done. In this study we retrospectively analyzed the performance of real-world alert transmissions from Medtronic devices using data from a query of the de-identified Medtronic CareLinkTM United States database (Medtronic, Minneapolis, MN). We limited our query to legacy transceivers and excluded Bluetooth transceivers owing to their differing transmission technology and to better reflect the current landscape of devices worldwide. Alert transmission time, defined as the interval from when the alert occurred to when it was received by the network, was analyzed overall, by device type, and by alert event. Lastly, using our results and data from previous studies, we calculated the expected improvement in alert transmission success and subsequent clinic burden with implementation of daily connectivity checks.
Methods
Data source
The Medtronic CareLink network is used to remotely monitor more than 2 million patients with a Medtronic CIED. Longitudinal data from the CareLink network, including all transmissions, is collected into a de-identified database system. A query of the de-identified United States database was conducted on July 19, 2021, by pulling a static data set using structured query language. The query identified parameters of interest for each alert transmission received by the CareLink network. The 2 primary parameters of interest were (1) the patient alert event date and time (representing when the CIED first identified an alert condition) and (2) the queue arrival date and time (representing when the CareLink network first received the alert transmission). The difference between these parameters provided the duration of time most relevant to this analysis. The query was limited to patients using the legacy 24950/24952 MyCareLinkTM patient transceivers, Medtronic’s most widely distributed transceiver, and excluded implantable loop recorders (ILR) and CIEDs actively using Bluetooth technology.
Data preparation
There were 2 primary steps involved in preparing the data for analysis: (1) adjusting for time zone differences between the 2 primary parameters of interest: the event alert date/time and the date/time for the arrival to the network server; and (2) identification and exclusion of devices with obvious clock drift, incorrect programming, or durations outside the relevant analysis window.
The patient alert event date and time was provided by the CIED, which had its own real-time device clock. The device clock did not account for time zone and was set to match the time zone in which the patient was expected to reside. For most patients this was the same time zone as the patient’s follow-up clinic. Although the patient’s time zone was not available owing to the de-identification process, the clinic’s time zone was queried and used as a surrogate for the patient device time zone.
The queue arrival date and time was provided by the CareLink network server that received the transmissions. All queue arrival dates and times were in Central Standard (Daylight) Time. The time zone information was used to identify the offset between the time zone’s standard time and Coordinated Universal Time equivalent, and the country was used to identify the offset between the time zone’s standard time and daylight time, as applicable, at the time of the interrogation and arrival. Each parameter was subsequently normalized to a single reference time zone equivalent to Coordinated Universal Time. Time zone adjustment was completed as part of the query step.
Alert transmission identification and exclusion
General criteria for exclusion on both the low end and high end of the duration range were established. All transmissions from CIEDs with an alert transmission time less than 10 minutes were excluded from the analysis to filter out CIEDs with inappropriately set clocks that would skew low or negative. On the high end of the range, individual transmissions with a duration from patient alert event to queue arrival of greater than 15 days were excluded, since the existing 15-day disconnected patient detection mechanism should result in discovery and resolution of a disconnected patient transceiver.
Study objectives
The main objective of this study was to assess patient connectivity through the lens of alert transmission time. Alert transmission time was defined as the duration from when the alert occurred to when the alert was received by the CareLink network. We also analyzed the distribution of alert transmissions over time and the number of alerts per day per patient, and compared alert transmission time between device types and between alert events. The rate of alerts per patient per year was determined by taking the total number of unique alerts for all patients and dividing by the total number of follow-up years, defined as the time from implant date to the date of their last transmission.
Simulation with daily connectivity check
We simulated a 200-patient clinic with or without daily connectivity checks. To determine the relative improvement in connectivity with daily connectivity checks, the following equation was used:
where (i) is the fraction of successful daily alert transmissions per day and (f) is the fraction of patients that are connected at any one time. Roughly 85% of patients are connected at any time, based on a connectivity failure rate of 15% determined from previous literature.1,8 A range of time to resolve each failed connection (9–55 min) and the mean clinic size (n = 5,758) were determined from previous studies.6,8
Statistical analysis
A calculated column was added to identify the transmission with the minimum time to receipt for each transmission. Additional calculated columns were added to enable filtering to 1 event per transmission for transmission-level analysis. Filtering was then used to apply the exclusions identified above. The remaining transmissions were analyzed from a graphical distribution perspective and a statistical perspective using Tibco Spotfire (Spotfire Software, Somerville, MA). The continuous variable (alert transmission time) was expressed as both the mean ± standard deviation, for purposes of statistical comparisons, and as the median, given that the data were not normally distributed. Alert transmission times were compared by device types (cardiac resynchronization therapy pacemakers and defibrillators [CRT-P/D], implantable cardioverter-defibrillators [ICD], implantable pulse generators [IPG]), alert events (21 identified in Table 3), and clinically relevant vs device/lead-related alerts using nonparametric tests owing to the uneven distribution of the data. To compare transmission times between alert events and device, a linear mixed-effects model was used, where alert events and device type were fixed effects and patient was a random effect to help control for variability in patient connectivity. A least squared means test, with adjustment for multiple comparisons, was used to compare alert transmission times between device types. For all tests, a P value ≤ .05 was considered significant. All statistical tests were performed using SAS Studio – Release 9.04.01 (SAS Institute Inc, Cary, NC).
Table 3.
Analysis of alert transmission durations by alert event
| Alert event | # Events | Median receipt | Mean receipt | SD receipt | P value† |
|---|---|---|---|---|---|
| AT/AF Burden | 99,987 | 6 | 12.6 | 25.1 | .2245 |
| Delivery of N Shocks | 35,519 | 6 | 9.7 | 10.5 | .2329 |
| Fast V Response | 25,391 | 6 | 13.7 | 30.2 | .0791 |
| Out of Range Subthreshold Lead Impedance | 23,793 | 6 | 15.3 | 33.3 | .0075 |
| Low Battery Voltage | 17,108 | 5.1 | 8.1 | 10.5 | .7317 |
| High RV Pacing % | 16,049 | 5.9 | 14.5 | 36.1 | .7528 |
| Monitored VT | 11,986 | 6 | 16.1 | 38.5 | .5954 |
| RV Lead Integrity | 6231 | 6 | 9 | 8.7 | .4557 |
| Fluid Detection | 4673 | 11.5 | 32.7 | 62.2 | <.0001 |
| Failed Alert Transmission | 3118 | 148 | 160.3 | 102 | <.0001 |
| RV Capture Management High Threshold | 2580 | 6.1 | 14 | 31.6 | .6071 |
| VF Detect or VF FVT via VF Rx Off | 2098 | 8.9 | 32.1 | 60.2 | <.0001 |
| Atrial Capture Management High Threshold | 1215 | 6 | 16.1 | 41.1 | .6289 |
| Exhaustion of Therapy Set | 1084 | 6 | 10.3 | 12.8 | .1866 |
| Device Circuit Error | 811 | 6.1 | 16.6 | 41.9 | .4849 |
| Lead Noise | 625 | 6.1 | 9.6 | 13 | .3362 |
| LV Capture Management High Threshold | 396 | 5.9 | 13.4 | 35 | .4326 |
| Excessive Charge Time | 107 | 7.8 | 10.3 | 9.6 | .4388 |
| Asynchronous Pacing Mode Since Midnight | 93 | 5.9 | 10.4 | 12.7 | .3782 |
| Charge Circuit Timeout | 45 | 5.7 | 8.1 | 4.9 | .9940 |
| Active Can Off and No SVC | 1 | 5 | 5 | . | .9428 |
AT/AF = atrial tachycardia/fibrillation; EOS = end of service; FVT = fast ventricular tachycardia; LV = left ventricle; RV = right ventricle; Rx = therapy; SVC = superior vena cava; VF = ventricular fibrillation; VT = ventricular tachycardia.
Determined by comparing individual means to the overall mean in a linear effects model (see Supplemental Table 3 for full results.)
Results
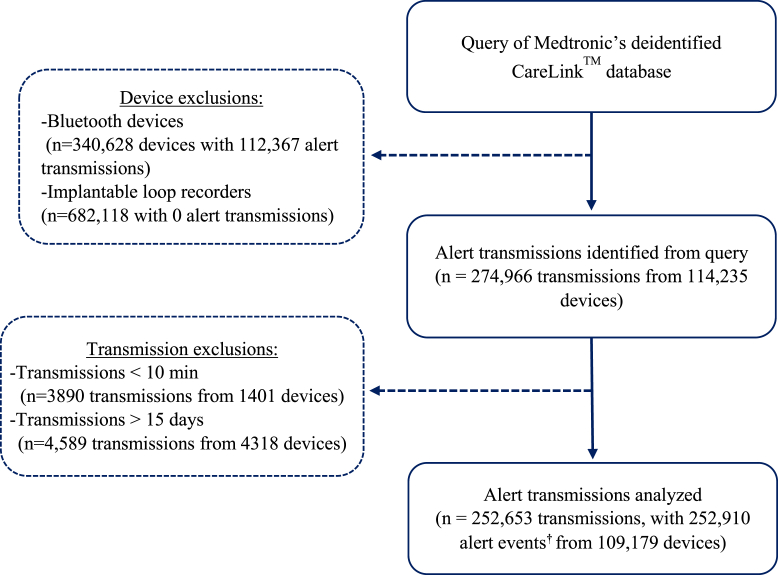
The initial query identified 274,966 alert transmissions from 114,235 devices between the dates of April 5, 2014, and July 16, 2021. After identification and exclusion of outliers, 252,653 alert transmissions containing 252,910 alert events from 109,179 devices remained and were used for the analysis (Figure 1). A breakdown of the CIED model, number of devices, and number of transmissions analyzed for each device model is provided in Supplemental Table 1. The Azure™ XT DR MRI dual-chamber pacemaker was the most frequently represented model in our analysis (Supplemental Table 1). What defines an alert varies by manufacturer and by device type. A detailed list of all the alert event types identified in the query, along with the number/types of devices associated with each alert, is provided in Supplemental Table 2.
Figure 1.
Exclusion/inclusion of alert transmissions used for analysis. Flow chart depicting the processing of data from the CareLink (Medtronic) network. For each step, we provided the number of transmissions and number of devices. †In some cases transmissions contained multiple alert events; the alert event that initiated the other alerts (ie, the most clinically relevant) was used as the identifier in these instances.
Analysis of alert transmission duration from all devices revealed a median time of 6.0 hours and an average time to receipt of 14.8 hours, with a large standard deviation of 34.0 hours (Table 1). Ninety percent of all transmissions were received within 22.2 hours, but this number varied by device type (Table 1). The mean alert transmission time was similar between ICDs and CRT-Ds (13.7 ± 29.4 hours vs 13.5 ± 30.2 hours, P = .0005), reaching significance owing to the large volume of transmissions in each group and between IPGs and CRT-Ps (17.0 ± 40.2 hours vs 17.2 ± 42.5 hours, P = .8156), whereas IPGs and CRT-Ps had significantly longer transmission times than ICDs and CRT-Ds (P < .0001 for all) (Tables 1 and 2). Median alert transmission times were identical across device types, and likely represent a more relevant assessment of transmission time, given the uneven distribution of the data (Table 1). The alert burden was also greater for CRT-P and IPG patients (1.93 and 2.07 alerts/y, respectively) than the burden for ICD and CRT-D patients (0.66 and 0.77 alerts/y, respectively) (Table 1).
Table 1.
Alert transmission characteristics by device type
| Overall | CRT-P | ICD | IPG | CRT-D | |
|---|---|---|---|---|---|
| Total alert transmissions | 252,653 | 15,017 | 87,620 | 74,524 | 75,492 |
| Mean alert transmission time (hours ± SD) | 14.8 ± 34.0 | 17.2 ± 42.5 | 13.7 ± 29.5 | 17.0 ± 40.2 | 13.5 ± 30.2 |
| Median alert transmission time (hours) | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Time to receive 90% of transmissions (hours) | 22.2 | 22.1 | 22.1 | 24.5 | 20.9 |
| Number alerts/year per patient | 0.92 | 1.93 | 0.66 | 2.07 | 0.77 |
CRT-D = cardiac resynchronization therapy defibrillator; CRT-P = cardiac resynchronization therapy pacemaker; ICD = implantable cardioverter-defibrillator; IPG = implantable pulse generator.
Table 2.
Least squared means comparison of alert transmission time between device types
| Comparison | Estimate | Standard error | P value | Adj P† |
|---|---|---|---|---|
| CRT-P vs ICD | 7.6454 | 0.2904 | <.0001 | <.0001 |
| CRT-P vs IPG | 0.3143 | 0.2706 | .2456 | .8156 |
| CRT-P vs CRT-D | 8.2237 | 0.2921 | <.0001 | <.0001 |
| ICD vs IPG | -7.3311 | 0.1753 | <.0001 | <.0001 |
| ICD vs CRT-D | 0.5783 | 0.1477 | <.0001 | .0005 |
| IPG vs CRT-D | 7.9094 | 0.1791 | <.0001 | <.0001 |
Abbreviations as in Table 1.
Adjusted P value to account for multiple comparisons.
Twenty-one unique alert events were identified in our query, with the most common being “AT/AF Burden,” which represented nearly half of all alert transmissions (Supplemental Table 2). In comparison of transmission times by alert event using a linear effects model, 4 alert events were significantly different from the overall mean (Table 3, Supplemental Table 3). Specifically, transmission times for “Fluid Detection” (32.7 ± 62.2 hours), “Ventricular Fibrillation (VF) Detect or Fast Ventricular tachycardia (FVT) via VF Rx Off” (32.1 ± 60.2 hours), and “Failed Alert Transmission” (160.3 ± 102 hours) alerts were all significantly longer than the overall mean (Table 3). Six alert events were common among all devices and shared a similar distribution of transmission times across all 4 device types (Supplemental Figure 1). Alert events defined as clinically relevant had significantly shorter transmission times compared to device/lead-related alerts (12.9 ± 26.8 hours vs 19.4 ± 46.8 hours; P < .0001) (Supplemental Table 3).
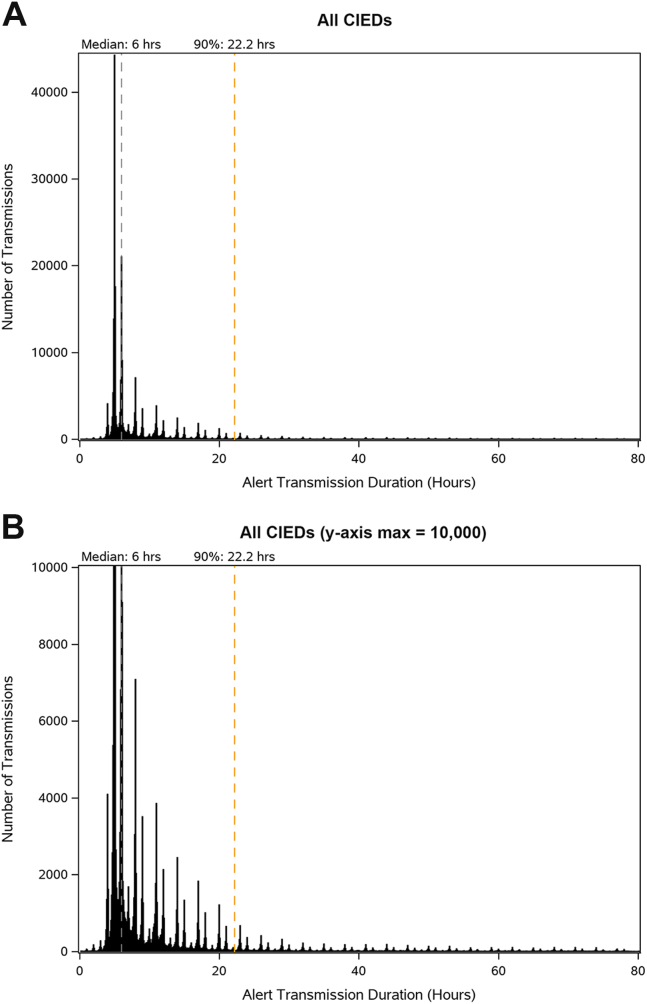
Graphing the distribution of alert transmissions received over time revealed a global peak at 5 hours with a long extinguishing tail (Figure 2A). A detailed view of the distribution of alert transmission times showed a pattern of peaks that is consistent with retry intervals that are automatically conducted by Medtronic patient transceivers (Figure 2B). As expected, when the distribution of alert transmission times are graphed for each device type, the distribution pattern of alert transmission times was similar between ICDs and CRT-Ds and between CRT-Ps and IPGs (Supplemental Figure 2). Lastly, a correlation analysis was performed to determine if implant age affected alert transmission times; and while older implants were associated with faster alert transmission times for CRT-Ds and ICDs, the opposite was true for IPGs and CRT-Ps (Supplemental Figure 3).
Figure 2.
Distribution of alert transmission times for all cardiac implantable electronic devices (CIEDs). A: Distribution of the duration of successful alert transmissions across all CIEDS. B: Detailed view of the distribution of alert transmission durations from (A) with shrunken y-axis (max = 10,000); gray dashed line represents the median time (50th percentile) and yellow dashed line represents the 90th percentile time.
To illustrate the potential impact of daily connectivity checks on clinic workload, we estimated the time needed to resolve disconnected patients on a single day. For an average CIED clinic of 5758 patients, daily connectivity checks would increase the number of successful alert transmissions in a single day by 8.51% (see equation in methods), at the expense of 129–792 staff hours (Table 4).
Table 4.
Simulation with daily connectivity check
| No daily connectivity check | With daily connectivity check | |
|---|---|---|
| Average U.S. clinic size6 | 5758 | 5758 |
| Alerts/day per patient (mean) | 0.0025 | 0.0025 |
| Alert transmissions attempted per day | 14.4 | 14.4 |
| Alert transmissions received per day | 13.09† | 14.20 |
| Failed daily connectivity checks per day1 | 0 | 863 |
| Time to resolve 1 connectivity check failure6,8 | 0 | 9–55 min |
| Time to resolve connectivity check failures per day | 0 | 129–792 h |
Based on 90.9% of all transmissions being received within 24 hours.
Discussion
In this analysis of CIED alerts transmitting to the CareLink network, we show that 90.9% of all alert transmissions were received within 24 hours. This is in line with the 87% success rate of daily transmissions from the TRUST trial using Biotronik’s Home Monitoring system and compares favorably with the 55% transmission success reported in the CONNECT trial.9,10 The CONNECT analysis included patients who never set up their monitor, which may have led to the low success rate compared to our study. The average alert transmission time for Medtronic devices was 14.8 hours, and the IN-TIME trial revealed similar times for Biotronik devices, with 83.1% of alerts being received in 1 day.11 To improve transmission success, Medtronic implements retry logic, and the distribution of alert transmissions in our analysis is consistent with these retry intervals. Alert transmission times for ICDs and CRT-Ds were ∼4 hours shorter than those from IPGs and CRT-Ps. The “Failed Alert Transmission” alert event had the longest mean transmission time at 160.3 hours, which is unsurprising, given that this alert signifies poor connectivity. “Fluid Detection” and “VF Detect or VF FVT via VF Rx Off” alert events had mean times more than twice the overall mean duration, which may be explained by many of those alerts either occurring in a healthcare setting or resulting in inpatient admission where the patient is out of range from their transceiver for an extended period. The median transmission times remained consistent across device types and across most alert events and is likely a better representation of alert transmission performance. For example, “Failed Alert Transmission” would signify connectivity issues that directly affect transmission times in almost all cases, which explains the similarity between the mean and median values. On the other hand, “Fluid Detection” may result in extended time away from the transceiver owing to a hospital visit in a minority of cases, thus the large discrepancy between the mean and median values.
The distribution of alert transmission times in Figure 2 is consistent with 2 overlaid signals. The first signal is a low-frequency distribution of peaks with a mode located at 5 hours and a long extinguishing tail. The second signal is a higher-frequency set of groupings spaced at approximately 1-hour intervals forming roughly triangular distributions around the center points of each peak from the first signal. These signals correlate with the expected retry intervals implemented by Medtronic CIEDs and by the 24950/24952 MyCareLink patient transceiver.
A transmission usually fails at 1 of 2 points: during CIED communication with the patient transceiver, or during transceiver communication with the CareLink network. Chokesuwattanaskul and colleagues showed that 63% of the data transmission delay was due to the MyCareLink transceiver not being in close enough proximity to the device, whereas the remaining 37% was caused by connectivity issues between the transceiver and the network.12 Muniyappa and colleagues found that only 31% of patients were completely adherent to remote monitoring.13 This is especially significant, given that the level of adherence to remote monitoring correlates with patient outcomes.2 Thus, patient compliance may be the single most important factor when it comes to connectivity and alert transmission success.
One method to improve patient compliance and connectivity would be implementing proactive daily connectivity checks, which would inevitably involve detection of false-positive disconnections. A previous study found an 85% patient connectivity rate and observed frequent short gaps in transmissions.1 The time to resolve connectivity issues can be anywhere from 9 minutes up to 55 minutes.1,8 This would place a considerable burden on clinics and require a substantial allocation of resources and training of appropriate staff.6,14 Biotronik’s Home Monitoring system implements a daily transmission schedule, but in a previous report it had a minimal impact on alert transmission time when compared to weekly or biannual schedules used by other manufacturers.15
The advancement of CIED technology is the most reasonable way to enhance the performance of remote monitoring transmissions. Bluetooth technology that connects the CIED directly to an app on the patient’s smart device has shown promise in early studies. App-based technologies like the MyCareLink Smart app significantly improve patient compliance, satisfaction, and transmission success.16, 17, 18 Taking this technology even further, BlueSyncTM completely eliminates external transceivers in favor of the smart device–enabled MyCareLink Heart app.19 The use of the MyCareLink Heart app for CRT-P monitoring increased transmission success from less than 70% with legacy technology to 95% with the app, while Abbott’s Confirm RxTM app showed similar improvements in patient compliance and transmission success.20,21 In a recent study, the MyLATITUDETM from Boston Scientific resulted in 99% patient compliance and 84% of patients feeling reassured by the app.16 Additionally, Boston Scientific’s smartphone myLUXTM app for ILRs showed an 86% daily connectivity rate.22 These results are not surprising, given that patients are more likely to be near their smartphone and bring it with them on vacation as opposed to external transceivers. Although these newer technologies have shown improvements in compliance and connectivity within a clinical trial setting that analyzed mostly scheduled transmissions, their performance in the real world is not well studied. This current study examined real-world alert transmissions as a surrogate for patient connectivity and showed favorable results, even when compared to previous prospective trials where participation and connectivity are more easily effectuated. However, our results still demonstrate issues with patient connectivity, which may partially be resolved with the advancement of Bluetooth, app-based technologies.
Despite the increased development and use of remote monitoring across the cardiac field, a clear definition or benchmark for “good connectivity” is lacking. This is likely due to connectivity relying on several variables, including patient compliance, access to communication infrastructure, patient proximity to the monitor, and age of the technology being used. Even intermittent connectivity is better than no connectivity at all, but an effort must be made to maximize connectivity for all patients.5 Over the next several decades, as technology improves and younger generations begin entering the pool of CIED recipients, app-based monitoring will become more prevalent as demand for such technology increases. However, for patients who currently have a CIED, there should be an emphasis on improving the existing technology and monitoring infrastructure to better reflect the improvements associated with app-based monitoring. In such case, remote monitoring can improve patient outcomes without increasing burden on already overstretched clinics.
Limitations
This study was limited to alert transmissions from wireless-enabled devices of a single manufacturer from a United States database. We excluded ILRs and Bluetooth-enabled CIEDs owing to their differing transmission models and novelty. Legacy telemetry devices are only in contact with the transceiver during an alert condition or scheduled transmission, whereas Bluetooth-enabled devices may be in constant communication with the transceiver. As previously mentioned, focusing on legacy devices more accurately represents the current global landscape of CIED technology. However, these categories of CIEDs should be evaluated in future analyses to compare alert transmission performance for legacy vs Bluetooth technology. An additional limitation was the use of de-identified data at a country level, limiting the specificity with which some adjustments could be made (eg, time zone adjustments may have been inaccurate in some cases). The database was also limited to alert transmissions that were eventually received by the network and thus did not include devices that were permanently disconnected from the monitor.
Conclusion
This study is the first in-depth analysis of alert transmission performance from Medtronic transceivers and presents a different approach to assessing patient connectivity. Overall, alert transmission times were acceptable across device types and alert events. A 90.9% daily success rate of alert transmissions demonstrates a high level of patient connectivity that compares favorably to previous studies. While opportunities exist to improve patient connectivity, daily connectivity checks provide only a modest incremental benefit at the cost of significant clinic burden. Newer technologies focused on Bluetooth, app-based remote monitoring provide a more logical solution to patient connectivity, and further studies should explore the ability of those technologies to improve remote monitoring care for CIED patients.
Acknowledgments
The authors would like to thank Kristen Cattin and Michael Galloway (Medtronic employees) for their expertise and time spent providing insightful review, and Luke Jacobsen (Medtronic employee) for verification of the statistical analyses performed.
Funding Sources
This work was sponsored by Medtronic.
Disclosures
EMC: honoraria, Medtronic; JCG, JL, TRH, DL: employees at Medtronic; TT: nothing relevant to disclose.
Authorship
All authors have met the current ICMJE guidelines for authorship.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.cvdhj.2023.03.003.
AppendixSupplementary data
s
References
- 1.Hindricks G., Taborsky M., Glikson M., et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 2.Varma N., Piccini J.P., Snell J., Fischer A., Dalal N., Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65:2601–2610. doi: 10.1016/j.jacc.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Slotwiner D., Varma N., Akar J.G., et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–e100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Burri H. Is there a future for remote cardiac implantable electronic device management? Arrhythm Electrophysiol Rev. 2017;6:109–110. doi: 10.15420/aer.2017:10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varma N., Love C.J., Michalski J., Epstein A.E. TRUST Investigators. Alert-based ICD follow-up: a model of digitally driven remote patient monitoring. JACC Clin Electrophysiol. 2021;7:976–987. doi: 10.1016/j.jacep.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Seiler A., Biundo E., Di Bacco M., et al. Clinic time required for remote and in-person management of patients with cardiac devices: time and motion workflow evaluation. JMIR Cardio. 2021;5 doi: 10.2196/27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma N., Love C.J., Schweikert R., et al. Automatic remote monitoring utilizing daily transmissions: transmission reliability and implantable cardioverter defibrillator battery longevity in the TRUST trial. Europace. 2018;20:622–628. doi: 10.1093/europace/eux059. [DOI] [PubMed] [Google Scholar]
- 8.Cronin E.M., Ching E.A., Varma N., Martin D.O., Wilkoff B.L., Lindsay B.D. Remote monitoring of cardiovascular devices: a time and activity analysis. Heart Rhythm. 2012;9:1947–1951. doi: 10.1016/j.hrthm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Crossley G.H., Boyle A., Vitense H., Chang Y., Mead R.H. CONNECT Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Varma N., Pavri B.B., Stambler B., Michalski J. TRUST Investigators. Same-day discovery of implantable cardioverter defibrillator dysfunction in the TRUST remote monitoring trial: influence of contrasting messaging systems. Europace. 2013;15:697–703. doi: 10.1093/europace/eus410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husser D., Christoph Geller J., Taborsky M., et al. Remote monitoring and clinical outcomes: details on information flow and workflow in the IN-TIME study. Eur Heart J Qual Care Clin Outcomes. 2019;5:136–144. doi: 10.1093/ehjqcco/qcy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chokesuwattanaskul R., Safadi A.R., Ip R., Waraich H.K., Hudson O.M., Ip J.H. Data transmission delay in Medtronic Reveal LINQTM implantable cardiac monitor: clinical experience in 520 patients. Journal of Biomedical Science and Engineering. 2019;12:391–399. [Google Scholar]
- 13.Muniyappa A.N., Raitt M.H., Judson G.L., et al. Factors associated with remote monitoring adherence for cardiovascular implantable electronic devices. Heart Rhythm. 2022;19:1499–1507. doi: 10.1016/j.hrthm.2022.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Afzal M.R., Nadkarni A., Niemet L., et al. Resource use and economic implications of remote monitoring with subcutaneous cardiac rhythm monitors. JACC Clin Electrophysiol. 2021;7:745–754. doi: 10.1016/j.jacep.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Soth-Hansen M., Witt C.T., Rasmussen M., Kristensen J., Gerdes C., Nielsen J.C. Time until diagnosis of clinical events with different remote monitoring systems in implantable cardioverter-defibrillator patients. Heart Rhythm. 2018;15:1648–1654. doi: 10.1016/j.hrthm.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Lavalle C., Magnocavallo M., Bernardini A., et al. A mobile app for improving the compliance with remote management of patients with cardiac implantable devices: a multicenter evaluation in clinical practice. J Interv Card Electrophysiol. 2022;64:257–264. doi: 10.1007/s10840-022-01207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantini N., Borne R.T., Varosy P.D., et al. Use of cell phone adapters is associated with reduction in disparities in remote monitoring of cardiac implantable electronic devices. J Interv Card Electrophysiol. 2021;60:469–475. doi: 10.1007/s10840-020-00743-9. [DOI] [PubMed] [Google Scholar]
- 18.Tarakji K.G., Vives C.A., Patel A.S., Fagan D.H., Sims J.J., Varma N. Success of pacemaker remote monitoring using app-based technology: does patient age matter? Pacing Clin Electrophysiol. 2018;41:1329–1335. doi: 10.1111/pace.13461. [DOI] [PubMed] [Google Scholar]
- 19.Roberts P.R., ElRefai M.H. The use of app-based follow-up of cardiac implantable electronic devices. Card Fail Rev. 2020;6:e03. doi: 10.15420/cfr.2019.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarakji K.G., Zaidi A.M., Zweibel S.L., et al. Performance of first pacemaker to use smart device app for remote monitoring. Heart Rhythm O2. 2021;2:463–471. doi: 10.1016/j.hroo.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilz R.R., Shaik N., Piorkowski C., et al. Real-world adoption of smartphone-based remote monitoring using the Confirm Rx insertable cardiac monitor. J Innov Card Rhythm Manag. 2021;12:4613–4620. doi: 10.19102/icrm.2021.120806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolen C., Rosman J., Manyam H., et al. Preliminary results from the LUX-Dx insertable cardiac monitor remote programming and performance (LUX-Dx PERFORM) study. Clin Cardiol. 2023;46:100–107. doi: 10.1002/clc.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
s